Abstract
Objectives: Many studies have examined the prognostic significance of the neutrophil-to-lymphocyte ratio (NLR) in oral cancer; however, the results are contradictory. We, therefore, conducted a meta-analysis aiming to clarify the prognostic value of the NLR in oral cancer patients. Methods: A literature search was conducted in the PubMed, Web of Science, and Embase databases. Stata version 12.0 was used for statistical analysis. Results: A total of 14 studies with 3216 patients were finally included. The results indicated that a high NLR was significantly associated with worse DFS (n=10, HR = 1.73, 95% confidence interval [CI] = 1.44–2.07, P<0.001). Similar results were observed for overall survival (OS) (n=9, HR = 1.61, 95% CI = 1.39–1.86, P<0.001). Moreover, a high NLR was also correlated with lymph node metastasis (n=7, odds ratio [OR] = 1.62, 95% CI = 1.32–1.98, P<0.001), advanced tumor stage (n=7, OR = 2.63, 95% CI = 2.12–3.25, P<0.001), T stage (n=6, OR = 3.22, 95% CI = 2.59–4.01, P<0.001), tumor differentiation (n=5, OR = 1.48, 95% CI = 1.03–2.11, P=0.033), and perineural invasion (n=4, OR = 1.83, 95% CI = 1.4–2.39, P<0.001). However, an elevated NLR was not correlated with gender. Conclusion: This meta-analysis showed that the NLR might be a potential independent prognostic factor in patients with oral cancer.
Keywords: meta-analysis, neutrophil- to-lymphocyte ratio, oral cancer, prognosis
Introduction
Oral cancer is a malignant neoplasia occurring in the lip or oral cavity, and is traditionally defined as squamous cell carcinoma (SCC) because 90% of cancers in the dental area originate from squamous cells [1]. Oral cancer is within the top 10 diagnosed cancers, and it is more prevalent in Asian countries, especially Southeast Asia [2]. Unfortunately, the incidence rate of oral cancer in Asia is still rising [2]. Despite progress in research and treatment in the past few decades, the survival of oral cancer patients has not substantially improved [3]. Prognostic biomarkers are essential for treatment because they can provide valuable information for prognosis prediction and therapeutic regimen selection. A variety of potential prognostic markers of oral cancer has been investigated and reported [3]. However, tissue specimens are needed to detect these molecular markers. In addition, due to the lack of follow-up studies, the clinical significance of these potential biomarkers cannot be guaranteed [3]. As a result, convenient and non-invasive prognostic biomarkers for oral cancer are still urgently needed.
It is well established that cancer has a close connection with inflammation [4]. Inflammatory responses play important roles at each step of tumor development, including initiation, progression, and metastasis [5]. The neutrophil-to-lymphocyte ratio (NLR) is an important hematological parameter that reflects inflammatory responses. Furthermore, the NLR is a readily available and inexpensive biomarker. The NLR has been reported to be a significant prognostic marker for a number of malignancies, including colorectal cancer [6], bladder cancer [7], breast cancer [8], ovarian cancer [9], and non-small-cell lung cancer [10]. Recently, many studies have also explored the prognostic and clinical significance of the NLR in oral cancer; however, the results have been inconclusive [11–16]. Therefore, to further verify the prognostic role of the NLR in oral cancer, we conducted a meta-analysis by pooling data from relevant studies.
Materials and methods
Search strategy
A systemic and comprehensive literature search was performed in the PubMed, Web of Science, and Embase databases up to November 2018. The following key terms were used: ‘oral cancer’ or ‘oral squamous cell carcinoma’ or ‘OSCC’ and ‘neutrophil-to-lymphocyte ratio’ or ‘NLR’. This meta-analysis was performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) [17].
Selection criteria
The inclusion criteria were as follows: (1) patients with a histologically confirmed diagnosis of oral cancer; (2) studies evaluated the association between the NLR and survival outcomes or clinicopathological characteristics in oral cancer; (3) a definite cut-off value of the NLR was used to stratify patients; (4) sufficient information was provided to allow the calculation of HRs and 95% confidence intervals (CIs) for survival analysis [18]; and (5) full-text articles published in English. The exclusion criteria were: (1) reviews, conference abstracts, letters, or case reports; (2) multiple cut-off values of the NLR were used; (3) animal studies; and (4) duplicate studies.
Data extraction and quality assessment
Two investigators independently extracted information from eligible studies using predesigned forms. The following data were extracted: first author’s name, year of publication, geographic location of study, sample size, age, treatment methods, study design, cut-off value, study end point, and HRs and 95% CIs for DFS and/or overall survival [OS]. Disagreements were resolved by consensus. The quality of the included studies was evaluated according to Newcastle-Ottawa Scale (NOS) [19]. The NOS contained three aspects: selection (4 points), comparability (2 points), and outcome assessment (3 points). Studies with a NOS score ≥6 were classified as high-quality studies.
Statistical analysis
The impact of the NLR on DFS and/or OS was measured by pooled HRs and their 95% CIs extracted from included studies. If an HR and its 95% CI were not directly presented, they were computed by methods described by Parmar et al.[18]. Odds ratios (ORs) and 95% CIs were utilized to estimate the association between NLR and clinicopathological features. Heterogeneity among studies was evaluated using χ2-based Q test and Higgins’ I2 statistic. If P<0.10 and/or I2>50%, indicating significant heterogeneity among studies, the random-effects model (DerSimonian–Laird method) was used. Otherwise, the fixed-effects model (Mantel–Haenszel method) was applied. Publication bias was quantified using Begg’s funnel plot and the Egger’s linear regression tests. The dose-response meta-analysis was performed by using generalized least squares trend estimation, according to the methods developed by Greenland and Longnecker [20]. All statistical analyses were performed with Stata software version 12.0 (Stata Corp, College Station, TX). P<0.05 was considered statistically significant.
Results
Literature search
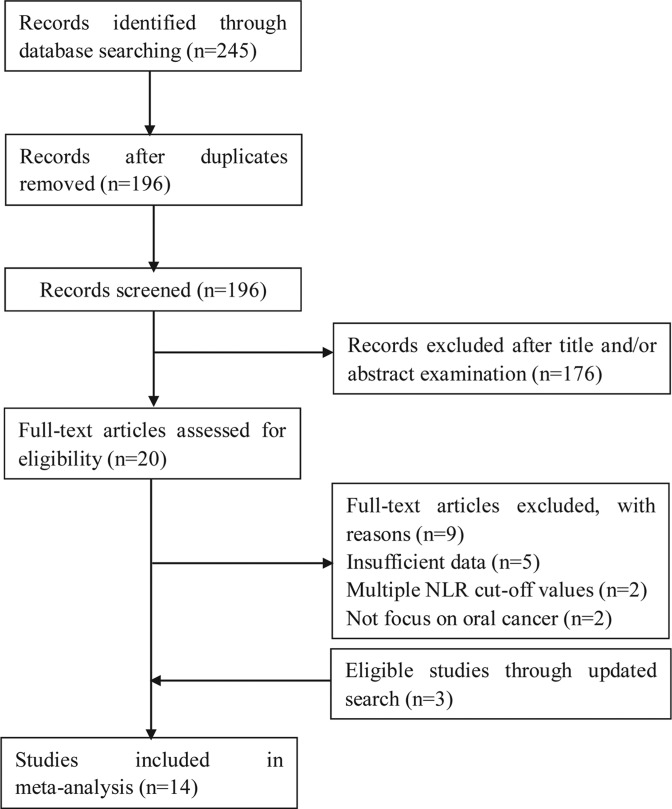
A total of 245 records were retrieved by the electronic database search. After removing duplicates, 196 studies were identified. Of these, 176 records were removed via title and abstract examination and 20 full-text studies were checked. Subsequently, nine studies were excluded because of insufficient data, multiple cut-off values, or a focus other than oral cancer. Three eligible studies were identified through updated search. Ultimately, 14 studies comprising 3216 patients were included in the meta-analysis. The selection process is shown in Figure 1.
Figure 1. Flow chart of study selection process.
Study characteristics
The basic characteristics of the included studies are shown in Table 1. The studies were published between 2013 and 2018 with sample sizes ranging from 68 to 613. All studies were retrospective. Twelve studies were conducted in Asia [11,13,14,16,21–28] and two were performed in Europe [12,15]. The cut-off values for the NLR ranged from 1.77 to 5. Ten studies [13–16,22–27] reported an association between the NLR and DFS, and nine studies [12,14,16,21–24,27,28] showed a correlation between the NLR and OS. Eight studies [11,15,16,21,23,24,26,28] presented data on the relationship between the NLR and clinicopathological factors. The NOS scores of the studies ranged from 6 to 8, with a median value of 7.
Table 1. Characteristics of all the studies included in this meta-analysis.
| Study | Year | Country/region | Number of patients | Study design | Age (years) Median/mean | Treatment | Study period | Cut-off value | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Acharya | 2017 | India | 68 | Retrospective | 48.75 | Surgery | 2011–2014 | 1.77 | 7 |
| Bobdey | 2017 | India | 471 | Retrospective | 50 | Mixed | 2007–2008 | 2.38 | 8 |
| Chen | 2016 | China | 306 | Retrospective | 55 | Surgery | 2004–2009 | 2.7 | 7 |
| Christina | 2016 | Austria | 144 | Retrospective | 58 | Chemoradiotherapy | 2004–2014 | 1.9 | 7 |
| Fang | 2013 | Taiwan | 226 | Retrospective | 52.47 | Surgery | 2007–2012 | 2.44 | 7 |
| Lee | 2017 | Taiwan | 396 | Retrospective | 53 | Surgery | 2006–2013 | 2.73 | 8 |
| Nakashima | 2016 | Japan | 124 | Retrospective | 67.2 | Mixed | 2003–2009 | 2.4 | 7 |
| Ong | 2017 | India | 133 | Retrospective | 51.92 | Surgery | 2009–2013 | 1.88 | 7 |
| Perisanidis | 2013 | Austria | 97 | Retrospective | NR | Mixed | 2001–2009 | 1.9 | 6 |
| Tsai | 2014 | Taiwan | 213 | Retrospective | 53 | Mixed | 2004–2011 | 5 | 7 |
| Wu | 2017 | Taiwan | 262 | Retrospective | 51 | Surgery | 2004–2011 | 2.95 | 7 |
| Park | 2018 | Korea | 69 | Retrospective | 62 | Mixed | 2007–2016 | 2.29 | 7 |
| Sano | 2018 | Japan | 94 | Retrospective | 94 | Surgery | 2007–2014 | 2.36 | 8 |
| Kao | 2018 | Taiwan | 613 | Retrospective | 53 | Surgery | 2005–2014 | 2.28 | 8 |
Abbreviations: NOS, Newcastle-Ottawa Scale; NR, not reported.
NLR and survival outcomes in oral cancer
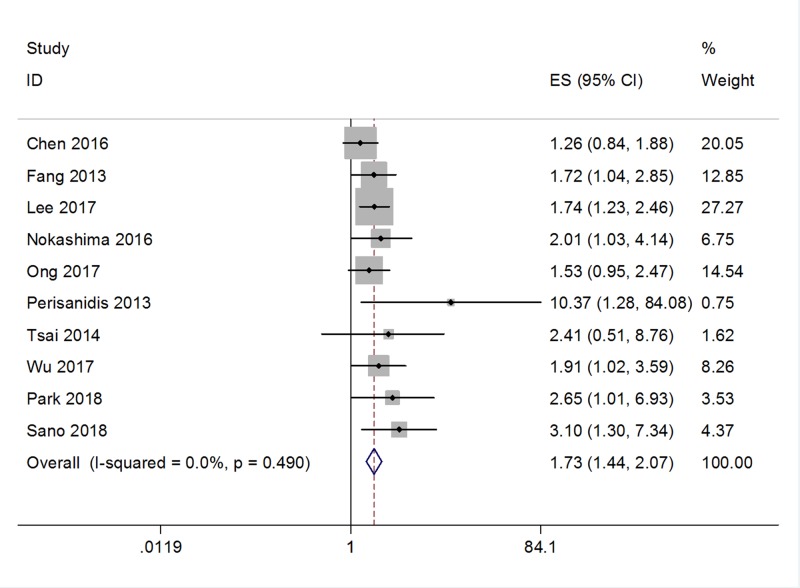
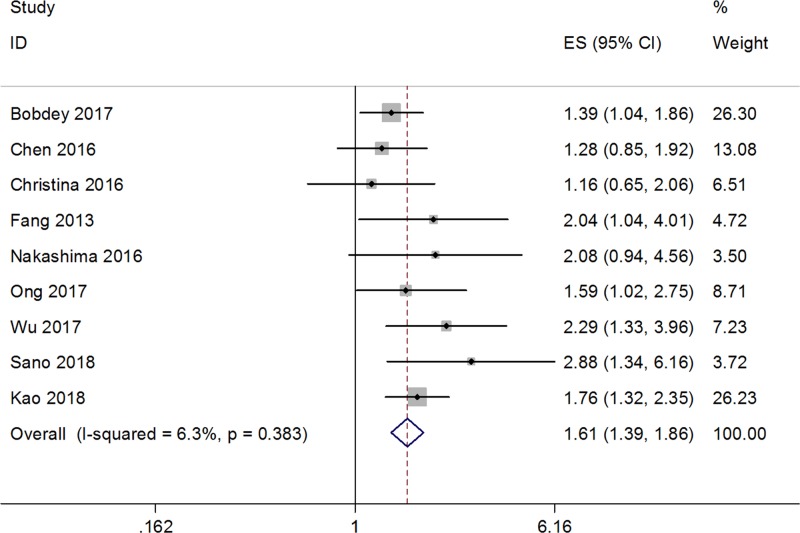
Ten studies [13–16,22–27] with a total of 1920 patients evaluated the prognostic value of the NLR on DFS. No significant heterogeneity was detected (I2 = 0, P=0.49; Figure 2); therefore, a fixed-effects model was used. The pooled results indicated an HR of 1.73, with 95% CI = 1.44–2.07, and P<0.001. Next, data from nine studies [12,14,16,21–24,27,28] were merged to explore the impact of the NLR on OS. Significant heterogeneity was not observed (I2 = 6.3%, P=0.383; Figure 3), so a fixed-effects model was applied. The combined HR was 1.61, with 95% CI = 1.39–1.86 and P<0.001. Taken together, the pooled data demonstrated that a high NLR was associated with poor DFS and OS in oral cancer.
Figure 2. Forest plot depicting association between NLR and DFS in oral cancer.
Figure 3. Forest plot depicting association between NLR and OS in oral cancer.
NLR and clinicopathological characteristics in oral cancer
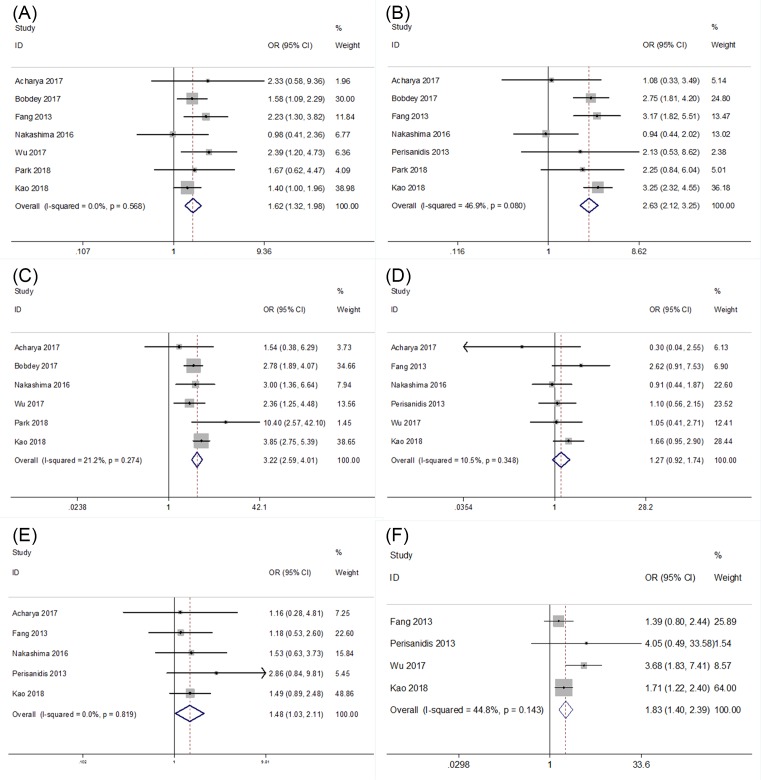
Ten studies [11,15,16,21,23,24,26,28] investigated the relationship of the NLR and clinicopathological characteristics, including lymph node metastasis, T stage, tumor stage, perineural invasion, gender and tumor differentiation. As shown in Figure 4 and Table 2, the results showed that a high NLR was significantly associated with lymph node metastasis (n=7, OR = 1.62, 95% CI = 1.32–1.98, P<0.001), advanced tumor stage (n=7, OR = 2.63, 95% CI = 2.12–3.25, P<0.001), T stage (n=6, OR = 3.22, 95% CI = 2.59–4.01, P<0.001), tumor differentiation (n=5, OR = 1.48, 95% CI = 1.03–2.11, P=0.033) and perineural invasion (n=4, OR = 1.83, 95% CI = 1.4–2.39, P<0.001). However, an elevated NLR was not correlated with gender (Figure 4 and Table 2).
Figure 4. The relationship of the NLR and clinicopathological characteristics.
Forest plots depicting correlations between NLR and (A) lymph node metastasis, (B) tumor stage, (C) T stage, (D) gender, (E) tumor differentiation, and (F) perineural invasion.
Table 2. Meta-analysis of the association between NLR and clinicopathlogical factors in oral cancer.
| Features | Number of studies | OR (95% CI) | P | Heterogeneity | Effects model | Publication bias | ||
|---|---|---|---|---|---|---|---|---|
| I2(%) | P | Begg’s P | Egger’s P | |||||
| Lymph node metastasis (positive vs negative) | 5 | 1.76(1.36–2.28) | <0.001 | 0 | 0.452 | FEM | 0.806 | 0.858 |
| T stage (T3-T4 vs T1-T2) | 4 | 2.64(1.96–3.55) | <0.001 | 0 | 0.839 | FEM | 0.308 | 0.31 |
| Tumor stage (advanced vs early) | 5 | 2(1.22–3.27) | 0.006 | 54.6 | 0.066 | REM | 0.624 | 0.274 |
| Perineural invasion (positive vs negative) | 3 | 2.36(1.07–5.22) | 0.034 | 59.2 | 0.086 | REM | 1 | 0.676 |
| Gender (male vs female) | 5 | 1.11(0.75–1.63) | 0.602 | 6.3 | 0.371 | FEM | 0.806 | 0.731 |
| Differentiation (poor vs good/moderate) | 4 | 1.46(0.89–2.41) | 0.135 | 0 | 0.672 | FEM | 0.734 | 0.574 |
| Lymph node metastasis (positive vs negative) | 7 | 1.62(1.32–1.98) | <0.001 | 0 | 0.568 | FEM | 0.3 | 0.523 |
| Tumor stage (advanced vs early) | 7 | 2.63(2.12–3.25) | <0.001 | 46.9 | 0.08 | FEM | 0.23 | 0.095 |
| T stage (T3-T4 vs T1-T2) | 6 | 3.22(2.59–4.01) | <0.001 | 21.2 | 0.274 | FEM | 0.707 | 0.918 |
| Gender (male vs female) | 6 | 1.27(0.92–1.74) | 0.146 | 10.5 | 0.348 | FEM | 0.707 | 0.428 |
| Differentiation (poor vs good/moderate) | 5 | 1.48(1.03–2.11) | 0.033 | 0 | 0.819 | FEM | 0.806 | 0.709 |
| Perineural invasion (positive vs negative) | 4 | 1.83(1.4–2.39) | <0.001 | 44.8 | 0.143 | FEM | 0.734 | 0.446 |
Abbreviations: FEM, fixed-effects model; REM, random-effects model.
Dose-response meta-analysis
Combined RRs comparing highest with lowest NLR were 2.34 (95% CI 1.49–3.25) for DFS, 2.73 (95% CI 1.62–4.01) for OS.
Publication bias
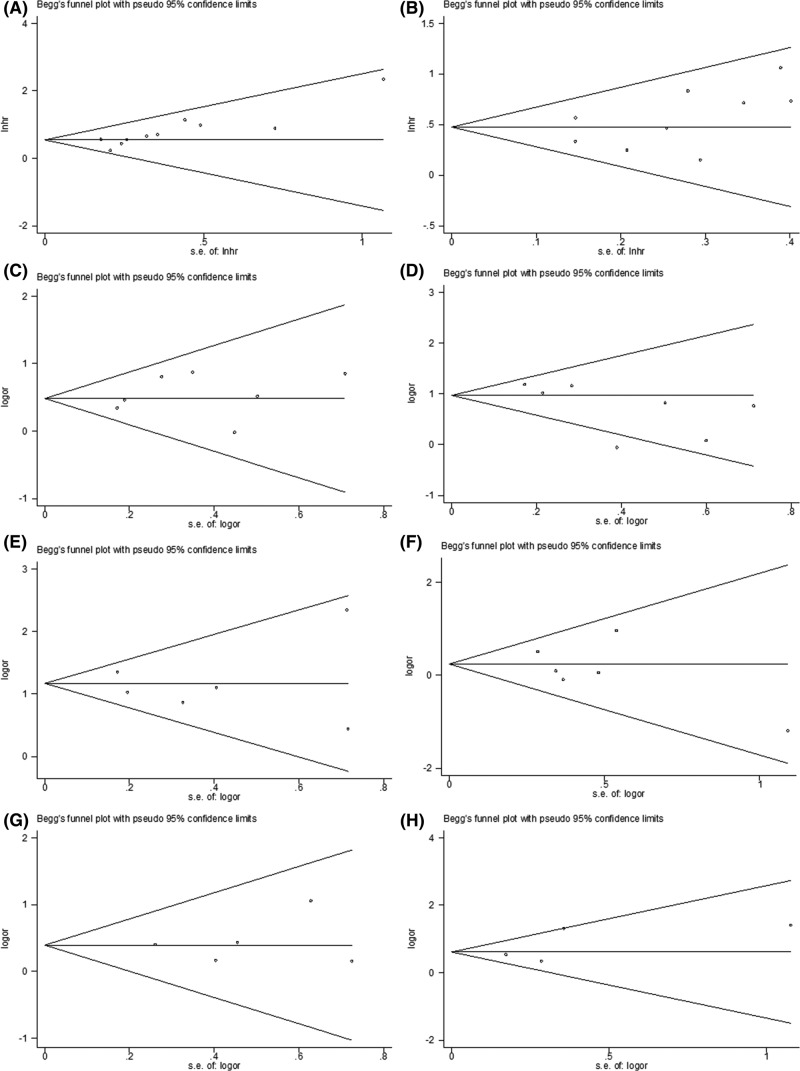
We used the Begg’s test and Egger’s test to estimate potential publication bias. The results of publication bias are listed in Table 2. The funnel plots are shown in Figure 5. All P values of publication bias were >0.05, suggesting that there was no evidence of publication bias in this meta-analysis.
Figure 5. The potential publication bias estimation.
Funnel plots on (A) DFS, (B) OS, (C) lymph node metastasis, (D) tumor stage, (E) T stage, (F) gender, (G) tumor differentiation, and (H) perineural invasion.
Discussion
This meta-analysis incorporating data from 14 studies showed that a high NLR was associated with poor DFS and OS in oral cancer patients. Furthermore, an elevated NLR was also significantly associated with lymph node metastasis, higher T stage, advanced tumor stage, tumor differentiation and perineural invasion. The present study collected the most recent data and provided relatively objective results concerning the prognostic role of the NLR in oral cancer.
Increased evidence indicates the important role of inflammation in tumorigenesis [29]. Due to the involvement of inflammatory responses in cancer, hematological parameters can provide important implications for cancer treatment and prognosis. Neutrophilia, a common occurrence in cancer patients, is often accompanied by relative lymphocytopenia [30]. Therefore, the NLR could be used as a simple index of systemic inflammatory responses in cancer patients. The NLR was first reported as a prognostic factor in colorectal cancer [31]. Walsh et al. found that a pre-operative NLR ≥5 was correlated with worse OS and cancer-specific survival in colorectal cancer patients receiving surgery [31]. Subsequently, the prognostic significance of the NLR was reported in various cancers [32]. With good accessibility and low cost, the NLR is a promising prognostic factor in daily practice. The biological validity underlying the NLR has also been investigated. In the tumor microenvironment, neutrophils can play a protumoral role by secreting matrix metalloproteinase 9 to facilitate carcinogenesis [33]. Moreover, neutrophils can also release factors that accelerate tumor cell proliferation [34]. Conversely, lymphocytes are known to impede malignant progression, and the infiltration of various subtypes of lymphocytes into tumor tissues has been demonstrated to predict increased survival in multiple cancers [35–37].
Other meta-analyses have also reported the prognostic value of the NLR in various cancers [38]. Chen et al. showed that the NLR was associated with poor OS in breast cancer [39]. Gu et al. reported that an elevated pretreatment NLR was correlated with both poor OS and PFS in non-small-cell lung cancer [40]. In addition, Tang et al. also demonstrated that a high NLR was significantly linked with detrimental long-term outcomes and clinicopathological parameters in patients with biliary tract cancer [41]. Our findings in the present study were in accordance with those of previous studies. It is noteworthy that a comprehensive meta-analysis including 100 studies explored the prognostic value of the NLR in various cancers [38]. That study was published in 2014, and only one study [15] on oral cancer was included. In the past several years, many new studies on the NLR and oral cancer have been published, and the current analysis contained 11 studies. Therefore, this is the most recent meta-analysis regarding the relationship between the NLR and oral cancer. A recent study [42] also investigated the association between the NLR and survival in oral cancer. That study conducted by Wang et al. [42] was performed well and concluded that a high NLR was correlated with poor DFS and OS in oral SCC, which was in accordance with our results. Our study also investigated the relationships between the NLR and clinical factors in oral cancer, whereas Wang’s study did not. We investigated the relationship of the NLR and clinicopathological characteristics, including lymph node metastasis, T stage, tumor stage, perineural invasion, gender and tumor differentiation. We found that the NLR was significantly associated with lymph node metastasis, T stage, advanced tumor stage, tumor differentiation and perineural invasion. These results provide implications for clinicians in practice. Therefore, our study was more comprehensive and provided more information on this issue.
However, there are several limitations to this meta-analysis. First, eligible studies used different cut-off values for the NLR; therefore, the definition of the high NLR group differed between studies. Second, most studies were conducted in Asia. Although this is in accordance with the high incidence of oral cancer in Asia, it may hinder the clinical use of the NLR in patients of other ethnicities. Third, confounders were not adjusted. Other pathological conditions such as cardiovascular disease, liver disease and infection can also influence the NLR. Because all eligible studies were retrospective cohort studies, the confounders may cause selection bias.
In conclusion, this meta-analysis showed that a higher NLR was associated with worse survival outcomes and several clinicopathological parameters in oral cancer. The NLR might be a potential independent prognostic factor in patients with oral cancer.
Abbreviations
- DFS
Disease-free survival
- HR
Hazard ratio
- NLR
neutrophil-to-lymphocyte ratio
- NOS
Newcastle-Ottawa Scale
- OS
overall survival
- OR
odd ratio
- RRs
Relative risks
- SCC
squamous cell carcinoma
Author contribution
Y.Y. and R.L. designed the study; drafted and revised the manuscript; performed statistical analysis. F.R., R.G., and P.Z. performed statistical analysis and critically revised the manuscript. Y.Y., R.L., and F.R. were responsible for data interpretation and critically revised the manuscript. R.G. and P.Z. were responsible for designing the study; data interpretation, and critically revised the manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of China [Grant number 81703054]; Key scientific research project of higher education of Henan Province, China [Grant number 17A350012 and 19A310004]; Key Science and Technology Program of Henan Province, China [Grant number 172102310614, 182102310259 and 182102310436]; and Doctoral Foundation of Xinxiang Medical University [Grant number XYBSKYZZ201506].
References
- 1.Lingen M.W., Kalmar J.R., Karrison T. and Speight P.M. (2008) Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 44, 10–22 10.1016/j.oraloncology.2007.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao S.V.K., Mejia G., Roberts-Thomson K. and Logan R. (2013) Epidemiology of oral cancer in asia in the past decade—an update (2000-2012). Asian Pac. J. Cancer Prev. 14, 5567–5577 10.7314/APJCP.2013.14.10.5567 [DOI] [PubMed] [Google Scholar]
- 3.Rivera C., Oliveira A.K., Costa R.A.P., De Rossi T. and Leme A.F.P. (2017) Prognostic biomarkers in oral squamous cell carcinoma: a systematic review. Oral Oncol. 72, 38–47 10.1016/j.oraloncology.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 4.Coussens L.M. and Werb Z. (2002) Inflammation and cancer. Nature 420, 860–867 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grivennikov S.I., Greten F.R. and Karin M. (2010) Immunity, inflammation, and cancer. Cell 140, 883–899 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J., Zhang H.Y., Li J., Shao X.Y. and Zhang C.X. (2017) The elevated NLR, PLR and PLT May predict the prognosis of patients with colorectal cancer: a systematic review and metaanalysis. Oncotarget, 8 (40), 68837–68846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X., Du P. and Yang Y. (2017) The clinical use of neutrophil-to-lymphocyte ratio in bladder cancer patients: a systematic review and meta-analysis. Int. J. Clin. Oncol., 22 (5), 817–825 10.1007/s10147-017-1171-5 [DOI] [PubMed] [Google Scholar]
- 8.Ethier J.L., Desautels D., Templeton A., Shah P.S. and Amir E. (2017) Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 19, 2 10.1186/s13058-016-0794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z., Gu J.H., Guo C.S., Li X.H. and Yang W.C. (2017) Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival of epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Oncotarget 8, 46414–46424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu X.B., Tian T., Tian X.J. and Zhang X.J. (2015) Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci. Rep. 5, 9 10.1038/srep12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acharya S., Rai P., Hallikeri K., Anehosur V. and Kale J. (2017) Preoperative platelet lymphocyte ratio is superior to neutrophil lymphocyte ratio to be used as predictive marker for lymph node metastasis in oral squamous cell carcinoma. J. Investigative Clin. Dent. 8, e12219 10.1111/jicd.12219 [DOI] [PubMed] [Google Scholar]
- 12.Christina E.C., Cornelia C. and Edgar S. (2016) Neoadjuvant radiotherapy plus radical surgery for locally advanced stage III/IV oral cancer: Analysis of prognostic factors affecting overall survival. Oral Oncol. 60, 1–7 10.1016/j.oraloncology.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 13.Lee C.C., Huang C.Y., Lin Y.S.. et al. (2017) Prognostic performance of a newstaging category to improve discrimination of disease-specific survival in nonmetastatic oral cancer. Jama Otolaryngol. Head Neck Surg. 143, 395–402 10.1001/jamaoto.2016.3802 [DOI] [PubMed] [Google Scholar]
- 14.Ong H.S., Gokavarapu S., Wang L.Z., Tian Z. and Zhang C.P. (2017) Low pretreatment lymphocyte-monocyte ratio and high platelet-lymphocyte ratio indicate poor cancer outcome in early tongue cancer. J. Maxillofac. Surg. 75, 1762–1774 10.1016/j.joms.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 15.Perisanidis C., Kornek G., Poschl P.W.. et al. (2013) High neutrophil-to-lymphocyte ratio is an independent marker of poor disease-specific survival in patients with oral cancer. Med. Oncol. 30, 334, 10.1007/s12032-012-0334-5 [DOI] [PubMed] [Google Scholar]
- 16.Wu C.N., Chuang H.C., Lin Y.T., Fang F.M., Li S.H. and Chien C.Y. (2017) Prognosis of neutrophil-to-lymphocyte ratio in clinical early-stage tongue (cT1/T2N0) cancer. OncoTargets Ther. 10, 3917–3924 10.2147/OTT.S140800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. and Grp P. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–W264 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 18.Parmar M.K., Torri V. and Stewart L. (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 17, 2815–2834 10.1002/(SICI)1097-0258(19981230)17:24%3c2815::AID-SIM110%3e3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- 19.Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M. and Tugwell P. (2006) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 20.Berlin J.A., Longnecker M.P. and Greenland S. (1993) Meta-analysis of epidemiologic dose-response data. Epidemiology 4, 218–228 10.1097/00001648-199305000-00005 [DOI] [PubMed] [Google Scholar]
- 21.Bobdey S., Ganesh B., Mishra P. and Jain A. (2017) Role of monocyte count and neutrophil-to-lymphocyte ratio in survival of oral cancer patients. Int. Arch. Otorhinolaryngol. 21, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S., Guo J.B., Feng C.J., Ke Z.F., Chen L.H. and Pan Y.P. (2016) The preoperative platelet-lymphocyte ratio versus neutrophil-lymphocyte ratio: which is better as a prognostic factor in oral squamous cell carcinoma? Ther. Adv. Med. Oncol. 8, 160–167 10.1177/1758834016638019 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Fang H.Y., Huang X.Y., Chien H.T.. et al. (2013) Refining the role of preoperative C-reactive protein by neutrophil/lymphocyte ratio in oral cavity squamous cell carcinoma. Laryngoscope 123, 2690–2699 10.1002/lary.24105 [DOI] [PubMed] [Google Scholar]
- 24.Nakashima H., Matsuoka Y., Yoshida R.. et al. (2016) Pre-treatment neutrophil to lymphocyte ratio predicts the chemoradiotherapy outcome and survival in patients with oral squamous cell carcinoma: a retrospective study. BMC Cancer 16, 10.1186/s12885-016-2079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai Y.D., Wang C.P., Chen C.Y.. et al. (2014) Pretreatment circulating monocyte count associated with poor prognosis in patients with oral cavity cancer. Head Neck 36, 947–953 10.1002/hed.23400 [DOI] [PubMed] [Google Scholar]
- 26.Park Y.M., Oh K.H., Cho J.G.. et al. (2018) A prognostic scoring system using inflammatory response biomarkers in oral cavity squamous cell carcinoma patients who underwent surgery-based treatment. Acta Otolaryngol. 138, 422–427 10.1080/00016489.2017.1404640 [DOI] [PubMed] [Google Scholar]
- 27.Sano Y., Kogashiwa Y., Araki R.. et al. (2018) Correlation of inflammatory markers, survival, and COX2 expression in oral cancer and implications for prognosis. Otolaryngol. Head Neck Surg. 158, 667–676 10.1177/0194599817745284 [DOI] [PubMed] [Google Scholar]
- 28.Kao H.K., Lofstrand J., Loh C.Y.. et al. (2018) Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci. Rep. 8, 13081 10.1038/s41598-018-31498-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balkwill F., Charles K.A. and Mantovani A. (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7, 211–217 10.1016/j.ccr.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 30.Dionigi R., Dominioni L., Benevento A.. et al. (1994) Effects of surgical trauma of laparoscopic vs open cholecystectomy. Hepatogastroenterology 41, 471–476 [PubMed] [Google Scholar]
- 31.Walsh S.R., Cook E.J., Goulder F., Justin T.A. and Keeling N.J. (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 91, 181–184 10.1002/jso.20329 [DOI] [PubMed] [Google Scholar]
- 32.Guthrie G.J.K., Charles K.A., Roxburgh C.S.D., Horgan P.G., McMillan D.C. and Clarke S.J. (2013) The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit. Rev. Oncol. Hematol. 88, 218–230 10.1016/j.critrevonc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 33.Ardi V.C., Kupriyanova T.A., Deryugina E.I. and Quigley J.P. (2007) Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 104, 20262–20267 10.1073/pnas.0706438104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang W. and Ferrara N. (2016) The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol. Res. 4, 83–91 10.1158/2326-6066.CIR-15-0313 [DOI] [PubMed] [Google Scholar]
- 35.Fridman W.H., Pages F., Sautes-Fridman C. and Galon J. (2012) The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 36.Kawai O., Ishii G., Kubota K.. et al. (2008) Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 113, 1387–1395 10.1002/cncr.23712 [DOI] [PubMed] [Google Scholar]
- 37.Rusakiewicz S., Semeraro M., Sarabi M.. et al. (2013) Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 73, 3499–3510 10.1158/0008-5472.CAN-13-0371 [DOI] [PubMed] [Google Scholar]
- 38.Templeton A.J., McNamara M.G., Seruga B.. et al. (2014) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl. Cancer Inst. 106, 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 39.Chen J., Deng Q., Pan Y.. et al. (2015) Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer. FEBS Open Bio. 5, 502–507 10.1016/j.fob.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu X.B., Tian T., Tian X.J. and Zhang X.J. (2015) Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci. Rep. 5, 12493 10.1038/srep12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang H., Lu W., Li B., Li C., Xu Y. and Dong J. (2017) Prognostic significance of neutrophil-to-lymphocyte ratio in biliary tract cancers: a systematic review and meta-analysis. Oncotarget 8, 36857–36868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y., Wang P., Andrukhov O.. et al. (2018) Meta-analysis of the prognostic value of the neutrophil-to-lymphocyte ratio in oral squamous cell carcinoma. J. Oral Pathol. Med. 47, 353–358 10.1111/jop.12688 [DOI] [PubMed] [Google Scholar]