Abstract
Within the field of neurology, there has been limited discussion of how to best respect patient autonomy in patients presenting with an acute stroke, who often have impairments in language and cognition. In addition to performing a detailed neurologic examination and providing a thorough timeline of their current presentation and medical history, these patients and their families are then asked to quickly make critical medical decisions regarding acute stroke therapies (thrombolysis and endovascular therapy). These discussions are often limited by time constraints and inadequate opportunities for patient education regarding acute stroke care. This article discusses some of the challenges of preserving patient autonomy in patients presenting with acute stroke and the advent of a stroke advance directive (Coordinating Options for Acute Stroke Therapy [COAST]) aimed to overcome these obstacles.
General overview of patient autonomy
The preservation of patient autonomy is a highly valued principle in health care today. Patient autonomy is defined as an individual's right to make decisions about his or her own medical treatment options. These decisions must be free from controlling interferences of others and limitations, such as an inadequate understanding of medical care, that can prevent meaningful decision-making.1 Respect for patient autonomy is particularly challenging in the field of stroke neurology, as patients often present without warning with catastrophic impairments in language or cognition, preventing them from making informed decisions about health care interventions. Recognition of these challenges has led to the inclusion of respect for patient autonomy as a key concern in recent biomedical ethics literature.1,2
One strategy to preserve patient autonomy is the use of advance directives. By specifying care preferences or naming a surrogate decision-maker in the form of a written document in advance, patients can maintain some degree of autonomy during periods of incapacity.3 Physician Orders for Life-Sustaining Treatment forms and living wills are commonly utilized advance directives that allow patients to document their wishes regarding medical care, and these forms typically document preferences for cardiopulmonary resuscitation, artificial nutrition, and intensive care. Advance directives are often discussed in a primary care setting, preferably with the patient's surrogate decision-maker present. Copies of advance directives are given to the patient and placed into the medical record, where they are readily accessible to future health care clinicians.
Stroke-specific considerations in preserving patient autonomy
Despite recent progress in available stroke interventions, including improved outcomes with endovascular therapy up to 24 hours in certain patients,4,5 advance directives are underutilized in the field of stroke neurology. In a recent observational cohort of 143 patients who died during their hospitalization from acute stroke, only 29.4% of these patients had written and signed an advance directive.6 This is particularly alarming given that stroke is currently the fifth leading cause of death and a leading cause of serious long-term disability in the United States.7,8
Acute stroke care poses several obstacles regarding the preservation of patient autonomy. Patients presenting with new focal neurologic deficits (e.g., weakness, numbness, vision changes, speech/language impairments, neglect) often find themselves in a stressful and fast-paced unfamiliar environment in the emergency department. In addition to being asked to perform a dizzying array of neurologic examination maneuvers, these patients and their families are simultaneously being asked to provide a detailed timeline of their current presentation, medical history, and baseline functional status, as well as make decisions regarding treatment or research options. The rationale for this rapid management strategy is that in the field of stroke neurology, “time is brain.”9 This widely utilized phrase highlights the fact that brain tissue is rapidly and irretrievably lost as a stroke progresses. It is estimated that patients on average lose 1.9 million neurons each minute in which a stroke is left untreated,10 making it critical that therapeutic interventions (e.g., thrombolysis, endovascular therapy) be emergently pursued to prevent permanent neurologic impairment. Although these interventions have been shown to reduce long-term disability and are currently standard of care,4,5,11 they also pose potential risks including intracranial hemorrhage, which must be carefully considered.
Aphasia, hemispatial neglect, and anosognosia pose unique challenges to the preservation of patient autonomy in stroke patients. Considering aphasia is an impairment of language comprehension or production, these patients have limited ability to communicate medical care decisions and are often unable to provide consent for stroke interventions. Similarly, patients with hemispatial neglect, who have impaired ability to process and perceive stimuli on one side of their body, or anosognosia (a component of neglect in which patients lack self-awareness of their deficits), may have reduced capacity to make decisions about acute stroke treatment. The inability for stroke patients to communicate and perceive their illness requires stroke clinicians to rely on a patient's previously written or verbalized wishes for medical care.
Acute stroke intervention preferences are seldom discussed and documented in advance and many patients and their families are unfamiliar with medical terms such as thrombolysis and endovascular therapy. The time-sensitive nature of acute stroke intervention limits the opportunity to have detailed discussions of relevant evidence pertaining to acute stroke treatment options. Time constraints and limited knowledge of a patient's preferences can lead to rushed and ill-informed decision-making by health care surrogates.
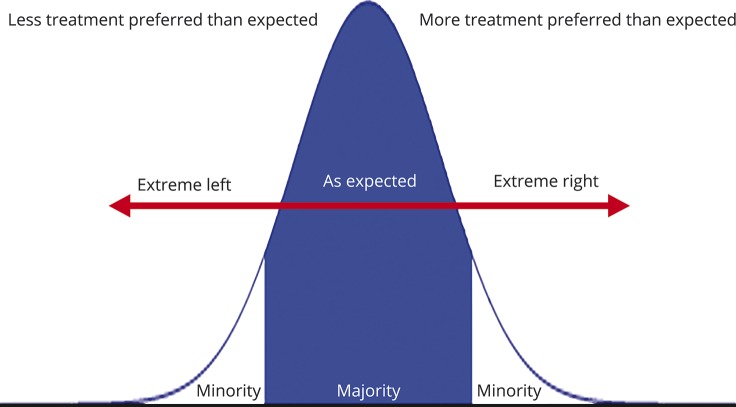
If patients are given the opportunity to document their preferences for acute stroke treatment options in a nonemergent setting, the general expectation is that most patients would prefer acute stroke interventions that are available, when indicated. However, there may be a small proportion of patients who opt for medical care that is contrary to generally accepted practice, as is their right. This assumption would result in a normal distribution of patient preferences consisting of a majority who prefer acute stroke treatment based on clinician discretion, a minority who may not want any treatment, and a minority who may always want treatment despite clinician discretion (figure 1). These preferences are not always elicited in an emergent setting when the decision to treat is time-sensitive. Stroke clinicians currently attempt to strike a balance between the most efficient and comprehensive acute care, but this may not always be patient-centered if prior wishes are unknown.
Figure 1. Theoretical distribution of patient preferences for acute stroke interventions.
Theoretical model of patient preference distribution showing a majority of individuals opting for standard of care treatment and a minority of patients opting for treatment outside current practice guidelines.
Strategies for preserving patient autonomy in stroke
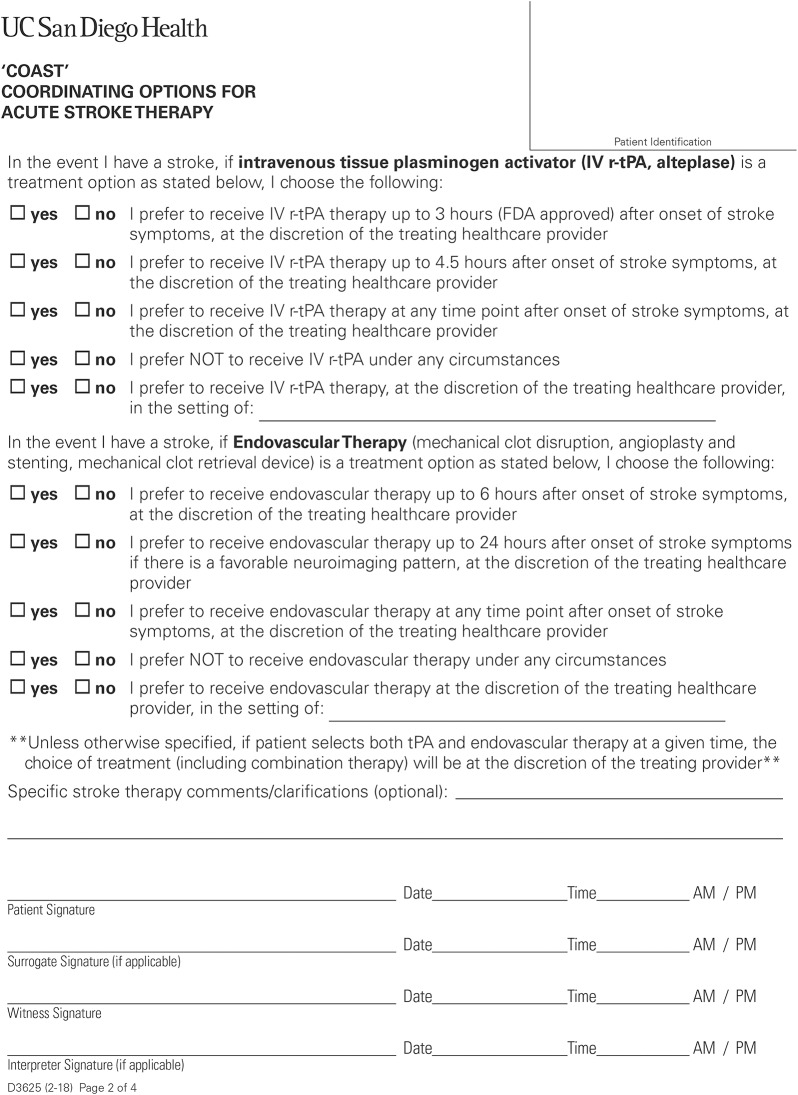
One solution to preserving patient autonomy in acute stroke care is the advent of a stroke advance directive. An advance directive for acute stroke therapy was created at the University of California, San Diego (UCSD) in 2015 titled COAST (Coordinating Options for Acute Stroke Therapy).12 This 4-page form allows patients to document their preferences regarding acute stroke treatment interventions, as well as participation in clinical stroke trials, in a nonurgent setting and in advance of a potential stroke (figure 2 and figure e-1, http://links.lww.com/CPJ/A69). The first 2 pages detail the purpose/content of the form and outline potential stroke treatment preferences for IV thrombolysis and endovascular therapy. The last 2 pages allow patients to document their wishes regarding participation in ongoing stroke research clinical trials (known as the COAST-R portion, for research trials).
Figure 2. Coordinating Options for Acute Stroke Therapy (COAST) form reprinted with permission from University of California San Diego Health, 2018 (Page 2 of four-page form).
COAST is unique in that it is a designation of patient preference rather than an order for these interventions. As such, stroke intervention preferences can be overridden at the time of a stroke by the patient, a health care surrogate, or a clinician based on change of wishes, or specific medical indications that may not have been accounted for at the time of documentation. The treating clinician can reference a patient's COAST form at the time of an acute stroke to help guide decision-making. Patients do not dictate their own treatments outside the bounds of medical appropriateness. Bedside clinicians make medical decisions based on the facts of the case, medical appropriateness, and patient preference. COAST simply allows the patient to make those prespecified preferences known ahead of time.
When completing the COAST form, available treatment options and preferences are discussed between patients and stroke clinicians. This may be done at a stroke clinic visit when there is not the time pressure of an emergency situation. It may also be completed during an inpatient hospitalization (perhaps after acute care moments are completed, while awaiting other studies, and typically just prior to a patient's discharge). This is an important time to discuss COAST as up to 25% of strokes in the United States are recurrent.13 An accompanying fact sheet is provided and discussed with each patient offered a COAST form to keep patients educated on current acute stroke treatment options. This fact sheet is updated when changes are made to stroke guidelines (as occurred in 2018) or when major new evidence is reported.14 As these conversations are limited by the degree of each patient's background medical knowledge, it is critical that stroke education and COAST discussions be tailored to the unique needs of each individual patient.
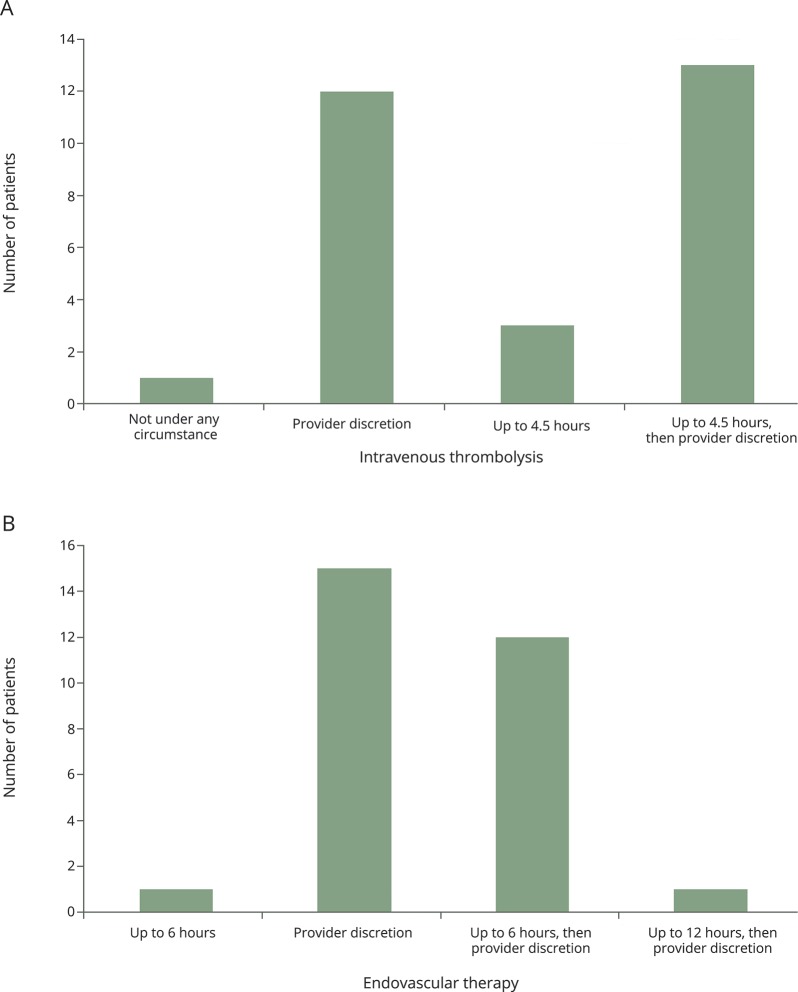
The COAST form has been in use at UCSD and initial results have been assessed. Pilot data were gathered to investigate the actual distribution of patient preferences related to potential acute stroke interventions (note that there was no option for endovascular therapy up to 24 hours on the COAST form because data were not published at the time of pilot data collection) (figure 3). In this pilot phase, COAST form completion was offered only to current inpatients admitted for a stroke or TIA, or outpatients in one of our stroke clinics. A total of 29 COAST forms were completed, taking an average of 11 minutes (range 5–15 minutes) to discuss and complete. Regarding thrombolytic preferences, we found that 96.6% (28/29) of patients would want IV recombinant tissue plasminogen activator (rtPA) up to 4.5 hours despite knowing that the Food and Drug Administration–approved window was only 3 hours, and 3.5% (1/29) of patients refused IV rtPA. For endovascular therapy, 100% (29/29) preferred endovascular therapy, but only 44.8% (13/29) preferred endovascular therapy up to 6 hours, while 51.2% (15/29) opted for endovascular therapy at any time under the discretion of the treating clinician. No patient (0/29) refused endovascular therapy. The COAST form is incorporated into the electronic medical record so that it is readily available to stroke clinicians in case of future stroke. Patients are given a copy of COAST and are instructed to keep this form with them just as they would with other advance directives should they require acute care at a different hospital. We also found that discussing acute stroke interventions outside of the emergency setting seems to improve patient education, allowing patients and their families to make well-informed decisions. Future analysis is pending regarding this perception.
Figure 3. Actual distribution of patient preferences for acute stroke interventions.
Bar graphs depict actual distribution of patient preferences for (A) IV thrombolysis and (B) endovascular therapy (n = 29).
Based on our observational pilot data results, it is evident that stroke clinicians can provide more tailored recommendations to patients by better understanding their personal goals of care. COAST has a powerful benefit to the majority of patients who would want an acute stroke intervention, which could encourage a clinician to treat in case the clinician may not be generally inclined to treat (figure 1). Likewise, COAST has a powerful benefit for the minority of patients with specific opinions about stroke interventions that may be contrary to common acute stroke practice (figure 1). This is especially seen in stroke patients who are at risk of developing language/cognitive disabilities and, with COAST, are effectively given a voice to express their medical care preferences to their future stroke clinicians. We are currently in the process of increasing the number of patients who complete a COAST form at UCSD to further assess a more robust distribution of patient preferences at a single center. We also aim to observe whether COAST has an effect on stroke treatment times and acute stroke treatment frequency, while assessing the actual number of patients who have a COAST form who return to UCSD for stroke code and stroke readmission.
A unique situation in which the COAST stroke advance directive has been utilized is in patients presenting with TIA who are at high risk of having a stroke during their hospital admission or soon thereafter. For instance, patients with critical intracranial/extracranial stenosis who are admitted to the hospital often have pressure-dependent examinations in which transient neurologic deficits fluctuate with changes in blood pressure or head of bed elevation, both of which can alter cerebral blood flow. One patient in particular was an 86-year-old woman with a history of atrial fibrillation admitted to our institution with multiple transient episodes of left-sided weakness and dysarthria who was found to have a distal right middle cerebral artery occlusion. The location of her occlusion was not amenable to endovascular intervention. On 2 separate occasions during her hospitalization, she became symptomatic (NIH Stroke Scale score 11), followed by complete resolution of her symptoms with fluid hydration and maintaining the head of bed flat. A COAST advance directive was completed while she was asymptomatic. She later developed permanent symptoms, but was able to be treated with IV thrombolysis within 10 minutes of symptom onset, as her preferences had already been thoroughly discussed and documented via the COAST form.
Another unique group of high-risk patients are those presenting to hospitals requiring evaluation by telestroke clinicians and subsequent transfer to comprehensive stroke centers for higher level of care. Patients requiring escalation of care via transfer to a more comprehensive center may be at high risk for neurologic worsening during their hospital course or during transfer. Discussing stroke advance directives on initial evaluation prior to transfer may streamline care upon arrival to a comprehensive stroke center should neurologic worsening occur.
For a stroke advance directive to be helpful and resource-efficient, targeting the appropriate patient population is paramount. Currently, the COAST advance directive is discussed with patients presenting to UCSD with stroke or stroke-related conditions (i.e., TIA, symptomatic intracranial/extracranial stenosis) in both the inpatient and outpatient settings. These patients have already had an ischemic event and are at high risk of developing a stroke in the future. If stroke advance directives become common practice and more resources are made available for dissemination, then ideally they would become a component of primary stroke prevention discussions as well. This would require identifying patients at high risk of future stroke by primary care clinicians and neurologists. One identification strategy may be by utilizing available stroke risk prediction tools,15,16 which predict the probability of future stroke based on risk factors (e.g., age, sex, blood pressure, diabetes mellitus, cigarette smoking, prior cardiovascular disease, atrial fibrillation, and left ventricular hypertrophy). The benefit of targeting patients in a primary care setting is that individuals with limited access to medical care, who are often at highest risk of stroke, would receive improved education on stroke prevention and acute stroke interventions. Increased resources and penetrance of the COAST advance directive may help decrease the number of future strokes and streamline systems of care when a stroke does occur.
The preservation of patient autonomy continues to challenge modern health care systems that strive to provide efficient and patient-centered care. Available therapeutic interventions in the field of stroke neurology are rapidly expanding.4,5 While this proves to be an exciting time for stroke clinicians and their patients, these advances increase the complexity of medical decision-making. Additional burden is often placed on the health care surrogate, who is tasked with speaking on behalf of the patient on an issue that may not have been previously discussed.
Despite stroke being the fifth leading cause of death in the United States,7 advance directives are underutilized in the field of stroke neurology. Given the aging population and the fact that the risk of stroke more than doubles with each successive decade after age 55 years,17 we can expect the issue of preserving patient autonomy in stroke to become even more relevant in years to come. The COAST stroke advance directive allows patients to communicate their acute intervention preferences in advance of future stroke and helps clinicians facilitate compassionate, ethical, and patient-centric care.
Author contributions
Kevin McGehrin: manuscript concept and initial draft. Ilana Spokoyny: revision of manuscript for intellectual content. Brett C. Meyer: revision of manuscript for intellectual content. Kunal Agrawal: revision of manuscript for intellectual content.
Study funding
No targeted funding reported.
Disclosure
K. McGehrin and I. Spokoyny report no disclosures. B.C. Meyer receives research support from NIH. K. Agrawal reports no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Varelius J. The value of autonomy in medical ethics. Med Health Care Philos 2006;9:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Entwistle V, Carter SM, Cribb A, McCaffery K. Supporting patient autonomy: the importance of clinician-patient relationships. J Gen Intern Med 2010;25:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spoelhof GD, Elliott B. Implementing advance directives in office practice. Am Fam Physician 2012;85:461–466. [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 5.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso A, Dorr D, Szabo K. Critical appraisal of advance directives given by patients with fatal acute stroke: an observational cohort study. BMC Med Ethics 2017;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics. Mortality in the United States, 2014. NCHS data brief, no 229 [online]. Available at: cdc.gov/nchs/data/databriefs/db229.pdf. Accessed July 13, 2017. [Google Scholar]
- 8.Mozzafarian D, Benjamin EJ, Go AS, et al. ; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2016 update: a report from the American Heart Association. Circulation 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 9.Gomez C. Time is brain. J Stroke Cerebrovasc Dis 1993;3:1–2. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL. Time is brain: quantified. Stroke 2006;37:263–266. [DOI] [PubMed] [Google Scholar]
- 11.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 12.Spokoyny I, Cederquist L, Clay B, Meyer BC. COAST (Coordinating Options for Acute Stroke Therapy): an advance directive for stroke. J Clin Ethics 2015;26:2016–2211. [PubMed] [Google Scholar]
- 13.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2017 update: a report from the American Heart Association. Circulation 2017;135:e229–445. [Google Scholar]
- 14.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 15.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham study. Stroke 1991;22:312–318. [DOI] [PubMed] [Google Scholar]
- 16.Nobel L, Mayo NE, Hanley J, Nadeau, Daskalopoulou SS. MyRisk_Stroke calculator: a personalized stroke risk assessment tool for the general population. J Clin Neurol 2014;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ovbiagele B, Goldstein LB, Higashida RT, et al. Forecasting the future of stroke in the United States. Stroke 2013;44:2361–2375. [DOI] [PubMed] [Google Scholar]