Abstract
Background
We sought to determine the prevalence of pseudobulbar affect (PBA) in a large MS population and assess its association with disability and symptom severity.
Methods
North American Research Committee on MS (NARCOMS) registry participants completed the Center for Neurologic Study-Lability Scale (CNS-LS), a validated 7-question self-report measure of PBA. A composite PBA score was derived from the sum of responses to the 7 questions. We categorized individuals as PBA-positive (PBA[+]) if they had a composite score ≥17 without current depression. Participants also reported their demographic characteristics and their clinical characteristics using Patient-Determined Disease Steps and Performance Scales. We compared clinical and disease characteristics for PBA(+) responders with those without PBA using descriptive statistics and multivariable multinomial logistic regression.
Results
Of the 8,136 responders, 574 (7%) had scores ≥17 on the CNS-LS; however, only 200 (2.5%) individuals had scores ≥17 without comorbid depression, of whom only 22 (11%) reported a diagnosis of PBA. PBA(+) individuals tended to be younger (mean [SD] 53.4 [11.0] vs 57.2 [10.3] years), non-white (13% vs 9%), and have lower socioeconomic status (≤$30,000 annual income: 28% vs 22%). In multivariable models, PBA(+) was associated with increased odds of more severe cognitive impairment (moderate vs mild disability OR: 1.37; 95% CI: 1.01, 1.84).
Conclusions
Our findings suggest that the prevalence of PBA in MS is low, but similar symptoms may co-occur or overlap with depression, highlighting the importance of concomitant assessment of mood when evaluating potential PBA. PBA may be associated with cognitive impairment in people with MS.
Pathologic laughing or crying, or pseudobulbar affect (PBA), is a socially debilitating condition that primarily affects people with neurologic diseases including MS.1–3 Characterized by sudden, brief, exaggerated, and uncontrollable expressions of laughing or crying, PBA is burdensome for affected persons. Emotional displays may be incongruent with mood, such as when anger is expressed as laughter. In some situations, emotional displays may be exaggerated to a contextually appropriate emotional trigger.
Detailed information regarding the prevalence of PBA in MS or its associations with disease characteristics is lacking. Prevalence estimates for PBA in MS are highly variable, ranging from 7% to 52%; several estimates are from studies conducted before 1970.4–8 More recent studies evaluating the prevalence and characteristics of PBA in MS used relatively small samples and/or included relatively homogeneous patient populations, which limit generalizability.4 Furthermore, although PBA is considered pathologically distinct from psychiatric comorbidities like depression, both conditions could result in seemingly similar symptom presentations, and many previous studies did not evaluate mood concomitantly with symptoms of PBA.
Therefore, we evaluated the prevalence and characteristics of the PBA in a large population of people with MS using a validated measure of PBA and including concomitant assessment of internal emotional state. Specifically, we evaluated the association between PBA and other MS symptoms, including cognitive impairment, which we hypothesized would be more severe in individuals with PBA.
Methods
Study population
The study included participants in the North American Research Committee on MS (NARCOMS) registry, which has enrolled >38,000 individuals with self-reported MS. In a previous validation study, >98% of self-reported MS cases were physician confirmed.9 Upon enrollment, participants provide date of birth, race, sex, and age at MS symptom onset. Semiannually thereafter, participants update their demographic information including household income and provide information relating to MS-related disease status, symptoms and disability, and disease-modifying therapy use in the past 6 months.
Standard protocol approvals, registrations, and patient consents
At the time of the survey, the NARCOMS registry was approved by the Institutional Review Board at the University of Alabama at Birmingham. Participants agree to the use of their information for research.
MS status
Registry participants report disability using patient-determined disease steps (PDDS), which strongly correlates with the physician-assessed Expanded Disability Status Score (r = 0.78).10 Participants also report impairment in 8 domains (mobility, hand function, vision, fatigue, cognition, bladder/bowel, sensory, and spasticity) using the validated Performance Scales (PS)11 and in 3 additional domains (depression, pain, and tremor) using other validated “functionality” scales.12–14 Each domain is rated from 0 (normal) to 5 (total disability), except for mobility (which is rated from 0 to 6). Participants reporting scores ≥2 on the depression scale were classified as clinically depressed; a previous validation study demonstrated high sensitivity (0.87) and specificity (0.92) for depression when applying this cut-point vs a score ≥21 on the Clinical Epidemiology Studies Depression Scale; the positive predictive value for a lifetime history of physician-diagnosed depression was 86.9%.14 The depression instrument demonstrates adequate convergent and divergent validity. Another study compared the depression instrument with the Beck Depression Inventory II and showed 70% sensitivity and 90% specificity for detecting severe depression.15 Greater cognitive impairment on the PS cognition scale correlates with lower processing speed measured using a version of the Symbol Digit Modalities Test (r = −0.33; 95% CI: −0.49 to −0.16).16 Participants also report whether they had experienced (1) a relapse or (2) whether they felt that MS symptoms were “gradually worsening” in the past 6 months.
Assessment and characterization of PBA
In the Fall 2012 survey, participants completed the Center for Neurologic Study-Lability Scale (CNS-LS),17 a validated self-report measure of pathologic laughing and crying that consists of 7 questions, answered using a 5-point Likert scale, rated from 1 (“Applies Never”) to 5 (“Applies Most of the Time”). Responses across questions were summed to derive a total PBA score. In a previous study, scores ≥17 (without comorbid depression) correctly diagnosed patients with PBA 89% of the time, with high sensitivity (0.94) and specificity (0.83).18 Depression and PBA can co-occur17; however, given that we were using self-reported data, we were conservative for our primary analysis and categorized PBA-positive (PBA[+]) as those individuals who reported a composite score ≥17 on the CNS-LS questionnaire and who did not report being currently depressed (scores <2 on depression functionality scale) for our primary analyses. We also considered a relaxed definition of PBA(+) as those who reported a score ≥17 on the CNS-LS questionnaire, regardless of depression status. In the original validation of the CNS-LS conducted in patients with amyotrophic lateral sclerosis (ALS), investigators did not exclude depressed individuals and found a modest association between CNS-LS and depression severity scores, which suggests that overall CNS-LS scores may at least partially separate PBA from depression in neurologic disease populations.17 Therefore, we considered this relaxed definition of PBA (CNS-LS ≥ 17, any depression status) as the upper limit of PBA prevalence in our sample. We also considered other non-MS-specific5 cut-points for CNS-LS scores to support comparisons with other studies, by defining PBA(+) as those with CNS-LS scores ≥13 and ≥21. Participants also reported whether a physician had ever diagnosed them with PBA and the year of PBA diagnosis; PBA treatment was not captured.
Statistical analysis
We excluded responders who did not report physician-confirmed MS (n = 181) and those with >15% missing responses on the CNS-LS (n = 378). For included individuals with missing values (n = 46), we imputed responses using multiple imputation by chained equations for 5 imputations.19
We summarized sample characteristics using mean (SD), median (interquartile range [IQR]), and frequency (percent) across the distribution of CNS-LS scores and by physician-diagnosed PBA. We tested for differences in the distribution of responses to CNS-LS questions and the distribution of composite CNS-LS scores (overall, subscale for pathologic sadness/crying, subscale for pathologic happiness/euphoria) by depression status (nondepressed vs depressed) using a Wilcoxon rank-sum test.
We assessed the association between the PDDS, subscales of the PS and NARCOMS functionality scales for pain and tremor with CNS-LS-defined PBA(+) or physician-diagnosed PBA using proportional odds models adjusted for age (linear term), sex, disease duration (approximate quartiles), income as a measure of socioeconomic status (SES; <$30,000, $30,000–$50,000, $50,000–$100,000, >$100,000), and race (white, non-white). Primary analyses adjusted for income rather than education as a measure of SES because income tends to better approximate SES in people with MS20; however, sensitivity analyses also evaluated models adjusted for education as a proxy for SES. Separate analyses also adjusted for disease-modifying therapy use (yes, no). We assessed deviations from proportionality (nonparallel lines) for included covariates graphically and using likelihood ratio tests. When testing of this assumption indicated evidence of a potential violation, we conducted sensitivity analyses allowing for nonproportionality (for violating covariates) using partial-proportional odds models. We fit similar multinomial regression models assessing the association between CNS-LS-defined PBA(+) and disability severity by categorizing PDDS as mild (<2), moderate (2–5) or severe (≥6). We also assessed whether CNS-LS-defined PBA(+) was associated with prevalence of relapse in the previous 6 months (binary; yes, no) and gradual feeling of symptoms worsening (binary; yes, no) using a logistic regression model (adjusted for age, sex, duration of symptoms, and income). All analyses were computed separately within each imputation, and results were pooled using Rubin's rules.19
Statistical analyses were conducted using SAS Version 9.4 (Cary, NC) and R Version 3.2.2 (r-project.org/).
Data availability
The data sets generated and analyzed during this study are held by the NARCOMS registry (narcoms.org).
Results
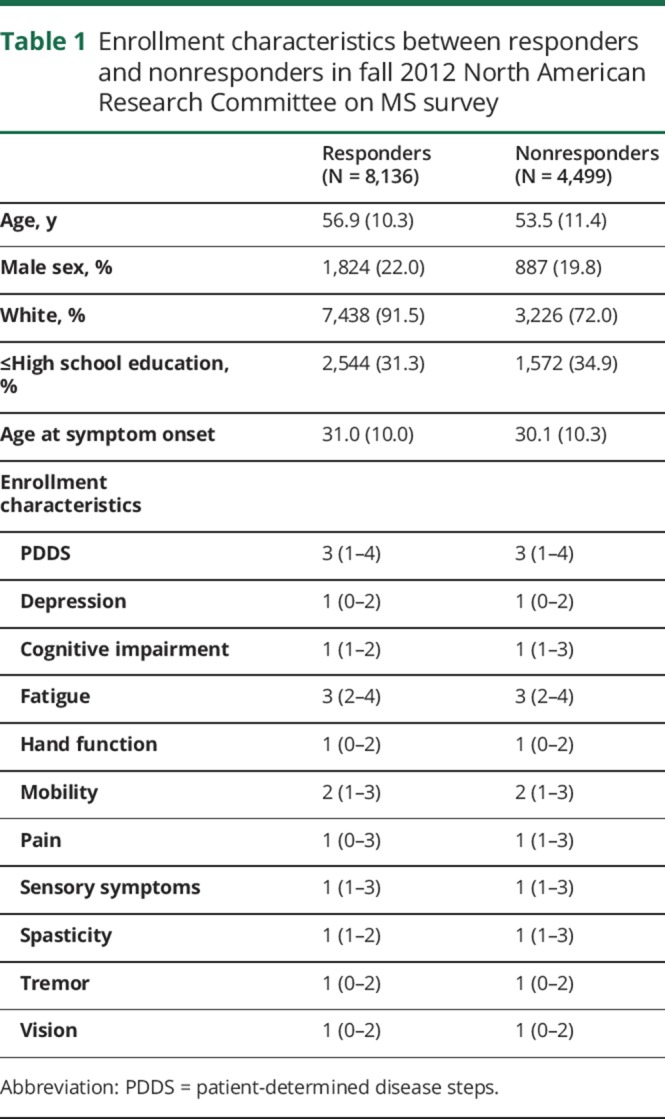
Of 12,635 active registry participants, 8,597 (68%) responded to the survey and 8,136 met the inclusion criteria. Relative to nonresponders, responders tended to be older (table 1; 56.9 [SD: 10.3] vs 53.5 [11.4] years), were more likely to be male (22% vs 19%), white (92% vs 72%), and to be more educated (beyond high school education: 68.7% vs 65.1%). Responders and nonresponders were similar with respect to severity of disability, depression, and cognitive impairment.
Table 1.
Enrollment characteristics between responders and nonresponders in fall 2012 North American Research Committee on MS survey
CNS-LS scores
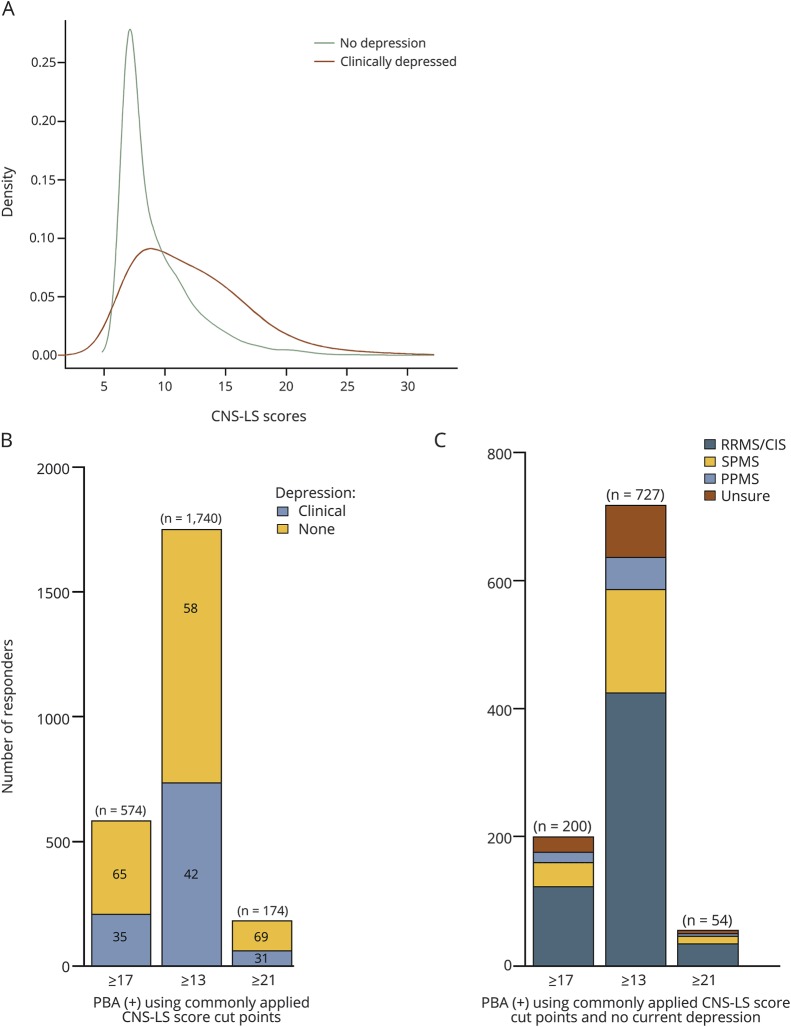
The median (IQR) CNS-LS score was 9 (7–12). The distribution of CNS-LS scores differed between those who did (median [IQR] CNS-LS Score: 11 [9–15]) and did not meet criteria for depression (8 [7–10]; p < 0.001; figure 1A). This difference was particularly pronounced when restricting to CNS-LS responses corresponding to aspects of pathologic crying and sadness and was less notable for the CNS-LS responses corresponding to aspects of pathologic happiness and euphoria (figure e-1, links.lww.com/CPJ/A49; for CNS-LS sadness/crying subscale: median [IQR] for depressed vs nondepressed; 6 [4–8] vs 3 [3–5]; p < 0.0001; for CNS-LS happiness/euphoria subscale: median [IQR] for depressed vs nondepressed; 4 [4–7] vs 4 [4–5]; p < 0.0001).
Figure 1. Distribution of Center for Neurologic Study-Lability Scale scores in the NARCOMS population.
(A) Distribution of CNS-LS scores among North American Research Committee on MS participants between depressed vs nondepressed individuals. (B) Number of responders with CNS-LS score cut-points that are commonly applied to determine pseudobulbar affect (PBA) by depression status. For CNS-LS score cut-points of ≥17, 200/574 report not currently being depressed, for CNS-LS score cut-points of ≥13, 727/1,740 report not currently being depressed, and for CNS-LS score cut-points of ≥21, 54/174 report not currently being depressed. (C) Responders meeting criteria for pseudobulbar affect with commonly applied cut-points (CNS-LS scores ≥17 [n = 200], ≥13 [n = 727], and ≥21 [n = 54] and no depression) by disease subtype.
Prevalence of PBA (PBA[+])
Using CNS-LS responses, 574 (7%) participants reported scores ≥17, of whom 200 (34%) met our conservative criteria for PBA(+), because they also were not currently depressed (figure 1B), yielding a prevalence of 2.4%. Using other non-MS-specific cut-points, 1,740 (21%) participants reported CNS-LS scores ≥13 and 154 (2%) reported scores ≥21. Of the 1,740 with CNS-LS scores ≥13, 727 (42%) did not report current depression, and of the 174 with CNS-LS scores ≥13, 54 (31%) did not report current depression. Most PBA(+) participants had relapsing-remitting MS, regardless of the CNS-LS cut-point applied (figure 1C). As a CNS-LS score ≥17 was developed and validated specifically in an MS-population,18 we subsequently present findings using this specific cut-point to define the presence of PBA.
Characteristics of PBA(+) participants
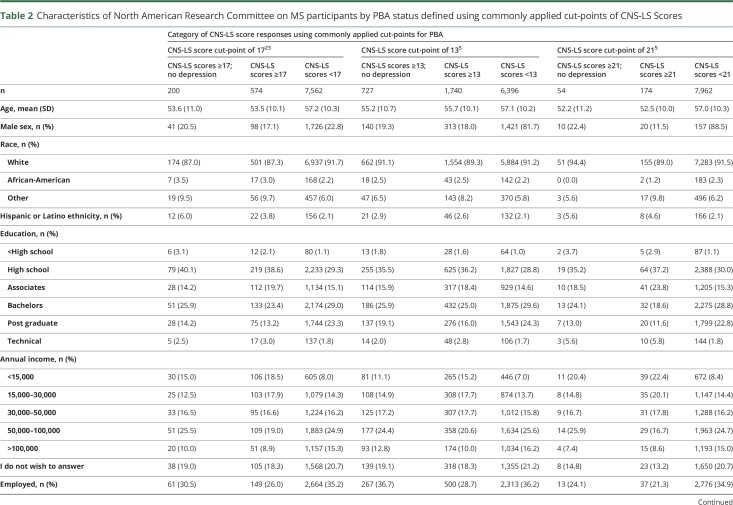
Respondents meeting the more stringent criteria for PBA(+) (CNS-LS scores ≥17 and no depression; n = 200) tended to be younger, non-white and have less education and income compared with non-PBA(+) responders and an earlier age at onset (table 2). Disease-modifying therapy use (overall or specific classes) was not associated with PBA(+). Demographic and MS-characteristics were generally similar when we applied other cut-points in CNS-LS scores to define PBA(+) (table 2).
Table 2.
Characteristics of North American Research Committee on MS participants by PBA status defined using commonly applied cut-points of CNS-LS Scores
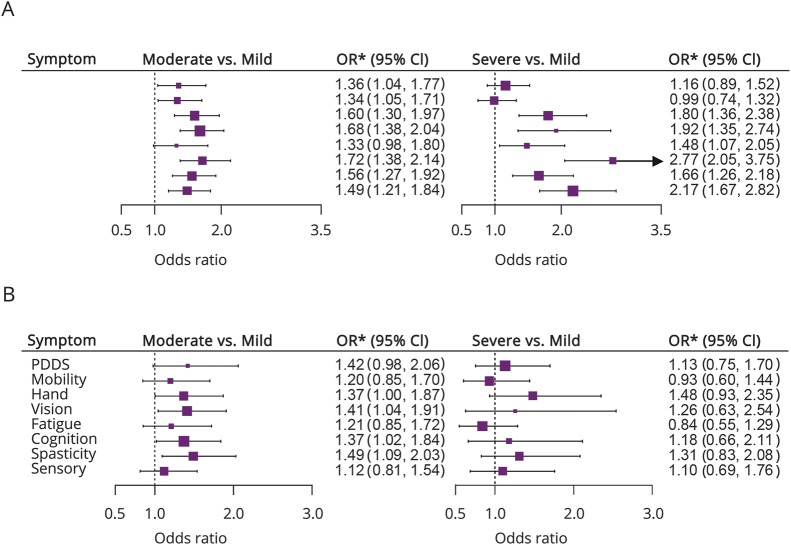
Those meeting the more stringent criteria for PBA(+) participants (CNS-LS scores ≥17 and no depression; n = 200) had on average greater disease severity across a range of symptoms (figure 2; table e-1, links.lww.com/CPJ/A50). Consistent with our hypothesis, PBA(+) was associated with increased odds of moderate vs mild self-reported cognitive impairment (OR: 1.37; 95% CI: 1.01–1.84) in multivariable models. It was also associated with an adjusted increased prevalence odds of moderate vs mild impairment in vision (OR: 1.36; 95% CI: 1.04–1.91), hand function (OR: 1.37; 95% CI: 1.01–1.87), and spasticity (OR: 1.49; 95% CI: 1.10–2.03). PBA(+) was also associated with self-reported relapse in the previous 6 months (1.74; 95% CI: 1.26–2.41) and marginally with an increased risk of moderate vs mild disability, as measured by PDDS (OR: 1.42; 95% CI: 0.98–2.03). Results assessing the association between PBA(+) defined using this strict criteria and severe vs mild symptom impairment were generally qualitatively similar in direction with OR for moderate vs mild symptom impairment but did not attain statistical significance, possibly due to small numbers within the severe categories. Similar findings were also observed for 1-unit increases in MS symptom scales from proportional odds models, and in models additionally adjusted for disease-modifying therapy use (data not shown). PBA(+) was not associated with a feeling of gradual symptom worsening in the past 6 months (OR: 1.05; 95% CI: 0.78–1.42).
Figure 2. Association between pseudobulbar affect (PBA) and disease and MS symptom Severity.
(A) PBA is defined as the CNS-lability scale (CNS-LS) score ≥17 (574 cases). (B) PBA is defined as a CNS-LS score ≥17 and no current depression is defined (200 cases of PBA). *PDDS, ORs, and 95% CIs are estimated from a multinomial model adjusted for age, symptom duration (quartiles), sex, race (white, African-American, other), and income (<$30,000, $30,000-$50,000, ≥$50,000, I prefer not to answer). CI = confidence interval; OR = odds ratio; PDDS = patient-derived disease steps.
The observed associations strengthened when we relaxed criteria for PBA(+) and did not exclude those with depression from our definition (CNS-LS ≥ 17 and any depression status; n = 574), most notably for tests comparing severe vs mild symptom severity. In multivariable models additionally adjusted for depression severity, PBA(+) was associated with increased odds of severe vs mild self-reported impairment in cognition (figure 2; OR: 2.77; 95% CI: 2.04–3.75), fatigue (OR: 1.48; 95% CI: 1.07–2.05), hand function (OR: 1.80; 95% CI: 1.36–2.38), vision (OR: 1.92; 95% CI: 1.34–2.75), sensory (OR: 2.17; 95% CI: 1.68–2.82), and spasticity domains (OR: 1.66; 95% CI: 1.26–2.18). The observed associations for PBA(+) using the relaxed criteria (CNS-LS ≥ 17 and any depression status; n = 574), were stronger when we did not adjust for depression severity, highlighting the importance of accounting for affective state (table 3).
Table 3.
Characteristics of North American Research Committee on MS participants ever-diagnosed with PBA by a physician
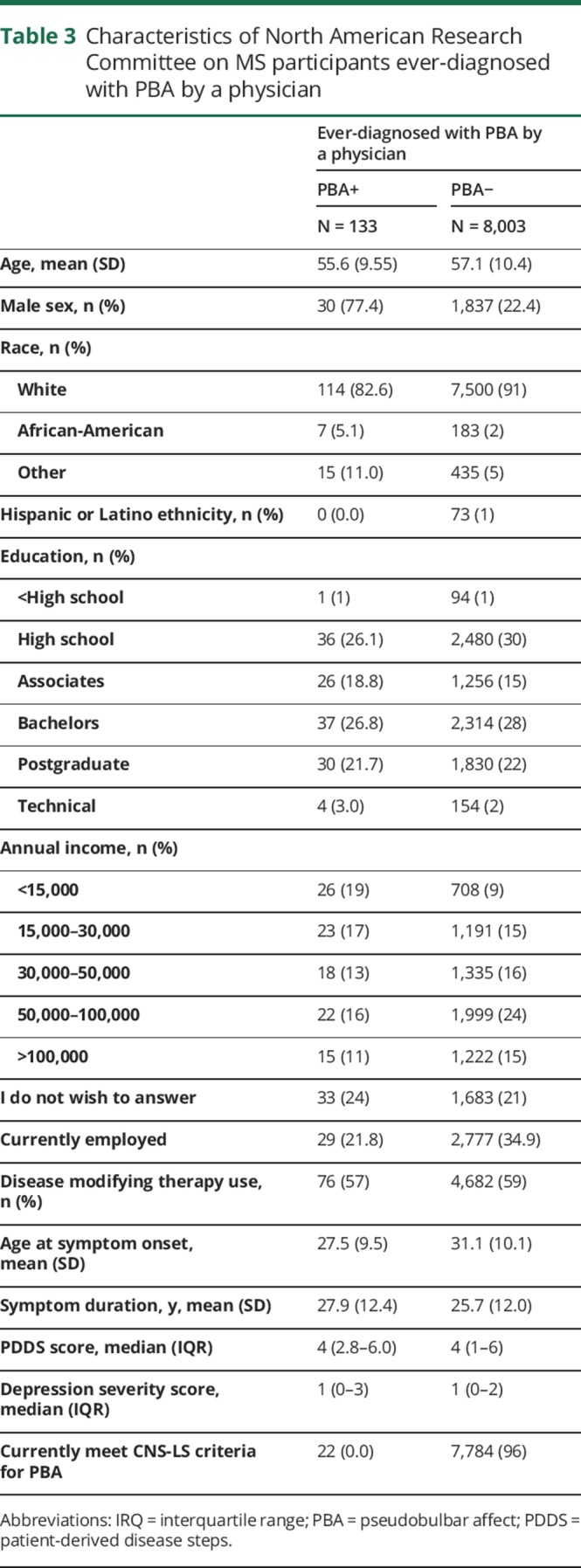
Physician-diagnosed PBA
Physician-diagnosed PBA was reported by 133 responders (figure e-2, links.lww.com/CPJ/A49). Responders with physician-diagnosed PBA were more likely to be non-white and have lower SES compared with responders without physician-diagnosed PBA (table 3). Those with physician-diagnosed PBA were, on average, aged 45.7 (9.8) years at PBA diagnosis and reported having symptoms related to PBA for a mean (SD) 9.1 (10.1) years at the time of survey completion. Seventeen percent (22 of 133) of these individuals reported a substantial current burden of CNS-LS symptoms (CNS-LS scores ≥ 17 and no depression).
Discussion
Using the CNS-LS alone without consideration of depression, 7.1% of participants met criteria on the CNS-LS for PBA. After excluding individuals with current depressed mood, the prevalence was 2.4%. Even with that conservative estimate, only 11% of those responders reported having been diagnosed with PBA by a physician. Notably, we also found an extensive overlap between individuals who reported high CNS-LS scores and those reporting current depression. The evaluation of PBA in a large sample of patients with MS with diverse demographic and disease characteristics and its assessment of the association between PBA and global disability and common MS symptoms such as fatigue or pain is novel. PBA was more prevalent in younger individuals and in those of non-white descent and with lower SES. The latter 2 groups are at an increased risk of depression and disability progression.20–24 Also, PBA was associated with self-reported cognitive difficulties, greater disability, spasticity, and vision-related symptoms.
Clinician-adjudication remains the gold-standard for the diagnosis of PBA. Formal diagnosis of PBA requires individuals to display a sudden loss of emotional control over the past month and demonstrate emotional responses to nonspecific stimuli that are incongruent with his or her mood state.25 Other questionnaires including the Pathological Laughing and Crying Scale (PLACS) also evaluate symptoms of PBA.26 In contrast to the CNS-LS, the PLACS is interviewer-administered. Feinstein et al.4 applied the PLACS survey and clinician adjudication in 159 people with MS. The prevalence of PBA was 10% and PBA was associated with a lower IQ, consistent with our findings of an association between PBA and self-reported cognitive impairment. Our findings are also consistent with reported associations between PBA and cognitive impairment in stroke, traumatic brain injury, and ALS.27–29
We found a strong association between high CNS-LS scores and depression, which was particularly strong among CNS-LS questions addressing pathologic crying/sadness. This suggests that a careful evaluation of mood is integral to evaluating symptoms potentially related to PBA, especially because therapeutic options exist for both conditions. It also highlights the difficulty in disentangling depression and PBA, particularly in an individual with known depression. Because depression is common in MS,14 a lack of systematic neuropsychiatric evaluation at the time of PBA assessment may have contributed to highly variable estimates of PBA prevalence in previous studies, which relied on questionnaires and did not account for comorbid depression.5
Assessment of the prevalence and characteristics of PBA in other neurologic conditions have also applied the CNS-LS; however, a uniform threshold for PBA across neurologic conditions is lacking. For example, a score ≥13 accurately predicted neurologists' clinical diagnosis in 82% of patients with ALS21 with relatively high specificity (81%); however, specificity was substantially lower (55%) in people with MS.22 Using CNS-LS scores ≥17 in people with MS, sensitivity (94%) and specificity (83%) were found to be markedly improved.22 Thus, differences in cutoffs applied to determine the presence or absence of PBA in previous studies of people with MS have likely contributed to the variable estimates of PBA prevalence. For example, the PBA Registry Series (PRISM)5 was a large sample of 5,290 clinic patients diagnosed with neurologic conditions including ALS, Alzheimer disease, Parkinson disease, stroke, traumatic brain injury, and MS that evaluated PBA prevalence in these populations. Using CNS-LS scores ≥13 and ≥21, the prevalence of PBA in MS patients was 45.8% and 12.0%, respectively; the cutoff ≥17 was not applied. No evaluation of neuropsychiatric symptoms was conducted in the PRISM study, so some responders may have been depressed without PBA (i.e., false positives).
A limitation of this study is the lack of information on treatment of PBA. Successful treatment and corresponding reduction in symptoms (and lower CNS-LS scores) may have resulted in an underestimation of PBA prevalence and contributed to the incongruity between PBA participants reporting physician-diagnosed PBA and current CNS-LS. However, the prevalence of physician-diagnosed PBA was low in our study, which suggests that the relatively low prevalence of PBA in our sample is not driven by treatment-alleviated PBA. Furthermore, we excluded individuals from our conservative estimates of PBA prevalence who report current depression. As such, our study did not quantify symptoms of PBA in which emotional responses are partially concordant with mood but exaggerated. This limitation may have also underestimated PBA prevalence. Using CNS-LS scores ≥17 and without considering depression status, PBA prevalence was 7%; therefore, this figure may represent an upper limit of PBA in our population because CNS-LS and depression severity scores are not perfectly correlated. Because the sensitivity of the NARCOMS depression scale in identifying individuals with severe depression is imperfect, those individuals may have been misclassified, thus meeting our more stringent criteria of PBA. However, because our study reports a relatively low prevalence of PBA (compared with previous studies), it is unlikely that a large portion of individuals with severe depression (and not PBA) were misclassified. We also lacked data regarding depression treatments, which may alleviate PBA and depressive symptoms and may have led to an underestimation of PBA prevalence. Because our study was cross-sectional and relied on self-report, we could not evaluate whether PBA is associated with cognitive impairment accrual. Also, MRI data were not available; so we could not evaluate the association between neuroimaging findings and PBA. Survey responders were largely older, white with long-standing MS, so findings may differ in other MS populations. However, recent prevalence studies suggest that demographic characteristics of our sample are consistent with those of a substantial portion of people living with MS in the United States.30 Strengths of our study include its large well-characterized sample with diverse disease characteristics. Furthermore, we also carefully evaluated the association between PBA and several burdensome MS symptoms while considering neuropsychiatric status.
PBA is a socially burdensome condition associated with reduced quality of life in individuals with MS. PBA is relatively rare but is also associated with greater disease severity in MS.3,5
Acknowledgment
NARCOMS is supported in part by the Consortium of Multiple Sclerosis Centers (CMSC) and the Foundation of the CMSC. Performance Scales Questions 9–16, Copyright Registration Number/Date: 233 TXu000743629/1996-04-04; assigned to DeltaQuest Foundation, Inc., effective October 1, 2005. U.S. 234 Copyright law governs terms of use.
Author contributions
K.C. Fitzgerald contributed to conception and design of the study, analysis of data and drafting the manuscript and figures. A. Salter and T. Tyry contributed to conception and design of the study, acquisition and analysis of data, and to critical review of the manuscript. R.J. Fox contributed to conception and design of the study, acquisition of data, and to critical review of the manuscript. G. Cutter contributed to conception and design of the study, acquisition and analysis of data, and to critical review of the manuscript. R.A. Marrie contributed to conception and design of the study, acquisition and analysis of data and drafting the manuscript and figures.
Study funding
NARCOMS is supported in part by the Consortium of Multiple Sclerosis Centers (CMSC) and The Foundation of the CMSC. This study was supported by a fellowship grant to K.C.F. from the CMSC's NARCOMS postdoctoral fellowship award.
Disclosure
K.C. Fitzgerald receives research funding the National MS Society and the Consortium of MS Centers in the form of postdoctoral fellowships. A. Salter and T. Tyry report no disclosures. R.J. Fox serves on scientific advisory boards for Actelion, Biogen Idec, and Novartis; serves on the editorial boards of Neurology and Multiple Sclerosis Journal; receives publishing royalties for Multiple Sclerosis and Related Disorders (Demos Medical, 2013); serves as a consultant for Actelion, Biogen, EMD Serono, Genentech, Novartis, and Teva; and receives research support from Novartis, Biogen, National MS Society, and Consortium of MS Centers. G. Cutter serves on scientific advisory boards for AMO Pharmaceuticals, Apotek, Gilead Pharmaceuticals, Horizon Pharmaceuticals, Modigenetech/Prolor, Merck, Merck/Pfizer, Opko Biologics, Neurim, Sanofi-Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva pharmaceuticals, NHLBI (Protocol Review Committee), and NICHD (OPRU oversight committee); has received speaker honoraria from Consortium of MS Centers and Teva; serves on the editorial boards of Multiple Sclerosis and Alzheimer's & Dementia: Translational Research & Clinical Interventions and as statistical consulting reviewer for Journal of the American Society of Nephrology; is President of Pythagoras, Inc. a private consulting company located in Birmingham AL; serves as a consultant for Atara Biotherapeutics, Bioeq GmBH, Cerespir Inc, Consortium of MS Centers (grant), Genzyme, Genentech, Innate Therapeutics, Jannsen Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Nivalis, Novartis, Opexa Therapeutics, Roche, Savara Inc., Somahlution, Teva pharmaceuticals, Transparency Life Sciences, and TG Therapeutics; works on studies funded to the Consortium of MS Centers subcontracted for analysis of NARCOMS Registry; receives/has received research support from NIH (NINDS, NIAID, NHLBI, NICHD, NIA, NIAMS, NIDDK), Consortium of MS Centers (CMSC), US Department of Defense, UAB/UCSD, Children's Hospital (Boston), and Myasthenia Gravis Foundation of America; receives stocks/stock options from Pythagoras, Inc.; and has participated in a medico-legal case. R.A. Marrie serves on the editorial boards of Neurology and Multiple Sclerosis Journal; has conducted clinical trials for Sanofi-Aventis; and receives research support from CIHR, Research Manitoba, the Waugh Family Chair in Multiple Sclerosis, Multiple Sclerosis Society of Canada, the National Multiple Sclerosis Society, Multiple Sclerosis Scientific Foundation, the Consortium of Multiple Sclerosis Centers, and Crohn's and Colitis Canada. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Prevalence of pseudobulbar affect (PBA) in people with MS is relatively low.
→ There is a strong overlap between PBA and depression in people with MS.
→ PBA is generally associated with greater disease severity and may be associated with more severe cognitive impairment.
References
- 1.Parvizi J, Arciniegas DB, Bernardini GL, et al. Diagnosis and management of pathological laughter and crying. Mayo Clin Proc 2006;81:1482–1486. [DOI] [PubMed] [Google Scholar]
- 2.Miller A, Pratt H, Schiffer RB. Pseudobulbar affect: the spectrum of clinical presentations, etiologies and treatments. Expert Rev Neurother 2011;11:1077–1088. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag 2013;9:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feinstein A, Feinstein K, Gray T, O'Connor P. Prevalence and neurobehavioral correlates of pathological laughing and crying in multiple sclerosis. Arch Neurol 1997;54:1116–1121. [DOI] [PubMed] [Google Scholar]
- 5.Brooks BR, Crumpacker D, Fellus J, Kantor D, Kaye RE. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One 2013;8:e72232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pratt RT. An investigation of the psychiatric aspects of disseminated sclerosis. J Neurol Neurosurg Psychiatry 1951;14:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langworthy OR, Kolb LC, Androp S. Disturbances of behavior in patients with disseminated sclerosis. Am J Psychiatry 1941;98:243–249. [Google Scholar]
- 8.MSAA survey: Pseudobulbar affect in multiple sclerosis (2010). Available at: http://www.mymsaa.org/PDFs/PBA_MSAA_Survey_Report_Final_0112.pdf. Accessed March 29, 2018. [Google Scholar]
- 9.Marrie RA, Cutter G, Tyry T, Campagnolo D, Vollmer T. Validation of the NARCOMS registry: diagnosis. Mult Scler 2007;13:770–775. [DOI] [PubMed] [Google Scholar]
- 10.Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol 2013;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz CE, Vollmer T, Lee H. Reliability and validity of two self-report measures of impairment and disability for MS: North American Research Consortium on Multiple Sclerosis Outcomes Study group. Neurology 1999;52:63–70. [DOI] [PubMed] [Google Scholar]
- 12.Marrie RA, Goldman M. Validation of the NARCOMS registry: tremor and coordination scale. Int J MS Care 2011;13:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrie RA, Cutter G, Tyry T, Hadjimichael O, Vollmer T. Validation of the NARCOMS Registry: pain assessment. Mult Scler 2005;11:338–342. [DOI] [PubMed] [Google Scholar]
- 14.Marrie RA, Cutter G, Tyry T, Campagnolo D, Vollmer T. Validation of NARCOMS depression scale. Int J MS Care 2008;10:81–84. [Google Scholar]
- 15.Devitt M, Foley FW, Miller RJ, et al. Use of single screening question from NARCOMS to detect severe depression in multiple sclerosis. Int J MS Care 2008;10:11–13. [Google Scholar]
- 16.Fitzgerald KC, Salter A, Tyry T, Fox RJ, Cutter G, Marrie RA. Validation of the SymptoMScreen with objective and clinician-assessed outcomes. Paper presented at: Consortium of Multiple Sclerosis Center Annual Meeting; May 21, 2018; Nashville, TN. Abstract EG08. [Google Scholar]
- 17.Moore SR, Gresham LS, Bromberg MB, Kasarkis EJ, Smith RA. A self report measure of affective lability. J Neurol Neurosurg Psychiatry 1997;63:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith RA, Berg JE, Pope LE, Callahan JD, Wynn D, Thisted RA. Validation of the CNS emotional lability scale for pseudobulbar affect (pathological laughing and crying) in multiple sclerosis patients. Mult Scler 2004;10:679–685. [DOI] [PubMed] [Google Scholar]
- 19.Little R, Rubin D. Statistical Analysis With Missing Data. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 20.Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler 2008;14:1091–1098. [DOI] [PubMed] [Google Scholar]
- 21.Kister I, Chamot E, Bacon JH, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology 2010;75:217–223. [DOI] [PubMed] [Google Scholar]
- 22.Ventura RE, Antezana AO, Bacon T, Kister I. Hispanic Americans and African Americans with multiple sclerosis have more severe disease course than Caucasian Americans. Mult Scler 2017;23:1554–1557. [DOI] [PubMed] [Google Scholar]
- 23.Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol 2017;13:375–382. [DOI] [PubMed] [Google Scholar]
- 24.Khan O, Williams MJ, Amezcua L, Javed A, Larsen KE, Smrtka JM. Multiple sclerosis in US minority populations. Neurol Clin Pract 2015;5;132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poeck K. Pathophysiology of emotional disorders associated with brain damage. In: Handbook of Clinical Neurology. Amsterdam, Netherlands: North Holland Publishing Company; 1969:343–367. [Google Scholar]
- 26.Robinson RG, Parikh RM, Lipsey JR, Starkstein SE, Price TR. Pathological laughing and crying following stroke: validation of a measurement scale and a double-blind treatment study. Am J Psychiatry 1993;150:286–293. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Teng F, Chen Y, et al. Clinical features and related factors of poststroke pathological laughing and crying: a case-control study. J Stroke Cerebrovasc Dis 2016;25:556–564. [DOI] [PubMed] [Google Scholar]
- 28.Thakore NJ, Pioro EP. Laughter, crying and sadness in ALS. J Neurol Neurosurg Psychiatry 2017;88:825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haiman G, Pratt H, Miller A. Brain responses to verbal stimuli among multiple sclerosis patients with pseudobulbar affect. J Neurol Sci 2008;271:137–147. [DOI] [PubMed] [Google Scholar]
- 30.Wallin MT, Culpepper WJ, Campbell J, et al. The prevalence of multiple sclerosis in the United States: a population-based healthcare database approach. Poster presented at: European Conference for the Treatment and Research on Multiple Sclerosis; October 26, 2017; Paris, France. Abstract P344. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed during this study are held by the NARCOMS registry (narcoms.org).