Abstract
Squamous cell esophageal cancer is common throughout East Africa, but its etiology is poorly understood. We investigated the contribution of alcohol consumption to esophageal cancer in Kenya, based on a hospital-based case-control study conducted from 08/2013 to 03/2018 in Eldoret, Western Kenya. Cases had an endoscopy-confirmed esophageal tumor whose histology did not rule out squamous cell carcinoma. Age and gender frequency-matched controls were recruited from hospital visitors/patients without digestive diseases. Logistic regression was used to calculate odds ratios (ORs) and their 95% confidence intervals (CI) adjusting for tobacco (type, intensity) and 6 other potential confounders. A total of 422 cases (65% male, mean at diagnosis 60 (SD 14) years) and 414 controls were included. ORs for ever-drinking were stronger in ever-tobacco users (9.0, 95% CI: 3.4, 23.8, with few tobacco users who were never drinkers) than in never-tobacco users (2.6, 95% CI: 1.6, 4.1). Risk increased linearly with number of drinks: OR for >6 compared to >0 to ≤2 drinks/day were 5.2 (2.4, 11.4) in ever-tobacco users and 2.1 (0.7, 4.4) in never-tobacco users. Although most ethanol came from low ethanol alcohols (busaa or beer), for the same ethanol intake, if a greater proportion came from the moonshine chang’aa, it was associated with a specific additional risk. The population attributable fraction for >2 drinks per day was 48% overall and highest in male tobacco users. Alcohol consumption, particularly of busaa and chang’aa, contributes to half of the esophageal cancer burden in Western Kenya.
Keywords: Esophageal cancer, Africa, Kenya, alcohol, spirits
Introduction
Esophageal cancer (EC) is the sixth most common cause of cancer deaths worldwide.1 Its incidence is characterized by a peculiar geographical distribution, with the highest incidence rates in the Asian EC belt. Another high incidence area is the African EC corridor,2 which includes Kenya where EC is the third most common cancer in both sexes. In 2012, Kenya’s age-standardized incidence rates per 100,000 were estimated to be 20.5 in men and 15.1 in women, compared to 5.9 globally.1 In this East African country, a predicted 4500 people will be diagnosed with EC in 2020. The burden is predominantly esophageal squamous cell carcinoma (ESCC), has a high proportion of young cases3 and prognosis is poor.
The etiology of Kenya’s EC burden has scarcely been studied, with the exception of a small case-control study (147 ESCC cases).4 However, a recent review found that many established ESCC risk factors have a high prevalence or high exposure levels in certain African population groups.2 Alcohol (ethanol), through it being metabolized to acetaldehyde, is an established human carcinogen (IARC Group 1)5 and its effects on ESCC act multiplicatively with tobacco6, a product that contains acetaldehyde among many other carcinogens. Alcohol is a major suspected contributor to Kenya’s ESCC burden. Kenya’s National Authority for the Campaign against Alcohol and Drug Abuse (NACADA) data show moderate prevalence of alcohol (20.7%) and tobacco consumption (11.2%) nationally, but prevalence is much higher in Western Kenya, especially in men (54%, compared to 9% in women), in lower socioeconomic groups, and in tobacco users.7 Moreover, levels of consumption are high – drinkers get drunk on 60% of drinking occasions.8 The majority of consumption is of traditional alcohols, namely busaa, a locally brewed beer (2–7% ethanol), and stronger distillations known as chang’aa or kumikumi (18–53% ethanol).8, 9 These local spirits are known to cause morbidity and acute death from poisoning, a great concern to both the Kenyan people and the government.10–12
African ESCC research was active in the 1970–80’s, led by Cook, Burkitt and McGlashan amongst others, and was recently revived.13–15 With regards to alcohol, positive associations with ESCC were found in South Africa, acting synergistically with tobacco, but the latter habit was the larger contributor.13, 16–21 Alcohol has also been associated with EC in Zimbabwe20 and in Kenya with both EC4 and ESCC’s precursor high grade dysplasia.22 Ecological observations were also made, in which McGlashan’s 1969 Gut publication drew attention to a specific home distillation of maize husks and sugar, known as kachasu in central Africa, akin to gongo in Tanzania and the aforementioned chang’aa in Kenya.23 In this work, he noted that kachasu consumption in eastern Zambia coincided with that region’s high incidence, whereas EC was apparently absent from northern Zambia where millet beer was consumed. These observations fueled hypotheses that alcohol-ESCC risks were not only due to ethanol, but also to other correlates, such as nutritional deficiencies,19 or to constituents or contaminants, such as mycotoxins, nitrosamines, polycyclic aromatic hydrocarbons or adulteration with pesticides, formaldehyde and battery acid.24 However, the strength of the ethanol-ESCC risk gradient, which is established to reach a 7-fold relative risk for 100 grams of ethanol per day,25 has not to our knowledge been investigated in East Africa.
Recognizing the need for detailed alcohol exposure assessment across the complex mix of alcohols consumed in Africa, in this article we present the first detailed analysis of alcohol in relation to ESCC in the West Kenyan ESCCAPE (ESCC African Prevention Research) case-control study.
Methods
Study design
In a joint collaboration between Moi University and IARC, a case-control study of EC was conducted at the Moi Teaching and Referral Hospital (MTRH), situated in Eldoret, Uasin Gishu County, western Kenya and whose catchment area stretches 150 km to the Ugandan border. The study was funded in two phases, with negligible protocol changes for the present analyses: a pilot phase from August 2013 to September 2014 and the main study from October 2015 to end March 2018. Cases were patients aged ≥ 18 years presenting with suspected incident first primary EC at the MTRH endoscopy unit where a pinch biopsy was taken and whose histology confirmed ESCC (90%) or did not rule it out (10%, e.g. tumor visualized at endoscopy but biopsy specimen was insufficient for evaluation).
We aimed to recruit an equal number of controls from the population from which cases arose. To do this, due to uncertainties in referral probabilities, controls were selected from adults aged ≥18 years who also made the journey to MTRH, i.e. 20% were hospital visitors and 80% hospital in and out-patients, recruited from MTRH out-patients, surgery, medical, orthopedic and ophthalmology wards. Hospital patients attending for digestive disease or cancer, or those with more than 3 nights in MTRH were not eligible. Controls were age and sex frequency matched to the cases’ observed distribution, who were approached at random from patients or visitors having a long waiting time when they could complete the study.
Consecutive patients were recruited, with a target of at least 400 cases and 400 controls, enabling 80% power to detect odds ratios as small was 1.5 for exposures with 30% prevalence. Participation rates were 96% in cases and 92% in controls. Representativeness of controls was examined by comparing their sociodemographic characteristics with that of indirectly age and gender standardized Kenyan Demographic Health Survey (KDHS) 2014 data26.
Questionnaire
Consenting participants completed a face-to-face interview in the participant’s vernacular with trained interviewers, provided a blood sample and had anthropometry measurements taken. Data were immediately entered into a tablet with a preloaded Open Data Kit questionnaire. For the primary exposure variable, ever-alcohol use was self-reported intake of at least 1 drink per week for 6 months. Ever drinkers were divided into current drinkers, who had not quit within the past 2 years, and past drinkers. This extended period was utilized due to more cases reported having quit within the past 2 years (among busaa-drinkers, 18% of cases and 13% of controls quit within the past 2 years), potentially due to early symptoms of the cancer. For ever-drinkers, we assessed consumption of each of 5 alcohol types, for which the average ethanol percentage and drink volume assumed hereafter were: busaa27 (4.5% ethanol, 1 drink=500 mL, ethanol/drink=17.8 g), commercial beer (5% ethanol, 1 drink=500 mL, ethanol/drink=19.8 g), chang’aa27 (ethanol 40%, 1 drink = 30 mL, ethanol /drink=9.5 g), commercial spirits (as per current sales: 35% ethanol, 1 drink = 30 mL, ethanol /drink=8.3 g) and other alcohols as specified by the participant. For each category, we obtained data on the average number of drinks on a weekday and on a weekend day, as well as age at first and last consumption. Three percent (n=16) of drinkers specified drinking other alcohols, which were muratina (a honey mead, serving size 250 mL, 5% ethanol28) and mnazi (coconut palm wine, 8% ethanol) and were incorporated into the total ethanol amounts. In the main study only, we asked chang’aa drinkers of their preferred chang’aa strength and whether they were ever temporarily blinded by the drink – an occasional side-effect of methanol.
We calculated average daily alcohol intakes, in line with previous publications and for the facilitation of risk communication to individuals on a meaningful time-scale. The average number of drinks per day was calculated for each type of alcohol (5/7 × no. drinks/weekday + 2/7 × no. drinks/weekend-day) and then summed across types to give the average number of drinks/day. Average daily ethanol consumption (g/day) was calculated for each alcohol type using standard formulae5 (drinks/day x volume/drink x ethanol% x 0.79), and summed to calculate the daily average.
Data collected on confounders and potential effect modifiers were extensive. For the present analyses, we included age, ethnicity, education, socio-economic status, hot beverage consumption, family history of esophageal cancer, tobacco use (smoking and smokeless) – the exact definitions of which are provided in tables.
Ethical approval
Written informed consent was sought after a full explanation of the study, which was approved by the Institutional Research and Ethics Committee of Moi University (000921) and by IARC (IEC 14–15). Participants received no payment for their involvement, but for cases the 1,000 Kenya shilling fee for histological results was paid for by the study.
Statistical analysis
Measures of the exposure of interest, alcohol consumption, are: use (current/past/never), ethanol consumption (g/week), duration of use, drinks per week, overall and by type. Odds ratios (ORs) and their 95% confidence intervals (CI) of EC associated with each exposure were estimated using logistic regression models. In model 1 (basic model), ORs were first estimated with adjustment for interviewer, phase (pilot/main) and the design factors of gender (binary) and age (continuous) to account for small imbalances in age-gender frequency matching. Thereafter, the fully adjusted model (Model 2) was further adjusted for education, ethnicity, hot beverage drinking, family history of EC, and tobacco type (smoking/ oral/ nasal/ multiple and smoking/use intensity) (model 2). Missing data on any of these confounders was rare (<2%), and were included by including a missing category. A priori effect modifiers of the alcohol (ever/never)-ESCC association were tobacco use (ever/never), age (<50/≥50 years, due to different latency periods), gender and hot beverage consumption as per recent findings29, tested using a single interaction term (exposure x ever-alcohol) added to model 2. To examine the ethanol-ESCC dose-response relationship, the fitted association was plotted graphically overlaid with Bagnardi et al.’s dose-response meta-analysis25: log(RR) = 0.05593(ethanol/day)–0.00789 (ethanol/day) x (log(ethanol/day)). Finally, for significant associations and assuming causality, we used the method of Greenland and Drescher30 to estimate the population attributable fraction (%), overall and by gender, associated with drinking more than 1 drink/day (using model 2’s parameters). This PAF is the estimated proportion of all ESCC cancer patients which would not have occurred if those patients who drank more than 2 drinks per day had been never drinkers. We also provide the PAFs attributed to drinking more than 2 drinks per day compared to drinking >0 to <2 drinks per day in Supplementary Table 1. All analyses were conducted in Stata version 14.0.
Results
Participant and alcohol consumption characteristics
Of 461 suspected EC cases interviewed, 36 EC adenocarcinomas, and one each of Kaposi sarcoma, leiomyoma and papilloma were excluded, resulting in 422 included cases and 414 controls. Most participants were of Kalenjin or Luhya ethnicities, 65% of cases were male and the majority were Christian (Table 1). Mean age at diagnosis was 60 years (6.7% <40 years). Cases had less education, larger family sizes and more were farmers than controls, but the latter’s distribution did not differ to KDHS data. The median distance of home village to the recruitment hospital was 54 km in cases and 47 km in controls.
Table 1:
Characteristics of cases and controls
| Cases N=422 N (column %) |
Controls N=414 N (column %) |
KDHS 2014a | ||
|---|---|---|---|---|
| Genderb | Male | 275 (65) | 253 (61) | - |
| Female | 147 (35) | 161 (39) | ||
| Ageb (years) at diagnosis / interview | Mean (SD) | 59.6 (14) | 57.0 (15) | - |
| IQR | 49–69 | 45–68 | ||
| Ethnic group | Kalenjin | 242 (57) | 218 (53) | - |
| Luhya | 100 (24) | 88 (21) | ||
| Luo | 34 (8) | 31 (7) | ||
| Kikuyu | 17 (4) | 29 (7) | ||
| Kisii | 6 (1) | 25 (6) | ||
| Other | 23 (5) | 23 (6) | ||
| Religion | Protestant | 240 (57) | 266 (64) | 71% |
| Catholic | 153 (36) | 131 (32) | 20% | |
| Muslim | 2 (1) | 3 (1) | 7% | |
| None | 26 (6) | 12 (3) | 2% | |
| Other/No answer | 1 (<1) | 2 (<1) | <1 | |
| Education (score) | None (1) | 102 (24) | 94 (23) | 25% |
| Some primary (2) | 161 (38) | 106 (26) | 25% | |
| Complete primary (3) | 82 (19) | 84 (20) | 23% | |
| Some (4)/ Complete secondary (5) | 58 (14) | 82 (20) | 19% | |
| Technical college (6) /University (7) | 19 (5) | 47 (11) | 8% | |
| Mean (SD) education score | 2.5 (1.4) | 3.0 (1.7) | 3.2 | |
| Number of children | Mean (SD) | 6.2 (3.6) | 6.0 (3.4) | 5.5 Western Kenya / 6.1 Rift valley |
| Occupation | Farmer | 281 (67) | 223 (54) | 39% |
| Other | 141 (33) | 190 (46) | ||
Kenya Demographic and Health Survey (KDHS) 2014 national (unless region specified) estimates weighted to age and gender distribution of this study’s controls;
Frequency-matched design factors.
Among controls, the prevalence of ever-drinking was 56% in men and 26% in women (Table 2). Controls’ drinking status (never/past/current) did not differ by several potential confounders, notably age, ethnicity, education, family history of EC or hot beverage drinking temperature. In contrast, tobacco and alcohol habits clustered: 85% of tobacco users - mostly male smokers and female tobacco chewers - were ever-drinkers compared to 25% of never-tobacco users. Among smokers, smoking intensity is low (median 5 cigarettes/day). Men drank twice as much as their female counterparts, in terms of drinks per day or ethanol intake and started drinking 4 years earlier. In terms of alcohol type, over two-thirds of drinkers consumed traditional alcohols (busaa and/or chang’aa). The greatest source of ethanol was busaa in 39% of male controls, beer for 43%, chang’aa for 11% and commercial spirits for 7%, whilst in women the corresponding percentages were 62%, 24%, 14% and 0% respectively (not in tables).
Table 2:
Alcohol consumption in controls, overall and by socio-demographic factors
| Category | Alcohol consumption distribution N (row %a) | p-valueb | |||
|---|---|---|---|---|---|
| Never | Past | Current | |||
| All | 231 (56) | 67 (16) | 116 (28) | - | |
| Gender | Men | 112 (44) | 47 (19) | 94 (37) | <0.001 |
| Women | 119 (74) | 20 (12) | 22 (14) | ||
| Age at interview (years) | 18-<40 | 36 (67) | 6 (11) | 12 (22) | 0.18 |
| 40-<50 | 41 (55) | 7 (10) | 26 (35) | ||
| 50-<60 | 57 (56) | 15 (15) | 30 (29) | ||
| 60-<70 | 55 (57) | 18 (19) | 23 (24) | ||
| 70+ | 42 (48) | 22 (24) | 25 (28) | ||
| Ethnicity | Kalenjin | 119 (55) | 38 (17) | 61 (28) | 0.89 |
| Luhya | 49 (56) | 12 (14) | 27 (31) | ||
| Other | 63 (58) | 17 (16) | 28 (26) | ||
| Education | None | 51 (54) | 16 (17) | 27 (29) | 0.92 |
| Some primary, no secondary | 110 (57) | 31 (67) | 49 (26) | ||
| Secondary or higher | 69 (53) | 20 (16) | 40 (32) | ||
| Family history of EC* | No | 212 (55) | 62 (16) | 109 (28) | 0.87 |
| Yes | 10 (50) | 4 (20) | 6 (30) | ||
| Hot beverage drinking temperature | Warm | 51 (49) | 21 (20) | 32 (31) | 0.19 |
| Hot | 159 (57) | 42 (15) | 80 (28) | ||
| Very hot | 21 (72) | 4 (14) | 4 (14) | ||
| Religion | Catholic | 168 (63) | 44 (17) | 54 (20) | <0.001 |
| Protestant | 59 (45) | 22 (17) | 50 (38) | ||
| Other | 4 (24) | 1 (6) | 12 (71) | ||
| Tobacco use (men) | Never | 98 (66) | 18 (12) | 32 (22) | <0.001 |
| Past | 6 (15) | 22 (55) | 12 (31) | ||
| Current | 8 (12) | 7 (11) | 50 (77) | ||
| Tobacco use (women) | Never | 113 (84) | 11 (8) | 11 (8) | <0.001 |
| Past | 4 (31) | 6 (46) | 3 (23) | ||
| Current | 2 (15) | 3 (23) | 8 (62) | ||
| Drinking habits among ever drinkers | |||||
| Men | Women | ||||
| Age first drank | Years (median, IQR) | 22 (20–28) | 26 (20–30) | ||
| Years since quittingc | Years (median, IQR) | 16 (7–26) | 11 (5–35) | ||
| Ever drank each alcohol type | Busaa | 99 (71%) | 31 (74%) | ||
| Chang’aa | 90 (65%) | 26 (62%) | |||
| Commercial beer | 90 (65%) | 11 (26%) | |||
| Spirits | 52 (37%) | 5 (12%) | |||
| Drinks per day, if a drinker of this type of alcohol | Busaa | 1.7 (0.6–2.6) | 1.3 (0.6–2.4) | ||
| Chang’aa | 1.3 (0.7–2.7) | 1.1 (0.8–1.7) | |||
| Commercial beer | 1.1 (0.7, 2.9) | 1.3 (0.6, 5) | |||
| Spirits | 1.2 (0.6–2.0) | 1.3 (0.6–1.6) | |||
| All types | 3.4 (1.4–7.1) | 1.7 (0.9, 4.3) | |||
Percentages are calculated among non-missing values. Data were complete except for family history of EC (N=11 missing) and education (n=1 missing).
p-value from chi-squared test.
in past drinkers, of whom 46 were male and 20 were female controls.
ESCC ORs associated with measures of alcohol consumption are shown in table 3. In minimally adjusted analyses compared to never drinkers, past and current drinkers had 3.1 and 4.6-fold increased ESCC risks respectively, however analyses of joint alcohol-tobacco status indicated effect modification by tobacco (p=0.02). Among never-tobacco users, ever-drinkers had a 2.6-fold increased ESCC risk, whereas the OR was 9.0 in ever-tobacco users. In contrast, there was no evidence of an interaction by gender (p=0.11) or hot beverage consumption (p=0.32). When stratifying by age, ORs were stronger over age 50: in never tobacco users, ORs for past and current drinking were 1.5 (0.7, 3.1) under 50 and 3.0 (1.5, 5.8) over 50, whilst in ever tobacco users they were 2.8 (1.6, 4.0) and 7.6 (4.7, 12.3) respectively (not in tables, p=0.005 for alcohol*age). This interaction was considered to reflect the exposure accumulation rather than a biological interaction, so it was not carried further. The remaining results are presented separately by tobacco use, adjusted for intensity and tobacco type in ever-tobacco users.
Table 3:
Odds ratios (OR) and 95% confidence intervals (CI) for the association of alcohol consumption with esophageal cancer risk in Kenya: effects of drinking intensity, duration and quitting, stratified by ever-tobacco use
| Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
| Minimal adjustmenta | Full adjustmentb, stratified by ever tobacco use | ||||||
| All participants | Never tobacco users | Ever tobacco users | |||||
| Categories/unit | Number of cases/controls | OR (95% CI) | Number of cases/controls | OR (95% CI) | Number of cases/controls | OR (95% CI) | |
| Alcohol drinker ever/never | Never drinker | 111/231 | 1 | 102/211 | 1 | 8/18 | 1 |
| Ever drinker | 311/183 | 4.1 (2.9, 5.7) | 72/71 | 2.6 (1.6, 4.1) | 238/110 | 9.0 (3.4, 23.8) | |
| Alcohol drinker current/past/never | Never drinker | 111/ 231 | 1 | 102/211 | 1 | 8/18 | 1 |
| Past drinker | 88/ 67 | 3.1 (2.0, 4.8) | 26/29 | 2.2 (1.1, 4.2) | 62/38 | 6.9 (2.5, 19.6) | |
| Current drinker | 223/ 116 | 4.6 (3.2, 6.6) | 46/42 | 2.8 (1.7, 4.9) | 177/72 | 10.1(3.8, 27.2) | |
| Joint alcohol-tobacco status | Never alcohol never tobacco | 103/ 211 | 1 | 102/211 | 1 | - | - |
| Ever tobacco, never alcohol | 8/ 20 | 0.7 (0.3, 1.7) | - | - | 8/18 | 1 | |
| Ever alcohol, never tobacco | 72/ 72 | 2.4 (1.6, 3.8) | 72/71 | 2.6 (1.6, 4.1) | - | - | |
| Ever alcohol, ever tobacco | 239/ 111 | 5.0 (3.5, 7.2) | - | - | 238/110 | 9.0 (3.4, 23.8) | |
| Number of alcoholic drinks per day | Never drinker | 111/231 | 1 | 102/211 | 0.5 (0.3, 0.9) | 8/18 | 0.2 (0.1, 0.6) |
| >0–2 | 80/72 | 2.4 (1.6, 3.7) | 31/33 | 1 | 49/37 | 1 | |
| >2–4 | 64/41 | 3.9 (2.4, 6.4) | 14/17 | 1.4 (0.5, 3.0) | 49/24 | 1.9 (0.9, 4.1) | |
| >4–6 | 52/26 | 5.5 (3.1, 9.7) | 9/8 | 1.4 (0.5, 5.6) | 43/18 | 2.6 (1.1, 5.8) | |
| >6 | 115/44 | 7.9 (4.9, 12.8) | 18/13 | 2.1 (0.7, 4.4) | 97/31 | 5.2 (2.4, 11.4) | |
| Per drink in drinkers up to 20 drinks/day | 1.13 (1.06, 1.19) | 1.06 (0.95, 1.20) | 1.16 (1.07, 1.26) | ||||
| Ethanol (g/day) | Never | 111/231 | 1 | 102/211 | 0.4 (0.2, 0.9) | 8/18 | 0.3 (0.1, 0.9) |
| 0-<20 | 58/54 | 2.2 (1.4, 3.5) | 25/23 | 1 | 33/29 | 1 | |
| 20-<50 | 83/47 | 4.2 (2.7, 6.7) | 16/22 | 1.0 (0.4, 2.5) | 66/25 | 3.4 (1.6, 7.5) | |
| 50-<100 | 82/42 | 5.4 (3.3, 8.9) | 18/13 | 1.7 (0.6, 4.5) | 64/29 | 3.4 (1.5, 7.6) | |
| ≥100 | 88/40 | 6.8(4.1, 11.4) | 13/13 | 1.3 (0.5, 3.8) | 75/27 | 6.5 (2.6, 16.1) | |
| Per category* | 311/183 | 1.6 (1.3, 2.0) | 64/71 | 1.3 (0.8, 2.0) | 238/110 | 1.9 (1.4, 2.6) | |
| Per 10 g/day if <150g/day | 27/165 | 1.14 (1.07, 1.21) | 59/66 | 1.07 (0.95, 1.21) | 209/97 | 1.19 (1.09, 1.30) | |
| Drinking duration (years) | Never drinker | 111/231 | 1 | 102/211 | 0.5 (0.3, 1.0) | 8/18 | 0.1 (0.1, 0.4) |
| < 15 | 63/49 | 3.3 (2.1, 5.3) | 20/24 | 1 | 43/25 | 1 | |
| 15–24 | 45/38 | 2.8 (1.7, 4.7) | 13/13 | 1.2 (0.4, 3.3) | 32/24 | 0.7 (0.3, 1.5) | |
| 25–39 | 106/48 | 5.6 (3.6, 8.8) | 18/23 | 1.0 (0.4, 2.6) | 87/25 | 2.2 (1.0, 4.7) | |
| 40+ | 97 / 48 | 4.7 (2.9, 7.6) | 21 / 11 | 2.9 (1.1, 8.1) | 76 / 36 | 0.8 (0.4, 1.9) | |
| Per 10 years in drinkers | 311/183 | 1.12 (0.98, 1.28) | 1.38 (1.03, 1.86) | 1.06 (0.89, 1.27) | |||
| Years since quitting drinking | Never drinker | 111/231 | 1 | 102/211 | 1 | 8/18 | 1 |
| Quit 30+ years ago | 27/16 | 3.6 (1.8, 7.3) | 3/13 | 3.4 (1.0, 11.9) | 19/11 | 10.0 (3.7, 26.8) | |
| 10 to 29 years ago | 48/24 | 5.0 (2.8, 8.8) | 15/11 | 3.7 (1.5, 9.0) | 33/13 | 9.9 (3.1, 31.5) | |
| 2 to 10 years ago | 13/26 | 1.3 (0.6, 2.7) | 8/5 | 0.6 (0.2, 2.3) | 10/13 | 3.6 (0.9, 13.5) | |
| Current drinker | 223/116 | 4.7 (3.3, 6.7) | 46/42 | 2.9 (1.7, 4.9) | 176/72 | 10.0 (3.7, 26.8) | |
| Age first started drinking (years) | Never drinker | 111/231 | 1 | 102/211 | 0.5 (0.3, 1.1) | 8/18 | 0.2 (0.1, 0.4) |
| ≥ 30 years | 74/51 | 3.2 (2.0, 5.0) | 22/25 | 1 | 51/25 | 1 | |
| 21 to <30 years | 79/55 | 3.9 (2.4, 6.1) | 17/25 | 1.0 (0.4, 2.5) | 62/30 | 1.6 (0.8, 3.4) | |
| 18 to <21 years | 94/50 | 4.5 (2.9, 7.1) | 20/14 | 2.5 (1.0, 6.6) | 74/36 | 1.2 (0.6, 2.4) | |
| <18 years | 64/27 | 5.6 (3.2, 9.5) | 13/7 | 2.6 (0.8, 8.5) | 51/19 | 1.7 (0.8, 3.7) | |
Minimal adjustment = Adjusted for continuous age, and binary variables: gender, study phase and interviewer.
Full adjustment adjusted = Minimal adjustment plus categorical variables: tobacco use (never/current/past), tobacco type (never/smoking/oral/nasal/multiple types), tobacco intensity (0/1–4/5–9/10–14/15+ cigarettes per day), ethnic group (Kalenjin, Luhya, other), family history of EC (yes/no/NK), hot beverages (very hot, hot, warm) and continuous linear variables: age, education level, and type (hand/commercial/oral).
ESCC risk increased with number of drinks per day (table 3). In ever-tobacco users, drinking over 6 drinks per day, compared to drinking up to 2 drinks per day, was associated with an OR of 5.2, whereas the corresponding OR was 2.1 in never-tobacco users. Positive trends were also found for ethanol consumption, which was twice as strong in ever-tobacco than in never-tobacco users in which it was borderline significant. Figure 1 shows ORs for ethanol consumption, which reached the same order of magnitude as those reported in the overlaid published dose-response relationship. However, in the Kenya study, the difference in risk between light and never drinkers is particularly steep, and even more so in tobacco users.
Figure 1:
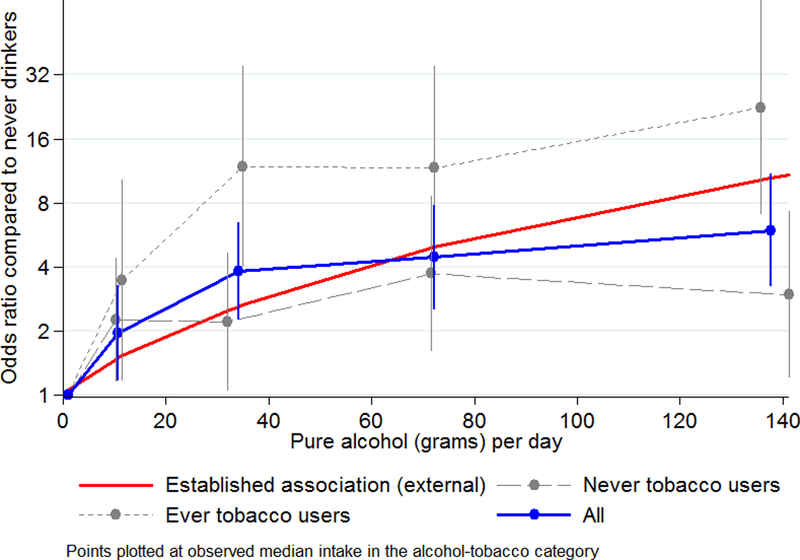
Association of alcohol drinking with squamous cell esophageal cancer in West Kenya: grams of alcohol per week: overall, in ever-tobacco users and in never-tobacco users, plotted alongside the Bagnardi et al.’s dose-response meta-analysis curve
Increased ESCC risks were lower in past than in current drinkers. In never smokers, ORs compared to never drinking were 1.8 for past and 2.7 for current drinkers and for ever-smokers were 6.7 and 9.0 respectively. ESCC risk increased with earlier age at commencing drinking, but did not increase monotonically with more recent alcohol cessation (Table 3). Duration of drinking showed a linear trend only in never tobacco users.
Among drinkers, ethanol sources differed by case/control status. More cases than controls had traditional drinks (busaa or chang’aa) as the greatest source (respectively, 79% v 50% in men, and 89% v 75% in women), in particular chang’aa (greatest ethanol source for 31% v 11% of male cases v controls, and in women 39% v 14%). Correspondingly, for the same amount of ethanol intake, drinkers who had 10 percentage points more ethanol consumed as chang’aa had a 16% (95%CI: 7, 27) higher ESCC risk (Table 4). We considered whether this effect was due to an underestimation of chang’aa’s ethanol content, by repeating analyses using a 50 mL serving and 55% ethanol, and the association remained. Finally among chang’aa drinkers, compared to controls, more cases preferred strong or very strong chang’aa (50% (66/133) v 41% (12/29), p=0.42) and more admitted or declined to answer whether the drink had ever caused blindness (34%=45/133 v 17%=5/29, p=0.08).
Table 4:
Odds ratios (OR) and 95% confidence intervals (CI) for esophageal cancer associated with alcohol type, expressed as the origin of ethanol origi risk in Kenya: effects of drinking intensity, duration and quitting, stratified by ever-tobacco use Associations by alcohol type, in ever-alcohol drinkers
| Categorical | Numbers of cases / controls | Median ethanol/day Cases, controls |
OR (95% CI), Model 2a + adjusted for grams of ethanol categories | |
|---|---|---|---|---|
| % ethanol (g) from busaa | 0-<10% | 44/56 | 43, 19 | 1 |
| 10-<50% | 128/58 | 71, 94 | 3.0 (1.6, 5.5) | |
| ≥50% | 136/68 | 53, 36 | 2.4 (1.3, 4.4) | |
| % ethanol (g) from chang’aa | 0-<5% | 48/69 | 30, 25 | 1 |
| 5–24% | 84/56 | 111, 98 | 1.8 (1.0, 3.5) | |
| ≥ 25% | 178/57 | 51, 32 | 2.9 (1.7, 5.1) | |
| % ethanol (g) from commercial beer | 0 | 197/81 | 41, 28 | 1 |
| >0-<50 | 81/52 | 103, 89 | 0.6 (0.3, 1.0) | |
| ≥50% | 32/49 | 107, 35 | 0.3 (0.2, 0.6) | |
| % ethanol (g) from spirits | 0 | 255/125 | 52, 32 | 1 |
| >0-<10 | 34/16 | 125,127 | 1.4 (0.6, 3.0) | |
| ≥10% | 21/41 | 103, 55 | 0.3 (0.2, 0.6) | |
| Continuous (not adjusted for each other): | Median % in cases, controls | Median % in cases, controls | Per 10% increase in ethanol from each type of drink** | |
| Men | Women | |||
| % ethanol from busaa | 44, 32 | 50, 65 | 1.05 (0.98, 1.13) | |
| % ethanol from chang’aa | 28, 14 | 35, 16 | 1.16 (1.07, 1.27)b | |
| % ethanol from commercial beer | 32, 0 | 0, 0 | 0.85 (0.78, 0.93) | |
| % ethanol from spirits | 0, 0 | 0, 0 | 0.75 (0.60, 0.92) | |
As specified in Table 3
Persists when ethanol g/day is adjusted as linear effect
In sensitivity analyses by type of control, increased EC risks for alcohol were significant regardless of control type, but were smaller in magnitude with hospital inpatients than for outpatients or visitors: OR of alcohol without tobacco 1.8 (1.1, 3.1) using inpatients, 2.4 (1.4, 4.2) outpatients and 2.6 (1.5, 4.5) with hospital visitors as controls. Whilst for ever-alcohol and tobacco ORs were 3.6 (2.3, 5.8) for inpatients, 4.0 (2.5, 6.5) outpatients and 4.8 (3.0, 7.9) visitors.
Population attributable fractions (PAF)
PAFs were calculated for drinking over 2 drinks per day, using never drinkers as the reference and are displayed overall and by population subgroups in Figure 2 (and Supplementary Table 1, additionally for a reference of 0 to <2 drinks per day). PAFs for >2 drinks/day were 48% overall, which was higher in men than women, and was concentrated in over 50-year olds and in tobacco users. Under age 50, alcohol had little contribution to the female ESCC burden.
Figure 2:
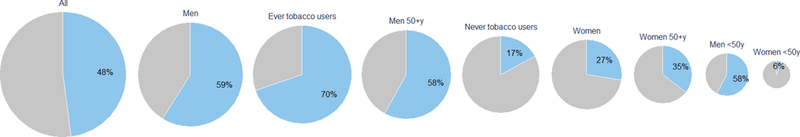
Pie charts of population attributable fractions of ESCC for alcohol (drinking >2 drinks per day compared to never drinking) in Western Kenya, overall and by age, gender and tobacco status. Each pie chart is sized proportional to the number of ESCC patients in that subgroup.
Discussion
This Kenyan case-control study is the first comprehensive assessment of the role of alcohol in this common poor prognosis cancer in East Africa, squamous cell esophageal carcinoma. We found a strong positive dose-response relationship with the number of drinks consumed per day and with ethanol intake, which was present in the smaller group of never-tobacco users, and stronger in the majority group of tobacco-users. Most ethanol came from traditional alcohols, specifically busaa. Fewer drinkers consumed chang’aa, however consumption of this local spirit was associated with a stronger increased risk than consumption of the same amount of ethanol from other alcohol types. Risks were higher the earlier drinking started in life and in current than in past drinkers.
The findings were remarkably consistent with previous findings, especially for the dose-response trend with daily ethanol consumption which broadly agreed with published meta-analyses25, lending credibility to the overall results. Differences to external findings were apparent, however, in the magnitude of the relative risk between light and never drinkers for which the present study found a much steeper risk differential than previous studies. This possibly reflects contaminants of alcohol, reporting or selection bias (both discussed later) or alternatively, the composition of the reference group may differ. In the European and North American context, ‘never’ drinkers (of at least 1 drink per week for 6 months) includes only a small proportion of true lifetime abstainers31 whereas in the African context drinking exhibits an ‘all-or-nothing’ behavior, therefore potentially creating a larger gap between never and light drinkers. Consistent with the IARC monograph5, we observed effect modification of alcohol with tobacco use, however whereas previous interactions were between heavy use of both carcinogens, Kenya’s intensity of tobacco smoking (or other use) was relatively low. In the Kenyan setting, exposures to the constituents of tobacco that are responsible for such an interaction may occur through non-tobacco routes, e.g. polycyclic aromatic hydrocarbons from household air pollution from indoor cooking/heating using biomass fuels. In the same vein, the IARC monograph commented on limited data on the effect of alcohol in never-tobacco users, whereas we saw an increased risk for ever drinkers. We did not, however, have power in to assess the dose-response relationship in this group. The present study did not find an interaction of alcohol with hot beverage drinking, in contrast to recent findings in the Kadoorie cohort.29 A protective effect of quitting was seen, but a linear trend with time-since-quitting was not found as in previous pooled analyses.32
Previous work showed no material differences in ESCC risk by type of alcoholic beverage.5 Our findings were broadly similar, except for a suggestion of a specific increased risk associated with the locally distilled chang’aa. This finding needs further study, but could potentially be due to its strength as its variable ethanol content reaching 60% or more, far exceeding commercially regulated spirits elsewhere. Locally, this spirit is known to burn the throat and cause lip hypopigmentation. Notwithstanding, chang’aa was not the chief contributor to ethanol intake, rather it was busaa or beer. With respect to drinking any alcohol type, regardless of tobacco use, ORs in light compared to never drinkers were unexpectedly large and unlikely to be entirely due to ethanol. Local alcohols may have other constituents, such as aflatoxin, lead, and nitrosamines, that possibly confer additional risks, but this should not detract from the large PAF for alcohol, which was not affected by this never/light drinker difference. Selection bias may have contributed to this light/ever drinking difference, as ORs were strongest in hospital visitors and lower, but still significant, in hospital inpatients. Due to this potential bias, we cannot be certain of the effect of alcohol in never-tobacco users.
The study had several strengths, but also weaknesses inherent to the design. For cases, systematic case recruitment over several years and histological confirmation ensured unbiased inclusion and little outcome misclassification. The choice of control population is a major challenge in settings where reliable population registration does not exist and referral biases may be large. However, as we conducted the study outside of a national referral hospital in a larger metropolitan city (e.g. Nairobi), our setting helped to ensure similar catchment populations of cases and controls, at least geographically. Nevertheless, selection bias may have influenced the results. The decline in the alcohol-ESCC ORs when comparing cases to hospital inpatients (expected underestimated ORs), and the highest OR for cases compared to hospital visitors (expected overestimate) demonstrates the presence of bias, thus the combination of control types is hoped to provide a less biased overall result. Hospital in or outpatients may have higher prevalence of alcohol consumption than the unknown underlying prevalence in the general population, as – although we excluded some digestive diseases – their hospital stay or outpatient visit may have been related to causes (e.g. accident) associated with alcohol consumption; on the other hand, visitors accompanying patients may have had a lower prevalence as heavier drinkers may be less likely to travel as a visitor to the hospital. Social desirability bias may also have inflated the associations, if controls – in this culture, women in particular - underreported alcohol exposures. ORs were not lower in women than in men suggesting modest if any influence of such a bias.
There remain many research gaps on ESCC in Kenya. For alcohol specifically, the relative proportions of the ALDH2 variant alleles are not known in East African populations. The ALDH2 gene influences the rate of ethanol metabolism to acetaldehyde and can influence both alcohol intake or increase exposure to acetaldehyde. Additionally little is known of congeners in traditionally manufactured alcohols, which are likely to include acetaldehyde itself and for chang’aa methanol causing temporary blindness. Larger sample sizes will be needed to examine the effect of alcohol in the absence of tobacco, or for the effect of pure drinkers of each alcohol type. Other effect modifiers suggested are thermal injury, which were not found in the present study, and poor nutritional status but we had limited power to investigate them. Beyond alcohol, there is still a considerable proportion of ESCC and consequently of years of life lost due to ESCC in men, in women, and at young ages that were not attributed to alcohol which need further investigation.
The public health implications of the findings are substantial. The PAFs of ESCC due to drinking 2 or more drinks per day compared to never drinking were near 50%, or one-third compared to drinking >0 to <2 drinks per day, which represents a large preventable disease burden in absolute numbers, even if the percentage is smaller than in low ESCC incidence populations, e.g. a PAF of 75% for smoking and heavy drinking was found in Australian men33. In Kenya, the high PAF arises from excessive consumption amongst drinkers who are also tobacco users. Due to other adverse societal and health problems, chang’aa consumption is already being tackled by Kenyan authorities and should aid in reducing ESCC incidence. Consumption of other alcohols also needs to be reduced as they contributed to a greater extent to ethanol intake. In conclusion, reductions in alcohol consumption, as promoted by WHO’s 2010 Global Strategy to Reduce the Harmful Use of Alcohol and the WHO Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013–2020, should lead to multiple health benefits that are key to controlling this common fatal cancer in Kenya.
Supplementary Material
Novelty and impact:
We investigated the role of commercial and locally manufactured alcohols in esophageal cancer in Kenya, a common cancer in East Africa. Alcohol was a large contributor to the cancer burden, particularly in men. Most ethanol came from the traditional brew busaa; and a particularly strong increased esophageal cancer risk was seen for the traditional spirit chang’aa. Alcohol consumption needs to be reduced in Kenya to prevent this common fatal cancer.
Acknowledgments
The authors would like to thank all the participants who took part in this study.
Funding: This work was supported by the International Agency for Research on Cancer (IARC), NIH/NCI grant R21CA191965, an IARC-UICC Development Fellowship to SKM and an IARC post-doctoral fellowship to DM partially supported by the European Commission FP7 Marie Curie Actions – People – Co-funding of regional, national and international programs (COFUND).
Abbreviations and definitions
- ASR
Age-standardized incidence rate
- busaa
local alcoholic drink, brewed from sorghum, maize or millet-flour, 4.5% ethanol
- chang’aa
moonshine (illicit) spirit distilled from maize husks, 20–60% ethanol, 40% assumed
- EC
Esophageal cancer
- ESCC
Esophageal squamous cell carcinoma
- ESCCAPE
Esophageal Squamous Cell Cancer African PrEvention research
- KDHS
Kenyan Demographic and Health Survey
- kumi kumi
Illegal liquor made from sorghum, maize or millet-flour, and a commonly used alcohol cost unit (10 Kenyan shilling)
- MTRH
Moi Teaching and Referral Hospital, Eldoret, Kenya
- NACADA
Kenyan National Authority for the Campaign against Alcohol and Drug Abuse
- PAF
Population attributable fraction (if drinkers of >2 drinks/day were never drinkers)
Footnotes
Competing interests
The authors declare no competing interests.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015; 136(5). [DOI] [PubMed] [Google Scholar]
- 2.McCormack VA, Menya D, Munishi MO, Dzamalala C, Gasmelseed N, Leon Roux M, Assefa M, Osano O, Watts M, Mwasamwaja AO. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: A review of setting-specific exposures to known and putative risk factors. International Journal of Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawsey SP, Tonui S, Parker RK, Fitzwater JW, Dawsey SM, White RE, Abnet CC. Esophageal cancer in young people: a case series of 109 cases and review of the literature. PLoS One. 2010; 5(11): e14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel K, Wakhisi J, Mining S, Mwangi A, Patel R. Esophageal Cancer, the Topmost Cancer at MTRH in the Rift Valley, Kenya, and Its Potential Risk Factors. ISRN Oncol. 2013; 2013: 503249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IARC. Personal Habits and Indoor Conbustions. IARC Monographs Volume 100E A Reivew of Human Carcinogens. Lyon: IARC; 2012. Available from: http://monographs.iarc.fr/ENG/Monographs/vol100E/mono100E-1.pdf. [Google Scholar]
- 6.Castellsague X, Munoz N, De Stefani E, Victora CG, Castelletto R, Rolon PA, Quintana MJ. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999; 82(5): 657–64. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi R, Wilunda C, Magutah K, Mwaura-Tenambergen W, Wilunda B, Perngparn U. Correlates of alcohol consumption in rural western Kenya: A cross-sectional study. BMC Psychiatry. 2017; 17(1): 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo TQ, Oeltmann JE, Odhiambo FO, Beynon C, Pevzner E, Cain KP, Laserson KF, Phillips-Howard PA. Alcohol use, drunkenness and tobacco smoking in rural western Kenya. Trop Med Int Health. 2013; 18(4): 506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papas RK, Sidle JE, Wamalwa ES, Okumu TO, Bryant KL, Goulet JL, Maisto SA, Braithwaite RS, Justice AC. Estimating alcohol content of traditional brew in Western Kenya using culturally relevant methods: the case for cost over volume. AIDS and Behavior. 2010; 14(4): 836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngunjiri J, Wanyoro C, Mutavi L, Mutua K, Macharia A, Karanja J. Poison drinks kill 56 in five counties as more than 70 in hospital. Daily Nation. 14 May, 2014. 6 May. [Google Scholar]
- 11.Mwaura M, Jebet V. Police begin crackdown on illegal brews, arrest suspects. Daily Nation. July 3, 2015. [Google Scholar]
- 12.Mosoku G Kenya declares total war on killer alcohol as crisis worsens. The Standard. 3 July, 2015. [Google Scholar]
- 13.Sewram V, Sitas F, O’Connell D, Myers J. Tobacco and alcohol as risk factors for oesophageal cancer in a high incidence area in South Africa. Cancer Epidemiol. 2016; 41: 113–21. [DOI] [PubMed] [Google Scholar]
- 14.Robertson MA, Harington JS, Bradshaw E. The cancer pattern in Africans at Baragwanath Hospital, Johannesburg. Br J Cancer. 1971; 25(3): 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGlashan ND, Bradshaw E, Harington JS. Cancer of the oesophagus and the use of tobacco and alcoholic beverages in Transkei, 1975–6. Int J Cancer. 1982; 29(3): 249–56. [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw E, Schonland M. Oesophageal and lung cancers in Natal African males in relation to certain socio-economic factors. An analysis of 484 interviews. British journal of cancer. 1969; 23(2): 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradshaw E, Schonland M. Smoking, drinking and oesophageal cancer in African males of Johannesburg, South Africa. British journal of cancer. 1974; 30(2): 157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burrell RJ. Esophageal cancer among Bantu in the Transkei. Journal of the National Cancer Institute. 1962; 28: 495–514. [PubMed] [Google Scholar]
- 19.Segal I, Reinach SG, de Beer M. Factors associated with oesophageal cancer in Soweto, South Africa. British journal of cancer. 1988; 58(5): 681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vizcaino AP, Parkin DM, Skinner ME. Risk factors associated with oesophageal cancer in Bulawayo, Zimbabwe. British journal of cancer. 1995; 72(3): 769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook P Cancer of the oesophagus in Africa. A summary and evaluation of the evidence for the frequency of occurrence, and a preliminary indication of the possible association with the consumption of alcoholic drinks made from maize. British journal of cancer. 1971; 25(4): 853–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mwachiro MM, Burgert SL, Lando J, Chepkwony R, Bett C, Bosire C, Abnet CC, Githanga J, Waweru W, Giffen CA, Murphy G, White RE, Topazian MD, Dawsey SM. Esophageal Squamous Dysplasia is Common in Asymptomatic Kenyans: A Prospective, Community-Based, Cross-Sectional Study. Am J Gastroenterol. 2016; 111(4): 500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGlashan ND. Oesophageal cancer and alcoholic spirits in central Africa. Gut. 1969; 10(8): 643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey K, Kinney J, Eckman M, Nassar A, Mehta K. Chang’aa Culture and Process: Detecting Contamination in a Killer Brew. Procedia Engineering. 2015; 107: 395–402. [Google Scholar]
- 25.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. British journal of cancer. 2015; 112(3): 580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Survey KDH. The Demographic Health Survey of Kenya. 2014. [Google Scholar]
- 27.Papas RK, Sidle JE, Wamalwa ES, Okumu TO, Bryant KL, Goulet JL, Maisto SA, Braithwaite RS, Justice AC. Estimating alcohol content of traditional brew in Western Kenya using culturally relevant methods: the case for cost over volume. AIDS Behav. 2010; 14(4): 836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nout MJR. Aspects of the manufacture and consumption of Kenyan traditional fermented beverages. Wageningen: Unveristy of Wageningen; 1981. [Google Scholar]
- 29.Yu C, Tang H, Guo Y, Bian Z, Yang L, Chen Y, Tang A, Zhou X, Yang X, Chen J, Chen Z, Lv J, Li L, China Kadoorie Biobank Collaborative G. Hot Tea Consumption and Its Interactions With Alcohol and Tobacco Use on the Risk for Esophageal Cancer: A Population-Based Cohort Study. Ann Intern Med. 2018; 168(7): 489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993; 49(3): 865–72. [PubMed] [Google Scholar]
- 31.Rehm J, Irving H, Ye Y, Kerr WC, Bond J, Greenfield TK. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am J Epidemiol. 2008; 168(8): 866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehm J, Patra J, Popova S. Alcohol drinking cessation and its effect on esophageal and head and neck cancers: a pooled analysis. Int J Cancer. 2007; 121(5): 1132–7. [DOI] [PubMed] [Google Scholar]
- 33.Pandeya N, Olsen CM, Whiteman DC. Sex differences in the proportion of esophageal squamous cell carcinoma cases attributable to tobacco smoking and alcohol consumption. Cancer Epidemiol. 2013; 37(5): 579–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


