Abstract
The naturally occurring compound α-pinene induces cell cycle arrest and antitumor activity. We examined effects of α-pinene on cell cycle regulation in hepatocellular carcinoma cells (HepG2) cells to establish a foundation for its development as a novel treatment for hepatocellular carcinoma (HCC). HepG2 cells treated with α-pinene exhibited dose-dependent growth inhibition as a result of G2/M-phase cell cycle arrest. Cell cycle arrest was associated with down-regulated cyclin-dependent kinase 1 (CDK1) and miR-221 levels and up-regulated levels of CDKN1B/p27, γ-H2AX, phosphorylated ATM, phosphorylated Chk2 and phosphorylated p53. Our observations are consistent with a model in which α-pinene inhibits miR221 expression, which leads to G2/M-phase arrest and activation of CDKN1B/p27-CDK1 and ATM-p53-Chk2 pathways that suppress human hepatoma tumor progression. Additionally, α-pinene was found to trigger oxidative stress and induce apoptosis of HepG2 cells. α-pinene, therefore, represents a potential chemotherapeutic compound for the treatment of HCC.
Keywords: α-pinene, anti-hepatoma carcinoma, apoptosis, in vitro, miR-221, miRNA
Introduction
The incidence of hepatocellular carcinoma (HCC), a highly malignant cancer, is increasing dramatically with annual new cases estimated at 600,000 worldwide [1,2]. Relatively, few cases can be successfully treated by surgery and the 5-year survival rate for HCC is less than 9% [3]. This is a very low treatment efficacy compared with other major malignancies. Therefore, it is an urgent need to identify novel, effective chemotherapeutic drugs to improve HCC patients’ survival and life quality.
The naturally occurring compound α-pinene (Figure 1) can be readily isolated from pine needles and reportedly exhibits antitumor [4,5], antimycotic [6,7] (Jeong, 2007 #13; Pichette, 2006 #12; Pichette, 2006 #12), and antianaphylactic [8] activities. Of particular interest, α-pinene has shown potential as an inhibitor of cell proliferation, inducing cell cycle arrest and suppressing angiogenesis in various cancers through effects on multiple molecular targets and signaling pathways [9].
Figure 1. The chemical structure of α-pinene.
HepG2 cells are derived from human hepatoblastoma and have high degree of malignancy. And their intrinsic activity of drug-metabolizing enzymes is stable and does not decrease with the increasing passages [10]. We examined the molecular mechanisms underlying α-pinene antihepatoma activity using HepG2 cells, laying a foundation for the development of α-pinene as a novel anticancer drug.
miRNAs are a small, noncoding RNAs that function in post-transcriptional regulation of gene expression. miRNAs are known to regulate the expression of oncogenes and tumor suppressor genes, and they have thus become the focus of research on anticancer drug mechanisms [7,8]. miR-221 is a key regulatory miRNA [9], the expression of which is increased in HCC, compared with normal hepatic tissue. miR-221 plays an important role in HCC tumorigenesis, possibly through specific down-regulation of CDKN1B/p27 [11,12]. Indeed, CDKN1B/p27 is a direct target of miR-221 [13] and when miR-221 is increased the expression of CDKN1B/p27 is down-regulated [12]. While CDKN1kB/p27 is thought to regulate the G1/S phase transition, research has shown that CDKN1B/p27 can bind to and inhibit the CDK1/cyclin B1 complex to block the cell cycle at G2/M phase [11]. Additionally, an active cyclin-CDK protein kinase complex promotes phosphorylation of a variety of proteins involved in cell cycle regulation. Two categories of CDK inhibitors (CDKIs) are recognized: the p16 family including p16, p15, p18, and p19 that specifically inhibit CDK4 and CDK6; and the p21 family including p21, CDKN1B/p27, and p57 that exhibit broad-spectrum CDK inhibition [14]. Thus, inhibition of miR-221 expression, thereby increasing CDKN1B/p27 activity might effectively inhibit HCC development.
We examined whether α-pinene might act to regulate the expression of miR-221 and relevant signaling pathways impacting cell cycle dynamics in response to DNA damage involved in HCC development. In response to DNA damage, activated ATM rapidly phosphorylates p53 on Ser15. Phosphorylated p53 dissociates from MDM2 and binds transcription factor CBP/300 which leads to acetylation of the carboxyl-terminal lysine 382 residue of p53 and completion of the damage-repair process [15,16]. ATM also activates Chk2 in response to DNA damage signals following exposure to ionizing radiation or chemotherapeutic agents [17]. We used Western blot analysis, immunofluorescence detection, and qPCR to examine cell cycle-related key regulatory factors (miR-221, CDKN1B/p27, cyclin-dependent kinase 1 [CDK1], γ-H2AX, H2AX, phos-ATM [Ser1981], ATM phos-Chk2 [Thr68], Chk2 and phos-p53) in HepG2 cells.
Reactive oxigen species (ROS) not only play an important role in redox regulation during normal physiological functions but are highly reactive molecules that have the potential to cause cellular damage, including damaging DNA, RNA, and proteins and degrading essential cellular molecules [18], which will lead to cells death. The mechanisms of ROS-induced apoptosis typically include receptor activation, caspase activation, Bcl-2 family proteins, and mitochondrial dysfunction [19]. Additionally, ROS-induced apoptosis is associated with decreased generation of glutathione (GSH) levels and the loss of redox homeostasis [20]. N-acety1-L-cysteine (NAC) is an amino with a sulfhydry1 group, it promotes the generation of glutathione, the principle intracellular antioxidant molecule. Thus, it acts as a direct ROS scavenger [21,22]. We performed experiments with DCFH-DA method and Annexin V-FITC/PI to examine whether the increasing of intracellular ROS levels would lead to the apoptosis of HepG2 cells. These studies provide novel molecular insights into the biological basis of potential anticancer efficacy of α-pinene.
Materials and methods
Materials
The following reagents were used: α-pinene, resveratrol (RSV), MTT (Sigma–Aldrich, St Louis, MO, U.S.A.); TRIzol Reagent (Life Technologies, Inc., Rockville, MD, U.S.A.); LipfofectamineTM 3000 Reagent (Invitrogen, CA, U.S.A.); Prime ScriptTM RT Reagent Kit and SYBR® Premix Ex TaqTMII (Takara Bio, Otsu, Japan). miR-221 and U6 specific primers for reverse transcription and PCR were purchased from Ribobio CO. LTD, Guangzhou, China. CDKN1B/p27 was purchased from Abcam, Cambridge, U.S.A. γ- H2AX, H2AX, phos-ATM (Ser1981), ATM, phos-Chk2 (Thr68), Chk2, p53, and phos-p53 were purchased from Cell Signaling Technology Inc., U.S.A.
Cell culture
Liver cancer HepG2 cells, breast cancer MCF-7 cells, lung cancer A549 cells, and neuroma cancer PC-12 cells were obtained from the China Center for Type Culture Collection of Wuhan University. Cells were cultured in DMEM containing 10% new-born calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin and incubated at 37°C in a humidified atmosphere containing 5% of CO2. Log phase cells were collected after several passages. DMSO concentration was maintained below 0.1%.
MTT assay
Cells in logarithmic phase were harvested, adjusted to 5 ×104 cells/ml, and seeded into 96-well culture plates at 100 μl per well. At the beginning, cells were exposed to 0, 2, 4, 8, 16, 32, 64, 128, 256 μmol/l or higher concentrations of α-pinene for 24 h. RSV was used as a positive control for anti-HCC activity and added to a concentration of 128 μmol/l [23]. After treatment, 5 mg/ml of MTT was added and cells were incubated at 37°C in the darkness for 24 h. After discarding the supernatant, 75 μl of DMSO was added and plates were placed on a rotary shaker for 15 min. A Bio-Rad iMark microplate reader (Richmond, CA, U.S.A.) was used to determine the absorbance of each well at 570 nm.
Cell cycle analysis
Flow cytometry (FCM) was used to determine cell cycle distribution. Briefly, after treatment with 0, 16, 32, or 64 μmol/l of α-pinene for 24 h, HepG2 cells were harvested and fixed in 70% ethanol overnight at 4°C. Cells were subsequently resuspended in 0.5 ml and 50 mg/l PI staining solution, kept in the darkness at room temperature for 30 min, and analyzed using a BD Accuri™ C6 Plus System (BD Biosciences, San Jose, U.S.A.). The cell cycle distribution was determined using ModFit LTTM software.
Quantitative real-time PCR analysis
HepG2 cells cultured in six-well plates were treated with 64 μmol/l α-pinene for 24 h. TRIzol reagent was used to extract total RNA according to standard procedure. Prime ScriptTM RT Reagent Kit (Takara Bio, Otsu, Japan) with Oligo dT primer or Bulge-LoopTM miRNA qRT-PCR (Ribobio CO. LTD, Guangzhou, China) with Bulge-LoopTM specific primer were used for reverse transcription. Quantitative PCR was performed using aCFX96 real-time PCR Detection System and standard conditions as described for SYBR® Premix Ex TaqTM II (Takara Bio, Otsu, Japan). Experiments were performed in triplicate. Samples were normalized to internal controls and fold changes were calculated based on the formula 2−ΔΔCT. RNU6B(U6) was used as internal control for miRNA and β-actin as internal control for mRNAs. Primers were based on Genebank sequences and designed using Express Primer 310 software. BLAST was used to confirm homology. CDKN1B/p27, CDK1M, and β-actin primers were synthesized by the LiuheHuada company, Beijing, China. Primer sequences: CDKN1B/p27 (forward, 5′- AAA CTA CAG GTC AAG TGG TAGC CA -3′, reverse, 5′- GTC TGT AGT AGA ACT CGGG -3′), CDK1 (forward, 5′- CCA GGA GTT ACT TCT ATG CCT GA-3′, reverse, 5′- TCC TGC ATA AGC ACA TCC TGA -3′), β-actin (forward, 5′- TCA CCC ACA CTG TGC CCA TCT ACGA-3′, reverse, 5′- CAG CGG AAC CGC TCA TTG CCA ATGG -3′).
Cell transfection
LipofectamineTM 3000 was used to introduce miR-221 mimic into HepG2 cells. HepG2 cells were seeded into six-well plates and 70–90% confluent at the time of transfection. LipofectamineTM 3000 and miR-221 mimic were diluted in Opti-MENTM Medium. Diluted LipofectamineTM 3000 and miR-221 mimic were combined at a 1:1 ratio and incubated at room temperature for approximately 15 min. The complex was added to the HepG2 cells for 6–16 h at 37 °C. Subsequently, α-pinene was added to the cells at a concentration of 64 μmol/l and incubated for another 24 h. Cells were then harvested for qPCR analysis.
Western blot analysis
HepG2 cells treated with 0, 16, 32, or 64 μmol/l of α-pinene for 24 h were harvested by incubating cells in ice-cold cell lysis buffer for Western blot analysis (Beyotime Biotechnology, Shanghai, China). Protein concentration was determined by using a BCA kit (Beyotime Biotechnology, Shanghai, China) according to manufacturer’s instructions. Approximately 20 μg of total protein was separated by 12% SDS/PAGE and transferred to PVDF membrane. The membrane was blocked using 5% (w/v) nonfat milk at room temperature for 1 h, then incubated with primary antibodies (specific for CDKN1B/p27, CDK1, γ- H2AX, H2AX, phos-ATM [Ser1981], ATM, phos-Chk2 [Thr68], Chk2 or phos-p53) in 5% nonfat milk overnight at 4°C. Secondary antibodies conjugated with horseradish peroxidase were diluted 1:2000 in 5% nonfat milk and incubated for 1 h at room temperature. Antibody signals were detected by ECL and analyzed using ImageJ software. β-actin was used as a protein-loading control.
Immunofluorescence analysis
Cells were fixed in precooled (−20°C) acetone-methanol solution for 15 min. Primary antibodies anti-γH2AX (upstate, 0.5 μg/ml) and antiphospho-ATM (S1981) (cell signaling, 1:100) were incubated at 4°C overnight. After washing, secondary antibodies FITC-Donkey antirabbit IgG (1:200, Jackson Immuno Research Lab) and Rhodamine-Donkey antimouse IgG (1:200, Jackson Immuno Research Lab) were incubated at room temperature for 1 h. The cells were then covered with VECTASHIELD mounting medium containing DAPI (VECTOR Lab Inc., Burlingame, CA94010). Cells were visualized using a fluorescence microscope (Carl Zeiss, Axiovert 200).
ROS measurement
Cellular ROS was measured with DCFH-DA, which was employed to measure ROS production. HepG2 cells were treated with 0, 16, 32, 64 μmol/l of α-pinene, and 10 mmol/l of NAC+64 μmol/l α-pinene (pretreated with 10 mmol/l NAC for 2 h and then exposed to 64 μmol/l of α-pinene) for 24 h. DCFH-DA was then added to a final concentration of 10 μmol/l and incubated for 20 min at 37°C. Fluorescence intensities were monitored by fluorescence microscopy (Carl Zeiss, Axiovert 200), and the images were semiquantitatively analyzed with ImageJ.
Apoptosis analysis
FCM was used to analyze early and late phases of apoptotic cells. Briefly, after treatment with 0, 16, 32, 64 μmol/l of α-pinene, and 10 mmol/l NAC+64 μmol/l of α-pinene for 24 h, HepG2 cells were collected and washed twice with cold PBS. After resuspending in Binding Buffer and FITC-Annexin V for 15 min, PI staining solution was added. The cells were kept in the darkness and analyzed using a BD Accuri™ C6 Plus System (BD Biosciences, San Jose, U.S.A.) within 30 min.
Statistical analysis
All experiments were repeated at least three times. Data were analyzed using SPSS 19.0 software (SPSS 19.0 for Windows; SPSS, Inc. Chicago, IL, U.S.A.) and presented as mean ± S.D. for three separate experiments. Differences amongst three or more groups were analyzed by one-way ANOVA for multiple comparisons. P<0.05 was considered significant.
Results
α-pinene induced the cancer cells toxicity
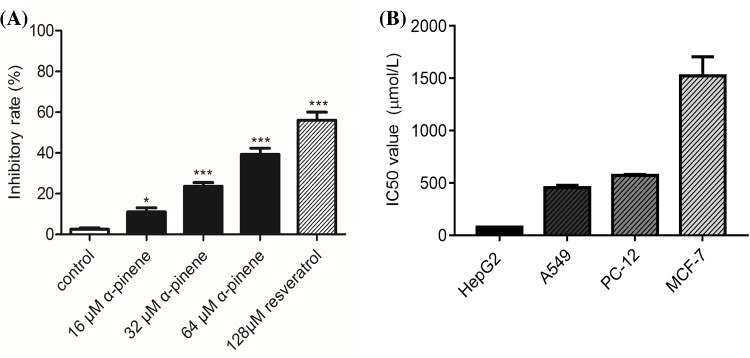
According to the MTT assay results, after exposed to α-pinene for 24 h, as shown in Figure 2A, α-pinene inhibited the proliferation of HepG2 cells in a dose-dependent manner. At a concentration of 64 μmol/l α-pinene inhibited HepG2 cells proliferation by approximately 39.3 ± 4.2%. The proliferation activities of the cells were inhibited in an order from strong to weak, which were HepG2 cells, A549 cells, PC-12 cells, and MCF-7 cells. And the IC50 value of a-pinene on each cell lines was displayed in Figure 2B. So we chose HepG2 cells for the subsequent research.
Figure 2. Inhibitory rate and the IC50 value of α-pinene on tumor cells.
(A) Inhibitory effects of α-pinene on HepG2 cell proliferation. MTT assay results. HepG2 cells were treated for 24 h with 0, 16, 32, 64 μmol/l of α-pinene. Positive control cells were treated with 128 μmol/l resveratrol. (B) The IC50 value of a-pinene on HepG2 cells, A549 cells, PC-12 cells and MCF-7 cells.
α-pinene induced G2/M phase cell cycle arrest
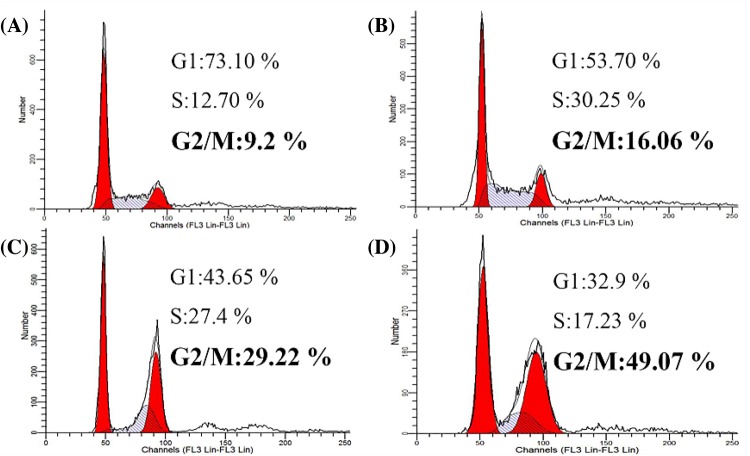
FCM was used to analyze the cell cycle distribution of HepG2 cells following 24 h of treatment with increasing concentrations of α-pinene. As shown in Figure 3, treatment resulted in arrest at G2/M phase, and the percentage of HepG2 cells at G2/M was 9.2, 16.06, 29.22, and 49.07% in the 0, 16, 32, and 64 μmol/l α-pinene treatment groups, respectively.
Figure 3. Cell cycle distribution following treatment with α-pinene.
(A) Nontreated control cells. (B) Treatment with 16 μmol/l α-pinene for 24 h. (C) Treatment with 32 μmol/l of α-pinene for 24 h. (D) Treatment with 64 μmol/l of α-pinene for 24 h.
Effects of α-pinene treatment on miR-221 and CDKN1B/p27-CDK1 signaling pathway components
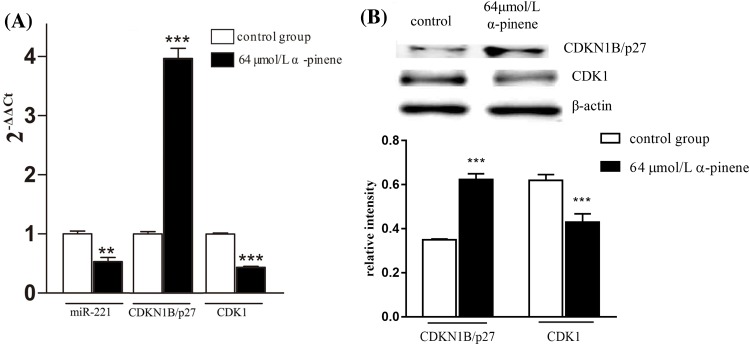
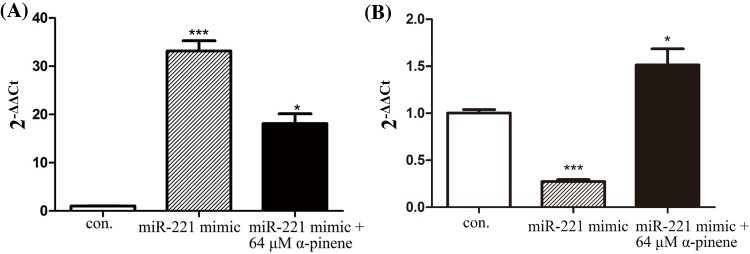
To better understand the molecular mechanisms by α-pinene induced G2/M phase cell cycle arrest in HepG2 cells, we analyzed miR-221 and CDKN1B/p27-CDK1 signaling pathway dynamics. As shown in Figure 4, α-pinene treatment led to miR-221 and CDK1 down-regulation, while CDKN1B/p27 was up-regulated. To further study effects of α-pinene on miR-221 and CDKN1B/p27, we introduced a miR-221 mimic into HepG2 cells by transfection and treated the cells with 64 μmol/l α-pinene for 24 h. As shown in Figure 5A, in contrast with control cells, miR-221 in transfected cells was markedly up-regulated. Nevertheless, α-pinene treatment was associated with miR-221 down-regulation in both control and transfected cells. As shown in Figure 5B, in HepG2 cells transfected with miR-221 mimic the expression of CDKN1B/p27 was significantly down-regulated. Treatment with α-pinene led to up-regulated CDKN1B/p27 expression.
Figure 4. Effects of α-pinene on expression of miR-221, CDKN1B/p27, and CDK1 in HepG2 cells.
(A) qPCR analysis of α-pinene induced changes in miR-221, CDKN1B/ p27, and CDK1 expression. (B) Western blot analysis of α-pinene induced changes in CDKN1B/p27 and CDK1 protein level. Results represent three independent experiments. **P<0.01 and ***P<0.001 (compared with control).
Figure 5. Effects of α-pinene on expression of miR-221 and CDKN1B/p27 in HepG2 cells transfected with miR-221 mimic.
(A) Effects of α-pinene on expression of miR-221 in HepG2 cells transfected with miR-221 mimic. (B) Effects of α-pinene on expression of CDKN1B/p27 in HepG2 transfected with miR-221 mimic. Results represent three independent experiments. *P<0.05, and ***P<0.001; n.s: not significant (compared with control).
Effects of α-pinene treatment on ATM-p53-Chk2 signaling pathway components
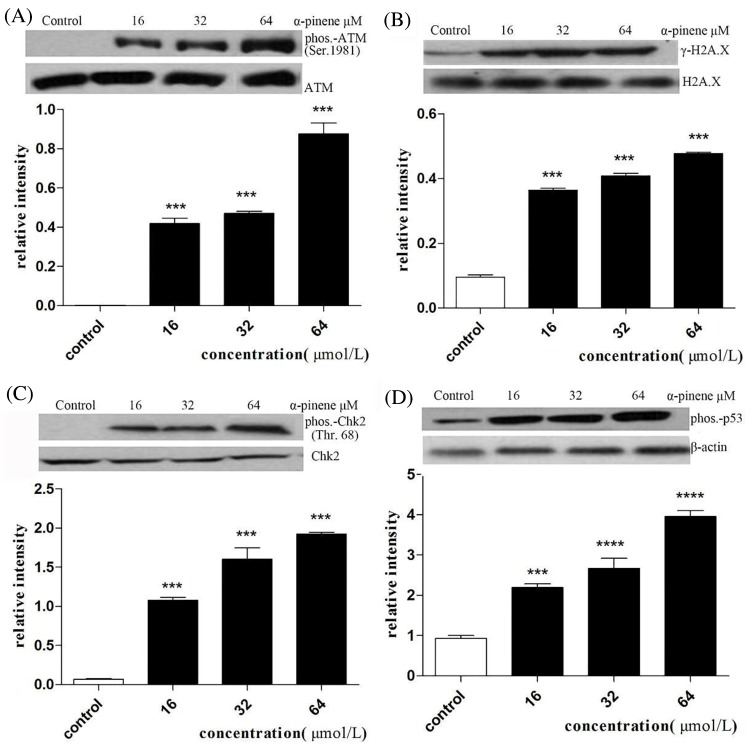
Western blot analysis of proteins involved in cell cycle regulation was performed to better understand the mechanism of α-pinene induced G2/M phase arrest of HepG2 cells. As shown in Figure 6, phosphorylated ATM (Ser1981), γ-H2AX, phosphorylated p53 and phosphorylated Chk2 (Thr68) exhibited dose-dependent up-regulation following α-pinene treatment of HepG2 cells.
Figure 6. Effects of α-pinene on expression of phosphorylated ATM (Ser1981), γ-H2AX, phosphorylated p53 and phosphorylated Chk2 (Thr68)in HepG2 cells.
(A) relative abundance of γ-H2AX compared with H2AX (B) relative abundance of phosphorylated ATM (Ser1981) compared with ATM (C) relative abundance of phosphorylated Chk2 (Thr68) compared with Chk2 (D) relative abundance of phosphorylated p53 compared with β-actin. Results represent three independent experiments.***P<0.001, ****P<0.0001 (compared with control).
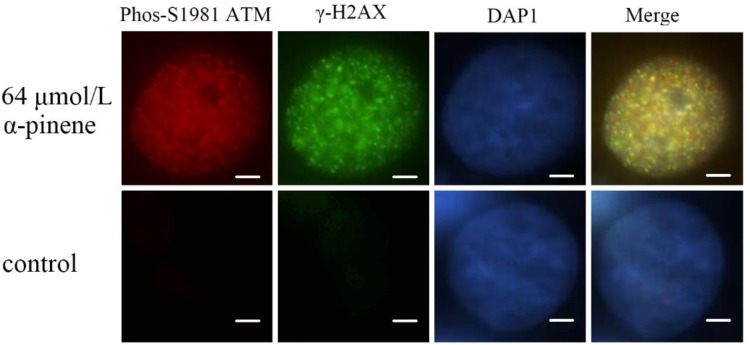
Immunofluorescence analysis was used to further clarify effects of α-pinene treatment on ATM and H2AX proteins in HepG2 cells. As can be seen in Figure 7, the nuclear localization of phosphorylated-ATM (S1981) and γ-H2AX in α-pinene treated HepG2 cells was much more pronounced than in nontreated control cells.
Figure 7. α-pinene treatment led to increased ATM (S1981) phosphorylation and γ-H2AX nuclear foci.
HepG2 cells were treated with DMSO (control) or 64 μmol/l of α-pinene for 24 h, followed by double immunofluorescence staining for ATM (S1981) phosphorylation (red), and γ-H2AX (green). Nuclei were counterstained with DAPI (blue). Scale bars represent 5 μm.
α-pinene triggered oxidative stress and induced apoptosis
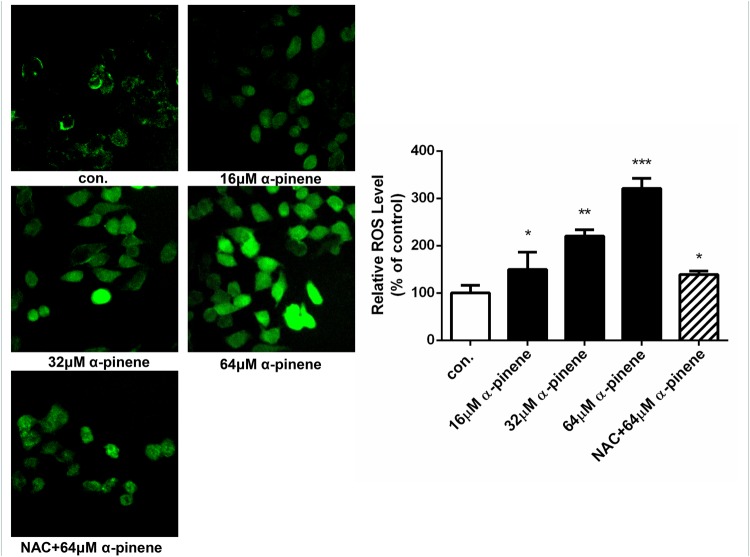
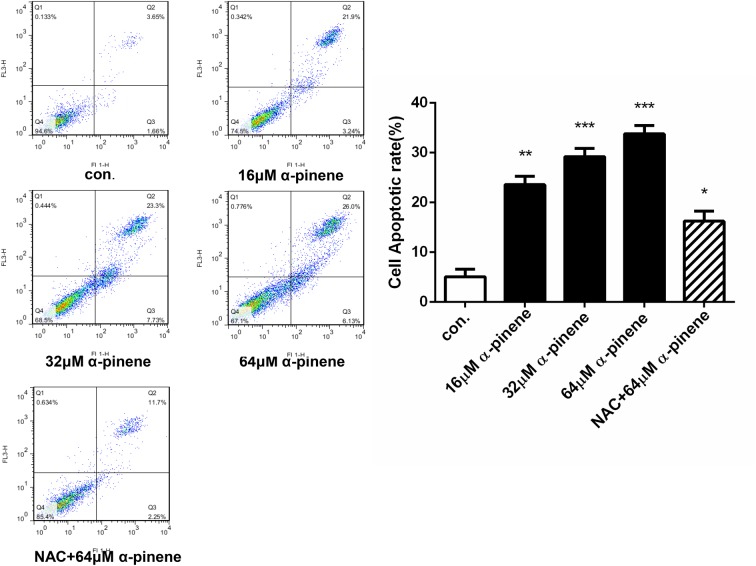
The cytoyoxic effects of α-pinene on HepG2 cells are probably mediated through ROS production, which is an important intracellular signal of cell proliferation, cell death, and homeostasis, etc. As shown in Figure 8, the brightness of fluorescence and ROS level increased in a concentration-dependent manner, and the differences were significant. When the cells were protected by NAC, the brightness and ROS level decreased clearly. From Figure 9, after treated with various concentrations (0, 16, 32, and 64 μmol/l) of α-pinene, the HepG2 cells apoptotic rate increased significantly, and in comparison with the 64 μmol/l α-pinene treatment group, the apoptotic rate of NAC protective group decreased markedly.
Figure 8. HepG2 cells were exposed to 0, 16, 32, 64 μmol/l α-pinene, and NAC+64 μmol/l α-pinene for 24 h.
α-pinene induced ROS level increased and pretreated with NAC decreased the ROS level. Results represent three independent experiments. *P<0.05, **P<0.01, and ***P<0.001, n.s: not significant (compared with control).
Figure 9. HepG2 cells were exposed to 0, 16, 32, 64 μmol/l α-pinene, and NAC+64 μmol/l α-pinene for 24 h.
α-pinene elicited apoptosis in HepG2 cells and pretreated with NAC had protective effective. Results represent three independent experiments. *P<0.05, **P<0.01, and ***P<0.001; n.s: not significant (compared with control).
Discussion
HCC, the predominant primary liver cancer, is the second most common cause of cancer death around the world [24]. However, the conventional therapies perform great side effect on human body. Therefore, it is imperative to find effectively drugs with fewer side effect for the malignant tumor. In this work, α-pinene displayed a promising miR-221 regulation, cell cycle arrest, ROS production increasing, and proapoptosis effect on HepG2 cells, suggesting that α-pinene impacts cell cycle dynamics in response to DNA damage and induces cytotoxic effect involved in HCC development. The results of the present study may provide a new option for the treatment of HCC.
In the present study, α-pinene was shown to induce G2/M phase cell cycle arrest in HepG2 cells, consistent with previous studies using a different HCC cell line, Bel-7402 [5]. However, previouse study showed that RSV arrested GepG2 cells in S phase [25], they showed different mechanism on the growth inhibition of GepG2 cells, so we did not used it as a positive control in the subsequent experiments. The G2/M checkpoint is the final opportunity for the repairment of damaged DNA prior to mitosis. To further explore the mechanism of α-pinene effects on HepG2 cells, we analyzed changes induced by α-pinene treatment on miR-221, CDKN1B/p27, CDK1, γ-H2AX, H2AX, phosphorylated ATM (Ser1981), ATM, phosphorylated Chk2 (Thr68), Chk2, and phosphorylated p53, all of which have roles in G2/M phase cell cycle regulation.
We observed α-pinene treatment-induced down-regulation of miR-221 and CDK1 and up-regulated expression of CDKN1B/p27. Transfection of HepG2 cells with a miR-221 mimic successfully increased miR-221 levels and led to decreased CDKN1B/p27. However, transfected cells treated with 64 μmol/l of α-pinene exhibited down-regulated miR-221 levels and up-regulation of CDKN1B/p27. This may indicate that α-pinene activates the CDKN1B/p27-CDK1 signaling pathway by the inhibition of pathway inhibitor miR-221, leading to G2/M-phase cell cycle arrest. Our previous studies showed that α-pinene reduces the expression of cyclinB1 and CDK1 proteins in tumor tissue [4,5]. To determine whether this is related to the α-pinene induced miR-221-mediated effects on CDKN1B/p27-CDK1 pathway, activation observed in the current study will require further analysis.
We also found evidence that α-pinene treatment induced dose-dependent up-regulation of γ-H2AX, phosphorylated ATM (S1981), phosphorylated CHK2 (T68), and phosphorylatedp53. These observations suggest that α-pinene can activate the ATM-p53-Chk2 signaling pathway to induce G2/M phase arrest, thereby inhibiting HepG2 cell proliferation.
Elevated levels of ROS are thought to be oncogenic, causing damage to DNA, proteins and lipids, promoting genetic instability, and tumorigenesis [26]. α-pinene increased ROS production in a dose-dependent manner. When pretreated with NAC, the ROS production increased less than only treated with 64 μmol/l of α-pinene. Apoptosis is a programmed cell death mediated through the death receptor-mediated extrinsic pathway and the mitochondrial-mediated intrinsic pathway. Our study revealed that α-pinene induced the HepG2 cells apoptosis in a dose-dependent manner, and NAC has a protective effect. These results indicated that the mechanism of α-pinene induced apoptosis of HepG2 cells may be related to oxidative stress.
The current study provides promising in vitro evidence that α-pinene effectively modulates miR-221 levels and activates the CDKN1B/p27-CDK1 and ATM-p53-Chk2 pathways, leading to G2/M phase cell cycle arrest of HepG2 cells. In addition, α-pinene increases ROS production and induces apoptosis in GepG2 cells. The pharmacokinetics of α-pinene in liver remains unclear so far, much more work employing additional cellular, and in vivo animal model studies will be needed to verify the translational potential of α-pinene as a chemotherapeutic agent useful for the treatment of HCC.
Acknowledgments
We sincerely thank Prof. Lianbao Ye for expert technical advice.
Abbreviations
- ATM
Ataxia Telangiectasia Mutated
- CDK1
cyclin-dependent kinase 1
- CDKI
CDK inhibitor
- DCFH-DA
2′,7′-Dichlorodihydrofluorescein diacetate
- DMSO
Dimethyl sulfoxide
- GSH
generation of glutathione
- HCC
hepatocellular carcinoma
- HepG2
hepatocellular carcinoma cell
- NAC
N-acety1-L-cysteine
- PI
Propidium Iodide
- ROS
reactive oxigen species
- RSV
resveratrol
Funding
This work was supported by funding from the Science and Technology Planning Project of Guangdong Province, China [grant numbers 2016A020215159, 2016A020215156].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
W.C. designed the present study. Q.X. and M.L. performed the experiments, analyzed and interpreted the data, and drafted the manuscript. M.Y. and J.Y. performed the experiments and helped to interpret the data. J.X., X.L., and F.W. helped to interpret the data. In addition, all authors have read and approved the manuscript.
References
- 1.Siegel R., Ma J., Zou Z. and Jemal A. (2014) Cancer statistics, 2014. CA-Cancer J. Clin. 64, 9–29 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., Siegel R.L., Lin C.C., Mariotto A.B., Kramer J.L. and Rowland J.H. (2016) Cancer treatment and survivorship statistics, 2016. CA-Cancer J. Clin. 66, 271–289 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 3.Mcglynn K.A., Petrick J.L., London W.T., Mcglynn K.A., Petrick J.L. and London W.T. (2015) Global epidemiology: an emphasis on demographic and regional variability. Clin. Liver Dis. 19, 223–238 10.1016/j.cld.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W.Q., Liu Y., Li M., Mao J., Zhang L. and Huang R. (2015) Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharmacol. Sci. 127, 332–338 10.1016/j.jphs.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 5.Chen W.Q., Xu B., Mao J.W., Wei F.X., Li M. and Liu T. (2014) Inhibitory effects of α-pinene on hepatoma carcinoma cell proliferation. Asian Pac. J. Cancer Prev. 15, 3293–3297 10.7314/APJCP.2014.15.7.3293 [DOI] [PubMed] [Google Scholar]
- 6.Pichette A., Larouche P.L., Lebrun M. and Legault J. (2006) Composition and antibacterial activity of Abies balsa-mea essential oil. Phytother. Res. 20, 371–373 10.1002/ptr.1863 [DOI] [PubMed] [Google Scholar]
- 7.Jeong S.I., Lim J.P. and Jeon H. (2007) Chemical composition and antibacterial activities of the essential oil fromAbieskoreana. Phytother. Res. 21, 1246–1250 10.1002/ptr.2229 [DOI] [PubMed] [Google Scholar]
- 8.Nam S.Y., Chung C.K., Seo J.H., Rah S.-Y., Kim H.-M. and Jeong H.-J. (2014) The therapeutic efficacy of α-pinene in an experimental mouse model of allergic rhinitis. Int. Immunopharmacol. 23, 273–282 10.1016/j.intimp.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Guo S. and Liu X. (2014) Synergistic antitumor effect of α-pinene and β-pinene with paclitaxel againstnon-small-cell lung carcinoma (NSCLC). Drug Res. (Stuttg) 3, 113–119 [DOI] [PubMed] [Google Scholar]
- 10.Yao X.F. and Zhong L.F. (2007) Application of human hepatoma cell line HepG2 and its progress in the detection of genotoxicants. Shijie Huaren Xiaohua Zazhi 15, 145–150 [Google Scholar]
- 11.Yang N., Ekanem N.R., Sakyi C.A. and Ray S.D. (2014) Hepatocellular carcinoma and microRNA: new perspectives on therapeutics and diagnostics. Adv. Drug. Deliv. Rev. 81, 62–74 10.1016/j.addr.2014.10.029 [DOI] [PubMed] [Google Scholar]
- 12.Sethi S., Li Y. and Sarkar F.H. (2013) Regulating miRNA by natural agents as a new strategy for cancer treatment. Curr. Drug Targets 14, 1167–1174 10.2174/13894501113149990189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Moralli S., Tarrado-Castellarnau M., Miranda A. and Cascante M. (2013) Targeting cell cycle regulation in cancer therapy. PharmacolTher 138, 255–271 [DOI] [PubMed] [Google Scholar]
- 14.Dai Y. and Grant S. (2003) Cyclin-dependent kinase inhibitors. Curr. Opin. Pharmacol. 5, 235. [DOI] [PubMed] [Google Scholar]
- 15.Fernandezcapetillo O., Chen H.T., Celeste A., Ward I., Romanienko P.J. and Morales J.C. (2002) DNA damage-induced G2/M checkpoint activation by histone H2AX and 53BP1. Nat. Cell Biol. 4, 993 10.1038/ncb884 [DOI] [PubMed] [Google Scholar]
- 16.Chen L., Gilkes D.M., Pan Y., Lane W.S. and Chen J. (2005) ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage. EMBO J. 24, 3411–3422 10.1038/sj.emboj.7600812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata A., Barton O., Noon A.T., Dahm K., Deckbar D. and Goodarzi A.A. (2010) Role of ATM and the damage response mediator proteins 53BP1 and MDC1 in the maintenance of G(2)/M checkpoint arrest.. Mol. Cell. Biol. 30, 3371–3383 10.1128/MCB.01644-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang K.M. and Kim BM. (2014) ω-Hydroxyundec-9-enoic acid induces apoptosis through ROS-mediated endoplasmic reticulum stress in non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 448, 267–273 10.1016/j.bbrc.2014.04.111 [DOI] [PubMed] [Google Scholar]
- 19.Ryter S.W., Kim H.P., Hoetzel A.. et al. (2007) Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 9, 49–89 10.1089/ars.2007.9.49 [DOI] [PubMed] [Google Scholar]
- 20.Lu C. and Armstrong J.S. (2007) Role of calcium and cyclophilin D in the regulation of mitochondrial permeabilization induced by glutathione depletion, Biochem. Biophys. Res. Commun. 363, 572–577 10.1016/j.bbrc.2007.08.196 [DOI] [PubMed] [Google Scholar]
- 21.Zafarullah M., Li W.Q. and Sylvester J. (2003) Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sciences: CMLS 60, 6–20 10.1007/s000180300001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwingmann C. (2006) Metabolic insights into the hepatoprotective role of N-acetylcysteine in mouse liver. Hepatology 43, 454–463 10.1002/hep.21075 [DOI] [PubMed] [Google Scholar]
- 23.Ma X.D., Yan F., Ma A.D.. et al. (2006) Resveratrol induces HepG2 cell apoptosis by depolarizing mitochondrial membrane. Nan Fang Yi Ke Da Xue Xue Bao 26, 406–413 [PubMed] [Google Scholar]
- 24.Torre L. A. et al. , Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 25.Zhou R., Fukui M. and Choi H.J. (2009) Induction of a reversible, non-cytotoxic S-phase delay by resveratrol: implications for a mechanism of lifespan prolongation and cancer protection. Br. J. Pharmacol. 158, 462–474 10.1111/j.1476-5381.2009.00268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moloney J.N. and Cotter T.G. (2017) ROS signalling in the biology of cancer[C]//Seminars in cell & developmental biology. Academic Press: London, UK: [DOI] [PubMed] [Google Scholar]