Abstract
Background
A number of neuroimaging studies on human addicts have revealed that abuse of Methamphetamine (METH) can induce neurodegenerative changes in various brain regions like the cerebral cortex and cerebellum. Although the underlying mechanisms of METH-induced neurotoxicity have been studied, the cellular and molecular mechanisms of METH-induced neurotoxicity remain to be clarified. Previous studies implicated that cannabinoid type 1 receptors (CB1Rs) exert neuroprotective effects on several models of cerebral toxicity, but their role in METH-induced neurotoxicity has been rarely investigated. Moreover, the cerebellum was considered as a potential target to evaluate the effects of cannabinoids on locomotion activity as the CB1Rs are most widely distributed in the molecular layer of cerebellum. Therefore, the present study was carried out to evaluate whether neurodegeneration induced in the cerebellum tissue implicated in locomotion deficit induced by METH.
Methods
In the current study, open field test was used to examine locomotor activity. Using hematoxylin and eosin (H&E) staining, morphology of the cerebellar vermis was investigated after repeated exposure to METH. Then, the effects of CB1Rs antagonist [SR17141A, 10 mg/kg, intraperitoneally (IP)] and CB1Rs agonist [WIN55, 212-2 (WIN), 3 mg/kg] against METH-induced neurodegeneration and locomotor deficit were assessed.
Findings
The results of the present study demonstrated that repeated exposure to METH increased cerebellar degeneration level as compared to the saline and dimethyl sulfoxide (DMSO) groups. In addition, METH-treated rats showed hyperactivity as compared to the saline and DMSO groups. Pretreatment with WIN significantly attenuated neurodegeneration and hyperactivity induced by METH.
Conclusion
The findings of this study provided evidence that CB1Rs may serve as a therapeutic strategy for attenuation of METH-induced locomotor deficits.
Keywords: Neurodegeneration, Methamphetamine, Cerebellum, CB1 receptor
Introduction
Methamphetamine (METH) is an illegal psychostimulant with high addictive potential consumed worldwide.1 The use of METH is a growing public health problem due to its association with neuropsychiatric disorders and sever health complications.2 METH is the second extensively abused drug following cannabinoids3 that is abused by 25 million individuals around the world.2 Several lines of evidence suggest that METH abuse causes neurodegenerative changes in the human brain.4 It has been shown that METH induces excessive release of dopamine (DA), subsequently causing depletion of dopamine in various brain regions including the cerebellum, basal ganglia, and other regions involved in motor activity.5,6 Clinical and basic studies indicate that METH-induced changes in dopaminergic system have been associated with locomotor deficit among rodents.5,6
These locomotor complications might be somewhat related to drug-induced neurotoxic effects, which include damage to the serotonergic and dopaminergic terminals and also neuronal apoptosis in regions involved in motor activity of the brain.6,7 The cerebellum constitutes functional loop pathways with various brain regions involved in addiction including prefrontal cortex (PFC), basal ganglia, and limbic system. In addition, the vermis of the cerebellum connects to dopaminergic neurons in the ventral tegmental area (VTA) and substantia nigra.8 Moreover, dopaminergic neurons from the VTA projected to the vermis, forming a reciprocal midbrain-cerebellar circuit.8 All of these anatomical findings indicated the cerebellum involvement in functional networks affected by METH. Furthermore, evidence has indicated that endocannabinoids (ECBs) are involved in signaling mechanisms in several brain regions related to motor activity.
Cannabinoid receptors (CBRs) are observed throughout the central nervous system (CNS), particularly high densities in the cerebellum and basal ganglia9 and most of the neuropsychological actions of ECBs can be mediated through the CB1 receptors (CB1Rs).10 Indeed, cannabinoids influence both motor and cognitive performances.11,12 In addition, there is reciprocal regulation between the ECBs and dopaminergic systems that indicated neuroprotective action of cannabinoids in locomotor defects.
Evidence highlights the key role of the cerebellum in drug dependency and suggests that the cerebellum may be intertwined with brain processes involved in drug abuse. In addition, the presence of high density CBRs on the axons and terminals of the glutamatergic granule cells of the cerebellum suggests a modulatory role for the cerebellum in motor activities.13,14 The exact mechanisms involved in METH-induced neurotoxicity and also the role of CBRs on such impairments have not been fully clarified. Therefore, the researchers in this study aimed to investigate the capability of activation of CBRs to augment destructive effects of METH on locomotor activity and also neurodegeneration and possible ameliorative impacts of antagonism of CB1R.
Methods
In the current study, male Wistar rats with a weight of 130-150 g at the beginning of the training were used. The animals were housed in a temperature-controlled room with a temperature of 23 ± 1 °C that was maintained on a standard 12-hour light/12-hour dark cycle with food and water available. In addition, the animals were allowed to adapt to the laboratory conditions for at least 1 week before experiments and were handled for 5 minutes per day during this adaptation period. The experiments were carried out between 8.00 AM and 16.00 PM The experimental protocol was approved by the research and ethics committee of the Faculty of Science, Kerman University of Medical Sciences, Kerman, Iran.
METH hydrochloride was purchased from Sigma Aldrich (St Louis, MO, USA) and dissolved in 0.9% saline solution (5 mg/kg). SR141716A [N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide hydrochloride] (10 mg/kg)15,16 was purchased from Tocris (Cookson, UK) and was dissolved in a mixture of sterile saline and dimethyl sulfoxide (DMSO, 9:1 v/v). WIN 55,212-2 (10-de]-1,4-benzoxazin-6-yl]-1-naphtalenylmethanone) mesylate (3 mg/kg). All injections were given intraperitoneally (IP). Fresh solutions of all drugs were prepared in an injection volume of 1 ml/kg prior to the experiments.
The animals were randomly divided into 7 groups (n = 11, in each group): Saline, DMSO, WIN55, 212-2, SR141716A, METH, METH + WIN55, 212-2, and METH + SR141716A. In the Saline, DMSO, and METH groups, animals received saline (0.5 ml/rat) and 30 minutes after received saline or DMSO (0.5 ml/rat) or METH (5 mg/kg) once daily for 3 days. In the WIN55 and 212-2 group, the rats received CB1Rs agonist (WIN55, 212-2, 3 mg/kg). In SR141716A group, the animals received CB1R antagonist (SR141716A, 10 mg/kg) 30 minutes before saline administration once daily for 3 days. The animals in the METH + WIN55 and 212-2 group received WIN55 3 mg/kg 30 minutes before METH administration. Finally in the METH + SR141716 A group, the rats received SR141716A 10 mg/kg 30 minutes before METH administration. All injections were given IP.
The locomotion activity of rats were recorded automatically and then analyzed using Ethovision (version 7, Noldus Information Technology). The apparatus consisted of a square arena (90 × 90 × 30 cm3) made of Plexiglas, and its floor was divided into 16 squares by lines, allowing the definition of central and peripheral areas. At the beginning of the trial, each rat was put in the center of the arena and its activity was recorded for 5 minutes, and the following behavioral parameters were then scored: total distance moved (cm), total duration of mobility (s), immobility (s), and rate of rearing. At the end of each trial, the experimental chamber was thoroughly cleaned.17,18
The association of changes of locomotion activity following repeated exposure to METH alone or in combination with CB1R agonist and antagonist with neurodegeneration induced in cerebellum tissue was evaluated in the present study. 24 hours after last treatment, the animals were deeply anaesthetized under diethyl ether and their brains were quickly removed and vermis of cerebellum was rapidly separated on an ice-cold, fixed in 10% formalin and dehydrated. A cortical coronal slice containing the vermis of cerebellum was embedded in paraffin, serially sectioned (4 μm), and then dewaxed. The slides were dewaxed and boiled (in a 600 W microwave oven) at 120 °C for 10 minutes. The slides were incubated at room temperature for 20 minutes and then washed in phosphate-buffered saline (PBS), exposed to hydrogen peroxide 0.03%, and washed in PBS.19 Tissue blocks were stained with hematoxylin and eosin (H&E) to observe the cerebellum tissue morphology.19 Random 10 visions were chosen for each section. Tissue sections were observed by light microscope (Olympus CX31) connected to a camera. All the fields of interest were counted in each section at a magnification of 400X, giving 2 to 4 × 104 cells per section.20
Statistical analysis was performed using the GraphPad Prism 6. Data analysis using Kruskal-Wallis test indicated an abnormal distribution and expressed as median and interquartile range. P < 0.050 was considered statistically significant (n = 11 in each group, data were expressed as mean ± SEM).
Results
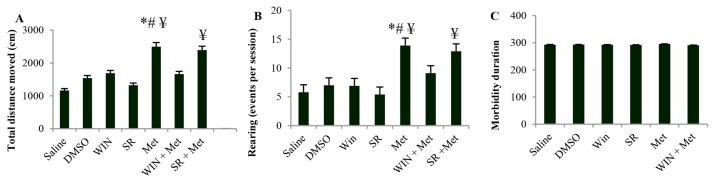
In the present study, open-field test was conducted to examine the effect of METH alone or in combination with CB1Rs agonist/CB1R antagonist on locomotor activity. Horizontal motor activity and vertical activity were expressed as total distance moved and rearing response rate, respectively. Total distance moved and rearing response rate among the animals that received METH significantly increased compared to the saline and DMSO groups. Pretreatment with CB1Rs agonist significantly decreased the METH-induced hyperactivity. Pretreatment with CB1Rs antagonist had no effect on METH-induced hyperactivity as compared to the METH. Administration of the same doses of CB1Rs agonist and antagonist alone had no effect on locomotor activity (Figure 1, A and B). There was no significant difference in mobility duration among the 7 groups (Figure 1, C). Administration of the same doses of CB1Rs agonist and antagonist alone had no effect on total distance moved, rearing response rate, and mobility duration.
Figure 1.
Effect of repeated exposure to Methamphetamine (METH) alone or in combination with cannabinoid type 1 receptor (CB1R) agonist and antagonist on locomotor activity in open field test. Total distance moved (A), rearing response rate (B) and mobility duration (C). *P < 0.001 indicated significant difference from the saline, #P < 0.001 significant difference from the vehicle treated group, and ¥P < 0.001 significant difference from the WIN, SR and WIN + METH treated groups. [WIN 55, 212-2 (WIN), SR141716A (SR) and Methamphetamine (METH), n = 11 in each group, data were expressed as mean ± SEM].
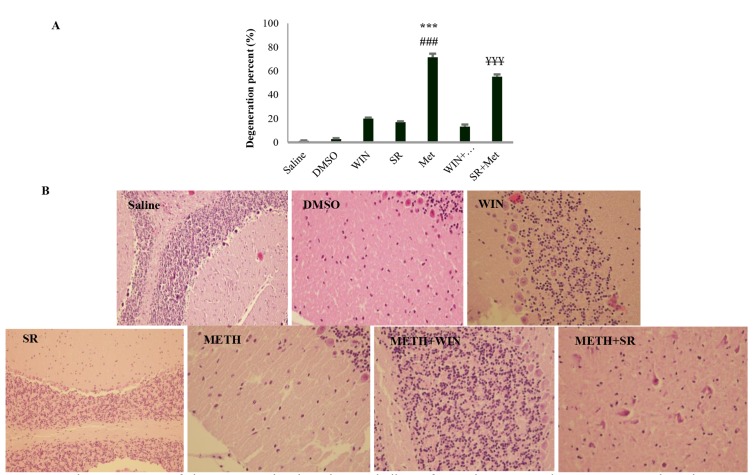
Moreover, the possible role of WIN 55, 212-2, and SR 141716A in METH-induced neurodegeneration was investigated. The study results indicated that repeated exposure to METH significantly increased neurodegeneration in the vermis of cerebellum. METH in combination with CB1Rs agonist (WIN55, 3 mg/kg) 30 minutes before METH administration once daily for 3 days significantly decreased METH-induced neurodegeneration.
Administration of CB1Rs antagonist (SR141716A, 10 mg/kg) in combination with METH had no effect on METH-neurodegeneration. Administration of the same doses of CB1Rs agonist and antagonist alone had no effect on neurodegeneration (Figure 2, A).
Figure 2.
The percentage of degeneration level in the cerebellum of rats that repeated exposure to Methamphetamine (METH) alone or in combination with cannabinoid type 1 receptor (CB1R) agonist [WIN55, 212-2 (WIN)] and antagonist [SR17141A (SR)]. Data describing percentage of degeneration level were not normally distributed, hence were represented as medians with interquartile ranges as a box and maxima/minima as whiskers. (B) represents histological images of neurons from the cerebellar cortex after repeated exposure to METH alone or in combination with CB1R agonist and antagonist. Most neurons from the cerebellum of Saline, DMSO, WIN, and SR-treated rats have normal morphology, however, several degenerated cells can be seen for the METH-treated groups. All the fields of interest were counted in each section at a magnification of 200X in SR group and 400X in other groups, giving 2 to 4 × 104 cells per section. ***P < 0.001 indicated significant difference from the saline, ###P < 0.001 significant difference from the vehicle treated group, and ¥¥¥P < 0.001 significant difference from the METH+WIN treated group.
Figure 2B has vividly illustrated histological images of neurons from the cerebellar cortex after repeated exposure to METH alone or in combination with CB1R agonist and antagonist. Most neurons from the cerebellum of saline, DMSO, WIN, and SR-treated rats had normal morphology, however for the METH-treated groups, several degenerated cells could be seen (Figure 2, B).
Discussion
The present study showed that repeated exposure to METH significantly increased locomotor activity and caused hyperactivity. The study results appeared to approve some previous findings. Similarly, Mendieta et al. have reviewed METH induced hyperlocomotion among rats.21 In contrast to the present results, findings of some previous studies like Curtin et al. have shown that a high dose of METH (30 mg/kg) among mice attenuated locomotor activity in open field test.5 Although the neural mechanisms of METH neurotoxicity have yet to be identified, studies have suggested that functional long-lasting alteration in the neurons of cerebellum can be linked with movement impairments.22,23
Pretreatment with CB1Rs agonist (WIN 55, 212-2) significantly decreased hyperactivity and METH-induced locomotion impairments while CB1R antagonist had no effect on motor impairments induced by METH. In contrast with the results of the present study, DeSanty and Dar reported that intracerebellar injection of CB1R agonist (CP55,940) cause motor incoordination and intracerebral injection of SR141716A nearly eliminated the motor incoordination elicited by CP55,940.24 These controversies are probably due to different factors affecting METH-neurotoxicity including, dose of drug injected, route and interval between injection and length of exposure to drugs.
The present study also showed that repeated exposure to METH and pretreatment with CB1Rs agonist significantly increased and decreased METH-induced neurodegeneration, respectively. Growing bodies of evidence have shown that METH can induce alterations on dopaminergic system markers.23 Curtin et al. reported that METH-induced changes in dopaminergic system have been associated with locomotor impairment among rodents.5 METH-induced changes in dopamine level might influence GABAergic and glutamatergic transmission down-stream and induce motor activity deficits.23 Moreover, it was shown that METH can cause deleterious effects to brain structures like the cortex, that has very little dopaminergic innervation.25 METH is a cationic lipophilic molecule that can diffuse into mitochondria and be sustained by mitochondria26 and mitochondrial damage can contribute to METH-neurodegeneration.7 METH neurotoxicity may cause via increase in extracellular glutamate and activation of N-methyl-D-aspartate (NMDA) receptors.27 METH has been shown to cause disruption of blood brain barrier (BBB) at the levels of the cortex and cerebellum.28
Recent studies have shown microglial activation as an early event in the neurotoxicity cascade that was initiated by METH. Specifically, METH causes strong microglial response in the areas of the brain showing dopaminergic degeneration.29 Microglial activation is essential for neuron survival, however, excessive activation of microglia cause secretion of several cytokine, reactive oxygen, and nitrogen species and prostaglandins (PG) that well result in neurodegeneration.30 In addition, microglial cells might potentiate METH-neurodegeneration via release of superoxide radicals and NO.31 Generally, several lines of evidence demonstrated that several factors, including excitotoxicity, inflammatory responses, hyperthermia, oxidative stress, and mitochondrial dysfunction mediate dopaminergic degeneration and apoptosis induced by METH.4
The present study also showed that pretreatment with CB1Rs agonist significantly decreased METH-induced neurodegeneration and hyperactivity. Neuroprotective mechanism of ECBs may include the ability of ECBs to inhibit the release of the excitatory neurotransmitter, glutamate. It is well known that ECBs inhibit the release of glutamate via triggering the signaling pathways of presynaptic CB1R.32 Some cannabinoid agonists have been reported to have various effects on other receptors compared to CBRs (e.g. dopamine receptors), therefore the co-localization and close relationship between cannabinoid and dopamine receptors seemed to contribute greatly to the protective effects of the CBRs agonists against the METH-induced toxicity.33 Other neuroprotective mechanisms of cannabinoids include attenuated oxidative damage by acting as scavengers of reactive oxygen species and enhancing endogenous antioxidant defenses.23 In overall, these findings suggested that the ECBs modulated neurotoxicity properties of METH and could attenuated METH-toxicity through activation of CB1R that interact with dopaminergic and glutamatergic systems.34,35
Conclusion
Although precise mechanisms of METH induced neurotoxicity have not been yet fully elucidated, the current study was an attempt to investigate the probable interaction of endocannabinoid receptors (ECBRs) and METH in pathophysiology of METH abuse. The findings of the study suggested that CB1Rs play a key role in attenuation of METH induced locomotor impairments and neurodegeneration. In addition, the results may also provide a new way for the development of novel modalities for METH abusers.
Acknowledgments
Funding for this study was provided by Kerman University of Medical Sciences as a grant for the PhD thesis conducted by Effat Ramshini.
Footnotes
Conflicts of Interest
The Authors have no conflict of interest.
REFERENCES
- 1.Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther. 2011;132(3):215–41. doi: 10.1016/j.pharmthera.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jan RK, Kydd RR, Russell BR. Functional and structural brain changes associated with methamphetamine abuse. Brain Sci. 2012;2(4):434–82. doi: 10.3390/brainsci2040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010;58(1):38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, Hanson GR. Methamphetamine/amphetamine abuse and risk of Parkinson's disease in Utah: A population-based assessment. Drug Alcohol Depend. 2015;146:30–8. doi: 10.1016/j.drugalcdep.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tulloch IK, Afanador L, Baker L, Ordonez D, Payne H, Mexhitaj I, et al. Methamphetamine induces low levels of neurogenesis in striatal neuron subpopulations and differential motor performance. Neurotox Res. 2014;26(2):115–29. doi: 10.1007/s12640-014-9456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CW, Ping YH, Yen JC, Chang CY, Wang SF, Yeh CL, et al. Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol Appl Pharmacol. 2007;220(3):243–51. doi: 10.1016/j.taap.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Schweighofer N, Doya K, Kuroda S. Cerebellar aminergic neuromodulation: Towards a functional understanding. Brain Res Brain Res Rev. 2004;44(2-3):103–16. doi: 10.1016/j.brainresrev.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ma YL, Weston SE, Whalley BJ, Stephens GJ. The phytocannabinoid Delta(9)-tetrahydrocannabivarin modulates inhibitory neurotransmission in the cerebellum. Br J Pharmacol. 2008;154(1):204–15. doi: 10.1038/bjp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bortolato M, Frau R, Bini V, Luesu W, Loriga R, Collu M, et al. Methamphetamine neurotoxicity increases brain expression and alters behavioral functions of CB(1) cannabinoid receptors. J Psychiatr Res. 2010;44(14):944–55. doi: 10.1016/j.jpsychires.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Rustay NR, Wahlsten D, Crabbe JC. Influence of task parameters on rotarod performance and sensitivity to ethanol in mice. Behav Brain Res. 2003;141(2):237–49. doi: 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- 12.Arjmand S, Vaziri Z, Behzadi M, Abbassian H, Stephens GJ, Shabani M. Cannabinoids and tremor induced by motor-related disorders: Friend or foe? Neurotherapeutics. 2015;12(4):778–87. doi: 10.1007/s13311-015-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vazquez-Sanroman D, Leto K, Cerezo-Garcia M, Carbo-Gas M, Sanchis-Segura C, Carulli D, et al. The cerebellum on cocaine: Plasticity and metaplasticity. Addict Biol. 2015;20(5):941–55. doi: 10.1111/adb.12223. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez-Franco J, Bartolome-Martin D, Alonso B, Torres M, Sanchez-Prieto J. Cannabinoid type 1 receptors transiently silence glutamatergic nerve terminals of cultured cerebellar granule cells. PLoS One. 2014;9(2):e88594. doi: 10.1371/journal.pone.0088594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbassian H, Esmaeili P, Tahamtan M, Aghaei I, Vaziri Z, Sheibani V, et al. Cannabinoid receptor agonism suppresses tremor, cognition disturbances and anxiety-like behaviors in a rat model of essential tremor. Physiol Behav. 2016;164(Pt A):314–20. doi: 10.1016/j.physbeh.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Abbassian H, Whalley BJ, Sheibani V, Shabani M. Cannabinoid type 1 receptor antagonism ameliorates harmaline-induced essential tremor in rat. Br J Pharmacol. 2016;173(22):3196–207. doi: 10.1111/bph.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabani M, Nazeri M, Parsania S, Razavinasab M, Zangiabadi N, Esmaeilpour K, et al. Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology. 2012;33(5):1314–21. doi: 10.1016/j.neuro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Razavinasab M, Shamsizadeh A, Shabani M, Nazeri M, Allahtavakoli M, Asadi-Shekaari M, et al. Pharmacological blockade of TRPV1 receptors modulates the effects of 6-OHDA on motor and cognitive functions in a rat model of Parkinson's disease. Fundam Clin Pharmacol. 2013;27(6):632–40. doi: 10.1111/fcp.12015. [DOI] [PubMed] [Google Scholar]
- 19.Abbasloo A, Wiens V, Hermann M, Schultz T. Visualizing tensor normal distributions at multiple levels of detail. IEEE Trans Vis Comput Graph. 2016;22(1):975–84. doi: 10.1109/TVCG.2015.2467031. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Kim SJ, Kim GE, Lee WJ, Hong WK, Baik GH, et al. Calcium polystyrene sulfonate induced colonic necrosis in patient with chronic kidney disease. Korean J Gastroenterol. 2010;55(4):261–5. doi: 10.4166/kjg.2010.55.4.261. [DOI] [PubMed] [Google Scholar]
- 21.Mendieta L, Granado N, Aguilera J, Tizabi Y, Moratalla R. Fragment C domain of tetanus toxin mitigates methamphetamine neurotoxicity and its motor consequences in mice. 2016;19(8) doi: 10.1093/ijnp/pyw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, et al. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127(Pt 2):363–70. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- 23.Caligiuri MP, Buitenhuys C. Do preclinical findings of methamphetamine-induced motor abnormalities translate to an observable clinical phenotype? Neuropsychopharmacology. 2005;30(12):2125–34. doi: 10.1038/sj.npp.1300859. [DOI] [PubMed] [Google Scholar]
- 24.DeSanty KP, Dar MS. Cannabinoid-induced motor incoordination through the cerebellar CB(1) receptor in mice. Pharmacol Biochem Behav. 2001;69(1-2):251–9. doi: 10.1016/s0091-3057(01)00539-1. [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158(3):377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 26.Salo R, Fassbender C, Iosif AM, Ursu S, Leamon MH, Carter C. Predictors of methamphetamine psychosis: History of ADHD-relevant childhood behaviors and drug exposure. Psychiatry Res. 2013;210(2):529–35. doi: 10.1016/j.psychres.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res. 2012;1456:14–21. doi: 10.1016/j.brainres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse. 2006;60(7):521–32. doi: 10.1002/syn.20324. [DOI] [PubMed] [Google Scholar]
- 29.Thomas DM, Francescutti-Verbeem DM, Liu X, Kuhn DM. Identification of differentially regulated transcripts in mouse striatum following methamphetamine treatment-an oligonucleotide microarray approach. J Neurochem. 2004;88(2):380–93. doi: 10.1046/j.1471-4159.2003.02182.x. [DOI] [PubMed] [Google Scholar]
- 30.Cadet JL, Jayanthi S, Deng X. Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review. Neurotox Res. 2005;8(3-4):199–206. doi: 10.1007/BF03033973. [DOI] [PubMed] [Google Scholar]
- 31.Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151(2):533–43. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polissidis A, Chouliara O, Galanopoulos A, Naxakis G, Papahatjis D, Papadopoulou-Daifoti Z, et al. Cannabinoids negatively modulate striatal glutamate and dopamine release and behavioural output of acute D-amphetamine. Behav Brain Res. 2014;270:261–9. doi: 10.1016/j.bbr.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Carroll CB, Zeissler ML, Hanemann CO, Zajicek JP. Delta(9)-tetrahydrocannabinol (Delta(9)-THC) exerts a direct neuroprotective effect in a human cell culture model of Parkinson's disease. Neuropathol Appl Neurobiol. 2012;38(6):535–47. doi: 10.1111/j.1365-2990.2011.01248.x. [DOI] [PubMed] [Google Scholar]
- 34.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert GL, Kim HJ, Waataja JJ, Thayer SA. Delta9-tetrahydrocannabinol protects hippocampal neurons from excitotoxicity. Brain Res. 2007;1128(1):61–9. doi: 10.1016/j.brainres.2006.03.011. [DOI] [PubMed] [Google Scholar]