To the Editor,
Bronchiolitis is the leading cause of hospitalization in U.S. infants, among which approximately 15% require intensive care.1 Emerging evidence suggest a complex interplay between viral infection, airway microbiota, and host immune response in the pathobiology of bronchiolitis.2 Vitamin D compounds have pleiotropic regulatory roles on both innate and adaptive immune responses.3 Low levels of 25-hydroxyvitamin D (25OHD), the major circulating form of vitamin D, have been found to be associated with higher risk and severity of acute respiratory infections (ARIs) in children.4 Recent studies also have reported an association of maternal5 and cord blood6 vitamin D levels with infant gut microbiota. Yet, there have been no studies investigating interactions between circulating 25OHD and airway microbiota on ARI severity in children. We have previously reported that infants with Haemophilus-dominant nasopharyngeal microbiota profile are at highest risk for intensive care use during bronchiolitis hospitalization.1 In the current analysis, we examined the same cohort of infants with bronchiolitis to investigate the interactions between serum 25OHD and the nasopharyngeal microbiota with regard to bronchiolitis severity.
This is an analysis of the data from the 35th Multicenter Airway Research Collaboration (MARC-35) cohort study, a multicenter prospective cohort study of infants hospitalized for bronchiolitis (i.e., severe bronchiolitis). The details of the study design and analysis may be found in the Online Supplement. Briefly, 17 sites across 14 U.S. states enrolled 1,016 infants (aged <1 year) hospitalized for bronchiolitis (Table E1). Bronchiolitis was defined according to the American Academy of Pediatrics guidelines.7 The institutional review boards at all participating sites approved the study. Informed consent was obtained from the infants’ parent or legal guardian.
Background and clinical data were collected via structured interview and chart reviews. Serum and nasopharyngeal airway samples were collected within 24 hours of hospitalization using standardized protocols.1 Serum total 25OHD levels were quantified by immunoassays. Nasopharyngeal samples were analyzed for microbiota using 16S rRNA gene sequencing. Nasopharyngeal microbiota profiles were derived by using partitioning around medoids (PAM) unbiased clustering with the use of weighted UniFrac distance.1
For the current analysis, the total 25OHD levels were dichotomized based on the median level into lower (<26.5 ng/ml) and higher (≥26.5 ng/ml) groups. The primary outcome was intensive care use, defined as intensive care unit admission or use of mechanical ventilation (continuous positive airway pressure or intubation) during bronchiolitis hospitalization. To test for a statistical interaction between serum total 25OHD and nasopharyngeal microbiota profiles – with regard to intensive care use – random-effects models were constructed accounting for the between-hospital differences (e.g., the differences in intensive care use) and adjusting for 12 patient-level covariates. As the models indicated a significant interaction, the analysis was then stratified by total 25OHD status. Data were analyzed using R version 3.4.4.
Of 1,016 infants with bronchiolitis, 1,005 (99%) met the quality control requirements for 16S rRNA gene sequencing. The median age was 3.2 months (IQR 1.6–5.9) and 60% were male. The median serum total 25OHD level was 26.5 ng/ml (IQR 18.0–33.1 ng/ml). Altogether 161 (16%) infants had intensive care use during a bronchiolitis hospitalization. The baseline characteristics of infants by 25OHD status are shown in Table E2. Compared with infants with higher 25OHD levels, those with lower 25OHD levels were younger and more likely to have household siblings and breastfeeding, but less likely to have a history of breathing problems and antibiotic use (all P<0.05).
In the nasopharyngeal samples, a total of 24 phyla and 379 genera were detected with predominance of three genera: Streptococcus (31%), Moraxella (30%), and Haemophilus (20%). The nasopharyngeal microbiota characteristics differed by 25OHD status (Table 1). Infants with lower 25OHD levels had significantly reduced richness and Shannon index than those with higher 25OHD levels (both P<0.05). While the abundance of most common genera did not differ significantly between the two groups (adjusted P>0.05, except for Staphylococcus), the microbiota profiles differed significantly by 25OHD status (P=0.04).
Table 1.
Nasopharyngeal microbiota of infants hospitalized for bronchiolitis by serum total 25-hydroxyvitamin D status
| Serum total 25OHD levels |
|||
|---|---|---|---|
| Characteristic | Lower (<26.5 ng/ml), n=498 |
Higher (≥26.5 ng/ml), n=507 |
P-value |
| Richness | |||
| Number of genera, median (IQR) | 15 (8–24) | 17 (10–24) | 0.04 |
| Alpha-diversity | |||
| Shannon index, median (IQR) | 0.88 (0.50–1.37) | 0.99 (0.62–1.45) | 0.02 |
| Relative abundance of 10 most abundant genera, mean (SD) | |||
| Streptococcus | 0.33 (0.31) | 0.29 (0.28) | 0.15† |
| Moraxella | 0.28 (0.34) | 0.32 (0.34) | 0.15† |
| Haemophilus | 0.20 (0.32) | 0.20 (0.30) | 0.98† |
| Prevotella | 0.02 (0.06) | 0.03 (0.07) | 0.15† |
| Neisseria | 0.02 (0.06) | 0.03 (0.08) | 0.07† |
| Staphylococcus | 0.03 (0.11) | 0.01 (0.06) | 0.009† |
| Corynebacterium | 0.02 (0.07) | 0.01 (0.07) | 0.43† |
| Alloprevotella | 0.01 (0.06) | 0.01 (0.04) | 0.98† |
| Veillonella | 0.01 (0.03) | 0.01 (0.03) | 0.20† |
| Gemella | 0.01 (0.04) | 0.01 (0.02) | 0.98† |
| Microbiota profiles | 0.04 | ||
| Haemophilus-dominant profile | 94 (18.9) | 99 (19.5) | |
| Moraxella-dominant profile | 96 (19.3) | 124 (24.5) | |
| Streptococcus-dominant profile | 159 (31.9) | 124 (24.5) | |
| Mixed profile | 149 (29.9) | 160 (31.6) | |
Abbreviations: 25OHD, 25-hydroxyvitamin D; IQR, interquartile range; SD, standard deviation
Benjamini-Hochberg false-discovery rate adjusted P-value accounting for multiple comparisons
There was a significant interaction between serum total 25OHD levels and nasopharyngeal microbiota profiles on the risk of intensive care use (Pinteraction=0.02), indicating heterogeneity in the microbiota-severity association. Indeed, among infants with lower 25OHD levels, Haemophilus-dominant microbiota profile was associated with a significantly higher risk of intensive care use (OR 3.08, 95%CI 1.31–7.25, P=0.01) compared to Moraxella-dominant profile (Figure 1). In contrast, there were no significant microbiota-severity associations among those with higher 25OHD levels (all P>0.20; Table E3). Similarly, in the sensitivity analysis using different cut-offs for 25OHD levels, Haemophilus-dominant profile was associated with a significantly higher risk of intensive care use only among infants with lower 25OHD levels (Tables E4 and E5).
Figure 1. Associations between nasopharyngeal microbiota profiles and risk of intensive care use in infants hospitalized for bronchiolitis by serum total 25-hydroxyvitamin D (25OHD) status.
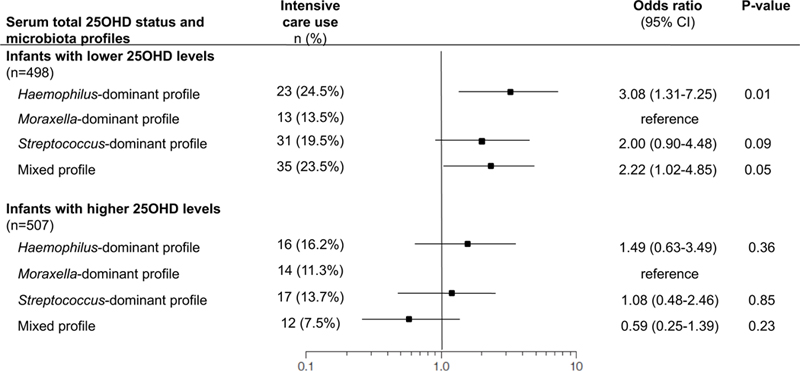
Random-effects model accounting for patient clustering at the hospital-level and adjusting for 12 factors (age, sex, race/ethnicity, gestational age, siblings at home, breastfeeding, history of breathing problems, lifetime history of systemic antibiotic use, weight at hospitalization, serum LL-37 level, and virology [RSV, rhinovirus]) was constructed for each strata – i.e., infants below or above the median serum total 25OHD levels (26.5 ng/ml). Moraxella-dominant profile was used as the reference. Full models are presented in Table E3.
In this multicenter prospective cohort study of 1,005 infants with bronchiolitis, we found significant associations of circulating total 25OHD levels with nasopharyngeal microbiota composition. Furthermore, there was a significant interaction between 25OHD levels and nasopharyngeal microbiota with regard to bronchiolitis severity. Specifically, the association of Haemophilus-dominant nasopharyngeal microbiota profile (compared to Moraxella-dominant profile) with higher severity was restricted to infants with lower 25OHD levels.
Previous studies have reported that both maternal and cord blood 25OHD levels are associated with infant gut microbiota.5,6 In this study, we observed that infants with lower 25OHD levels had specific microbiota structures in the nasopharyngeal airway – e.g., a lower diversity and likelihood of Moraxella-dominant profile. Consistent with our findings, lower abundance of Moraxella has been reported to associate with a higher risk of ARIs in early childhood.8 Our findings corroborate these earlier epidemiological reports, and extend them by demonstrating, for the first time, the interrelations between circulating 25OHD, airway microbiota, and clinical outcomes in infants with bronchiolitis.
The observed heterogeneity in the microbiota-bronchiolitis severity association – i.e., only lower 25OHD levels were associated with higher severity – is consistent with the emerging evidence. A recent meta-analysis of individual participant data from 25 randomized controlled trials demonstrated that protective effects of vitamin D supplementation against ARIs were seen in individuals with low baseline 25OHD levels.9 Regardless, the mechanisms underlying the observed heterogeneity remain to be elucidated. It is possible that 25OHD contributed, through upregulation of antimicrobial peptide LL-37, to the microbiota-severity association. However, the association remained significant after adjusting for serum LL-37 levels. Alternatively, low 25OHD levels are related to up-regulation of proinflammatory cytokines and an impaired epithelial barrier, leading to enhanced inflammation and dysbiosis.3 Furthermore, we have previously shown that lower circulating 25OHD levels associate with specific nasopharyngeal metabolomic signature – e.g., enriched proinflammatory lipids that can serve as mediators in the microbiome-host interactions in the airways.2
This study has potential limitations. First, we examined nasopharyngeal microbiota. Yet, studies have shown reliable correlation between upper and lower airway microbiota.10 Serum 25OHD and nasopharyngeal microbiota were measured at a single time-point during severe illness and there was no healthy control group. However, the goal was to examine the interrelations between circulating 25OHD, nasopharyngeal microbiota, and severity among infants hospitalized for bronchiolitis, not the development of bronchiolitis. The observed associations do not necessarily prove causality and might be explained by unmeasured confounders. However, in the current study, we rigorously adjusted for patient-level confounders, including age, prematurity, and breastfeeding, which may be associated with 25OHD levels, airway microbiota, and risks of severe ARI. Lastly, our inferences may not be generalizable to mild-to-moderate ARI. However, they remain directly relevant to the large population with severe bronchiolitis.
In summary, we found a significant interaction between serum total 25OHD levels and nasopharyngeal microbiota on bronchiolitis severity. Haemophilus-dominant nasopharyngeal microbiota profile was associated with an increased risk for intensive care during bronchiolitis hospitalization only in infants with lower 25OHD levels. While the causal inference remains premature, our data should facilitate further investigations into the complex interplay between early-life exposures (e.g., vitamin D), airway microbiome, and pathobiology of bronchiolitis.
Supplementary Material
Acknowledgments:
We thank the MARC-35 study hospitals and research personnel for their ongoing dedication to bronchiolitis and asthma research (Table E1 in the Online supplement). We also thank Janice A. Espinola, MPH, Ashley F. Sullivan, MS, MPH, and Courtney N. Tierney, MPH, for their many contributions to the MARC-35 study.
Financial support: The current analysis was supported by grants UG3 OD-023253, U01 AI-087881, R01 AI-134940, and R01 AI-137091 from the National Institutes of Health (Bethesda, MD, USA). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Toivonen was supported by the Finnish Medical Foundation and Päivikki and Sakari Sohlberg Foundation.
Footnotes
Conflicts of interest:The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Hasegawa K, Mansbach JM, Ajami NJ, et al. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur Respir J 2016;48:1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasegawa K, Stewart CJ, Celedon JC, Mansbach JM, Tierney C, Camargo CA Jr. Circulating 25-hydroxyvitamin D, nasopharyngeal airway metabolome, and bronchiolitis severity. Allergy 2018;73:1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanhere M, Chassaing B, Gewirtz AT, Tangpricha V. Role of vitamin D on gut microbiota in cystic fibrosis. J Steroid Biochem Mol Biol 2018;175:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo CA Jr. Vitamin D, acute respiratory infection, and asthma/chronic obstructive pulmonary disease. In: Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, eds. Vitamin D Fourth ed. Cambridge, MA: Elsevier Academic Press; 2018. pp. 1096–1120. [Google Scholar]
- 5.Talsness CE, Penders J, Jansen E, Damoiseaux J, Thijs C, Mommers M. Influence of vitamin D on key bacterial taxa in infant microbiota in the KOALA Birth Cohort Study. PLoS One 2017;12:e0188011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sordillo JE, Zhou Y, McGeachie MJ, et al. Factors influencing the infant gut microbiome at age 3–6 months: Findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol 2017;139:482–491.e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014;134:e1474–1502. [DOI] [PubMed] [Google Scholar]
- 8.Biesbroek G, Tsivtsivadze E, Sanders EA, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med 2014;190:1283–1292. [DOI] [PubMed] [Google Scholar]
- 9.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh RL, Kaestli M, Chang AB, et al. The microbiota in bronchoalveolar lavage from young children with chronic lung disease includes taxa present in both the oropharynx and nasopharynx. Microbiome 2016;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


