Abstract
Calcium supplementation (1200 mg/day) did not significantly reduce colorectal adenomas in our recent randomized, controlled trial (Vitamin D/Calcium Polyp Prevention Study, VCPPS, 2004-2013) in contrast to our previous trial (Calcium Polyp Prevention Study, CPPS, 1988-1996). To reconcile these findings, we identified participant characteristics that differed between the study populations and modified the effect of calcium supplementation on adenomas or high-risk findings (advanced or multiple adenomas). Compared to the CPPS, more participants in the VCPPS were obese (body mass index (BMI) ≥30 kg/m2; 37.5% vs. 24.4%) and fewer had normal BMI (BMI <25 kg/m2; 18.5% vs. 31%). BMI appeared to modify the effect of calcium supplementation on adenomas and especially on high risk-findings: in the VCPPS, there was a 44% reduction in high-risk findings among individuals whose BMI was normal (RR=0.56, 95% CI=0.25-1.26), but not among overweight (RR=1.11, 95% CI=0.63-1.94) or obese (RR=1.52, 95% CI=0.90-2.55) individuals (Pinteraction=0.03). Similarly, in the CPPS, there was a 54% reduction in high-risk findings among individuals whose BMI was normal (RR=0.46, 95% CI=0.27-0.77), but not among overweight (RR=0.86, 95% CI=0.54-1.38) or obese (RR=1.00, 95% CI=0.56-1.79) individuals (Pinteraction=0.03). Standardization of each trial’s findings to the BMI distribution in the other attenuated calcium’s protective effect on adenomas in the CPPS but enhanced it in the VCPPS. In conclusion, 1200 mg/day calcium supplementation may reduce risk of colorectal adenomas among those with normal BMI but not in overweight or obese individuals; and differences in BMI distribution partially account for the apparent difference in calcium efficacy between the two trials.
Keywords: calcium supplementation, colorectal adenoma, body mass index, clinical trial
INTRODUCTION
Considerable evidence from preclinical and observational studies suggests that calcium supplementation exerts a protective effect against colorectal neoplasia, both colorectal cancer and its main precursor lesion, colorectal adenomas 1–4. We conducted two randomized, double-blind, placebo-controlled trials of calcium supplementation (1200 mg/day elemental calcium as carbonate) for the prevention of new colorectal adenomas in individuals with a recent history of adenomas. These studies were conducted 16 years apart with disparate results. In the Calcium Polyp Prevention Study (CPPS; 1988–1996) calcium supplementation was associated with relative risk reductions of about 15% for adenomas and 27% for advanced adenomas (those with high-grade dysplasia, >25% villous features, a diameter ≥1 cm, or cancer) 5, 6. However, in the more recent Vitamin D/Calcium Polyp Prevention Study (VCPPS; 2004–2013), calcium supplementation had no demonstrable effect: there was a non-significant 5% relative risk reduction for adenomas and a non-significant 2% relative risk increase for advanced adenomas 7.
In the present work, we explored whether differences in participant characteristics between the two trials may help to explain the disparate results and clarify who may benefit from supplementation. The goals of this paper are to: (1) describe differences in participant characteristics between the two trial populations, (2) investigate the potential for these characteristics to modify the effect of calcium supplementation on risk of colorectal adenomas or high-risk findings and (3) if effect modification is present, to estimate how much of the discrepancy in the results of the two trials could be explained by differences in these characteristics.
METHODS
Study Populations and Designs
The two trials had similar designs, as detailed in our original publications 5, 7 and described briefly below.
CPPS Study Design: Participants were recruited between November 1988 and December 1992 from six academic medical centers and associated clinical practices located in CA, IA, MN, NC, NH, OH. Eligible patients had at least one colorectal adenoma removed at a qualifying colonoscopy within three months before recruitment, were in good general health, and had no familial colorectal cancer syndromes or indications/contraindications for the study agents.
At enrollment, participants completed questionnaires regarding demographics, medical history, medications, lifestyle habits, and diet and were asked to forgo use of calcium supplements or multivitamins containing calcium while on study treatment. Diet and alcohol intake were assessed using the semi-quantitative Block 1998 Food Frequency Questionnaire (110 food items) (https://nutritionquest.com/). Enrollment was followed by an approximately 3 month blinded run-in period designed to identify and exclude participants who took less than 80% of their run-in pills. At randomization, participants were assigned with equal probability to either placebo or calcium carbonate (1200 mg/day elemental calcium). Identical appearing study tablets containing placebo or study agents were to be taken twice a day. After randomization, interval questionnaires were administered every six months regarding participant adherence to study treatment, illnesses, and medication and supplement use. Study treatment ended in December 1996. Baseline serum 25-hydroxyvitamin D concentrations were measured in 2001 on stored specimens obtained at time of enrollment using a competitive protein binding radioimmunoassay kit from Nichols Institute Diagnostics that used the vitamin D binding protein as the binding agent and 3H-labeled 25-hydroxyvitamin D as the reporter 8.
VCPPS study design: The design of the VCPPS was as described above for the CPPS, with the following differences. Recruitment was between July 2004 and July 2008 and, in addition to the centers in the CPPS, five more centers located in CO, GA, PR, SC, and TX were included. Diet and alcohol intake were assessed using the semi-quantitative Block Brief 2000 Food Frequency Questionnaire (with a reduced food list of about 70 items) (https://nutritionquest.com/). In a partial factorial design, participants were randomly assigned with equal probability to placebo, calcium carbonate (1200 mg/day elemental calcium), vitamin D3 (1000 IU/day) or calcium + vitamin D (full factorial randomization). However, women who wanted calcium supplementation could elect to be randomly assigned to either calcium or calcium + vitamin D (two-group randomization). Study treatment ended in July 2013. Baseline 25-hydroxyvitamin D concentrations were measured between 2004–2008 using a competitive protein binding radioimmunoassay kit from Immunodiagnostic Systems that used an antibody to 25-hydroxyvitamin D as the binding agent and 125I-labeled 25-hydroxyvitamin D as the reporter 7.
In both studies, all participants provided written informed consent, and institutional review board approval was obtained at the Dartmouth Project Coordination Center and each participating clinical center.
Treatment Duration and Outcome Assessment
In the CPPS, participants were scheduled to undergo two follow-up colonoscopies: one and four years after the qualifying exam, with treatment ending at the time of the four year follow-up exam. In the original trial analysis, the period after the first exam up to and including the second exam was considered the main risk period 5. In the current analyses, adenoma outcomes for CPPS were assessed from randomization up to and including their year four follow-up exam to more closely resemble the study design used in the VCPPS (see below). All randomized participants with outcome data were included in the present analyses.
In the VCPPS, participants were scheduled to undergo a single follow-up colonoscopy either three or five years after the qualifying exam as recommended by the treating endoscopists, with treatment ending at the time of that follow-up exam. Adenoma outcomes in the VCPPS were assessed from randomization up to and including the year three or five follow-up exam. The primary analyses performed here included only full factorial participants randomized to placebo or to calcium alone, in order to exclude potential effects of vitamin D3 supplementation. In sensitivity analyses, all full factorial participants were included. However, women in the two-group randomization (who elected to receive calcium rather than being randomized to calcium treatment) were excluded from all analyses.
The same blinded study pathologist assessed adenoma outcomes in both trials. Advanced adenomas were defined as those with cancer, high-grade dysplasia, more than 25% villous features, or an estimated diameter of at least 1 cm. For statistical analyses, two outcomes were assessed: 1) adenomas, defined as the occurrence during follow-up of one or more adenoma of any type; and 2) high-risk findings, defined as the occurrence during follow-up of one or more advanced adenoma or of multiple small tubular adenomas (3 or more), which are both strong predictors of future risk for advanced colorectal neoplasia and cancer 9.
Statistical Analyses
We used multivariable generalized linear models for binary data to estimate adjusted risk ratios, risk differences, and confidence intervals for the occurrence of either: 1) adenomas or 2) high-risk findings (as defined above). Due to sample size limitations in sub-group analyses designed to assess effect measure modification, we used minimal adjustment for a set of pre-specified co-variates [age (continuous), sex (male or female), and race/ethnicity (self-reported non-Hispanic white or other)] reduced from those included in the original analyses of the treatment effects published previously 5–7).
For common factors that differed in prevalence between the two study populations and could be reliably compared, post-hoc subgroup analyses were conducted to examine whether they modified the effect of calcium supplementation: age, sex, race/ethnicity, smoking status, BMI, alcohol use, baseline high-risk findings, and use of aspirin or non-steroidal anti-inflammatory drugs (NSAIDs) during follow-up. Effect measure modification was assessed with the use of Wald tests or likelihood ratio tests (race/ethnicity or smoking) and multiplicative or additive interaction terms. For continuous variables (age, BMI, alcohol), trends were assessed over categories, using the median value of each category to calculate the p-value for interaction.
Participant characteristics (potential effect measure modifiers) that might partially explain the difference in overall treatment effect between trials were selected as those that had statistically significant interaction terms with the treatment variable (calcium vs. placebo) in both trials. Variables meeting this criterion were further assessed using interaction analyses10 with additional adjustment for potential confounders of their association with adenoma outcomes as well as using standardization methods (see below) to compare results across the two studies. To standardize the participant population in the CPPS or the VCPPS trial to the distribution of observed effect measure modifier in the other trial, we used inverse odds of sampling weights 11. Briefly, inverse odds of sampling weights use a form of model-based standardization 12 to transport the effects of an intervention in a given study population (in this case, the effects of calcium supplementation on adenoma risk in one of the two trials) to another target population (here, the other trial). These methods have been applied to a variety of randomized clinical trials when seeking to generalize or transport treatment effects to other target populations of interest 12–14.
All analyses were conducted according to the intent-to-treat principle regardless of participant adherence. Two-sided P values of <0.05 were considered to indicate statistical significance. The significance levels were not adjusted for multiple testing because tests for interaction are generally underpowered and this is an exploratory hypothesis-generating analysis. Analyses were conducted with STATA software, version 12 (StataCorp) and SAS, version 9.4.
RESULTS
Flow diagrams for participants from both studies that were included in the present analyses are shown in Figure 1. Among 930 randomized participants in the CPPS, 882 (95%) had data available for the outcome of adenomas and 856 (92%) for the outcome of high-risk findings (Figure 1). Among the subset of 834 participants randomized to placebo or calcium alone in the VCPPS, 761 (91%) had data available for the outcome of one or more adenomas and 751 (90%) for the outcome of high-risk findings (Figure 1).
Figure 1.
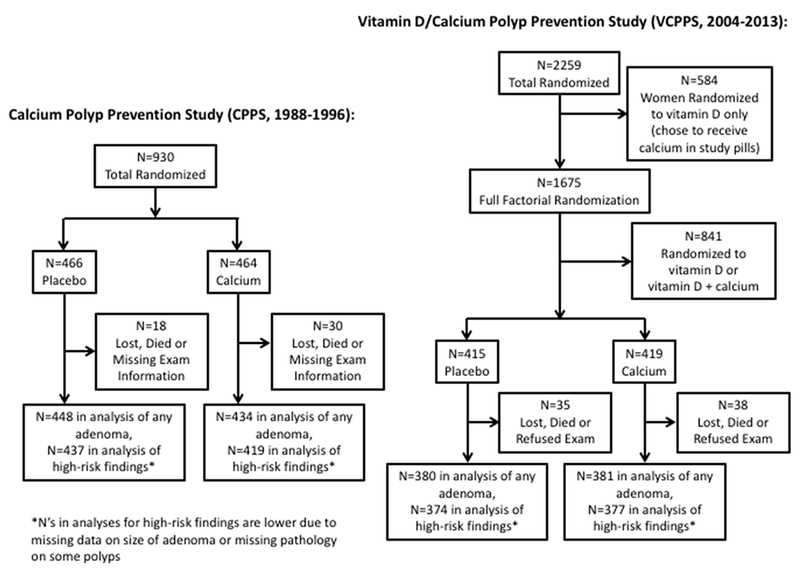
Participant Flowcharts
Selected baseline characteristics of the participants analyzed from the two trials are compared in Table 1. Participants in the VCPPS were, on average, three years younger (58 vs. 61 years old), and more likely to be male (85.3 vs. 72.3%). The overall racial and ethnic make-up of participants in the two trial populations was broadly similar, with 84-85% being non-Hispanic whites; however, there were slightly more Hispanics in the VCPPS than in the CPPS (5.1 vs 2.9%). Participants in the VCPPS were less likely to be current smokers (8.2 vs. 19.3%) and more likely to have >1 alcoholic drink per day (30.3 vs 19.2%). BMI was 1.8 kg/m2 higher on average among participants in the VCPPS than in the CPPS (29.2 vs. 27.4 kg/m2, P<0.001), such that participants in the VCPPS were more likely to be obese (BMI ≥30 kg/m2, 37.5 vs. 24.4%) and less likely to have normal BMI (BMI <25 kg/m2, 18.5 vs 31%). Also, participants in the VCPPS were less likely to have high-risk adenoma findings at their baseline colonoscopy (28.1 vs. 45.7%). Although baseline serum 25-hydroxyvitamin D concentrations were assessed in both studies, the values could not be directly compared since different methods were used for the measurements.
Table 1.
Selected Characteristics of Randomized Participants, by Study
| CPPS (N=930) |
VCPPS (N=834) |
P1 |
|||
|---|---|---|---|---|---|
| Placebo (N=466) | Calcium (N=464) | Placebo N=415 | Calcium N=419 | ||
| Baseline Characteristics | |||||
| Age, Mean (SD) | 61 (9.1) | 61 (9.1) | 58 (7.0) | 59 (7.0) | <0.0001 |
| Male, N (%) | 327 (70.2) | 345 (74.4) | 355 (85.5) | 356 (85.0) | <0.0001 |
| Race/ethnicity, N (%) | 0.12 | ||||
| White, Non-Hispanic | 405 (86.9) | 386 (83.2) | 352 (84.8) | 347 (83.2) | |
| Black, Non-Hispanic | 34 (7.3) | 41 (8.8) | 27 (6.5) | 32 (7.7) | |
| Hispanic | 14 (3.0) | 13 (2.8) | 19 (4.6) | 23 (5.5) | |
| Other | 13 (2.8) | 24 (5.2) | 17 (4.1) | 15 (3.6) | |
| Smoking, N (%) | <0.0001 | ||||
| Never | 157 (33.7) | 152 (32.8) | 187 (45.1) | 212 (50.6) | |
| Former | 224 (48.1) | 218 (47.0) | 193 (46.5) | 174 (41.5) | |
| Current | 85 (18.2) | 94 (20.3) | 35 (8.4) | 33 (7.9) | |
| BMI, N (%) | <0.0001 | ||||
| < 25 (normal) | 153 (32.9) | 135 (29.2) | 80 (19.3) | 74 (17.7) | |
| 25-<30 (overweight) | 213 (45.8) | 201 (43.4) | 187 (45.2) | 180 (43.0) | |
| ≥30 (obese) | 99 (21.3) | 127 (27.4) | 147 (35.5) | 165 (39.4) | |
| Alcohol Use, N (%) | <0.0001 | ||||
| None | 156 (34.3) | 153 (33.9) | 117 (30.1) | 117 (30.2) | |
| >0-≤1 drinks/day | 209 (45.9) | 214 (47.5) | 148 (38.1) | 159 (41.1) | |
| >1 drink/day | 90 (19.8) | 84 (18.6) | 124 (31.9) | 111 (28.7) | |
| High-Risk Findings2, N (%) | 213 (45.7) | 212 (45.7) | 104 (26.0) | 120 (30.2) | <0.0001 |
| Follow-up Characteristics | |||||
| Frequent Aspirin Use3, N (%) | 93 (20.2) | 71 (15.5) | 224 (54.6) | 230 (55.4) | <0.0001 |
| Frequent Non-Aspirin NSAID Use3, N (%) | 42 (9.1) | 64 (14.0) | 190 (46.3) | 204 (49.2) | <0.0001 |
Abbreviations: CPPS, Calcium Polyp Prevention Study; VCPPS, Vitamin D/Calcium Polyp Prevention Study; SD, standard deviation; BMI, body mass index in kg/m2; NSAID, non-steroidal anti-inflammatory drug.
P values for comparisons between the CPPS and the VCPPS; two-sample t tests for continuous variables and Pearson’s chi-squared tests for categorical variables.
High-risk findings at baseline colonoscopy refers to ≥1advanced adenoma or ≥3 adenomas of any type. Advanced adenomas are those with cancer, high-grade dysplasia, > 25% villous features, or diameter ≥1 cm. In the CPPS lost pathology was not recorded at baseline, so participants who had lost pathology may have been misclassified as having no high-risk findings.
Use reported on >50% of the participant’s interval questionnaires. In the CPPS participants were asked what prescription and over-the-counter drugs they were currently taking; aspirin and NSAID containing products were pulled from those drugs reported. In the VCPPS participants were specifically asked if they had taken any aspirin or NSAID products.
N’s for missing data: race/ethnicity, 2 (VCPPS); BMI, 2 (CPPS) 1 (VCPPS); alcohol use, 24 (CPPS) 58 (VCPPS); high-risk findings, 36 (VCPPS); Aspirin and NSAID use, 11 (CPPS) 9 (VCPPS).
Participant characteristics related to study participation, including length of follow-up on treatment, adherence to pill-taking and avoidance of personal calcium supplementation were generally similar in the two trials. The median (± standard deviation) follow-up time was 43.9 (± 10.4) months in the CPPS and 42.8 (± 13.1) months in the VCPPS, although the distribution was bi-modal in the VCPPS, reflecting the endoscopists’ recommendations for follow-up (Supplement Figure 1). In the CPPS, 75.7% of participants reported taking >80% of their study pills in their last year of treatment, compared with 72.3% of participants in the VCPPS. Avoidance of personal calcium supplementation was excellent in both trials: only 4 participants in the CPPS and 7 in the VCPPS reported taking >400 mg/day calcium in the last year of treatment. Frequent use of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) during study participation was much higher among VCPPS participants than among CPPS participants (55.0 vs. 17.9% and 47.8 vs. 11.5%, respectively; Table 1).
In the current analysis (adjusted for age, sex and race/ethnicity), calcium supplementation reduced the risk of adenomas and high-risk findings in the CPPS but not in the VCPPS, consistent with our prior published results 5–7. In the CPPS, calcium supplementation was associated with borderline statistically significant risk reductions of 13% for adenomas (RR=0.87, 95% CI=0.76–1.00) and 27% for high-risk findings (RR=0.73 95% CI=0.55–0.97). In the VCPPS, calcium supplementation was not associated with statistically significant risk reductions for adenomas (RR=0.94, 95% CI=0.81–1.10) or high-risk findings (RR=1.12, 95% CI=0.81–1.56).
We next assessed whether the participant characteristics included in Table 1 modified the effect of calcium supplementation on adenomas (Table 2) or high-risk findings (Table 3) in the two trials. The most notable findings were for BMI, which modified the effect of calcium supplementation such that the preventive effect on adenoma outcomes disappeared and became potentially harmful as BMI increased. Specifically, in the VCPPS, calcium supplementation was associated with a statistically significant 46% risk reduction for adenomas among individuals with normal BMI (RR=0.54, 95% CI=0.36–0.80), but not among those who were overweight (RR=0.95, 95% 0.74–1.21) or obese (RR=1.22, 95% CI=0.96–1.55) (Pinteraction=0.001) (Table 2). Similarly, in the VCPPS, calcium supplementation was associated with a non-significant 44% risk reduction for high-risk findings among individuals with normal BMI (RR=0.56, 95% CI=0.26–1.23), but not among those who were overweight (RR=1.091, 95% CI=0.62–1.91) or obese (RR=1.55, 95% CI=0.92–2.57) (Pinteraction=0.03) (Table 3). Similar trends were seen when full factorial VCPPS participants assigned to vitamin D treatment were included in the analyses (Supplement Table 1).
Table 2.
Subgroup Analyses of the Effect of Calcium Supplementation on Adenoma Recurrence1
| CPPS |
VCPPS |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Category | N/Total (%)2 | RR (95% CI)3 | Pint4 | N/Total (%)2 | RR (95% CI)3 | Pint4 |
| Overall | 421/882 (47.7) | 0.87 (0.76-1.00) | 356/761 (46.8) | 0.94 (0.81-1.10) | |||
| Age | <55 | 78/208 (37.5) | 0.90 (0.63-1.28) | 0.53 | 101/256 (39.5) | 1.12 (0.82-1.52) | 0.17 |
| 55-64 | 153/316 (48.4) | 0.75 (0.59-0.94) | 156/329 (47.4) | 0.92 (0.73-1.16) | |||
| 65+ | 190/358 (53.1) | 0.97 (0.80-1.18) | 99/176 (56.3) | 0.81 (0.62-1.06) | |||
| Sex | Male | 324/637 (50.9) | 0.89 (0.77-1.04) | 0.49 | 307/653 (47.0) | 0.91 (0.77-1.07) | 0.23 |
| Female | 97/245 (39.6) | 0.82 (0.60-1.14) | 49/108 (44.4) | 1.15 (0.75-1.76) | |||
| Race/ ethnicity |
NHW | 363/749 (48.5) | 0.87 (0.75-1.01) | 0.48 | 306/645 (47.4) | 0.96 (0.81-1.13) | 0.87 |
| NHB | 31/72 (43.1) | 1.23 (0.71-2.15) | 25/51 (49.0) | 0.81 (0.45-1.46) | |||
| Other | 27/61 (44.3) | 0.59 (0.33-1.07) | 24/63 (37.1) | 0.94 (0.49-1.81) | |||
| Smoking | Never | 126/291 (43.3) | 0.72 (0.55-0.95) | 0.34 | 170/370 (46.0) | 1.09 (0.88-1.37) | 0.35 |
| Former | 210/422 (49.8) | 0.90 (0.74-1.09) | 151/327 (46.2) | 0.82 (0.64-1.05) | |||
| Current | 85/169 (50.3) | 1.08 (0.79-1.47) | 35/64 (54.7) | 0.82 (0.51-1.31) | |||
| BMI | <25 | 145/274 (52.9) | 0.79 (0.63-1.00) | 0.29 | 65/140 (46.4) | 0.54 (0.36-0.80) | 0.001 |
| 25-29.9 | 176/389 (45.2) | 0.90 (0.72-1.12) | 148/336 (44.1) | 0.95 (0.74-1.21) | |||
| 30+ | 98/217 (45.2) | 0.93 (0.69-1.26) | 142/284 (50.0) | 1.22 (0.96-1.55) | |||
| Alcohol Use | None | 128/286 (44.8) | 1.06 (0.82–1.38) | 0.03 | 113/205 (55.1) | 0.87 (0.67–1.12) | 0.72 |
| >0 to ≤1 | 192/408 (47.1) | 0.88 (0.72–1.09) | 116/287 (40.4) | 1.10 (0.83–1.47) | |||
| >1 | 89/165 (53.9) | 0.67 (0.50–0.90) | 108/219 (49.3) | 0.91 (0.69–1.19) | |||
| Baseline High-Risk Findings5 | No | 202/476 (42.4) | 0.85 (0.68–1.05) | 0.68 | 228/520 (43.9) | 0.93 (0.77–1.13) | 0.94 |
| Yes | 219/406 (53.9) | 0.89 (0.74–1.07) | 110/208 (52.9) | 0.92 (0.71–1.19) | |||
| Frequent Aspirin Use6 | No | 337/719 (46.9) | 0.84 (0.71–0.98) | 0.31 | 158/340 (46.5) | 0.90 (0.72–1.14) | 0.68 |
| Yes | 81/159 (50.9) | 1.00 (0.73–1.36) | 196/418 (46.9) | 0.97 (0.79–1.19) | |||
| Frequent NSAID Use6 | No | 381/781 (48.8) | 0.87 (0.75–1.00) | 0.65 | 183/365 (50.1) | 1.06 (0.86–1.31) | 0.12 |
| Yes | 37/97 (38.1) | 0.95 (0.56–1.81) | 171/393 (43.5) | 0.83 (0.66–1.04) | |||
Abbreviations: CPPS, Calcium Polyp Prevention Study; VCPPS, Vitamin D/Calcium Polyp Prevention Study; RR, relative risk; 95% CI, 95% confidence interval; NHW, non-Hispanic white; NHB, non-Hispanic black; NSAID, non-steroidal anti-inflammatory drug.
Models are adjusted for age (continuous), sex (male/female), and race/ethnicity (Non-Hispanic white/other).
Adenoma recurrence refers to the development of ≥1 adenomas of any type.
Number of participants with outcome/total number of participants (% of participants with outcome).
Relative risks for calcium supplementation within each stratum.
Interactions were assessed with the use of Wald tests or likelihood ratio tests (race and smoking) and multiplicative interaction terms.
High-risk findings at baseline colonoscopy refers to ≥1advanced adenoma or ≥3 adenomas of any type. Advanced adenomas are those with cancer, high-grade dysplasia, >25% villous features, or diameter ≥1 cm.
Frequent aspirin use or non-aspirin NSAID use refers to use reported on >50% of interval questionnaires collected during study participation.
Table 3.
Subgroup Analyses of the Effect of Calcium Supplementation on High-Risk Findings1
| CPPS |
VCPPS |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Category | N/Total (%)2 | RR (95% CI)3 | Pint4 | N/Total (%)2 | RR (95% CI)3 | Pint4 |
| Overall | 158/856 (18.5) | 0.73 (0.55-0.97) | 121/751 (16.1) | 1.12 (0.81-1.56) | - | ||
| Age | <55 | 20/205 (9.8) | 0.80 (0.35-1.87) | 0.94 | 39/253 (15.4) | 1.47 (0.82-2.65) | 0.23 |
| 55-64 | 56/302 (18.5) | 0.63 (0.39-1.03) | 49/324 (15.1) | 1.06 (0.63-1.79) | |||
| 65+ | 82/349 (23.5) | 0.78 (0.53-1.16) | 33/174 (19.0) | 0.84 (0.45-1.58) | |||
| Sex | Male | 121/616 (19.6) | 0.84 (0.61-1.16) | 0.08 | 106/645 (16.4) | 1.01 (0.71-1.43) | 0.08 |
| Female | 37/240 (15.4) | 0.42 (0.22-0.83) | 15/106 (14.2) | 2.64 (0.91-7.72) | |||
| Race/ ethnicity |
NHW | 135/724 (18.7) | 0.69 (0.50-0.94) | 0.34 | 108/636 (17.0) | 1.10 (0.78-1.55) | 0.71 |
| Other | 23/132 (17.4) | 1.02 (0.47-2.19) | 13/111 (11.7) | 1.37 (0.47-3.95) | |||
| Smoking | Never | 37/287 (12.9) | 0.40 (0.20-0.78) | 0.12 | 53/364 (14.6) | 1.21 (0.73-2.01) | 0.76 |
| Former | 85/407 (20.9) | 0.79 (0.54-1.15) | 48/324 (14.8) | 1.21 (0.72-2.04) | |||
| Current | 36/162 (22.2) | 1.04 (0.57-1.90) | 20/63 (31.8) | 0.93 (0.47-1.82) | |||
| BMI | <25 | 56/264 (21.2) | 0.44 (0.26-0.74) | 0.02 | 24/137 (17.5) | 0.56 (0.26-1.23) | 0.03 |
| 25-29.9 | 61/378 (16.1) | 0.87 (0.55-1.39) | 44/334 (13.2) | 1.09 (0.62-1.91) | |||
| 30+ | 39/212 (18.4) | 1.02 (0.57-1.82) | 53/279 (19.0) | 1.54 (0.92-2.57) | |||
| Alcohol Use | None | 47/279 (16.9) | 0.66 (0.38-1.14) | 0.98 | 35/203 (17.2) | 0.93 (0.50-1.72) | 0.86 |
| >0 to ≤1 | 69/397 (17.4) | 0.82 (0.53-1.26) | 35/283 (12.4) | 1.81 (0.93-3.51) | |||
| >1 | 35/157 (22.3) | 0.76 (0.43-1.33) | 44/215 (20.5) | 1.09 (0.65-1.84) | |||
| Baseline High-Risk Findings5 | No | 53/462 (11.5) | 0.69 (0.40-1.20) | 0.71 | 71/513 (13.8) | 1.04 (0.67-1.60) | 0.82 |
| Yes | 105/394 (26.7) | 0.75 (0.54-1.05) | 43/207 (20.8) | 1.13 (0.66-1.94) | |||
| Frequent Aspirin Use6 | No | 129/700 (18.4) | 0.75 (0.55-1.03) | 0.66 | 50/335 (14.9) | 1.08 (0.65-1.82) | 0.95 |
| Yes | 29/152 (19.1) | 0.65 (0.31-1.34) | 69/413 (16.7) | 1.11 (0.72-1.71) | |||
| Frequent NSAID Use6 | No | 144/758 (19.0) | 0.82 (0.61-1.10) | 0.04 | 64/361 (17.7) | 1.15 (0.73-1.79) | 0.80 |
| Yes | 14/94 (14.9) | 0.26 (0.09-0.74) | 55/387 (14.2) | 1.06 (0.65-1.74) | |||
Abbreviations: CPPS, Calcium Polyp Prevention Study; VCPPS, Vitamin D/Calcium Polyp Prevention Study; RR, relative risk; 95% CI, 95% confidence interval; NHW, non-Hispanic white; NSAID, non-steroidal anti-inflammatory drug.
Models are adjusted for age (continuous), sex (male/female), and race/ethnicity (Non-Hispanic white/other).
High-risk findings refers to the development of ≥1 advanced adenoma or multiple (≥3) adenomas of any type. Advanced adenomas are defined as those with cancer, high-grade dysplasia, >25% villous features, or a diameter ≥1 cm.
Number of participants with outcome/total number of participants (% of participants with outcome).
Relative risks for calcium supplementation within each stratum.
Interactions were assessed with the use of Wald tests or likelihood ratio tests (race and smoking) and multiplicative interaction terms.
High-risk findings at baseline colonoscopy refers to ≥1advanced adenoma or ≥3 adenomas of any type. Advanced adenomas are those with cancer, high-grade dysplasia, >25% villous features, or diameter ≥1 cm.
Frequent aspirin use or non-aspirin NSAID use refers to use reported on >50% of interval questionnaires collected during study participation.
In the CPPS, there were similar trends in effect measure modification by BMI for adenomas, though this was not statistically significant (Pinteraction=0.29). Specifically, calcium supplementation was associated with a borderline statistically significant 21% risk reduction among individuals with normal BMI (RR=0.79, 95% CI=0.63–1.00), but not among those who were overweight (RR=0.90, 95% CI=0.72–1.12) or obese (RR=0.93, 95% CI=0.69–1.26) (Table 2). For high-risk findings, in the CPPS, calcium supplementation was associated with a statistically significant 56% risk reduction among individuals with normal BMI (RR=0.44, 95% CI=0.26–0.74), but not among those who were overweight (RR=0.87, 95% CI=0.55–1.39) or obese (RR=1.02, 95% CI=0.57–1.82) (Pinteraction=0.02) (Table 3). In both trials, controlling for smoking status and baseline blood 25-hydroxyvitamin D concentrations, which are negatively correlated with BMI (not shown), did not appreciably change the results in the analysis of the causal interaction of BMI with calcium supplementation (Table 4).
Table 4.
Interaction of BMI with the Effect of Calcium Supplementation on Adenoma Outcomes
| Placebo | Calcium | RR (95% CI)3 within BMI Strata |
Pint4 | |||
|---|---|---|---|---|---|---|
| N/Total (%)1 | RR (95% CI)2 | N/Total (%)1 | RR (95% CI)2 | |||
| CPPS – Adenoma Recurrence5: | ||||||
| BMI6 <25 | 86/146 (58.9) | 1.0 (Ref) | 59/128 (46.1) | 0.83 (0.65-1.07) | 0.84 (0.66-1.07) | 0.49 |
| BMI 25-<30 | 96/204 (47.1) | 0.80 (0.64-1.00) | 80/185 (43.2) | 0.73 (0.58-0.92) | 0.90 (0.72-1.13) | |
| BMI ≥30 | 45/97 (46.4) | 0.79 (0.60-1.05) | 53/120 (44.2) | 0.74 (0.57-0.97) | 0.95 (0.68-1.31) | |
| CPPS - High Risk Findings7: | ||||||
| BMI <25 | 41/142 (28.9) | 1.0 (Ref) | 15/122 (12.3) | 0.47 (0.27-0.82) | 0.48 (0.28-0.82) | 0.03 |
| BMI 25-<30 | 34/200 (17.0) | 0.62 (0.41-0.95) | 27/178 (15.2) | 0.52 (0.33-0.82) | 0.82 (0.51-1.33) | |
| BMI ≥30 | 17/94 (18.1) | 0.60 (0.34-1.05) | 22/118 (18.6) | 0.66 (0.40-1.08) | 1.12 (0.59-2.11) | |
| VCPPS – Adenoma Recurrence5: | ||||||
| BMI <25 | 44/74 (59.5) | 1.0 (Ref) | 21/66 (31.8) | 0.56 (0.38-0.82) | 0.54 (0.36-0.80) | 0.001 |
| BMI 25-<30 | 79/172 (45.9) | 0.79 (0.60-1.04) | 69/164 (42.1) | 0.73 (0.55-0.97) | 0.95 (0.74-1.22) | |
| BMI ≥30 | 59/133 (44.4) | 0.79 (0.59-1.05) | 83/151 (55.0) | 0.94 (0.72-1.24) | 1.21 (0.95-1.54) | |
| VCPPS - High Risk Findings7: | ||||||
| BMI <25 | 16/71 (22.5) | 1.0 (Ref) | 8/66 (12.1) | 0.57 (0.26-1.25) | 0.58 (0.26-1.28) | 0.04 |
| BMI 25-<30 | 22/172 (12.8) | 0.56 (0.31-1.02) | 22/162 (13.6) | 0.63 (0.35-1.14) | 1.13 (0.64-1.97) | |
| BMI ≥30 | 19/130 (14.6) | 0.67 (0.36-1.25) | 34/149 (22.8) | 1.01 (0.58-1.75) | 1.52 (0.90-2.56) | |
Abbreviations: CCPS, Calcium Polyp Prevention Study; VCPPS, Vitamin D/Calcium Polyp Prevention Study; BMI, body mass index in kg/m2; Pint, interaction p-value; RR, relative risk; 95% CI, 95% confidence interval.
Models are adjusted for age (continuous), sex (male/female), race/ethnicity (non-Hispanic white/other), smoking status (never/former/current), baseline serum 25(OH)D.
Number of participants with outcome/total number of participants (% of participants with outcome).
Relative risks compared to referent group (BMI<25, assigned to placebo).
Relative risks for calcium supplementation within each BMI stratum.
Interactions (calcium/placebo × BMI category) were assessed with the use of Wald tests and multiplicative interaction terms.
Adenoma recurrence refers to the development of ≥1 adenomas of any type.
Median BMI values for the three categories are: 22.9, 27.3 and 33.4 (CPPS); 23.3, 27.5, 34.2 (VCPPS)
High-risk findings refers to the development of ≥1 advanced adenoma or ≥3 adenomas of any type. Advanced adenomas are defined as those with cancer, high-grade dysplasia, >25% villous features, or a diameter ≥1 cm.
There was little evidence that other participant characteristics modified the effect of calcium supplementation on adenomas (Table 2) or high-risk findings (Table 3). Age, sex, race/ethnicity, smoking, baseline high-risk findings, and frequent aspirin use during study participation did not modify the effect of calcium supplementation on either outcome in either study (Pinteraction > 0.05). There was inconsistent evidence for effect modification by alcohol use and frequent non-aspirin NSAID use during the study. Increasing alcohol use was associated with a greater adenoma risk reduction from calcium supplementation in the CPPS (Pinteraction=0.03, Table 2) but not the VCPPS (Pinteraction=0.72, Table 2), and no effect modification was seen for high-risk findings in either trial (Table 3). Non-aspirin NSAID use was associated with a greater risk reduction for high-risk findings from calcium supplementation in the CPPS (Pinteraction=0.04, Table 2) but not in the VCPPS (Pinteraction=0.80, Table2) and no effect modification was seen for adenoma risk in either study (Table 2).
Finally, to estimate how much of the discrepancy in the results for calcium supplementation between the two trials could be explained by the difference in BMI distributions of the trial participants, we standardized the findings from the CPPS trial to the BMI distribution of the VCPPS and, alternatively, standardized the findings from the VCPPS to the BMI distribution of the CPPS (Table 5). After standardization of the CPPS findings to the higher BMI distribution of the VCPPS, the protective effects of calcium supplementation were attenuated: 1) there was a standardized relative risk reduction of 11% for adenoma risk (RR=0.89, 95% CI=0.77–1.03) compared to a 13% unstandardized relative risk reduction (RR=0.87, 95% CI = 0.76–1.00); 2) there was a standardized relative risk reduction of 19% for high-risk findings (RR=0.81, 95% CI=0.60–1.08) compared to a 28% unstandardized relative risk reduction (RR=0.72, 95% CI = 0.54–0.96). Conversely, after standardization of the VCPPS to the lower BMI distribution of the CPPS, the protective effect of calcium supplementation on adenomas was enhanced and the deleterious effect on high-risk findings was eliminated: 1) there was a standardized relative risk reduction of 14% for adenoma risk (RR=0.86, 95% CI=0.73–1.00) compared to a 5% unstandardized relative risk reduction (RR=0.95, 95% CI = 0.81–1.10); 2) there was a standardized relative risk reduction of 3% for high-risk findings (RR=0.97, 95% CI=0.70–1.35) compared to a 12% unstandardized relative risk increase (RR=1.12, 95% CI = 0.81–1.56).
Table 5.
Effect of Calcium Supplementation on Adenoma Outcomes: Unstandardized Analyses and Analyses Standardized to BMI
| Placebo | Calcium | Unstandardized2 |
Standardized3 |
|||
|---|---|---|---|---|---|---|
| N/Total (%)1 | N/Total (%)1 | RR (95% CI) | P | RR (95% CI) | P | |
| CPPS Study Findings: Unstandardized and Standardized to the BMI Distribution in the VCPPS | ||||||
| Adenoma Recurrence4 | 228/448 (50.9) | 193/434 (44.5) | 0.87 (0.76-1.00) | 0.05 | 0.89 (0.77-1.03) | 0.11 |
| High Risk Findings5 | 93/437 (21.3) | 65/419 (15.5) | 0.73 (0.54-0.97) | 0.03 | 0.82 (0.61-1.09) | 0.17 |
| VCPPS Study Findings: Unstandardized and Standardized to the BMI Distribution in the CPPS | ||||||
| Adenoma Recurrence4 | 183/380 (48.2) | 173/381 (45.4) | 0.95 (0.81-1.10) | 0.49 | 0.86 (0.73-1.00) | 0.05 |
| High Risk Findings5 | 57/374 (15.2) | 64/377 (17.0) | 1.12 (0.81-1.56) | 0.49 | 0.97 (0.70-1.35) | 0.87 |
Abbreviations: CCPS, Calcium Polyp Prevention Study; VCPPS, Vitamin D/Calcium Polyp Prevention Study; BMI, body mass index in kg/m2; RR, relative risk; 95% CI, 95% confidence interval; P, Wald test p-value.
All models are adjusted for age (continuous), sex (male/female), and race/ethnicity (non-Hispanic white/other).
Number of participants with outcome/total number of participants (% of participants with outcome).
Unstandardized RRs for calcium supplementation compared to placebo.
For the CPPS, the RRs for calcium supplementation compared to placebo were standardized to the BMI distribution of the VCPPS using inverse odds of sampling weights. For VCPPS, the RRs for calcium supplementation compared to placebo were standardized to the BMI distribution in the CPPS using inverse probability of treatment weights.
Adenoma recurrence refers to the development of ≥1 adenomas of any type.
High-risk findings refers to the development of ≥1 advanced adenoma or ≥3 adenomas of any type. Advanced adenomas are defined as those with cancer, high-grade dysplasia, >25% villous features, or a diameter ≥1 cm.
DISCUSSION
BMI was 1.8 kg/m2 higher, on average, among participants in our recent trial (VCPPS) compared to our prior trial conducted about 16 years earlier (CPPS), consistent with the overall trend in rising obesity rates in the United States 15. In both trials, BMI appeared to modify the effect of calcium supplementation on adenoma findings. Specifically, among those with normal BMI, calcium supplementation was associated with risk reductions of 21% and 46% for any adenoma and 56% and 44% for high-risk findings, in the CPPS and VCCP trials, respectively; but appeared to be largely ineffective, or to even increase risk, among individuals who were overweight or obese. Further, after standardization of the CPPS findings to the higher BMI distribution of the VCPPS, the overall protective effects of calcium supplementation on adenoma outcomes were attenuated. Conversely, after standardization of the VCPPS to the lower BMI distribution of the CPPS, the overall protective effect of calcium supplementation on any adenoma was enhanced and the deleterious effect on high-risk findings was eliminated. Thus, the higher BMI of participants in the VCPPS seems to partially explain why calcium supplementation was not effective at reducing overall colorectal adenoma risk in that study. Obesity is known to be associated with lower circulating 25-hydroxyvitamin D concentrations, possibly because of volumetric dilution, fat sequestration, or metabolic effects 16–18. However, BMI modified the effect of calcium supplementation in our trials independently of serum 25-hydroxyvitamin D concentrations, indicating that effect modification by BMI is not due to confounding by 25-hydroxyvitamin D concentrations.
A recent meta-analysis of observational studies found an approximately linear decrease in adenoma risk with increasing total or supplemental calcium intake that was greater in magnitude for high-risk adenomas 3. Results of similar magnitude were observed in a meta-analysis of randomized controlled trials of calcium supplementation for the prevention of colorectal adenomas, which included our CPPS 19. Among observational studies of adenoma risk, we found only one small cross-sectional study that reported results for effect modification by BMI 20. In agreement with the direction of our findings, higher total calcium intake was associated with a reduction in adenoma risk among individuals with BMI < 30 kg/m2 but not among those with BMI ≥ 30 kg/m2 (Pinteraction=0.24)20. Several large prospective observational studies of adenomas (e.g., 21–23) and colorectal cancer (e.g., 24, 25) did not report results for this interaction, but in light of our findings this would be worth exploring. However, in two recent studies there were weak suggestions of effect measure modification in the opposite direction to what we found. First, in a combined analysis of results from the Nurses’ Health Study and the Health Professionals Follow-up Study, total calcium intake appeared to be associated with the largest decrease in risk of distal colon cancer among those individuals with the highest BM (>30 kg/m2), but effect modification by BMI was not statistically significant (Pinteraction=0.15) 26. Second, motivated by our findings 7, Keum et al. re-examined meta-analytical data on the inverse associations of calcium intake with adenomas and CRC from prospective observational studies 27. In this work, when studies were classified based on the average BMI of the study populations into two groups (BMI < 25 kg/m2 and BMI ≥ 25 kg/m2), no significant evidence of heterogeneity by population BMI was found 27. When colon and rectal cancer were examined separately, the benefit of calcium appeared to be confined to studies with populations with higher average BMI (≥ 25 kg/m2) at both sites, in apparent contradiction to our results, although the heterogeneity in the findings by population BMI was not statistically significant 27. An important limitation of this work was the analysis of population average BMIs instead of participant-level BMI data 27. The reason(s) why the findings from these analyses of observational data differ from ours are unclear but could be related to confounding of calcium intake or imprecision in the measurement of BMI or calcium intake in the meta-analyses. Importantly, our studies utilized randomized assignments to a clearly defined calcium supplement (calcium carbonate, providing 1200 mg/day elemental calcium).
Two major mechanisms have been proposed for the protective effects of calcium against the development of colorectal neoplasia. First, calcium may bind secondary bile acids and fatty acids in the lumen of the colorectum, forming insoluble calcium salts that are excreted thereby reducing the carcinogenic effects of these bile acids on colonic epithelial cells 28–32. Second, calcium may bind apical membrane calcium-sensing receptors on colorectal enterocytes activating intracellular calcium signaling pathways that restrain proliferation and promote differentiation and apoptosis 33–37. It is possible that obesity could impact either or both of these mechanisms to modify the effect of calcium supplementation, or that other pathways may be involved. The association of obesity with increased colorectal carcinogenesis is well established and proposed mechanisms involve alterations in insulin/insulin-like growth factor, adipokines, signaling lipids, inflammatory cytokines and sex hormones 38–43. Interestingly, calcium intake and the calcium-sensing receptor have been implicated in the regulation of body weight, potentially involving decreased appetite, modulation of lipid metabolism in adipocytes, and increased fecal fat excretion 44, 45. Given increasing recognition of the role of bile acids as metabolic regulators 46, 47, their influence on the colorectum 32, and the interaction of obesity with the microbiome 48 (with the potential to influence the formation of secondary bile acids), we speculate that these interrelated pathways may be important for modifying the effect of calcium supplementation. Nonetheless, the specific mechanism(s) involved in the putative interaction between calcium and obesity in relation to colorectal carcinogenesis is/are unknown.
In addition to our BMI findings, there were suggestions that two other participant characteristics may also modify the effect of calcium supplementation on risk of colorectal adenomas, including NSAID use (as previously reported 49), and alcohol consumption. However, these suggestive findings were limited to the CPPS, where these interactions were found, and were in the opposite direction as would be needed to help explain the reduced effect of calcium supplementation in the VCPPS. Specifically, NSAID use and alcohol consumption were higher among participants in the VCPPS than in the CPPS but were associated with greater protective effects in the CPPS. Thus, these may be chance findings.
Although the study designs were almost identical, some of the differences in findings between the two studies may be partially explained by changes as a result of conducting the two studies 16 years apart, but only if the changes differentially impact the findings in the calcium vs placebo treatment groups. For example, increased use of high definition colonoscopes during the more recent VCPPS could have increased the detection of smaller adenomas in that study. This might contribute to an apparent reduction in calcium’s efficacy in the later trial if it acts primarily to reduce adenoma progression as opposed to initiation.
A major strength of our analysis is that we compared results from two clinical trials with randomized assignment to the same calcium intervention, thereby minimizing the potential for confounding and bias associated with observational analyses of self-reported calcium intake. Moreover, both trials were conducted by the Polyp Prevention Study Group consortium with similar procedures and target populations. The median treatment length was similar between the two trials (43–44 months), but the individual follow-up colonoscopy intervals differed (4 years in the CPPS vs. either 3 or 5 years in the VCPPS). Calcium supplementation appeared to be slightly more protective with the longer (5 year) follow-up in the VCPPS but the difference was not statistically significant 7. Also, we have measurements of baseline 25-hydroxyvitamin D concentrations within each study, which was used to rule out confounding in the assessment of the interaction of BMI with calcium supplementation.
There were also some limitations to the present analysis. There was likely some measurement error in the estimation of BMI, particularly in the earlier study: although, measurement of height and weight at enrollment was preferred, the actual source of these measurements was not captured in the CPPS, while in the VCPPS they were measured in about 75% of participants and self-reported by the rest. However, if self-reports tend to underreport BMI, then this would be expected to bias our results (for the interaction of BMI with calcium supplementation) towards the null. Additionally, there may be other unmeasured differences between the two study populations that would further explain the risk differences observed, such as a measure of central obesity (waist-to-hip ratio), which is more strongly related to colorectal cancer risk than is BMI 50. We had limited power to measure interactions because of sample size restrictions and did not adjust for multiple comparisons, so our analyses should be considered exploratory. Finally, this work was limited to analyses of adenoma endpoints and did not assess effects on serrated polyps.
In conclusion, the higher BMI of participants in our more recent VCPPS trial, than in our CPPS trial conducted 16 years earlier, may have contributed to the lack of an overall protective effect of calcium supplementation on risk of colorectal adenomas observed in the later trial. Further, although additional clinical and mechanistic studies are warranted, our findings suggest that normal weight individuals may benefit from supplementation with 1200 mg/day of calcium carbonate for the prevention of colorectal adenomas, while overweight and obese individuals may not.
Supplementary Material
Novelty and Impact Statement:
Calcium supplementation was ineffective against colorectal adenomas in a recent randomized trial in contrast to a similar trial that the authors conducted previously. Here, they examined participant characteristics that may help explain this discrepancy. They found that body mass index (BMI) was higher among participants in the more recent trial, and that calcium supplementation was less effective among individuals with higher BMI, which appears to partially explain why calcium was ineffective in the later trial.
Acknowledgements:
We would like to thank the participants and staff of the Calcium Polyp Prevention Study and the Vitamin D/Calcium Polyp Prevention Study for their valuable contributions.
Grant Sponsors:
This research was supported by the National Institutes of Health (NIH) grants CA046927 and CA098286 (to JAB) and HD084070 (to DW). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations used:
- CPPS
Calcium Polyp Prevention Study
- VCPPS
Vitamin D/Calcium Polyp Prevention Study
- BMI
body mass index
- RR
relative risk
- CI
confidence interval
- SD
standard deviation
- NSAID
non-steroidal anti-inflammatory drug
- NHW
non-Hispanic white
- NHB
non-Hispanic black
Footnotes
Conflicts of Interest:
Dr. Lund receives research support from the PhRMA Foundation to the University of North Carolina at Chapel Hill. Dr. Lund’s spouse is a full-time paid employee of GlaxoSmithKline (which markets calcium supplements).
Dr. Westreich is a consultant for Sanofi Pasteur pharmaceutical company.
Dr Ahnen serves on the Data and Safety Monitoring Committee for Cancer Prevention Pharmaceuticals, and the Speakers Bureau for Ambry Genetics.
Dr. Burke receives research support from Cancer Prevention Pharmaceuticals and Ferring Pharmaceuticals, and she is a consultant for Sucampo Pharmaceuticals, Salix Pharmaceuticals and Aries Pharmaceuticals.
Dr. Baron, along with Dartmouth College, holds a use patent for the chemopreventive use of calcium (currently not licensed, formerly licensed by Pfizer). Pfizer provided the study tablets at no cost to the VCCPS.
The remaining authors report no potential conflicts of interest.
REFERENCES:
- 1.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer 2009; 61: 47–69. [DOI] [PubMed] [Google Scholar]
- 2.Keum N, Aune D, Greenwood DC, et al. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. Int J Cancer 2014; 135: 1940–8. [DOI] [PubMed] [Google Scholar]
- 3.Keum N, Lee DH, Greenwood DC, et al. Calcium intake and colorectal adenoma risk: dose-response meta-analysis of prospective observational studies. Int J Cancer 2015; 136: 1680–7. [DOI] [PubMed] [Google Scholar]
- 4.Heine-Broring RC, Winkels RM, Renkema JM, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J Cancer 2015; 136: 2388–401. [DOI] [PubMed] [Google Scholar]
- 5.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med 1999; 340: 101–7. [DOI] [PubMed] [Google Scholar]
- 6.Wallace K, Baron JA, Cole BF, et al. Effect of Calcium Supplementation on the Risk of Large Bowel Polyps. J Natl Cancer Inst 2004; 96: 921–5. [DOI] [PubMed] [Google Scholar]
- 7.Baron JA, Barry EL, Mott LA, et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N Engl J Med 2015; 373: 1519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst 2003; 95: 1765–71. [DOI] [PubMed] [Google Scholar]
- 9.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136: 832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanderWeele TJ, Knol MJ. A Tutorial on Interaction. Epidemiologic Methods 2014; 3. [Google Scholar]
- 11.Westreich D, Edwards JK, Lesko CR, et al. Transportability of Trial Results Using Inverse Odds of Sampling Weights. Am J Epidemiol 2017; 186: 1010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: The ACTG 320 trial. Am J Epidemiol 2010; 172: 107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesko CR, Cole SR, Hall HI, et al. The effect of antiretroviral therapy on all-cause mortality, generalized to persons diagnosed with HIV in the USA, 2009–11. Int J Epidemiol 2016; 45: 140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong JL, Jonsson Funk M, LoCasale R, et al. Generalizing Randomized Clinical Trial Results: Implementation and Challenges Related to Missing Data in the Target Population. Am J Epidemiol 2018; 187: 817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. J Am Med Assoc 2016; 315: 2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drincic AT, Armas LA, Van Diest EE, et al. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012; 20: 1444–8. [DOI] [PubMed] [Google Scholar]
- 17.Earthman CP, Beckman LM, Masodkar K, et al. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 2012; 36: 387–96. [DOI] [PubMed] [Google Scholar]
- 18.Pourshahidi LK. Vitamin D and obesity: current perspectives and future directions. Proc Nutr Soc 2015; 74: 115–24. [DOI] [PubMed] [Google Scholar]
- 19.Shaukat A, Scouras N, Schunemann HJ. Role of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trials. Am J Gastroenterol 2005; 100: 390–4. [DOI] [PubMed] [Google Scholar]
- 20.Miller EA, Keku TO, Satia JA, et al. Calcium, dietary, and lifestyle factors in the prevention of colorectal adenomas. Cancer 2007; 109: 510–7. [DOI] [PubMed] [Google Scholar]
- 21.Massa J, Cho E, Orav EJ, et al. Total calcium intake and colorectal adenoma in young women. Cancer Causes Control 2014; 25: 451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh K, Willett WC, Wu K, et al. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol 2007; 165: 1178–86. [DOI] [PubMed] [Google Scholar]
- 23.Peters U, Chatterjee N, McGlynn KA, et al. Calcium intake and colorectal adenoma in a US colorectal cancer early detection program. Am J Clin Nutr 2004; 80: 1358–65. [DOI] [PubMed] [Google Scholar]
- 24.Park Y, Leitzmann MF, Subar AF, et al. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med 2009; 169: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SY, Murphy SP, Wilkens LR, et al. Calcium and vitamin D intake and risk of colorectal cancer: the Multiethnic Cohort Study. Am J Epidemiol 2007; 165: 784–93. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Keum N, Wu K, et al. Calcium intake and colorectal cancer risk: Results from the nurses’ health study and health professionals follow-up study. Int J Cancer 2016; 139: 2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keum N, Kim H, Giovannucci EL. Calcium as a chemopreventive agent against colorectal neoplasm: does obesity play a role? Cancer Causes Control 2017; 28: 853–6. [DOI] [PubMed] [Google Scholar]
- 28.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst 1984; 72: 1323–5. [PubMed] [Google Scholar]
- 29.Lapre JA, De Vries HT, Termont DS, et al. Mechanism of the protective effect of supplemental dietary calcium on cytolytic activity of fecal water. Cancer Res 1993; 53: 248–53. [PubMed] [Google Scholar]
- 30.Govers MJ, Termont DS, Lapre JA, et al. Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic cytotoxicity in humans. Cancer Res 1996; 56: 3270–5. [PubMed] [Google Scholar]
- 31.Steinbach G, Lupton J, Reddy BS, et al. Effect of calcium supplementation on rectal epithelial hyperproliferation in intestinal bypass subjects. Gastroenterology 1994; 106: 1162–7. [DOI] [PubMed] [Google Scholar]
- 32.Barrasa JI, Olmo N, Lizarbe MA, et al. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol In Vitro 2013; 27: 964–77. [DOI] [PubMed] [Google Scholar]
- 33.Buset M, Lipkin M, Winawer S, et al. Inhibition of human colonic epithelial cell proliferation in vivo and in vitro by calcium. Cancer Res 1986; 46: 5426–30. [PubMed] [Google Scholar]
- 34.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 2003; 3: 601–14. [DOI] [PubMed] [Google Scholar]
- 35.Chakrabarty S, Radjendirane V, Appelman H, et al. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res 2003; 63: 67–71. [PubMed] [Google Scholar]
- 36.Singh N, Promkan M, Liu G, et al. Role of calcium sensing receptor (CaSR) in tumorigenesis. Best Pract Res Clin Endocrinol Metab 2013; 27: 455–63. [DOI] [PubMed] [Google Scholar]
- 37.Rey O, Young SH, Jacamo R, et al. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J Cell Physiol 2010; 225: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J Nutr Biochem 2006; 17: 145–56. [DOI] [PubMed] [Google Scholar]
- 39.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med 2010; 61: 301–16. [DOI] [PubMed] [Google Scholar]
- 40.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011; 11: 886–95. [DOI] [PubMed] [Google Scholar]
- 41.Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci 2012; 1271: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louie SM, Roberts LS, Nomura DK. Mechanisms linking obesity and cancer. Biochim Biophys Acta 2013; 1831: 1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J, Morley TS, Kim M, et al. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 2014; 10: 455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Astrup A The role of calcium in energy balance and obesity: the search for mechanisms. Am J Clin Nutr 2008; 88: 873–4. [DOI] [PubMed] [Google Scholar]
- 45.Villarroel P, Villalobos E, Reyes M, et al. Calcium, obesity, and the role of the calcium-sensing receptor. Nutr Rev 2014; 72: 627–37. [DOI] [PubMed] [Google Scholar]
- 46.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap--bile acids in metabolic control. Nat Rev Endocrinol 2014; 10: 488–98. [DOI] [PubMed] [Google Scholar]
- 47.Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2014; 28: 573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–31. [DOI] [PubMed] [Google Scholar]
- 49.Grau MV, Baron JA, Barry EL, et al. Interaction of calcium supplementation and nonsteroidal anti-inflammatory drugs and the risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 2005; 14: 2353–8. [DOI] [PubMed] [Google Scholar]
- 50.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007; 16: 2533–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


