Abstract
Background:
Decreased cerebrospinal fluid (CSF) amyloid-β 1-40 (Aβ40) and amyloid-β 1-42 (Aβ42) and increased total and phosphorylated tau (t-tau, p-tau) concentrations have been described in cerebral amyloid angiopathy (CAA).
Objective:
Our aim was to analyze these biomarkers in patients with CAA-related inflammation (CAA-I).
Methods:
We prospectively recruited nine patients with acute phase CAA-I fulfilling Chung criteria. CSF was analyzed for t-tau, p-tau, Aβ42, and Aβ40. Data were compared to controls (n = 14), patients with Alzheimer’s disease (AD, n = 42), CAA (n = 10), and primary angiitis of the central nervous system (PACNS, n = 3).
Results:
For the CAA-I group, statistically significant differences were: lower Aβ42 (p = 0.00053) compared to the control group; lower t-tau (p = 0.018), p-tau (p < 0.001), and Aβ40 (p < 0.001) compared to AD; lower Aβ42 (p = 0.027) compared to CAA; lower Aβ42 (p = 0.012) compared to PACNS. Nearly significantly lower Aβ40 (p = 0.051) and higher t-tau (p = 0.051) were seen in CAA-I compared to controls.
Conclusion:
CSF biomarkers profile similar to that of CAA was observed in CAA-I (with even lower levels of Aβ42 compared to CAA). Based on our findings, high p-tau seems more specific for AD, whereas low Aβ42 differentiates CAA-I from CAA, PACNS, and controls, and low Aβ40 differentiates CAA-I from AD.
Keywords: Alzheimer’s disease, amyloid-β, cerebral amyloid angiopathy, cerebrospinal fluid, inflammation, tau
INTRODUCTION
In amyloid β (Aβ)-related angiitis of the central nervous system (CNS) (also called CAA-related inflammation, CAA-I), cerebral amyloid angiopathy (CAA) occurs in association with primary vasculitis of small- and medium-sized leptomeningeal and cortical arteries [1–6]. It has been suggested that CAA-I is triggered by vascular Aβ deposition followed by an Aβ-directed (auto)immune response, based on the presence of auto-antibodies against Aβ40 and Aβ42 and the clinical improvement most frequently encountered in response to immunosuppressive treatment. The apolipoprotein E (ApoE) ɛ4/ɛ4 genotype is predominant in CAA-I. In order to avoid brain biopsy, diagnostic criteria for probable CAA-I have been proposed by Chung et al. including all of the following: acute-subacute symptom onset, >40 years of age, at least one of the clinical features (headache, mental status, or behavioral change, focal neurological signs, seizures), patchy or confluent T2 or FLAIR hyperintensity, evidence of pre-existing CAA on susceptibility-weighted MRI sequences, and absence or neoplastic, infectious, or other cause [2].
Decreased cerebrospinal fluid (CSF) Aβ42 and Aβ40 and increased total and phosphorylated tau (t-tau and p-tau) concentrations have been described in CAA [7, 8]. In particular, Aβ40 levels seemed to be of clinical interest to differentiate CAA from AD. To the best of our knowledge, CSF concentrations of these biomarkers have been analyzed only once in a series of CAA-I patients [9]. The authors found significantly higher t-tau and p-tau, equivalent Aβ40, and non-significantly lower Aβ42 levels in the acute phase of CAA-I compared with controls. During the remission phase of CAA-I, all biomarker levels decreased to levels significantly lower than in the acute phase. In their study, the authors essentially compared anti-Aβ autoantibodies in patients with CAA-I, CAA, multiple sclerosis, and control subjects, but they did not compare biomarker (CSF Aβ42, Aβ40, t-tau, and p-tau) levels to CAA or AD patients. Our aim was to analyze CSF biomarkers in clinically and radiologic well characterized patients with acute phase CAA-I and to compare them with patients with CAA, AD, primary angiitis of the CNS (PACNS) patients, and control subjects.
METHODS
Between November 2011 and November 2014, we prospectively included nine patients with CAA-I according to the Chung criteria in the three participating centers (CHU Nîmes, France; CHU Montpellier, France; CH Narbonne, France). None of our CAA-I patients had prior cognitive impairment according to standard criteria [10].
Informed consent to participate in CSF assessments and analysis was obtained from all patients. Lumbar puncture and CSF analyses were performed in conditions and with techniques previously described [8]. Analyses in CAA-I patients were compared with CSF data of prospectively recruited AD patients (n = 42, all meeting the criteria of probable AD defined by the NINCDS-ADRDA), CAA patients (n = 10), and controls subjects (n = 14) previously enrolled for biomarker analyses in CAA and with patients with PACNS (n = 3) [8]. In this study, however, we only took into account CAA patients with lobar hemorrhage (and thus excluding patients with isolated superficial siderosis only considered by the modified Boston criteria) according to the classical Boston criteria. All control patients underwent MRI including GRE sequences showing absence of abnormalities seen in CAA or CAA-I. In AD patients at time of CSF analysis, mean, standard deviation, median, and range values were respectively 19/30, 5.5, 21/30, and 5-27/30 for the Mini-Mental State examination and 34.5, 22.5, 34.5, and 2–84 for disease duration (months).
Graphic results were presented as medians and interquartile ranges. Statistical pairwise comparisons were performed with the non-parametric Kruskal–Wallis test using the Conover post-hoc method [11]. The H-score corrected for ties is indicated in the text after the p-values.
RESULTS
Clinical, radiological, and CSF characteristics of the nine CAA-I patients are summarized in Table 1. Age between the CAA-I (n = 9), CAA (n = 10), AD (n = 42), and control group (n = 14) did not differ significantly (mean age 70, 77, 73, and 69, respectively). However, PACNS patients (n = 3, mean age 58, range 53–64) were significantly younger than all other groups. CSF analysis was performed in the acute phase in all CAA-I patients, after a mean of 7.5 weeks (range 0.5–44 weeks) after symptoms onset. In one patient (patient nr 3), CAA-I-related symptoms were preceded by a large acute symptomatic lobar hemorrhage a few months earlier. FLAIR and GRE imaging of CAA-I patients are shown in the Supplementary Figure 1. Only two patients (patients 3 and 4) had histological analysis (lobar hemorrhage surgery in patient 3 and biopsy performed for symptoms related to CAA-I in patient 4), confirming CAA-I. ApoE genotype was determined for all CAA-I patients (ɛ4/ɛ4 in patients 1, 2, 4, 7, and 8; ɛ2/ɛ3 in patients 3 and 5; ɛ3/ɛ4 in patient 6; ɛ3/ɛ3 in patient 9), but not systematically for the other groups. Treatment, given to seven patients (all including corticosteroid therapy), led to clinical and radiological improvement in all. The two remaining patients showed spontaneous improvement.
Table 1.
Clinical, radiological, CSF data, and treatment and effect of treatment in CAA-I patients
| Nr | Gender | Age | Symptoms | MB | LH | SS | Gado | Time LP | WBC | Protein | OCB | Treatment Effect |
| 1 | F | 65 | seizure, cognitive deficit | +++ | 0 | 0 | NP | 44 weeks | <3 | 0.45 | No | No / |
| 2 | F | 80 | confusion, aphasia, cognitive deficit | +++ | 0 | 0 | Mild lepto | 6 weeks | <3 | 0.28 | No | CS + |
| 3 | F | 70 | seizure, aphasia, cognitive deficit | 3 | 2 | FSS | Mild lepto | 2 weeks | 6 | 1.09 | Yes | CS + |
| 4 | M | 55 | headache, cognitive and visual deficit | +++ | 0 | 0 | Mild lepto | 2 weeks | <3 | 0.54 | No | CS/AZT/CPM + |
| 5 | F | 78 | apathy, gait disturb, confusion, cognitive deficit | +++ | 0 | DSS | No | 2 weeks | <3 | 1.12 | NP | CS + |
| 6 | F | 76 | confusion, apathy, left neglect | +++ | 0 | 0 | No | 10 weeks | <3 | 0.31 | No | CS + |
| 7 | M | 71 | apathy, confusion | +++ | 0 | FSS | Mild lepto | 4 weeks | <3 | 0.77 | NP | CS + |
| 8 | M | 66 | confusion | +++ | 0 | FSS | No | 3 weeks | <3 | 0.43 | NP | CS + |
| 9 | M | 68 | transient aphasia and faciobrachial paresthesias | +++ | 0 | FSS | Mild lepto | 0.5 week | <3 | 0.62 | No | No / |
Nr, patient number; MB, number of microbleeds; LH, lobar hemorrhage; SS, superficial siderosis; Gado, gadolinium enhancement on MRI; Time LP, delay between symptom onset and performance of lumbar puncture; WBC, number of white blood cells/mm3 in CSF; Protein, protein level (g/L) in CSF; OCB, oligoclonal bands; +++, innumerable (>25 microbleeds); FSS, focal superficial siderosis (superficial siderosis restricted to 3 or fewer sulci); DSS, disseminated superficial siderosis (superficial siderosis affecting at least 4 sulci); NP, not performed; Mild lepto, mild leptomeningeal enhancement; CS, corticosteroids; AZT, azathioprine; CPM, cyclophosphamide.
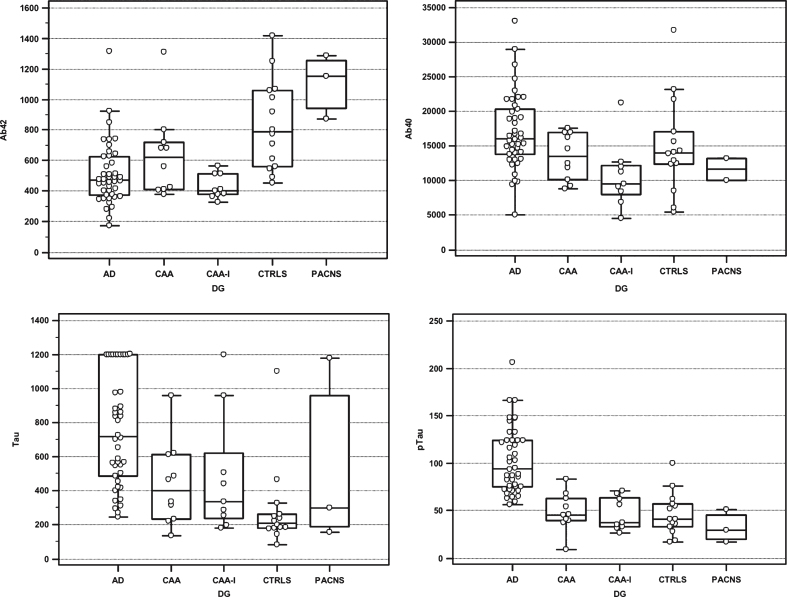
Results of CSF biomarkers in the different patient’s groups are shown in Fig. 1. Median values (in pg/mL) for the nine CAA-I patients were: t-tau 333 (range 179–1200), p-tau 37 (range 26–71), Aβ42 400 (range 326–563), and Aβ40 9457 (range 4486–21205).
Fig.1.
Box-and-whisker diagrams, presented as medians and interquartile ranges of CSF Aβ42, Aβ40, t-tau, and p-tau levels in the AD patients (n = 42), CAA patients (n = 10), CAA-I patients (n = 9), controls (n = 14), and PACNS patients (n = 3).
Compared to controls, CAA-I patients showed significantly lower Aβ42 (p < 0.001) levels, nearly significantly lower Aβ40 (p = 0.051) and higher t-tau (p = 0.051) levels, and comparable p-tau levels. When compared to AD, CAA-I showed significantly lower t-tau (p = 0.018), p-tau (p < 0.001), and Aβ40 (p < 0.001) levels, but not significantly lower Aβ42 (p = 0.35) levels. Compared to CAA, CAA-I had significantly lower Aβ42 (p = 0.027) but not significantly lower t-tau (p = 0.9), p-tau (p = 0.51), and Aβ40 (p = 0.10) levels. With respect to PACNS, CAA-I patients had significantly lower Aβ42 (p = 0.012) but not significantly lower Aß40 (p = 0.35) and higher t-tau (p = 0.78) and p-tau (p = 0.17). For AD, p-tau was the only biomarker significantly different (i.e., higher) compared to each other groups.
In the group of CAA-I group, biomarker values did not differ significantly between the ɛ4/ɛ4 and the non-ɛ4/ɛ4 patients.
One patient had a second CSF biomarker analysis (performed because of clinical and radiological relapse) three months after the first analysis/neurological episode (successfully treated by one month-lasting corticoid treatment). This second analysis showed 326 pg/ml (versus initial 286 pg/ml) for t-tau, 31 pg/ml for p-tau (versus initial 37 pg/ml), 416 pg/ml (versus initial 412 pg/ml) for Aβ42, and 9,799 pg/ml (versus initial 12633 pg/ml) for Aβ40. In this study, only CSF during the first episode was taken into account.
DISCUSSION
To the best of our knowledge, this is the first study to assess CSF Aβ42, Aβ40, t-tau, and p-tau levels in a series of CAA-I patients and to compare them with CAA, AD, PACNS patients, and controls. Based on our data, especially Aβ42 and Aβ40 levels seem to be of particular interest since the lowest levels of both biomarkers were found in the CAA-I group. Aβ42 seemed to be the most specific CAA-I biomarker when compared to the other disorders: indeed, we observed levels significantly lower than CAA, PACNS, and control subjects, and non-significantly lower than AD. However, Aβ42 and Aβ40 levels showed important overlap between the different amyloid-related conditions making interpretation in individual patients difficult. CSF biomarkers, particularly Aβ42, may vary with age. In our study, PACNS patients were significantly younger than the other groups, whereas the age between the CAA-I, CAA, AD, and control groups did not differ significantly. We performed statistical analyses comparing CAA-I patients with a subgroup of four controls with comparable age, and still found very significantly lower Aβ42 levels, making less likely that the lower Aβ42 levels in CAA-I compared to PACNS are only an age-dependent phenomenon. However, interpretation of biomarker levels in the PACNS group should be done with caution because of both a younger age and the limited number of these patients included in the study.
When dosing Aβ42 and Aβ40 levels, investigators and clinicians should bear in mind that amyloid may play a role (and thus potentially affect Aβ42 and Aβ40 levels) in other inflammatory and infectious CNS disorders usually not linked to primary amyloid-related pathophysiological processes (e.g., multiple sclerosis, human immunodeficiency virus) [12–15].
Based on our findings, when comparing CSF biomarkers in AD, CAA, CAA-I, PACNS, and control subjects, high p-tau seems to be the most specific biomarker for AD, whereas as mentioned above low Aβ42 can differentiate CAA-I well from CAA, PACNS, and controls while low Aβ40 can differentiate CAA-I from AD. Aβ concentrations in the CSF are the result of the dynamic equilibrium of brain production, clearance, and accumulation of Aβ. In CAA and CAA-I, both Aβ40 and Aβ42 are probably trapped in the cerebral vasculature, whereas in AD predominant deposition of Aβ42 in diffuse senile plaques probably leads to selective reduction of CSF Aβ42 levels. Our data suggest that entrapment of Aβ42 (and to a lesser degree Aβ40) seems to be even more severe in CAA-I than in CAA, although other mechanisms influencing Aβ42 and Aβ40 levels cannot be excluded.
Compared with a previous study performed in CAA-I patients and showing non-significantly lower Aβ42 levels in CAA-I acute phase as compared with controls, our study showed that Aβ42 levels were significantly lower in CAA-I patients (also analyzed also in the acute phase) [9]. In Piazza and colleagues’ study, Aβ42 (together with Aβ40, tau, and p-tau) levels decreased over time with lowest levels reached in the CAA-I remission phase. The time between symptom onset and CSF analysis seems to not explain the difference between the two studies since in our study CSF analysis was performed also in the acute phase and even earlier (mean of 7.5 weeks versus 3.8 months). Our CAA-I patients were slightly older than in the previous study (mean age of 70 versus 68), but this small age difference is probably insufficient to explain the difference found.
Comparing the biomarkers profile of CAA-I, CAA, and AD seems logical because of shared pathophysiological mechanisms. In daily practice, however, clinical and radiological abnormalities in CAA-I often suggest other differential diagnoses including vasculitis, tumor, and reversible posterior leukoencephalopathy syndrome. In our study, histology was available in a minority of CAA-I cases. Ideally, CSF biomarkers should be analyzed in a larger number of histologically proven CAA-I patients and compared to patients with other disorders mimicking CAA-I. If our observations can be confirmed, it might be interesting to integrate CSF data in the CAA-I diagnostic criteria in the future. Our study was mainly focused on AD biomarkers in CSF. In addition, dosing inflammatory markers (e.g., interleukin-6) and microglial-derived proteins (e.g., monocyte chemotactic protein-1 and YKL-40) might be interesting in order to see if these markers can help to differentiate inflammatory from non-inflammatory CNS disorders.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Mariella Lomma (Department of Biostatistics, Nimes University Hospital, 4 Rue du Pr Debré, 30029 Nîmes, France) for her help in editing assistance.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0621r2).
Appendix
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150621.
REFERENCES
- [1]. Scolding NJ, Joseph F, Kirby PA, Mazanti I, Gray F, Mikol J, Ellison D, Hilton DA, Williams TL, MacKenzie JM, Xuereb JH, Love S (2005) Abeta-related angiitis: Primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain 128, 500–515. [DOI] [PubMed] [Google Scholar]
- [2]. Chung KK, Anderson NE, Hutchinson D, Synek B, Barber PA (2011) Cerebral amyloid angiopathy related inflammation: Three case reports and a review. J Neurol Neurosurg Psychiatry 82, 20–26. [DOI] [PubMed] [Google Scholar]
- [3]. Melzer N, Harder A, Gross CC, Wölfer J, Stummer W, Niederstadt T, Meuth SG, Marziniak M, Grauer OM, Wiendl H (2012) CD4(+) T cells predominate in cerebrospinal fluid and leptomeningeal and parenchymal infiltrates in cerebral amyloid β-related angiitis. Arch Neurol 69, 773–777. [DOI] [PubMed] [Google Scholar]
- [4]. Salvarani C, Hunder GG, Morris JM, Brown RD Jr, Christianson T, Giannini C (2013) Aβ-related angiitis: Comparison with CAA without inflammation and primary CNS vasculitis. Neurology 81, 1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Danve A, Grafe M, Deodhar A (2014) Amyloid beta-related angiitis–a case report and comprehensive review of literature of 94 cases. Semin Arthritis Rheum 44, 86–92. [DOI] [PubMed] [Google Scholar]
- [6]. Schwab P, Lidov HG, Schwartz RB, Anderson RJ (2003) Cerebral amyloid angiopathy associated with primary angiitis of the central nervous system: Report of 2 cases and review of the literature. Arthritis Rheum 49, 421–427. [DOI] [PubMed] [Google Scholar]
- [7]. Verbeek MM, Kremer BP, Rikkert MO, Van Domburg PH, Skehan ME, Greenberg SM (2009) Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol 66, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Renard D, Castelnovo G, Wacongne A, Le Floch A, Thouvenot E, Mas J, Gabelle A, Labauge P, Lehmann S (2012) Interest of CSF biomarker analysis in possible cerebral amyloid angiopathy cases defined by the modified Boston criteria. J Neurol 259, 2429–2433. [DOI] [PubMed] [Google Scholar]
- [9]. Piazza F, Greenberg SM, Savoiardo M, Gardinetti M, Chiapparini L, Raicher I, Nitrini R, Sakaguchi H, Brioschi M, Billo G, Colombo A, Lanzani F, Piscosquito G, Carriero MR, Giaccone G, Tagliavini F, Ferrarese C, DiFrancesco JC (2013) Anti-amyloid β autoantibodies in cerebral amyloid angiopathy-related inflammation: Implications for amyloid-modifying therapies. Ann Neurol 73, 449–458. [DOI] [PubMed] [Google Scholar]
- [10]. American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th ed American Psychiatric Association, Washington, DC. [Google Scholar]
- [11]. Conover WJ (1999) Practical nonparametric statistics, 3rd editionJohn Wiley & Sons, New York. [Google Scholar]
- [12]. Chandra A (2015) Role of amyloid from a multiple sclerosis perspective: A literature review. Neuroimmunomodulation 22, 343–346. [DOI] [PubMed] [Google Scholar]
- [13]. Gentile A, Mori F, Bernardini S, Centonze D (2015) Role of amyloid-β CSF levels in cognitive deficit in MS. Clin Chim Acta 449, 23–30. [DOI] [PubMed] [Google Scholar]
- [14]. David MA, Tayebi M (2014) Detection of protein aggregates in brain and cerebrospinal fluid derived from multiple sclerosis patients. Front Neurol 5, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Achim CL, Adame A, Dumaop W, Everall IP, Masliah E, Neurobehavioral Research, Center (2009) Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol 4, 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150621.