Abstract
Background:
Theories of early stress exposure and allostatic load offer a lifespan perspective to adult health after prematurity based on these early stressors affecting endocrine and metabolic systems. In this study, we examine cardiovascular and metabolic risk by comparing two groups of preterm infants who experienced a full spectrum of neonatal illness and a term-born group at age 23.
Methods:
Of the 215 infants recruited at birth, (84%) participated at age 23. The cohort included 45 full term (FT), 24 healthy preterm (HPT), and 111 sick preterm (SPT) infants. Socioeconomic status (SES) was equivalent across groups. Cardiovascular and metabolic outcomes were: blood pressure (BP), fasting glucose and lipid profiles, weight, waist-hip ratio (WHR), and body mass index (BMI). Clinical and sub-clinical ranges were compared across neonatal groups and gender.
Results:
At age 23, the HPT and SPT groups had higher systolic BP compared to the FT group. The SPT group had lower weight compared to the FT and HPT groups.
No group differences were found on diastolic BP, glucose, total cholesterol, HDL, LDL, triglycerides, BMI or WHR. Preterm males had more systolic hypertension and low HDL than FT males. Former preterm males and females had high WHR ratios and BMI at 23 years. Subclinical pre-hypertensive rates were highest for the HPT female group, followed by the SPT females. Only one (4.2%) HPT adult male was clinically diabetic.
Conclusions:
As young adults, HPT and SPT infants had early indicators of cardiovascular risk, but no indicators of metabolic risk. There is utility in using clinical and sub-clinical ranges to identify early cardiovascular risk in early adulthood.
Keywords: prematurity, cardiovascular risk, Developmental Origins Theory
Introduction
Among the most intense experiences of adversity for infants is premature birth (World Health Organization, 2016). Prematurity affects 1 in 10 infants each year, numbering 15 million infants worldwide (World Health Organization, 2013). More than 70% of preterm infants spend time in NICUs where frequent, high-intensity, invasive medical procedures are required (Holsti, Grunau, Oberlander, & Whitfield, 2005). Often infants face a double jeopardy of prematurity and neonatal illness including bronchopulmonary dysplasia (BPD), necrotizing enterolitis (NEC), intraventricular hemorrhage (IVH)], and retinopathy of prematurity (ROP) compounded by stressful, painful medical procedures (March of Dimes Perinatal Data Center, 2011). Eleven percent of premature infants are small for gestational age (SGA) with possible morbidities (Regev & Reichman, 2004) while only 6% are considered ‘healthy’, experiencing fleeting or no complications.
The hypothalamic-pituitary-adrenal (HPA) axis is one primary mechanism thought to underlie the differential experience with adversity in early life. Higher levels of HPA reactivity to stress, if experienced on a chronic basis throughout childhood and early adulthood, has been associated with wear and tear on downstream biological processes resulting in negative health consequences health (McEwen & Seeman, 1999). The Developmental Origins of Health and Disease is one theory that emphasizes the experience of early life adversity on long-term outcomes (Barker, 1995; Boyce & Ellis, 2005). We take this perspective to examine indicators of cardiovascular and metabolic disease in a sample characterized by neonatal illness and birth weight who were prospectively followed though age 23 years.
Risk factors of cardiovascular disease associated with prematurity and LBW include hypertension, hyperlipidemia, glucose intolerance, and obesity (Hovi et al., 2007; Irving, Belton, Elton, & Walker, 2000; Sipola-Leppänen et al., 2015), and should be examined in former preterm infants into adulthood (de Koning, Merchant, Pogue, & Anand, 2007). This prospective, longitudinal study examined blood pressure (BP), fasting glucose, lipid profiles, and weight-related indicators [i.e., waist-hip ratio (WHR), waist circumference (WC), and body mass index (BMI)] in former preterm infants at age 23. Three groups were compared; two groups of premature infants who experienced the full spectrum of neonatal illness and a full-term group. Following well-known guidelines, clinical and sub-clinical cut-off values for systolic and diastolic BP, hyperglycemia, lipids and body fat were used for fine-grained examinations.
Methods
Participants
Two hundred and fifteen infants were recruited at birth in 1985–1989 from a Level-III NICU in a specialty hospital in the northeast United States. A priori recruitment criteria included neonatal illnesses, birth weight, absence of maternal mental illness, and primary English language. All preterm infants were <37 wga, had bw <1850 g, and were classified by neonatal illness. Healthy preterm infants (HPT; n=33) had no medical or neurological illness. Sick preterm infants (SPT; n=127) had medical illness [BPD (supplemental oxygen at 28 days), RDS (tachypnea, grunting, cyanosis in conjunction with abnormal chest x-ray and requiring ventilation with positive pressure), NEC (Stages II & III; Bell et al., 1978), sepsis [early-onset sepsis (EOS) within 72 hours; late-onset sepsis (LOS) is defined as culture-proven sepsis from 7 days of age to discharge and treated with antibiotics for at least 5 days], neurological illness [IVH, grade III & IV (Papile, Burstein, Burstein, & Koffler, 1978), meningitis (positive cerebrospinal fluid culture), shunted hydrocephalus], and/or SGA (Lubchenco, Hansman, & Boyd, 1966). Full-term infants (FT; n=55) were healthy, bw ≥2,500g , ≥38 wga, of uncomplicated labor and delivery and recruited at the same time as the preterm infants. Socioeconomic status (SES) was equally distributed within and across neonatal groups. Fewer than 10% of the parent(s) declined study participation.
Procedure
Neonatal data were collected from medical records. At age 23, fasting lipids and glucose were collected during a home visit. At the hospital research center, demographics, health history and physical assessment were completed. Informed consent was obtained from parents at recruitment and participants at age 23. A cash incentive was given. University and hospital Institutional Review Boards approved the study.
Measurements
At age 23, participants reported their gender and race. SES was measured (Hollingshead, 1975). The cardiovascular and metabolic risk indicators were BP, fasting glucose, lipids, and weight-related indicators (i.e., WHR, WC, BMI).
Blood Pressure.
After 5 minutes of seated rest using an appropriate cuff (Pickering, Hall, Appel, & et al., 2005), the BP was taken on the right arm using a calibrated manual aneroid sphygmomanometer in 5-minute intervals three times. The three reading average was used.
Glucose and Lipid Profile.
After an 8–12 hour fast, blood glucose and lipids (total cholesterol, HDL, LDL, triglycerides) were collected by finger stick (puncture depth of 0.085mm; (CardioCheck PA, 2007). Fasting glucose values were displayed in 15 seconds, and lipids in 2 minutes.
Body Mass Index and Waist-Hip Ratio.
A calibrated medical scale was used for weight and height by a wall chart (shoeless). Body fat percentage and BMI were recorded with the Omron Body Fat Analyzer (Omron Health Care Inc®, 2001). A 72” paper tape was used around narrowest part of the waist and the widest part of the hips (de Koning et al., 2007) for the WHR.
Clinical and Sub-Clinical Classification.
Following well-known guidelines, (American Heart Association, 2015) the systolic BP pre-hypertensive clinical cut-off was >140 mmHg and sub-clinical cut-off was 120–139 mmHg. The diastolic clinical cut-off was > 90 mmHg and sub-clinical cut-off was 80–89 mmHg. The hyperglycemic glucose cut-off was ≥126 mg/dL. Lipid clinical and sub-clinical classifications were: normal total cholesterol ≤199 mg/dL, borderline 200–239 mg/dL, high ≥240 mg/dL; HDL were high (good) ≥60 mg/dL, borderline 41–59 mg/dL, low (bad) ≤40 mg/dL; LDL were optimal/near optimal ≤129 mg/dL, borderline 130–159 mg/dL, high/very high ≥160 mg/dL; and triglycerides were normal ≤149 mg/dL, borderline 150–199 mg/dL, high/very high ≥200 mg/dL. Body fat percentage (i.e., BMI) was classified as low (underweight; <18.5), normal (healthy weight; 18.5–24.9), high (overweight; 25–29.9), and very high (obese/morbidly obese; >30). WHR >1.0 was classified as high risk (World Health Organization, 2006).
Statistical Analyses
Analysis of Variance (ANOVA) was used to analyze the group differences on the neonatal variables (bw, ga, neonatal risk, length of stay, oxygen). Chi-square was used to analyze group differences on neonatal diagnosis (BPD, IVH III/IV, meningitis, hydrocephalus). ANOVA within gender was used for cardiovascular and metabolic risk variables (BP, fasting glucose, lipid profiles, BMI, WHR) at age 23. A 3 × 2 (group × gender) Chi Square was used to analyze differences on clinical and sub-clinical parameters of cardiovascular and metabolic risk.
Results
Sample characteristics
One hundred, eighty participants (n=45 FT, n=24 HPT, n=111 SPT) of the original 215 completed a comprehensive assessment battery at age 23 (84% retention). Those who dropped from the study were 35 infants (9 HPT, 16 SPT,10 FT). No differences were found between those who participated at age 23 and those who dropped on bw, ga, Hobel neonatal risk (Hobel, Hyvarinen, Okada, & Oh, 1973), length of stay, oxygen use, BPD, NEC, sepsis, IVH, meningitis, hydrocephalus, SES, parent education, marital status, or race. More male infants (n=23) than female (n=12) dropped from the study at age 23, χ2 (1) = 4.3, p=.04.
At birth, the FT group had the highest bw and ga compared with the HPT and SPT groups (see Table 1). The SPT group had the highest neonatal risk score, longest length of stay, oxygen use, and occurrence of BPD, NEC, sepsis, and IVH III/IV. All levels of SES were equally represented within and across groups at birth and remained so at age 23. At age 23 (M=23.2, SD=1.0), 53% were female. Eighty-seven percent were White, 9% African-American, 1% Native American, 1% Asian, and 2% more than one race.
Table 1.
Neonatal Demographics of the Age 23 Sample (N=180)
| Neonatal Variables | Full-Term (n=45) | Healthy Preterm (n=24) | Sick Preterm (n=111) | P value | |||
|---|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |||||
| Birth Weight, g | a3386 | (406) | b1470 | (218) | c1207 | (327) | .001 |
| Gestational Age, wk | a39.8 | (0.86) | b31.0 | (1.7) | c29.7 | (2.7) | .001 |
| Hobel Neonatal Risk1 | c1.4 | (3.4) | b57.6 | (22.7) | a93.6 | (29.9) | .001 |
| Length of Stay, total days | c3.0 | (0.40) | b33.0 | (9.9) | a58.9 | (33.5) | .001 |
| Oxygen, total hours | b0 | (0.0) | b13.7 | (18.5) | a553.9 | (904.9) | .001 |
| n | % | n | % | n | % | ||
| Female | 26 | (57.8) | 14 | (58.3) | 56 | (50.5) | .62 |
| BPD | 0 | (0.0) | 0 | (0.0) | a27 | (24.3) | .001 |
| Necrotizing Enterocolitis | 0 | (0.0) | 0 | (0.0) | a13 | (11.7) | .013 |
| Sepsis | 0 | (0.0) | 0 | (0.0) | a17 | (15.3) | .003 |
| IVH III/IV | 0 | (0.0) | 0 | (0.0) | 12 | (10.8) | .09 |
| Meningitis | 0 | (0.0) | 0 | (0.0) | 4 | (3.6) | .28 |
| Shunted Hydrocephalus | 0 | (0.0) | 0 | (0.0) | 6 | (5.4) | .15 |
BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage.
Letters in the body of the table indicate significant differences between neonatal groups, a>b>c.
Cardiovascular Risk at Age 23 Years - Full Sample
The FT group had lower systolic BP (M =122.1, SD=11.7) compared to the premature neonatal groups [(HPT M130.4, SD=10.1; SPT M=127.3, SD=11.0), F (2,161) = 4.6, p=0.01]. The SPT group had lower weight (M=155.2 lbs., SD=40.8) compared to the FT (M=175.1 lbs., SD=48.8) and HPT (M=177.7 lbs., SD=76.2) neonatal groups, F (2,167) = 3.6, p=0.03; see Table 2). There were no differences across groups on diastolic BP, glucose, total cholesterol, HDL, LDL, triglycerides, BMI or WHR.
Table 2.
Anthropometric Measures of Cardiovascular Risk by Neonatal Group
| Neonatal Variables | Full-Term (n=45) | Healthy Preterm (n=24) | Sick Preterm (n=111) | P value |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| Weight, kg | b78.8 (48.8) | b79.9 (76.2) | a69.8 (40.8) | .029 |
| Height, cm | 169.9 (3.8) | 167.4 (3.3) | 164.9 (3.8) | .06 |
| Waist, cm | 92.5 (7.9) | 93.7 (10.7) | 86.6 (5.9) | .09 |
| Hip circumference, cm | a104.7 (5.3) | a102.9 (7.4) | b96.3 (4.9) | .002 |
| Waist-Hip Ratio, cm | 0.22 (0.13) | 0.23 (0.10) | 2.26 (0.09) | .63 |
| BMI | 27.6 (7.5) | 28.4 (10.9) | 25.7 (5.8) | .15 |
| Systolic BP, mmHg | a122.1 (11.7) | b130.4 (10.1) | b127.3 (11.0) | .011 |
| Diastolic BP, mmHg | 74.7 (7.2) | 76.7 (7.9) | 75.4 (7.3) | .58 |
| Glucose, mg/dL | 75.4 (16.8) | 83.3 (21.9) | 78.2 (18.4) | .26 |
| Cholesterol, mg/dL | 160.7 (36.3) | 161.5 (39.3) | 150.9 (32.8) | .18 |
| HDL, mg/dL | 46.7 (20.1) | 44.1 (17.8) | 44.2 (20.2) | .76 |
| LDL, mg/dL | 98.5 (30.3) | 106.6 (35.5) | 96.2 (27.9) | .53 |
| Triglycerides, mg/dL | 90.9 (47.3) | 92.8 (71.2) | 87.6 (53.9) | .89 |
BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Letters in the body of the table indicate significant differences between neonatal groups, a<b.
Cardiovascular Risk by Neonatal Group for Males
Blood Pressure.
The premature groups had more systolic BP pre-hypertensive and hypertensive males than those born FT. All HPT males had pre-hypertensive (n=6, 67%) or hypertensive (n=3, 33%) systolic BP, followed by 92% SPT (pre-hypertensive n=34, 68%; hypertensive n=12, 24%) compared to 87% of the FT group (pre-hypertensive n= 2, 75%; hypertensive n=2, 12%; χ2 (4) = 2.44, p > .05). For diastolic BP, the FT (n=6, 37%) and HPT (n=3, 33%) groups had more with pre-hypertensive diastolic BP risk compared to 20% of SPT (n=10; χ2 (4) = 3.07, p >.05. The diastolic pre-hypertensive BP rates were lower with 33–37% of HPT and FT respectively, and 20% of SPT in this range.
Glucose and Lipid Profile.
Only one HPT male was hyperglycemic. The lipid results had similar low clinical and subclinical results. For total cholesterol less than 8% of FT and SPT males had subclinical high levels and only one HPT male had high levels. For HDL, 31% of SPT (n=16) and 39% of FT (n=7) had subclinical low HDL. All HPT males (n=9) had low HDL, followed by 63% of SPT (n=32) and 50% of FT (n=9) males, χ2 (4) = 6.86, p >.05. One HPT male had high LDL. For triglycerides, two HPT males had high triglycerides compared to 17% of the SPT group with subclinical (n=6, 11%) and high triglycerides (n=3, 6%; χ2 (4) = 3.29, p>.05).
Body Mass Index and Waist-Hip Ratio.
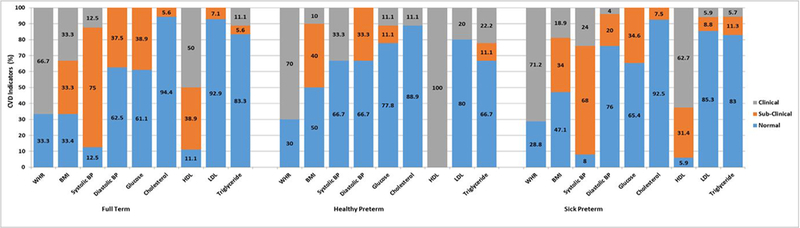
Four HPT males had high BMI and one had very high BMI. Thirty-four percent of SPT (n=18) had high BMI. An additional 19% (n=10) had very high BMI (χ2 (6) = 4.17, p >.05). Over 70% of HPT (n=7, 70%) and SPT males (n=37, 71%) were classified as high risk for WHR (see Figure 1; χ2 (2) = 0.13, p >.05).
Figure 1.
Clinical and sub-clinical cardiovascular risk indicators at age 23 by neonatal group for males only.
Note. CVD=Cardiovascular Indicators; WHR=Waist-Hip Ratio; BMI=Body Mass Index; BP=Blood Pressure; HDL=High Density Lipoprotein; LDL=Low Density Lipoprotein; *Healthy Preterm: Glucose, Hyperglycemic Male Healthy Preterm> Sick Preterm, Full Term, χ2(4)=9.6, p=.05.
Clinical & sub-clinical classifications. Body fat percentage (i.e., BMI) was classified as low (underweight; <18.5), normal (healthy weight; 18.5–24.9), high (overweight; 25–29.9), and very high (obese/morbidly obese; >30) and WHR greater than 1.0 was classified as high risk. Prehypertensive systolic BP sub-clinical cut-off was 120–139 mmHg and hypertensive systolic BP clinical cut-off was >140mmHg. (American Heart Association, 2015) Prehypertensive diastolic BP sub-clinical cut-off was 80–89mmHg and hypertensive diastolic BP clinical cut-off was >90mmHg. Clinical and sub-clinical classifications for lipid profiles were: total cholesterol was normal ≤199 mg/dL, borderline 200–239 mg/dL, high ≥240 mg/dL; HDL ranges were high (good) ≥60 mg/dL, borderline 41–59 mg/dL, low (bad) ≤40 mg/dL; LDL ranges were optimal/near optimal ≤129 mg/dL, borderline 130–159 mg/dL, high/very high ≥160 mg/dL; and triglycerides ranges were normal ≤149 mg/dL, borderline 150–199 mg/dL, high/very high ≥200 mg/dL.
Cardiovascular Risk by Neonatal Group for Females
Blood Pressure.
Subclinical pre-hypertensive rates were highest for the HPT female group (n =7, 63%), followed by SPT (n=28, 56%) compared to 43% of FT (n=9). Only one HPT and two SPT females had systolic BP hypertension. Less than 20% of all females had diastolic BP pre-hypertensive or hypertensive rates, χ2 (4) = 6.88, p > .05.
Glucose and Lipid Profile.
Females born preterm were not hyperglycemic, χ2 (4) = 4.88, p >. 05. Less than 8% of former preterm females had subclinical or high total cholesterol, χ2 (4) = 3.44, p > .05. For HDL, over 70% had either subclinical low (FT n=4, 16%; HPT n=2, 14%; SPT n=9, 17%) or low HDL (FT n=13, 52%; HPT n=8, 57%; SPT n=28, 53%; χ2 (4) = 0.14, p > .05). For LDL, only 1–2 HPT, SPT, and FT females had subclinical readings; while one SPT female had high/very high LDL. For the neonatal groups, more SPT (n=5, 9%) than HPT females (n=1) had high/very high triglycerides.
Body Mass Index and Waist-Hip Ratio.
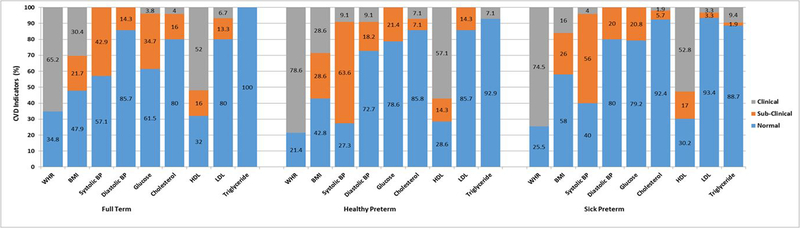
The preterm groups had higher WHR [HPT (n=11, 78%; SPT (n=38, 74%)] than the FT group (n=15; 65%; χ2 (2) = 0.98, p > .05). Over half (57%) of HPT females had very high and high BMI (n=8, 56%) while 42% (n=21) of SPT females had very high BMI and high BMI (see Figure 2; χ2 (6) = 8.90, p > .05).
Figure 2.
Clinical and sub-clinical cardiovascular risk indicators at age 23 by neonatal group for females only.
Note. CVD=Cardiovascular Indicators; WHR=Waist-Hip Ratio; BMI=Body Mass Index; BP=Blood Pressure; HDL=High Density Lipoprotein; LDL=Low Density Lipoprotein.
Clinical & sub-clinical classifications. Body fat percentage (i.e., BMI) was classified as low (underweight; <18.5), normal (healthy weight; 18.5–24.9), high (overweight; 25–29.9), and very high (obese/morbidly obese; >30) and WHR greater than 1.0 was classified as high risk. Prehypertensive systolic BP sub-clinical cut-off was 120–139 mmHg and hypertensive systolic BP clinical cut-off was >140mmHg. (American Heart Association, 2015) Prehypertensive diastolic BP sub-clinical cut-off was 80–89mmHg and hypertensive diastolic BP clinical cut-off was >90mmHg. Clinical and sub-clinical classifications for lipid profiles were: total cholesterol was normal ≤199 mg/dL, borderline 200–239 mg/dL, high ≥240 mg/dL; HDL ranges were high (good) ≥60 mg/dL, borderline 41–59 mg/dL, low (bad) ≤40 mg/dL; LDL ranges were optimal/near optimal ≤129 mg/dL, borderline 130–159 mg/dL, high/very high ≥160 mg/dL; and triglycerides ranges were normal ≤149 mg/dL, borderline 150–199 mg/dL, high/very high ≥200 mg/dL.
Discussion
Often young adult follow-up studies of preterm survivors are homogenous samples defined by bw or ga, excluding the impact of neonatal morbidity. Several long-term follow-up meta-analyses of premature infants report neurocognitive (Kerr-Wilson, Mackay, Smith, & Pell, 2012), academic (Aarnoudse-Moens, Weisglad-Kuperus, van Goudoever, & Oosterlaan, 2009), and mental health (Aarnoudse-Moens et al., 2009) sequelae directly associated with prematurity (Lemola, 2015). The compounded risk of the NICU experience, initial maternal separation, and subsequent health and developmental challenges affects the psychobiology of the body’s stress response that can be observed in dysregulation of the HPA axis. In another study of this cohort, we reported that prematurity and perinatal illness influenced the activity of the environmentally sensitive HPA axis system at age 23 (Winchester, Sullivan, Roberts, & Granger, 2018). There is growing evidence that chemical signals of the sensitive biological systems of the stress response contribute to allostatic load and is a mechanism underlying theories of early life adversity, such as DOaHD. Over time, these affect a developing metabolic system inducing later cardiovascular-related disease (Barker, 2007). At young adulthood, these can be seen in important risk factors of obesity, hypertension, hyperglycemia, and dyslipidemia (Smith & Ryckman, 2015).
This study compared cardiovascular risk in a well-characterized sample of young adults representing a full spectrum of neonatal risk associated with prematurity. While neonatal care has improved, 40% of low birth-weight preterm infants weighing between 500–1500 grams experience one or more serious morbidities (Horbar et al., 2017). While there were no statistically significant differences between the two preterm groups and the full term group at age 23, our data demonstrated more cardiovascular risk across preterm groups, compared to FT peers in many of the cardiovascular risk markers. Indicators of weight, systolic BP and HDL and sub-clinical indicators may identify those former preterm infants most at risk for later cardiovascular-related disease(s).
Those 23 year olds born prematurely who experienced a lengthy NICU stay and associated stress due to illness (SPT) had significantly lower weight than the term (FT) or healthy preterm (HPT) groups. Consistent with other studies, there were no significant differences across the three neonatal groups on WHR or BMI scores (Darlow, Horwood, Pere-Bracken, & Woodward, 2013; Evensen et al., 2009). Yet upon closer examination of clinical and subclinical ranges, both former preterm males and females (HPT, SPT) had high rates of clinical and sub-clinical classifications for WHR and BMI. Almost 3/4 of the HPT and SPT adult males and females had high risk for WHR, and at least half were overweight or obese. With approximately two-thirds (60%) overweight or obese (BMI >25), and 30% obese (BMI ≥ 30; Ogden, Carroll, Kit, & Flegal, 2014) these rates may foreshadow higher risk and health care costs for prematurely-born as they age.
Both HPT and SPT groups had higher systolic BP (mean difference: HPT 8.3mmHg; SPT 5.2mmHg) than FT peers. This is a larger mean difference than has been reported (Hovi et al., 2016; Parkinson, Hyde, Gale, Santhakumaran, & Modi, 2013). The magnitude of the difference matters because it increases the odds of later cardiovascular risk (Nelson, 2010). Given our sample size, we did not adjust for confounders which may show smaller mean differences, nonetheless, significance would likely remain (Parkinson et al., 2013; Tu, West, & Ellison, 2005). While our findings correspond with other reports of higher systolic BP in preterm LBW infants at young adulthood (Evensen et al., 2009; Hack, Schluchter, Cartar, & Rahman, 2005; Irving et al., 2000), they exceed the national average of prevalence rates for pre-hypertension and hypertension. In the U.S. prior to the new AHA guidelines (Flack, Calhoun, & Schiffrin, 2018), one in three adults (29%) or over 70 million are hypertensive (Nwankwo, Yoon, Burt, & Gu, 2013), including 11% of males and 6.8% of females ages 20–34 years (Mozaffarian et al., 2015). An additional 30% of adults have pre-hypertension (Nwankwo et al., 2013). In this sample, the majority of HPT and SPT males were pre-hypertensive (range 67%−68%) or hypertensive (range 24%−33%). The unexpected finding that 75% of FT males met the cut-off for pre-hypertension suggest that those born prematurely are not different from FT peers and raise warnings for later hypertension-related health conditions at young adulthood.
While other preterm follow-up studies (Hovi et al., 2007) have reported higher indexes of insulin resistance and glucose intolerance for infants with bw < 1500 grams, we found only one HPT adult male was clinically diabetic. Fasting glucose values did not differ across groups and there were no early indicators of glucose intolerance or type 2 diabetes in either preterm group. In the Helsinki Cohort, the relative risk of diabetes after age 40 adjusted for gestational age was RR = 1.59 (1.0–2.2; (Kajantie, Osmond, Barker, & Eriksson, 2010). Perhaps the risk of type 2 diabetes for former preterms increases at middle age.
The subclinical low HDL (31%) for males is consistent with prevalence rates for U.S. adult males (31.4%) and females (11.9%; (Carroll, Kit, & Lacher, 2012). All HPT males and 63% of SPT males had low HDL, while 20% HPT males had high LDL. One-third of HPT males had subclinical and clinically high triglycerides, compared to 17% of SPT males. For females, HPT and SPT groups had subclinical or low rates for HDL. These differ from those reported in a systematic review and meta-analysis of metabolic syndrome of premature infants as adults (Parkinson et al., 2013). However, the outcomes were measured at older ages (cholesterol 38 years; LDL 29.7 years) than the present sample. Thus, our findings lend support to the utility of the clinical and subclinical cut-off ranges in early adulthood so that individuals at higher risk can be identified early and monitored regularly to optimize healthy behaviors.
In an international study of adults born VLBW, BP was higher than controls, but unrelated to ga, maternal smoking, multiple pregnancy, ROP or BPD (Hovi et al., 2016). The diet preferences of the present cohort revealed less healthy dietary behaviors known to contribute to elevated cardiovascular disease at age 23 (Sharafi et al., 2016). Those born prematurely had higher preference for sweets, low preference for protein-enriched foods, wine, and spicy foods, lower food restraint, and high neophobia scores. Thus, this US sample of young adults born prematurely has health and dietary behavior indicators for cardiovascular risk.
We explored health behaviors known to impact risk and protections in life course trajectories (Snelgrove & Murphy, 2015). These included SES, smoking, alcohol, illicit drug use (marijuana (THC), cocaine, opiates, benzodiazepines, barbituates, methamphetamines, phencyclidine (PCP) psychedelics) exercise and activity. Social determinants represented by SES did not differ across the three neonatal groups at age 23 years. Only marijuana (THC) approached statistical significance (χ2 (2) = 5.83, p >.054) where 22% (n = 8) of the FT, 40% (n = 8) of the HPT and 16% (n = 16) of the SPT had a positive THC urine screen. Self-reported smoking was not different across the groups (χ2 (2) = 5.78, p >.05). Due to the low frequencies, further analysis was not possible. Participants self-reported their exercise and activity habits, however the different views on what constituted exercise and activity made group comparisons unreliable. In this prospective U.S. preterm follow-up, we examined the independent effects of neonatal history, but acknowledge that additional risks and protections can impact life course health trajectories.
Our findings may underestimate the early risk for males because more were lost to follow-up at age 23. The participants of this longitudinal study have been followed in research protocols since their birth and a small incentive was given to recognize the time and effort to participate. At age 23, the cash amount was not considered coercive, however we acknowledge that represents a possible source of bias. We did not include carotid artery intimal thickness or C-reactive protein (CRP) since sensitivity to CRP assays is suggested for individuals at least 40 years old (Goff et al., 2014). The psychobiology of neuroendocrine regulation as a mechanism for later cardiovascular risk bears further exploration in preterm samples. Additional mechanisms, such as the neuroendocrine stress-response (Winchester, Sullivan, Roberts, & Granger, 2016) and inflammatory (ter Wolbeek et al., 2015) systems, may help inform the clinical picture.
Key Messages.
Prematurity and Cardiovascular Risk at Early Adulthood
Prematurity affects 1 in 10 infants each year, numbering 15 million infants worldwide.
The hypothalamic-pituitary-adrenal (HPA) axis is one primary mechanism thought to underlie the differential experience with adversity in early life into later risk.
Our results suggest that weight, systolic BP and HDL and sub-clinical indicators may identify former prematurely born young adults who are most at risk for later cardiovascular-related disease(s).
Our findings lend support to the utility of the clinical and subclinical cut-off ranges so that former preterms at higher risk for dyslipidemia and other indicators of metabolic risk can be identified early and monitored regularly in order to optimize healthy behaviors.
Acknowledgement
The authors extend their appreciation to the participating parents and infants for their long-term commitment to the research study. The National Institutes of Health/National Institute of Nursing Research R01 003695 supported the study.
List of Abbreviatons
- NICU
Neonatal Intensive Care Unit
- wga
Weeks Gestational Age
- ga
Gestational Age
- BPD
Bronchopulmonary Dysplasia
- NEC
Necrotizing Enterocolitis
- EOS
Early Onset Sepsis
- LOS
Late Onset Sepsis
- IVH
Intraventricular Hemorrhage
- SGA
Small for Gestational Age
- FT
Full-Term
- BP
Blood Pressure
- WHR
Waist-Hip Ratio
- WC
Waist Circumference
- BMI
Body Mass Index
- g
Grams
- HDL
High-Density Lipids
- LDL
Low-Density Lipids
- LBW
Low Birth Weight
- VLBW
Very-Low-Birth-Weight
- HC
Hip Circumference
- HPT
Healthy Preterm
- SPT
Sick Preterm
- SES
Socioeconomic Status
- CRP
C-Reactive Protein
Footnotes
The authors have indicated they have no personal or financial potential conflicts of interest to disclose.
References
- American Heart Association. (2015). Symptoms, diagnosis, & monitoring of diabetes. Retrieved from http://www.heart.org/HEARTORG/Conditions/Diabetes/SymptomsDiagnosisMonitoringofDiabetes/Symptoms-Diagnosis-Monitoring-of-Diabetes_UCM_002035_Article.jsp
- Barker DJ (1995). The fetal and infant origins of disease. European Journal of Clinical Investigations, 25, 457–463. doi:10.1111/j.1365-2362.1995.tb01730.x [DOI] [PubMed] [Google Scholar]
- Barker DJ (2007). The origins of the developmental origins theory. Journal of Internal Medicine, 261, 412–417. doi:10.1111/j.1365-2796.2007.01809.x [DOI] [PubMed] [Google Scholar]
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, & Brotherton T (1978). Neonatal necrotizing enterocolitis: Therapeutic decisions based on clinical staging. Annals of Surgery, 187, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce TW, & Ellis BJ (2005). Biological sensitivity to context: I An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17, 271–301. doi:10.10170S0954579405050145 [DOI] [PubMed] [Google Scholar]
- CardioCheck PA. (2007). Point of care lipid management for health care professionals. Retrieved from http://www.cardiochek.com/professional/index2.asp.
- Carroll MD, Kit BK, & Lacher DA (2012). Total and high-density lipoprotein cholesterol in adults: National Health and Nutrition Examination Survey, 2009–2010. Atlanta, GA. [PubMed] [Google Scholar]
- Darlow BA, Horwood LJ, Pere-Bracken HM, & Woodward LJ (2013). Psychosocial outcomes of young adults born very low birth weight. Pediatrics, 132(6). doi:10.1542/peds.2013-2024 [DOI] [PubMed] [Google Scholar]
- de Koning L, Merchant AT, Pogue J, & Anand SS (2007). Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. European Heart Journal, 28(7), 850–856. doi:10.1093/eurheartj/ehm026 [DOI] [PubMed] [Google Scholar]
- Evensen KAI, Steinshamn S, Tjonna AE, Stolen T, Hoydal MA, Wisloff U,…Vik T (2009). Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood. Early Human Development, 85, 239–245. doi:10.1016/j.earlhumdev.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Flack JM, Calhoun D, & Schiffrin EL (2018). The new ACC/AHA Hypertension Guidelines for the prevention, detection, evaluation, and management of high blood pressure in adults. American Journal of Hypertension, 31(2), 133–135. doi:https://doi.org/10.1093/ajh/hpx207 [DOI] [PubMed] [Google Scholar]
- Goff DC, L.-J. DM, Bennett G, Coady S, D’Agostino RB, Gibbons R,…Wilson PWF (2014). 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 129 (suppl 2), S49–S73. doi:https://doi.org/10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- Hack M, Schluchter M, Cartar L, & Rahman M (2005). Blood pressure among very low birth weight (<1.5 kg) young adults. Pediatric Research, 58, 677–684. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Hyvarinen MA, Okada DM, & Oh W (1973). Prenatal and intrapartum high-risk screening: I. Prediction of the high-risk neonate. American Journal of Obstetrics and Gynecology, 117(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four factor index of social status. New Haven, CT: Yale University. [Google Scholar]
- Holsti L, Grunau RE, Oberlander TF, & Whitfield MF (2005). Prior pain induces heightened motor responses during clustered care in preterm infants in the NICU. Early Human Development, 81(3), 293–302. doi:10.1016/j.earlhumdev.2004.08.002 [DOI] [PubMed] [Google Scholar]
- Hovi P, Andersson S, Eriksson JG, Järvenpää A, Strang-Karlsson S, Mäkitie O, & Kajantie E (2007). Glucose regulation in young adults with very low birth weight. New England Journal of Medicine, 356, 2053–2063. [DOI] [PubMed] [Google Scholar]
- Hovi P, Vohr B, Ment L, Doyle LW, McGarvey L, Morrison KM,…Kajantie E (2016). Blood pressure in young adults born at very low birth weight: Adults born preterm international collaboration. Hypertension, 68, 880–887. doi:10.1161/HYPERTENSIONAHA.116.08167 [DOI] [PubMed] [Google Scholar]
- Irving RJ, Belton NR, Elton RA, & Walker BR (2000). Adult cardiovascular risk factors in premature babies. Lancet, 355, 2135–2136. doi:10.1016/S0140-6736(00)02384-9 [DOI] [PubMed] [Google Scholar]
- Kajantie E, Osmond C, Barker DJ, & Eriksson JG (2010). Preterm birth-A risk factor for type 2 diabetes? The Helsinki birth cohort study. Diabetes Care, 33, 2623–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubchenco LO, Hansman C, & Boyd E (1966). Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics, 37(3), 403–408. [PubMed] [Google Scholar]
- March of Dimes Perinatal Data Center. (2011). Special care nursery admissions. White Plains, NY: National Perinatal Information System/Quality Analytic Services. [Google Scholar]
- McEwen BS, & Seeman T (1999). Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Science, 896, 30–47. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M,…on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. (2015). Heart disease and stroke statistics-2015 update: A report from the American Heart [published online ahead of print December 16, 2015]. Circulation, e29–322. doi:doi:10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Nelson M (2010). Preterm birth-An emerging risk factor for adult hypertension? Seminars in Perinatology, 34(3), 183–187. doi:doi.org/10.1053/j.semperi.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Nwankwo T, Yoon SS, Burt V, & Gu Q (2013). Hypertension among adults in the US: National Health and Nutrition Examination Survey, 2011–2012. Hyattsville, MD: Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, & Flegal KM (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA, 311, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omron Health Care Inc®. (2001). Body fat analyzer instruction manual: Model HBF-306. Vernon Hills, IL: Retrieved from http://www.omronhealthcare.com. [Google Scholar]
- Papile LA, Burstein J, Burstein R, & Koffler H (1978). Incidence and evaluation of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. Journal of Pediatrics, 92(4), 529–534. [DOI] [PubMed] [Google Scholar]
- Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, & Modi N (2013). Preterm birth and the metabolic syndrome in adult life: A systematic review and meta-analysis. Pediatrics, 131(4), e1240–e1263. doi:10.1542/peds.2012-2177 [DOI] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, & et al. (2005). Recommendations for blood pressure measurement in humans and experimental animals: Part 1. Blood pressure measurement in humans: A Statement for professionals from the subcommittee of professional and public education of the American Heart Association Council on High Blood Pressure Research. Hypertension, 45(1), 142–161. [DOI] [PubMed] [Google Scholar]
- Regev RH, & Reichman B (2004). Prematurity and intrauterine growth retardation-double jeopardy? Clinics in Perinatology, 31(3), 453–473. [DOI] [PubMed] [Google Scholar]
- Sharafi M, Duffy VB, Miller RJ, Winchester SB, Heudo-Medina T, & Sullivan MC (2016). Dietary behaviors of adult born prematurely may explain future risk of cardiovascular disease. Appetite, 99, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipola-Leppänen M, Vääräsmäki M, Tikanmäki M, Matinolli H, Miettola S, Hovi P,… Kajantie E (2015). Cardiometabolic risk factors in young adults who were born preterm. American Journal of Epidemiology, 181(11), 861–873. doi:10.1093/aje/kwu443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, & Ryckman KK (2015). Epigenetic and developmental influences on the risk of obesity, diabetes, and metabolic syndrome. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 8, 295–302. doi:10.2147/DMSO.S61296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelgrove JW, & Murphy KE (2015). Preterm birth and social inequality: Assessing the effects of material and psychosocial disadvantage in a UK birth cohort. ACTA Obstetricia et Gynecologica Scandinavica, 94(7), 766–775. doi:10.1111/aogs.12648 [DOI] [PubMed] [Google Scholar]
- ter Wolbeek M, Kavelaars A, de Vries WB, Tersteeg-Kamperman M, Veen S, Kornelisse RF,… Heijnen CJ (2015). Neonatal glucococorticoid treatment: Long-term effects on hypothalamus-pituitary-adrenal axis, immunne sytem, and problem behavior in 14–17 year old adolescents. Brain, Behavior, and Immunity, 45, 128–138. doi:10.1016/j.bbi.2014.10.017 [DOI] [PubMed] [Google Scholar]
- Tu Y-K, West R, & Ellison GTH (2005). Why evidence for the fetal origins of adult disease might be a statistical artifact: The “reversal paradox” for the relation between birth weight and blood pressure in later life. American Journal of Epidemiology, 161(1), 27–32. [DOI] [PubMed] [Google Scholar]
- Winchester SB, Sullivan MC, Roberts MB, Bryce CI, & Granger D (2018). Long-term effects of prematurity, cumulative medical risk, and proximal and distal social forces on individual differences in diurnal cortisol at young adulthood. Biological Research for Nursing, 20(1), 5–15. doi: 10.1177/1099800417718955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester SB, Sullivan MC, Roberts MB, & Granger DA (2016). Prematurity, birth weight, and socioeconomic status are linked to atypical diurnal hypothalamic-pituitary-adrenal axis activity in young adults. Research in Nursing and Health, 39, 15–29. doi:10.1002/nur.21707 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2006). Global strategy on diet, physical activity and health. Retrieved from http://www.who.int/dietphysicalactivity/publications/facts/obesity/
- World Health Organization. (2013). Preterm birth (Fact sheet No. 363). Retrieved from http://www.who.int/mediacentre/factsheets/fs363/en/index.html.
- World Health Organization. (2016). Preterm birth Retrieved from http://www.who.int/mediacentre/factsheets/fs363/en/