Abstract
Background
Fatigue is a frequent disabling symptom in multiple sclerosis (MS), but its pathophysiology remains incompletely understood. This study aimed to explore the underlying neural basis of fatigue in patients with MS.
Methods
We enrolled 60 consecutive patients with MS and 60 healthy controls (HC) matched on age, sex, and education. Fatigue was assessed using the Portuguese version of the Modified Fatigue Impact Scale (MFIS). All participants underwent 3T brain MRI (conventional and diffusion tensor imaging [DTI] sequences). White matter (WM) focal lesions were identified and T1/T2 lesion volumes were computed. Tract-based spatial statistics were applied for voxel-wise analysis of DTI metrics fractional anisotropy and mean diffusivity (MD) on normal-appearing WM (NAWM). Using Freesurfer software, total and regional volumes of cortical and subcortical gray matter (GM) were calculated.
Results
Compared to HC, patients with MS scored significantly higher on MFIS (33.8 ± 19.7 vs 16.5 ± 15.1, p < 0.001). MFIS scores were not significantly correlated with T1/T2 lesion volumes, total GM volume, or any regional volume of cortical and subcortical GM. Significant correlations were found between global scores of MFIS and MD increase of the NAWM skeleton, including corona radiata, internal capsule, external capsule, corticospinal tract, cingulum, corpus callosum, fornix, superior longitudinal fasciculus, superior fronto-occipital fasciculus, sagittal stratum, posterior thalamic radiation, cerebral peduncle, and uncinate fasciculus.
Conclusions
In this study, fatigue was associated with widespread NAWM damage but not with lesion load or GM atrophy. Functional disconnection, caused by diffuse microstructural WM damage, might be the main neural basis of fatigue in MS.
Fatigue is reported by more than 80% of patients with multiple sclerosis (MS) during their disease course and drastically affects their quality of life.1 Fatigue can be defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities.”2 Although the clinical aspects of fatigue are well-recognized, its pathophysiologic mechanisms remain incompletely understood. Neuroimaging studies have yielded divergent results regarding a correlation between fatigue severity and MRI lesion load,3-6 number and volume of gadolinium-enhancing lesions,7 brain atrophy measurements,3,8,9 and diffuse damage to the normal-appearing brain tissues.10,11 Interestingly, several works have succeeded in establishing a link between fatigue and cortico-subcortical disconnection.4,12,13 However, most of these studies only used a single MRI technique and therefore did not assess the influence of all candidate brain regions and pathologic mechanisms. The advantage of combining MRI techniques is to get a more comprehensive view on pathophysiologic processes underpinning fatigue in MS.
Hence, the aim of this work was to explore the underlying neural basis of fatigue in patients with MS by means of a multimodal MRI approach assessing simultaneously the contribution of cortical gray matter (GM), subcortical GM, and white matter (WM) pathology.
Methods
Participants
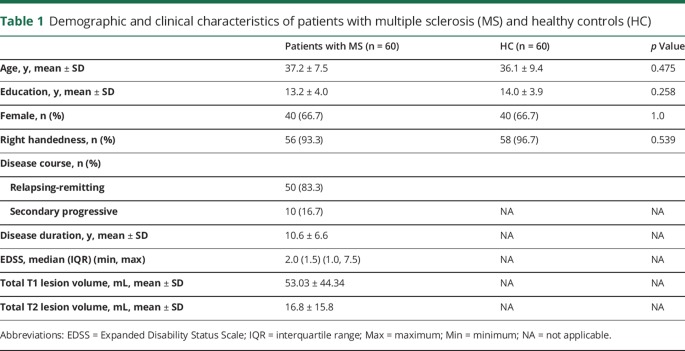
Participants included in this study correspond to the same dataset previously published by our group.14 It consists of 60 patients with MS regularly followed in our department and 60 healthy volunteers recruited from the community who served as healthy controls (HC), matched on age, sex, and education level (table 1).
Table 1.
Demographic and clinical characteristics of patients with multiple sclerosis (MS) and healthy controls (HC)
Patients were eligible if they were ≥18 and ≤55 years of age with relapsing-remitting or secondary progressive MS according to the McDonald criteria 2010.15 Participants were excluded if there was history of an additional neurologic, psychiatric (including clinically relevant depression), or systemic disorder. Other exclusion criteria included history of head injury with loss of consciousness; visual, auditory, or language impairment; or drug (including antipsychotic medication) or alcohol abuse. Participants who had stopped antidepressants up until 2 months before testing and patients with MS who had a relapse or used steroids within 2 months before evaluation were also excluded.
Standard protocol approvals, registrations, and patient consents
The local ethics committee approved this study and all participants gave written informed consent prior to participation.
Clinical assessment
A complete medical history and neurologic examination were performed in all patients. Expanded Disability Status Scale (EDSS)16 was used to evaluate neurologic disability. For HC, the medical history was collected through an in-person interview before assessment. We obtained the following clinical and demographic data: age, sex, handedness, years of education, disease duration, and current disease-modifying treatment.
Fatigue and depression assessment
Fatigue was assessed using the Portuguese version of the Modified Fatigue Impact Scale (MFIS).17,18 The test contains 21 items and the Portuguese version comprises 2 levels of fatigue: physical (MFISphy) and cognitive (MFIScog). The total score (MFIStotal) ranges from 0 to 84, with higher scores indicating more fatigue. The cutoff score above which the patient can be considered fatigued is 38. The Beck Depression Inventory (BDI)19,20 was used to determine the influence of depression on fatigue. The BDI comprises a self-reported response to a multi-choice questionnaire on 21 items, with a score ≥18 indicating clinically relevant depression.
MRI acquisition and analysis
All participants were examined on a 3T Siemens Magnetom TrioTim scanner (Erlangen, Germany) using a 12-channel birdcage head coil. Scans were placed in an axial-oblique orientation, parallel to the subcallosal line.21 The following acquisitions were obtained: (1) 2 high-resolution T1-weighted 3D magnetization prepared rapid acquisition gradient echo sequences; (2) sagittal 3D fluid-attenuated inversion recovery: repetition time (TR) 5 seconds, echo time (TE) 388 milliseconds, inversion time 1.8 seconds, field of view 250 × 250 mm2, yielding 160 slices with 1 × 1 × 1 mm3 voxel size; (3) diffusion tensor imaging (DTI): TR 7,800 milliseconds, TE 90 milliseconds, number of excitations 1; matrix, 96 × 96 × 63 contiguous axial slices; isotropic voxel resolution of 2 × 2 × 2 mm3; bandwidth of 1,628 Hz/pixel and echo spacing of 0.72 milliseconds. The DTI was acquired along 63 noncollinear directions (b = 1,000 s/mm2), with one scan without diffusion weighting (b = 0 s/mm2, b0). Of the 120 participants included in the study, 3 MRI datasets of the MS group were excluded due to major artefacts that precluded analysis.
A semiautomatic pipeline was used through FreeSurfer (version 5.3.0, surfer.nmr.mgh.harvard.edu) in a Linux (CentOS 6) platform22,23 to perform cortical surface reconstruction and volumetric segmentation. The cortical parcellation was based on the Desikan-Killiany atlas and volumes were corrected for the estimated total intracranial volume.14 As we did not find any significant lateral hemispheric differences regarding fatigue in a preliminary analysis, left and right hemispheric volumes were averaged in order to reduce the number of variables to the smallest possible set.14 The DTI images were processed using Oxford University's FMRIB Software Library (FSL, fmrib.ox.ac.uk/fsl) version 5.0.9, on a Linux-based platform, following the tract-based spatial statistics (TBSS) pipeline.14,24 A more detailed description of the methods used in MRI acquisition and analysis has been provided elsewhere.14,21
Statistical analysis
Group comparisons were performed using the t test for unpaired samples, the nonparametric Mann-Whitney U test, and χ2 test when appropriate. Correlations between MFIS scores and demographic, clinical, and MRI variables were examined using Pearson coefficients or Spearman rank order coefficient when appropriate. These analyses were conducted using SPSS for Windows version 20.0 (SPSS, Chicago). All tests performed were 2-tailed and the statistical threshold was set at p < 0.05, corrected through the false discovery rate for multiple testing.
Voxelwise statistics for each skeleton voxel were calculated using FSL's randomize tool, which combines permutation testing and general linear modeling: a 2-sample unpaired t test for patients with MS vs HCs' fractional anisotropy (FA) and mean diffusivity (MD), and a correlation analysis between MFIS scores and these diffusion metrics for the MS group, while treating age as a covariate of no interest. WM voxels with lesions were excluded from the analyses (after transforming the binary lesion masks to the same standard space as the FA images) and, therefore, only the normal-appearing WM (NAWM) was considered for the purpose of these analyses.
A total of 5,000 permutations were used with threshold-free cluster enhancement, corrected for multiple comparisons by controlling for family-wise error (FWE) rates.25 p Values <0.05 were considered statistically significant.
The skeletal regions with statistical significance were labeled anatomically by mapping the TBSS FWE-corrected statistical maps to the JHU-ICBM-DTI-81 WM atlas.26
The mean FA skeleton was also mapped onto the aforementioned atlas, and the total number of voxels per tract was calculated, which was used to determine the percentage of voxels with statistical significance within each labeled tract. The mean p values per labeled region were determined. The mean and SD of both diffusion metrics were obtained per participant and for each region.
Data availability
The datasets generated during or analyzed during the current study are available from the corresponding author on request.
Results
Sample characteristics
Patients with MS and HC did not differ significantly in educational level, age, or sex (table 1). The disease course was relapsing-remitting in 50 patients with MS (83.3%) and secondary progressive in 10 patients with MS (16.7%). The mean disease duration was 10.6 ± 6.6 years and the median EDSS score was 2.0 (interquartile range 1.5) (min, max 1.0, 7.5). All the patients in our study were receiving disease-modifying drugs. Analysis between different drug classes was not within the scope of this study.
Fatigue in patients with MS
Fatigue prevalence was 43.3% in patients with MS and 11.7% in HC. The prevalence of fatigue was not significantly different between the subgroups of patients with relapsing-remitting and secondary progressive MS (40% vs 60%, respectively, p = 0.244). Compared to HC, patients with MS scored significantly higher on physical (MFISphy), cognitive (MFIScog), and total (MFIStotal) dimensions of fatigue (19.4 ± 11.5 vs 7.8 ± 8.0, p < 0.001; 14.3 ± 10.1 vs 8.7 ± 7.8, p = 0.001; 33.8 ± 19.7 vs 16.5 ± 15.1, p < 0.001, respectively). In the MS group, scores on MFISphy, MFIScog, and MFIStotal positively correlated with scores on BDI (r = 0.546, r = 0.696, r = 0.676, p < 0.001). Only scores on MFISphy and MFIStotal correlated with EDSS (r = 0.427, p = 0.001; r = 0.296, p = 0.022). Age, sex, education level, and disease duration were not associated with fatigue.
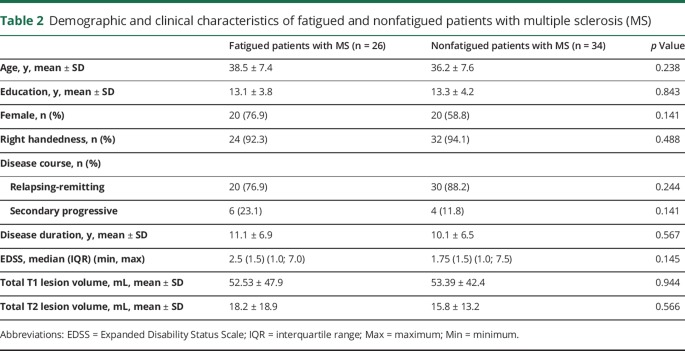
Comparing fatigued and nonfatigued patients with MS, we found no statistically significant differences in age, sex, education, disease course, disease duration, EDSS, or total T1 and T2 lesion volumes (table 2).
Table 2.
Demographic and clinical characteristics of fatigued and nonfatigued patients with multiple sclerosis (MS)
MRI correlates of fatigue in MS
Lesion load and GM volumes
MFIS scores (total, physical, and cognitive dimensions) were not significantly correlated with T1/T2 lesion volumes, total GM volume, or with any regional volume of cortical or subcortical GM (table e-1, links.lww.com/CPJ/A54).
Tract-based spatial statistics analysis
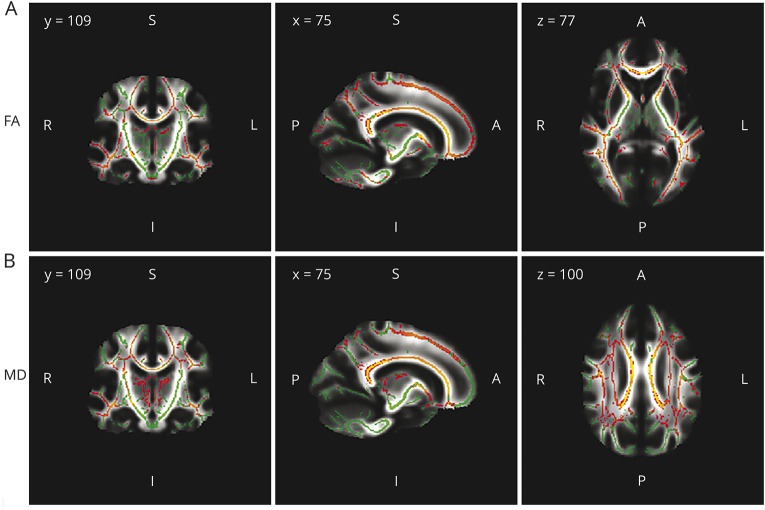
Compared with HC, patients with MS showed widespread abnormalities throughout the WM skeleton in both hemispheres defined by significant FA decrease or MD increase (figure 1).
Figure 1. White matter tracts.
White matter tracts with significant fractional anisotropy (FA) reduction (A) and mean diffusivity (MD) increase (B) in patients with multiple sclerosis relative to healthy controls. Significant regions are displayed in red/yellow (gradient of significance level red < yellow). Results are shown overlaid on the mean FA skeleton (A) or on the mean MD skeleton (B), both displayed in green (nonsignificant regions). White matter voxels with lesions were excluded and therefore only the normal-appearing white matter was considered for the analysis. p Values < 0.05 were considered statistically significant, corrected for multiple comparisons controlling for family-wise error rates.
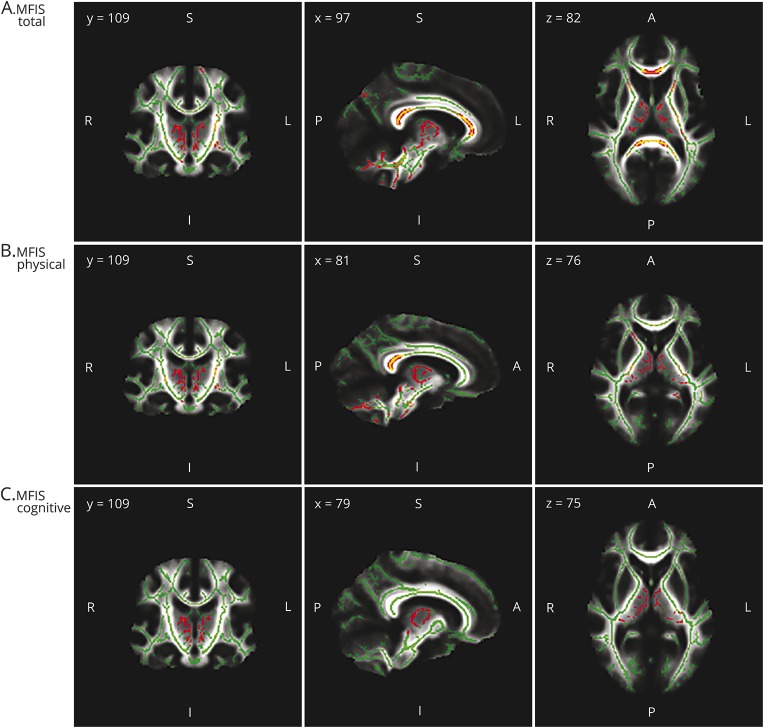
In patients with MS, the global score of fatigue (MFIStotal) and physical fatigue (MFISphy) were positively correlated with MD across widespread WM tracts of both hemispheres, including anterior and superior corona radiata, internal capsule (retrolenticular, anterior, and posterior limb), external capsule, corpus callosum (body, genu, and splenium), cerebral peduncles, corticospinal tractus, stria of fornix, cerebellar peduncles (superior, middle, and inferior), medial lemniscus, pontine crossing tract, superior fronto-occipital fasciculus, left posterior corona radiata, left posterior thalamic radiations, left sagittal stratum, left uncinate fasciculus, left cingulum of hippocampus, and left superior longitudinal fasciculus (figure 2, A and B).
Figure 2. White matter tracts significantly correlated with fatigue in patients with multiple sclerosis.
Significant regions correlated with Modified Fatigue Impact Scale (MFIS) total (A), physical (B), and cognitive (C), treating age as a covariate of no interest, are displayed in red/yellow (gradient of significance level red < yellow). Results are shown overlaid on the mean diffusivity skeleton, both displayed in green (nonsignificant regions). White matter voxels with lesions were excluded and therefore only the normal-appearing white matter was considered for the analysis. Cluster-based thresholding corrected for multiple comparisons controlling for family-wise error rates, with p values < 0.05 considered statistically significant.
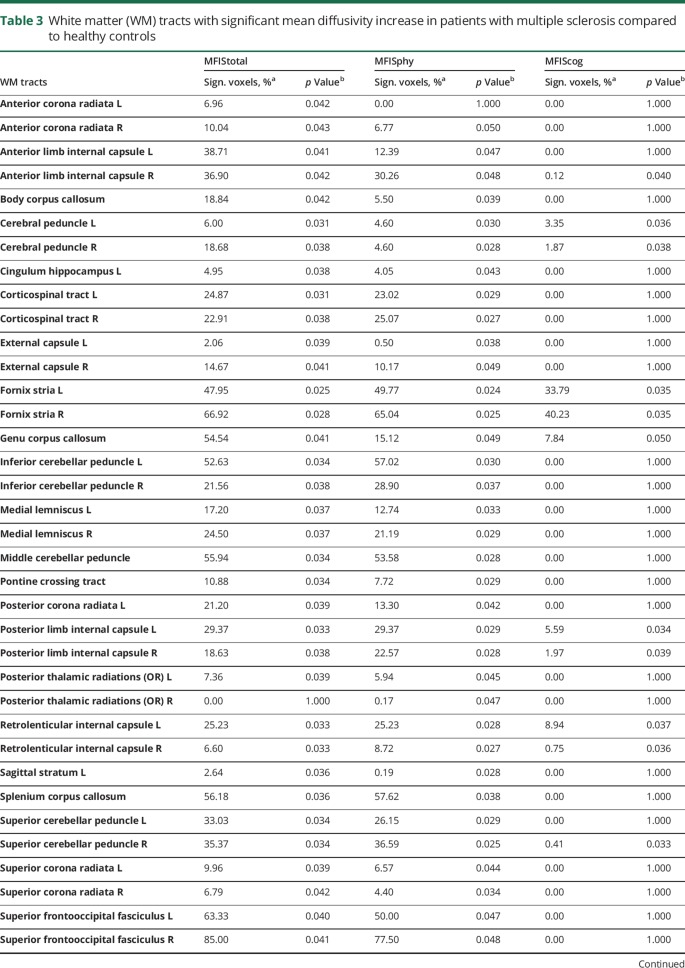
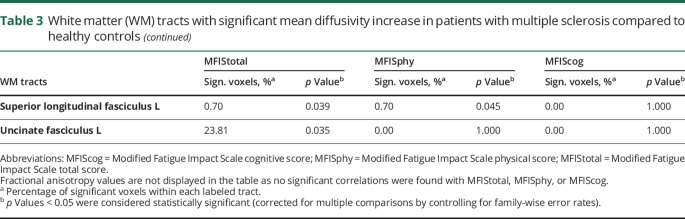
Cognitive fatigue (MFIScog) was positively correlated with MD of stria of fornix, genu of corpus callosum, cerebral peduncles, internal capsule (posterior limb and retrolenticular), right superior cerebellar peduncle, and right anterior limb of internal capsule (figure 2C). No significant correlations were found between FA and MFIStotal, MFISphy, or MFIScog (table 3).
Table 3.
White matter (WM) tracts with significant mean diffusivity increase in patients with multiple sclerosis compared to healthy controls
Discussion
In this study, we explored the contribution of GM and WM damage to fatigue in MS by means of combining two advanced MRI techniques: DTI with TBSS analysis to assess WM integrity and FreeSurfer to evaluate cortical and subcortical GM volumes. Our results suggest that fatigue is mainly related to widespread NAWM damage but not to conventional lesion load or GM atrophy.
In line with these findings, previous studies demonstrated that the disruption of neural circuits contributes to generating fatigue.12 In particular, several associative tracts, such as frontal and occipital fibers, the uncinate fasciculus, superior longitudinal fasciculus, external and internal capsules, and corpus callosum, were electively damaged in patients with MS. The involvement of fronto-frontal connections and frontal-connected associative tracts reinforces the role of the frontal lobes as one of the most important substrates of fatigue in MS, as previously suggested in other studies.4,11-13 The disruption of the corpus callosum deserves particular attention, as this structure is a crossroads of pathways linking not only both frontal lobes but also the presupplementary motor area and anterior cingulate region with the dorsolateral prefrontal cortex.27 Both executive and working memory tasks are associated with the activation of the anterior cingulate and dorsolateral prefrontal cortex in both hemispheres.28,29 Therefore, the association of fatigue with callosal pathway damage may be related to impaired communication between these structures.4 Callosal damage proven by structural MRI4 and by MRI spectroscopy30 has been previously associated with fatigue in MS.
The theory of cortico-subcortical disconnection underlying fatigue in MS is supported by a growing body of evidence coming not only from structural but also from functional MRI studies. Data from 73 patients who had undergone proton magnetic resonance spectroscopy imaging showed that the N-acetylaspartate-creatine ratio was significantly lower in the high-fatigue group, suggesting a widespread axonal dysfunction.30 Moreover, a functional MRI study has demonstrated impaired interactions between functionally related cortical and subcortical areas.31 Bisecco et al.13 conducted a study with a similar approach to the present one (i.e., DTI with TBSS whole-brain voxelwise analysis) and also reported an association between fatigue and widespread microstructural NAWM damage. These authors proposed that the less extensive increase of axial diffusivity observed in fatigued patients with MS suggested the presence of a diffuse axonal loss reflecting the effect of inflammation. However, in our study, the pattern of DTI measures correlated with MFIS (increased MD without significant correlation with FA) suggests that the NAWM damage underlying fatigue is probably more related to diffuse inflammation/edema than to irreversible axonal injury. Accordingly, one hypothesis for the pathophysiology of fatigue in MS is that the inflammatory milieu in the CNS might produce symptoms of fatigue by inducing functional alterations in the brain networks.32
Studies are inconsistent regarding the association between fatigue and total measures of lesion load and atrophy in MS. Two studies reported an association between fatigue and higher T1 and T2 lesion load3,4 and in one of these studies the authors also found an association between lower white matter and gray matter fraction and fatigue in MS.3 However, several other studies did not find any association between these fractions and fatigue, which is in line with our results.6,9,11,13,32 Possible explanations for these discrepancies include differences in patients' clinical characteristics (disease duration, concomitant presence of depression and cognitive impairment); use of different fatigue scales and sample sizes; sensitivity of the technique applied to different substrates of MS pathology; and methods used for the analysis (e.g., global vs regional assessment).11
Regarding the contribution of GM pathology to fatigue in MS, previous studies have suggested that the main contributor is multiregional damage rather than global brain damage.9,11,33 The regional analysis supported the role of specific brain regions in the pathogenesis of this symptom, particularly the thalamus,9,33 basal ganglia nuclei,33,34 and frontal,4,11,34 temporal,11 and posterior parietal8,34 cortices. On the contrary, in the current study, fatigue was not correlated with any regional volume of cortical and subcortical GM.
There are some limitations to this study to be considered. First, its cross-sectional design may interfere with group comparison and therefore limit the generalization of our findings. Nevertheless, the overlap with previous studies supports the validity of our results. Second, the patients had relatively mild disability (median EDSS score 2.0) and most of them had a relapsing-remitting subtype of MS. Therefore, we could not define if the results of the current study are fully applicable to all patients with MS. Third, the relationship between fatigue and treatment with different disease-modifying drugs was not explored. In addition, we did not assess whether the patients were on therapies for fatigue, which might have altered their fatigue scores. Finally, a functional MRI examination was not included in the present study. As a consequence, we could not analyze the functional correlates of the widespread NAWM damage.
Fatigue in its physical and cognitive dimensions was associated with diffuse lesion of NAWM but not with GM atrophy or conventional lesion load. This strengthens the hypothesis that the main neural basis of fatigue in MS might be a functional disconnection caused by diffuse microstructural damage of NAWM.
Author contributions
A.M. Novo: data acquisition, analysis and interpretation of data, wrote the original manuscript. S. Batista: study concept and design, study supervision, data acquisition, analysis and interpretation of data. C. Alves: MRI acquisition and analysis, interpretation of data. O.C. d'Almeida: MRI acquisition and analysis, interpretation of data. I.B. Marques: data acquisition and analysis. C. Macário: data acquisition, critical revision of the manuscript for important intellectual content. I. Santana: study design, study supervision, critical revision of the manuscript for important intellectual content. L. Sousa: study design, data acquisition, and critical revision of the manuscript for important intellectual content. M. Castelo-Branco: study design, MRI acquisition and analysis, critical revision of the manuscript for important intellectual content. L. Cunha: study concept and design, study supervision, critical revision of the manuscript for important intellectual content.
Study funding
This research was supported by a grant from Biogen. The sponsor did not participate in any aspect of the design or performance of the study, including the data collection, management, analysis, and interpretation or preparation of the manuscript.
Disclosure
A.M. Novo reports no disclosures. S. Batista serves on scientific advisory boards for Biogen and Novartis; has received speaker honoraria from Biogen, Novartis, Teva, Genzyme, and Merck Serono; serves on speakers' bureaus for Biogen, Novartis, Teva, Genzyme, and Merck Serono; and receives research support from Biogen. C. Alves reports no disclosures. O.C. d'Almeida has received research support from the Portuguese Foundation for Science and Technology. I.B. Marques receives research support from Tecnifar. C. Macário has received honoraria for serving on scientific advisory boards or speaking in scientific meetings of Teva, Merck Serono, Bayer, Genzyme, Biogen, and Novartis Pharma. I. Santana reports no disclosures. L. Sousa has received honoraria for serving on scientific advisory boards or speaking in scientific meetings of Teva, Merck Serono, Bayer, Genzyme, Biogen, and Novartis Pharma. M. Castelo-Branco has received research support from the Portuguese Foundation for Science and Technology. L. Cunha reports no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Patients with MS present a high prevalence of fatigue and score significantly higher than controls on physical, cognitive, and total dimensions of the MFIS.
→ The use of a multimodal MRI approach allows a more comprehensive view on pathophysiologic mechanisms underlying fatigue in MS.
→ Fatigue is mainly related to widespread NAWM damage but not to lesion load or gray matter atrophy.
→ Functional cortico-subcortical disconnection may be an important substrate of fatigue in MS.
References
- 1.Lobentanz IS, Asenbaum S, Vass K, et al. Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurol Scand 2004;110:6–13. [DOI] [PubMed] [Google Scholar]
- 2.Fatigue Guidelines Development Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998. [Google Scholar]
- 3.Tedeschi G, Dinacci D, Lavorgna L, et al. Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J Neurol Sci 2007;263:15–19. [DOI] [PubMed] [Google Scholar]
- 4.Sepulcre J, Masdeu JC, Goni J, et al. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler 2009;15:337–344. [DOI] [PubMed] [Google Scholar]
- 5.Van der Werf SP, Jongen PJ, Lycklama à Nijeholt GJ, Barkhof F, Hommes OR, Bleijenberg G. Fatigue in multiple sclerosis: interrelations between fatigue complaints, cerebral MRI abnormalities and neurological disability. J Neurol Sci 1998;160:164–170. [DOI] [PubMed] [Google Scholar]
- 6.Bakshi R, Miletich RS, Henschel K, et al. Fatigue in multiple sclerosis: cross-sectional correlation with brain MRI findings in 71 patients. Neurology 1999;53:1151–1153. [DOI] [PubMed] [Google Scholar]
- 7.Mainero C, Faroni J, Gasperini C, et al. Fatigue and magnetic resonance imaging activity in multiple sclerosis. J Neurol 1999;246:454–458. [DOI] [PubMed] [Google Scholar]
- 8.Pellicano C, Gallo A, Li X, et al. Relationship of cortical atrophy to fatigue in patients with multiple sclerosis. Arch Neurol 2010;67:447–453. [DOI] [PubMed] [Google Scholar]
- 9.Nourbakhsh B, Azevedo C, Nunan-Saah J, et al. Longitudinal associations between brain structural changes and fatigue in early MS. Mult Scler Relat Disord 2016;5:29–33. [DOI] [PubMed] [Google Scholar]
- 10.Codella M, Rocca MA, Colombo B, Rossi P, Comi G, Filippi M. A preliminary study of magnetization transfer and diffusion tensor MRI of multiple sclerosis patients with fatigue. J Neurol 2002;249:535–537. [DOI] [PubMed] [Google Scholar]
- 11.Rocca MA, Parisi L, Pagani E, et al. Regional but not global brain damage contributes to fatigue in multiple sclerosis. Radiology 2014;273:511–512. [DOI] [PubMed] [Google Scholar]
- 12.Pardini M, Bonzano L, Mancardi GL, Roccatagliata L. Frontal networks play a role in fatigue perception in multiple sclerosis. Behav Neurosci 2010;124:329–336. [DOI] [PubMed] [Google Scholar]
- 13.Bisecco A, Caiazzo G, d'Ambrosio A, et al. Fatigue in multiple sclerosis: the contribution of occult white matter damage. Mult Scler 2016;22:1676–1684. [DOI] [PubMed] [Google Scholar]
- 14.Batista S, d’Almeida OC, Afonso A, et al. Impairment of social cognition in multiple sclerosis: amygdala atrophy is the main predictor. Mult Scler 2017;23:1358–1366. [DOI] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 17.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 1994;21:9–14. [PubMed] [Google Scholar]
- 18.Gomes L. Validação da Versão Portuguesa da Escala de Impacto da Fadiga Modificada e da Escala de Severidade da Fadiga na Esclerose Múltipla. Braga: University of Minho; 2011. [Google Scholar]
- 19.Vaz Serra A, Pio Abreu JL. Aferição dos quadros depressivos: I: ensaio de aplicação do Inventário Depressivo de Beck a uma amostra Portuguesa de doentes deprimidos. Coimbra Médica 1973;20:623–644. [Google Scholar]
- 20.Vaz Serra A, Pio Abreu JL. Aferição dos quadros depressivos: II: estudo preliminar de novos agrupamentos sintomatológicos para complemento do Inventário Depressivo de Beck. Coimbra Médica 1973;20:713–736. [Google Scholar]
- 21.Batista S, Alves C, d’Almeida OC, et al. Disconnection as a mechanism for social cognition impairment in multiple sclerosis. Neurology 2017;89:38–45. [DOI] [PubMed] [Google Scholar]
- 22.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207. [DOI] [PubMed] [Google Scholar]
- 23.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I: segmentation and surface reconstruction. Neuroimage 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 26.Oishi K, Zilles K, Amunts K, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 2008;43:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci 2007;27:4839–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage 2005;26:471–479. [DOI] [PubMed] [Google Scholar]
- 29.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci 2003;4:829–839. [DOI] [PubMed] [Google Scholar]
- 30.Tartaglia MC, Narayanan S, Francis SJ, et al. The relationship between diffuse axonal damage and fatigue in multiple sclerosis. Arch Neurol 2004;61:201–207. [DOI] [PubMed] [Google Scholar]
- 31.Filippi M, Rocca MA, Colombo B, et al. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 2002;15:559–567. [DOI] [PubMed] [Google Scholar]
- 32.Biberacher V, Schmidt P, Selter RC, et al. Fatigue in multiple sclerosis: associations with clinical, MRI and CSF parameters. Mult Scler 2018;24:1115–1125. [DOI] [PubMed] [Google Scholar]
- 33.Bernitsas E, Yarraguntla K, Bao F, et al. Structural and neuronal integrity measures of fatigue severity in multiple sclerosis. Brain Sci 2017;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabrese M, Rinaldi F, Grossi P, et al. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing-remitting multiple sclerosis. Mult Scler 2010;16:1220–1228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during or analyzed during the current study are available from the corresponding author on request.