Abstract
Background
Very preterm (VPT) infants are at risk for altered growth, slower speed of processing (SOP) and hypertension. This study assesses the relationship between postnatal body composition (BC), neurodevelopment (indexed by SOP) and blood pressure (BP) in VPT infants.
Methods
34 VPT infants underwent weekly measurements and BC testing until discharge, and post-discharge at 4mos CGA and 4yrs. At post-discharge visits, SOP was assessed using visual evoked potentials and the NIH Toolbox; BP was also measured.
Results
In-hospital rate of weight, length and fat-free mass (FFM) gains were associated with faster SOP at 4yrs. Higher rate of gains in weight and FFM from discharge to 4mos CGA were associated with faster SOP at 4mos CGA, while higher fat mass (FM) gains during the same time were positively associated with BP at 4yrs. BC at 4yrs nor gains beyond 4mos CGA were associated with outcomes.
Conclusions
In VPT infants, early FFM gains are associated with faster SOP, whereas post-discharge FM gains are associated with higher BPs at 4yrs. This shows birth to 4mos CGA is a sensitive period for growth and its relation to neurodevelopmental and metabolic outcomes. Close monitoring and early nutritional adjustments to optimize quality of gains may improve outcomes.
Background
Postnatal growth and body composition of preterm infants is altered compared to intrauterine gains such that by term corrected gestational age (CGA), preterm infants have lower fat-free mass (FFM) and higher fat mass (FM) compared to term counterparts (1-3). Longterm neurodevelopment in preterm infants is affected in part by nutritional status and growth during hospitalization and early infancy. In particular, weight gain, head growth, linear growth, and FFM gains during hospitalization and up to 4 months CGA are associated positively with gross neurodevelopment at 18-24 months, where as growth beyond 4 months CGA has been shown to have minimal associations with neurodevelopmental outcomes (4-6). Early aggressive nutrition is also associated with brain development and outcomes at 12-24 months (7-10). This early, postnatal time period appears to be a sensitive stage for growth as it relates to the brain and neurodevelopment in preterm infants. Very preterm (VPT) infants are particularly vulnerable given that most or all of their third trimester, a period of marked brain development, is spent ex-utero.
One aspect of neurodevelopment particularly affected in preterm infants is speed of processing (SOP). SOP is delayed in preterm infants during early and mid-childhood (11-12). Processing speed goes on to affect daily functioning, academics and behavior through its mediation of executive functioning and attention (12,13) and is also related to full-scale IQ (11).
Our group previously published a pilot study showing that higher FFM at term and 4 months CGA is associated with faster SOP at 4 months CGA in a small group of preterm infants born <35 weeks (6). SOP, which reflects both synaptic integrity and myelination, is easily tested in infants and children using visual evoked potentials (VEPs). Latency to the peak of the P100 waveform indexes neuronal processing speed and maturation of the visual system and shortens (speeds up) throughout the third trimester, infancy, and early childhood as the visual pathway develops. These factors, as well as low intra-subject variability and ease of administration, make VEP a useful tool in assessing early brain development in at-risk infants and children (14). Older children are also able to complete task-based testing that assesses SOP.
Additionally, preterm infants are at risk for long-term metabolic abnormalities including hypertension (15); however, whether early catch-up growth and/or body composition changes alter this risk remains unclear (16). In preterm infants, some studies have shown that high BMI and weight gain during the first 12-18 months of life increases the risk of obesity during later childhood (17,18). Lapillionne et al. summarized a number of studies that show early growth of preterm infants is not associated with later hypertension, particularly under 8 months of age, while gains after this time period showed associations (8). Very few studies have evaluated the effect of in-hospital growth, and particularly body composition, on long-term metabolic outcomes.
The objective of the present study was to prospectively follow a cohort of very preterm infants (<32 weeks at birth) from birth to 4 years to evaluate the association between early body composition and long-term neurodevelopment, as assessed by SOP, as well as metabolic outcomes, as assessed by blood pressure (BP).
Methods
Thirty-four VLBW preterm infants were recruited from the University of Minnesota Masonic Children’s Hospital Neonatal Intensive Care Unit between April 2011 and November 2012. Inclusion criteria included birth weight <1500g, gestational age at birth <32 weeks, and size appropriate for gestational age (birth weight between the 10th and 90th percentile on the Fenton Curve (19)). Infants were excluded if there was presence of a congenital metabolic disease that would affect growth. The University of Minnesota Institutional Review Board approved the protocol and informed consent was obtained from a parent within the first 5 days of life.
Inpatient Data
Infants had weekly inpatient anthropometrics recorded and, once able to tolerate room air for >5 minutes, also underwent weekly body composition testing (median day of life 24.5 and median CGA 33.2 weeks). Weight was recorded to the nearest 1g, supine length was measured using an infant length board (Perspective Enterprises; Portage, MI), and OFC was measured with a flexible cloth tape measure (length and OFC recorded to the nearest 0.1cm). Body composition was measured using air displacement plethysmography (PEA POD, COSMED, Ltd.; Concord, CA), which provides a validated measurement of infant body composition, including preterm infants (20). Briefly, infant weight to the nearest 0.1g and volume were measured using the PEA POD and were used to calculate body density using known age and sex-specific FFM density values (21). A detailed description of the PEA POD’s measurement procedures, design, and operating principles are described elsewhere (22-25).
Data collected as covariates included nutritional intake and markers of illness. Caloric intake (kcal/kg/d) and protein intake (g/kg/d) were recorded by a NICU dietician. Cumulative nutritional deficit or surplus for the hospitalization was calculated from goals of 120 kcal/kg/d of energy and 4 g/kg/d of protein. Illness severity was measured using the Score for Neonatal Acute Physiology-II (SNAP-II), a validated illness severity and mortality risk score for neonates (26), on day 1. Also recorded was worst grade of intraventricular hemorrhage and retinopathy of prematurity, as well as number of days on positive airway pressure, oxygen, antibiotics, and IV/oral steroids.
Outpatient Data
Body composition (again via air displacement plethysmography using the PEA POD (COSMED, Ltd.) for infants and BOD POD with Pediatric Option (COSMED, Ltd.; Concord, CA) at 4 years) and VEPs were recorded at 4 months CGA and 4 years old. At 4 years old, children were also assessed using the NIH Toolbox Pattern Comparison Processing Speed Test Ages 3-6 (NTPST) (27) and blood pressure was measured using a Datascope Accutor Plus non-invasive automatic oscillometric blood pressure monitor. The average of two blood pressure measurements was used for analysis. At the 4 month CGA visit, a 64-electrode Sensor Net was used for VEP data acquisition; at the 4 year visit a 128-channel net was used. Nets are designed using an elastic tension structure with electrodes positioned in a geodesic pattern. Infants were seated in a darkened room and presented a visual stimulus of a pattern-reversing black-and-white checkerboard, presented by E-Prime software (Psychology Software Tools, Sharpsburg, PA). The pattern was comprised of ~1.5 × 1.5cm individual checks (1.4 × 1.4°) with two reversals per second for 50 trials. It was displayed on a 20” ViewSonic Graphics Series G225f monitor (ViewSonic, Walnut, CA) with a screen that was 39cm wide x 29cm tall so that the viewing field subtended to 33.4 × 23.5° at a viewing distance of 60cm. A research assistant, hidden behind the monitor and a cloth screen, provided auditory stimuli to keep the participants’ attention on the monitor if needed (14). Pattern-reversal VEP data was collected and recorded from ongoing EEG using the Geodesic EEG System 200 (Electrical Geodesics, Inc. (EGI), Eugene, OR), and NetStation 4.4.2 software (EGI) was used to measure scalp impedances (accepted if <50KΩ) and process the data (rereferenced to the vertex, amplified with a 0.1-100Hz bandpass and digitized at 250Hz).
Using NetStation 4.4.2 analysis software (EGI), EEG data were filtered with a 30-Hz lowpass filter, segmented to 500ms periods starting 100ms before the stimulus presentation, and baseline corrected to the average prestimulus voltage. Segments were then hand-edited for movement artifact, eye movement, and poor recordings. Per center protocol (28), trials were excluded if the number of electrodes rejected was >16% and participants with <12 accepted trials (out of 50) were excluded from further analysis (mean number of accepted trials at 4 months and 4 years was 35.7 and 38, respectively). For accepted trials, bad data on individual electrode was replaced using spherical spline interpolation and the average waveform at each electrode was calculated and re-referenced to the average reference. Latency of the P100 component was measured at Oz (at 4 months, the average of leads O1 and O2 were used to approximate Oz). If an individual electrode did not have an identifiable P100, data were treated as missing.
Data Analysis
Multiple linear regression was performed to assess the relationship between body composition and anthropometric measurements and SOP (using latency to P100 on the VEP, where shorter (smaller) latency indicates faster processing, and raw score from NTPST, where a higher score means faster processing) in all patients who had a 4 month VEP and longitudinal SOP data at 4 years. Covariates adjusted were CGA at visit and birth gestational age, as well as ROP stage for VEPs; we were unable to adjust for other clinical factors given the small sample size. Slope estimates with standard errors and P values were calculated. The associations between clinical factors (caloric and protein deficits, SNAP-II score, and days of antibiotics, steroids and positive-pressure ventilation) and SOP were assessed using Pearson partial correlation coefficients for nutritional deficits and SNAP-II, and Spearman partial correlation for steroid, antibiotic and positive-pressure ventilation exposure due to skewed distribution of data. For analysis of the relationship between body composition and blood pressure, linear regression analysis was performed, adjusting for sex, gestational age at birth, age at visit, and 4-year length. All analyses were performed using SAS (V9.4; SAS Institute, Cary, NC). Statistical significance was defined as P < 0.05.
Results
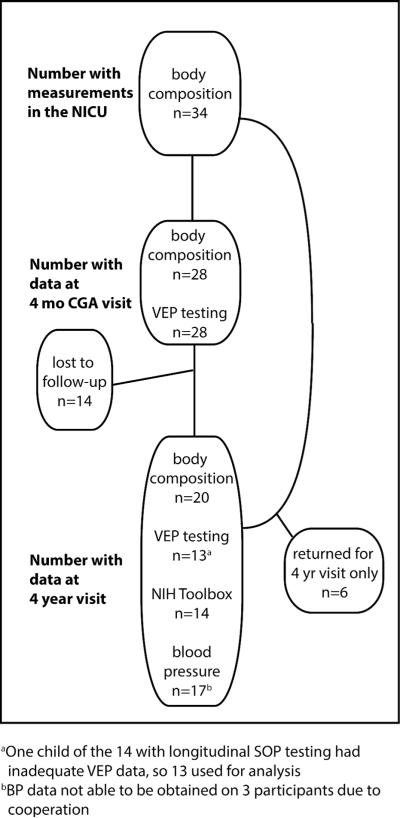
Infant characteristics for participants are included in Table 1. Of the 34 infants enrolled in the NICU with inpatient data, 28 returned for the 4 month visit and had adequate VEP data for analysis. Of those infants, 14 returned at 4 years for longitudinal SOP, body composition, and blood pressure testing. There were 6 infants who were followed in the NICU, missed the 4 month visit, and returned at 4 years for BC and BP analysis, and thus were included in that portion of the analysis only (Figure 1). There were some statistical differences in characteristics between that group of 6 infants who missed the 4 month visit and those who attended – they were younger at birth (26.2 weeks (SD 2.05) vs 29.0 weeks (SD 1.14), p=0.013) and experienced more complications of prematurity with higher rates of ROP (3 (50%) vs 1 (5.9%), p=0.03) and chronic lung disease (5 (83.3%) vs 1 (5.9%), p=0.012), higher caloric deficits (1759 kcal (SD 969) vs 674 kcal (SD 541), p=0.003), longer NICU stays (95.3 days (SD 31.5) vs 58.4 days (SD 16.4), p=0.02) and more days on positive pressure ventilation (55.8 days (SD 40) vs 16.1 days (SD 13.4), p=0.02). As expected, mean P100 latency decreased over time: (163 ms at 4 months CGA and 123 ms at 4 years). Early P100 latency did not predict later processing speeds (VEP or NTPST) in this small group of subjects.
Table 1.
Descriptive characteristics of 34 very preterm infants
| Variable | Dataa |
|---|---|
| Gestation age at birth (weeks) | 28.2 (2.17) |
| Sex (male) | 19 (55.9%) |
| Maternal race (non-hispanic white) | 26 (76.5%) |
| Birth weight (g) | 1094 (257.8) |
| Birth length (cm) | 36.56 (3.16) |
| Birth head circumference (cm) | 25.75 (2.34) |
| Calorie deficit at discharge (kcal) | 1018 (727.1) |
| Protein deficit at discharge (g) | 43.39 (23.79) |
| Inpatient days | 69.24 (28.02) |
| Age at first body composition testing (days)b | 24.5 (2-122) |
| CGA at first body composition testing (weeks)b | 33.2 (30.1-40.5) |
| SNAP score at day 1 | 10.32 (8.22) |
| Postnatal steroids (days) | 0 (0-68) |
| Antibiotic use (days) | 7 (0-75) |
| Positive pressure use (days) | 15 (0-114) |
| Intraventricular hemorrhage >grade 1 | 7 (20.6%) |
| Retinopathy of prematurity >stage 1 | 8 (23.5%) |
| Chronic Lung Diseasec | 9 (26.5%) |
Continuous variables expressed as mean (standard deviation) and categorical variables as n (percentage). Skewed data reported as median (range).
Excluding 2 participants who could not have inpatient body composition testing due to oxygen need.
Chronic lung disease defined as oxygen need at ≥36 weeks CGA.
Figure 1.
Flow diagram of study subject participation
Speed of processing at 4 months CGA and body composition
After adjusting for CGA at visit, GA at birth and ROP stage, higher rate of weight (p=0.04, r2=0.65) and FFM (p=0.05, r2=0.65) gains (g/wk) between term and 4 months CGA were associated with shorter latency to P100 (or faster SOP) (Table 2). Absolute weight and FFM at 4 months were also associated with shorter P100 latencies (slope estimate (SD) −0.02 (0.01), p=0.015, r2=0.64 and slope estimate (SD) −0.023 (0.01), p=0.05, r2=0.60 espectively). Conversely, fat mass and %body fat changes had no associations with SOP at 4 months.
Table 2.
The relationship of body composition and anthropometric changes with speed of processing and blood pressures
| VEP at 4 months CGAa | VEP at 4 yearsa | 4 year NIH Toolbox Processing Speed Testb |
Systolic Blood Pressure at 4 yearsc |
|||||
|---|---|---|---|---|---|---|---|---|
| Rate of change epoch | Slope (SE) | P-value | Slope (SE) | P-value | Slope (SE) | P-value | Slope (SE) | P-value |
| Inpatient: Weight (g/wk) Length (cm/wk) OFC (cm/wk) FFM (g/wk) FM (g/log(wk)) %BF (g/log(wk)) |
0.04 (0.91) 116.5 (139.6) 4.7 (229.5) 0.007 (1.26) −0.024 (0.16) −1.74 (9.5) |
0.96 0.41 0.98 0.99 0.88 0.86 |
0.84 (1.48) −37.56 (254.3) 47.08 (312.6) 0.68 (1.99) −0.078 (0.19) 0.75 (11.76) |
0.58 0.89 0.88 0.74 0.69 0.95 |
0.78 (0.31) 95.6 (43.2) 134.3 (73.2) 0.90 (0.36) 0.089 (0.06) 4.09 (4.18) |
0.03 0.05 0.09 0.03 0.17 0.35 |
−0.88 (0.64) −71.22 (92.6) 25.54 (145.7) 0.43 (0.82) −0.084 (0.09) −4.585 (4.81) |
0.19 0.46 0.86 0.61 0.36 0.36 |
| Term to 4 mo CGA: Weight (g/wk) Length (cm/wk) OFC (cm/wk) FFM (g/wk) FM (g/log(wk)) %BF (g/log(wk)) |
−0.31 (0.14) −4.67 (42.9) −105.9 (75.9) −0.43 (0.21) −0.32 (0.26) −8.3 (32.08) |
0.04 0.99 0.18 0.05 0.24 0.7 |
0.36 (0.28) −1.23 (44.89) 213.6 (125.7) 0.57 (0.32) −0.13 (0.95) 22.91 (62.9) |
0.24 0.98 0.13 0.12 0.89 0.73 |
−0.13 (0.1) −23.7 (16.7) 50.1 (60.8) −0.11 (0.14) −0.29 (0.21) −11.78 (13.18) |
0.26 0.19 0.43 0.47 0.2 0.39 |
0.22 (0.14) 6.69 (21.05) −66.47 (56.09) 0.039 (0.19) 0.83 (0.19) 45.56 (14.93) |
0.16 0.76 0.27 0.84 0.002 0.01 |
| 4 mo CGA to 1 yr: Weight (g/wk) Length (cm/wk) OFC (cm/wk) |
0.3 (0.63) 41.9 (162.9) −41.77 (464.2) |
0.65 0.81 0.93 |
−0.17 (0.25) 39.6 (75.1) −206.2 (141) |
0.5 0.61 0.2 |
0.37 (0.28) 14.53 (94.45) −98.14 (254.1) |
0.22 0.88 0.71 |
||
| 1 yr to 2 yr: Weight (g/wk) Length (cm/wk) OFC (cm/wk) |
−1.56 (1.79) 127.9 (314.5) 435.2 (521.3) |
0.43 0.7 0.45 |
0.25 (0.66) −62.8 (107.5) 231.8 (251.3) |
0.72 0.58 0.39 |
−0.43 (0.29) −19.06 (142.7) 481.78 (273.9) |
0.19 0.9 0.13 |
||
| 2 yr to 4 yr: Weight (g/wk) Length (cm/wk) OFC (cm/wk) |
−1.95 (1.47) −671.5 (316.4) −2686.3 (1778.5) |
0.26 0.09 0.21 |
0.27 (0.43) −82.8 (157.3) 214.6 (684.8) |
0.55 0.61 0.76 |
−0.2 (0.38) 105.92 (160.7) 892.75 (750.96) |
0.61 0.53 0.27 |
||
| 4 mo to 4 yr: Weight (g/wk) Length (cm/wk) OFC (cm/wk) FFM (g/wk) FM (g/log(wk)) %BF (g/log(wk)) |
0.83 (2.04) −50.34 (389.1) 21.88 (2859.9) 1.87 (1.96) −2.88 (3.3) 88.9 (522.76) |
0.7 0.9 0.99 0.39 0.41 0.87 |
0.58 (0.4) 73.66 (150.7) −63.65 (963.75) 0.48 (0.62) 0.54 (0.52) 78.93 (80.17) |
0.18 0.64 0.95 0.46 0.32 0.35 |
0.24 (0.5) −1.53 (248.1) 965.35 (984.8) 0.96 (0.94) −0.08 (0.75) −160.2 (129.56) |
0.64 0.99 0.35 0.33 0.91 0.24 |
||
Adjusted for birth gestational age (bGA), CGA at visit and ROP.
Adjusted for bGA and CGA at visit.
Adjusted for sex, bGA, CGA at visit, and length
Speed of processing at 4 years and body composition
There were no significant associations between body composition at any age and P100 latency at 4 years after adjusting for age at visit, GA at birth and ROP. However, early body composition changes were positively associated with processing speed as assessed by the NTPST after adjusting for age at visit and GA at birth. This was seen only in early gains in lean body mass: higher inpatient (birth to discharge) rates of increase in weight (g/wk), length (cm/wk) and FFM (g/wk) were positively associated with SOP on the NTPST (respective p-values: p=0.03, p=0.05, p=0.03; respective r2 values: r2=0.39, r2=0.34, r2=0.39) (Table 2). Neither body composition at 4 years, nor rates of gains from 4 months to 4 years were associated with SOP at 4 years.
Relationship of Clinical Factors to SOP
We explored the relationship between several clinical factors and their relationship with SOP at 4 months CGA and 4 years (adjusting for age at measurement and birth GA). Several clinical factors strongly correlated with early SOP: in-hospital caloric and protein deficits, as well as number of days of steroids and antibiotics had the strongest correlations; however these were no longer significant by 4 years (Table 3).
Table 3.
Relationship of clinical factors to SOP
| Calorie Deficit |
Protein Deficit |
SNAP Score Day 1 |
Steroid Days |
Antibiotic Days |
Positive Pressure Days |
||
|---|---|---|---|---|---|---|---|
|
4 month VEP |
Correlation P-value |
0.47 0.016 |
0.49 0.01 |
−0.06 0.77 |
0.44 0.02 |
0.55 0.004 |
0.39 0.05 |
|
4 year VEP |
Correlation P-value |
0.47 0.167 |
−0.31 0.38 |
−0.14 0.7 |
0.18 0.63 |
0.41 0.25 |
−0.38 0.28 |
|
NIH Toolbox SOP |
Correlation P-value |
−0.43 0.21 |
−0.09 0.8 |
−0.28 0.42 |
−0.51 0.13 |
−0.24 0.51 |
−0.14 0.70 |
Blood pressure at 4 years and body composition
Faster gains in FM and percent body fat (%BF) between term and 4 months CGA were associated with increased systolic blood pressure (SBP) at 4 years of age (FM p≤0.01, r2=0.78 and %BF p=0.01, r2=0.68) (Table 2). Higher absolute FM at 4 months CGA was also associated with increased SBP and diastolic blood pressure (DBP) at 4 years of age (4 month SBP: slope estimate (SD) 0.03 (0.01), p=0.04, r2=0.40; 4 month DBP: slope estimate (SD) 0.02 (0.006) p=0.03, r2=0.64). Neither growth nor body composition changes prior to term nor after 4 months CGA were associated with SBP or DBP at 4 years of age.
Discussion
In very preterm infants, goals of ex-utero growth and nutritional status are meant to mimic in-utero conditions (29); yet preterm infants continue to have altered body composition at term CGA compared to their term counterparts (1-3). Further, they often have subnormal neurodevelopment (30) and are at risk for hypertension (8) and other metabolic concerns. The present study shows that increased early growth and lean body mass gains (rather than catch up after 4 months CGA) are associated with faster speed of processing during infancy and early childhood in VPT infants. In addition, we report that early gains in adiposity are associated with higher blood pressure at 4 years.
Neurodevelopmental outcomes
Like other studies (4,5,17) our results show birth to 4 months CGA to be a critically sensitive time period for growth and nutrition and their relation to neurodevelopmental outcomes in preterm infants. In particular, increased inpatient rate of gain in weight, length (a surrogate for FFM) and FFM go on to positively affect SOP at 4 years of age. Daily life and school-related functions are affected by processing speed, as it impacts executive functioning skills and the ability to take in information and respond to it properly.
As shown by the r2 values, 65% of the variance of P100 latency (or SOP) at 4 months is accounted for by early FFM gains, birth gestational age, CGA at visit and ROP. By 4 years, this value decreases, yet it is noteworthy that early FFM remains significantly associated with 4 year SOP given the number of exposures and experiences that influence neurodevelopment and could diminish the effect.
Other clinical factors, such as nutritional deficits and exposure to steroids, antibiotics and positive pressure ventilation also appear to affect early brain development, as assessed by SOP. However, it seems these factors are overcome with time, as they were no longer associated with SOP by 4 years of age. This is unlike early FFM gains, which had an ongoing association. FFM indexes organ growth (31) and protein accretion (32). Protein status affects neuronal growth and differentiation, particularly during the rapid synaptogenesis occurring during the third trimester, thus impacting the scaffolding of the brain on which further growth and development is based (33). Some of the illness factors mentioned above are associated with inflammation (steroid need representing lung inflammation and antibiotics as a surrogate for inflammation related to sepsis). Illness and inflammation affect neurodevelopment through white matter injury (34,35) and suppression of insulin-like growth factor 1 (IGF-1), a protein important for neuronal growth and differentiation (36,37). Based on our results, it is possible the effects of inflammation on brain development may be partly overcome by improved scaffolding from early protein gains.
Metabolic outcomes
We also found increased gains in adiposity between term and 4 months CGA to be associated with higher blood pressure at 4 years of age, where as body composition changes prior to term did not show the same relationship to later blood pressure. Previous studies have mostly only looked at the association between early weight gain and later blood pressure, which have minimal associations, and linear growth, which may have some association (8). Body composition better represents the quality of growth, which is more likely to be related to metabolic outcomes. As shown by the r2 values, FM gains from discharge to 4 months CGA (along with birth gestational age, age at visit and length at 4 years) account for 78% of the variance in BP at 4 years, showing the clear impact on this outcome.
Metabolic outcomes, such as blood pressure, of formerly preterm infants are becoming an important topic of research. Much of the literature up until now has focused on IUGR term infants; however, this is not necessarily a comparable population to preterm infants undergoing altered extra-uterine growth. Though multiple large studies and reviews have shown that preterm infants are at risk for elevated blood pressure (15,38,39), reduced insulin sensitivity (40), and increased low-density lipoprotein (15), the influence of growth, diet, and neonatal illness on these outcomes are just now being evaluated. While the association between early body composition on later body composition or other metabolic markers was not within the scope of this paper, we have previously shown that FFM accretion between 1-2 weeks and 3-4 months CGA in preterm infants is related to preschool lean body mass, and this may lower the risk of later adverse metabolic outcomes (41). Thus, a focus on inpatient gains and optimizing individual early nutrition plans and growth so that minimal catch-up growth post-discharge is needed may help minimize long-term metabolic risk. In addition, our findings highlight the importance of monitoring quality of weight gain after discharge to allow optimization of neurodevelopment and diminish later metabolic risk.
One strength of this study is the group of VPT infants studied, as infants born <32 weeks are at the highest risk for growth and neurodevelopmental derangements. Further strengths are the longitudinal evaluation of both metabolic and neurodevelopmental outcomes; the length of follow-up out to 4 years, a time when outcomes are more predictive of adulthood outcomes; as well as data showing the influence of in-hospital body composition, growth and nutrition on long-term outcomes, a time period that has received less focus in the literature. The more we understand about the influence early changes and interventions have on long-term outcomes, the better we will be able to optimize our care of this neonatal population. The small cohort size is a weakness of this study, and larger longitudinal studies are needed to support the results. Also, a few of the sickest infants initially recruited in the study were unable to be included in the BC/SOP analysis due to continued oxygen requirement at 4 months of age (and thus unable to undergo body composition or SOP testing). This remains a population with lacking data due to inability to study their body composition, and yet a population at highest risk of altered body composition and suboptimal neurodevelopment. Body composition studies that include these infants would greatly add to the literature.
In summary, body composition is an important biomarker to follow in VPT infants and early body composition changes have long-lasting effects. Monitoring quality of weight gain in early infancy, and focusing early nutritional interventions on weight gain prior to 4 months and on FFM rather than FM gains may improve neurodevelopmental and metabolic outcomes.
Acknowledgments
Support was provided by the Amplatz McQuarrie Scholar Award through the Department of Pediatrics at the University of Minnesota and NIH grant P30 DK50456 to the Minnesota Obesity Center at the University of Minnesota and the Mayo Clinic.
Footnotes
The authors have no conflicts of interest to disclose related to this study.
Category of study: Clinical
References
- 1.Simon L, Frondas-Chauty A, Senterre T, Flamant C, Darmaun D, Roze JC. Determinants of body composition in preterm infants at the time of hospital discharge. Am J Clin Nutr 2014. July;100(1):98-104. [DOI] [PubMed] [Google Scholar]
- 2.Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics 2012;130(3):e640-9. [DOI] [PubMed] [Google Scholar]
- 3.Ramel SE, Gray HL, Ode KL, Younge N, Georgieff MK, Demerath EW. J Body composition changes in preterm infants following hospital discharge: comparison with term infants. Pediatr Gastroenterol Nutr 2011. September;53(3):333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belfort MB, Rifas-Shiman SL, Sullivan T, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics 2011. October;128(4):e899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramel SE, Demerath EW, Gray HL, Younge N, Boys C, Georgieff MK. The relationship of poor linear growth velocity with neonatal illness and two year neurodevelopment in preterm infants. Neonatology 2012;102:19–24. [DOI] [PubMed] [Google Scholar]
- 6.Pfister KM, Gray HL, Miller NC, Demerath EW, Georgieff MG. Exploratory relationship of fat-free mass to speed of brain processing in preterm infants. Pediatr Res 2013:74(5):576-83. [DOI] [PubMed] [Google Scholar]
- 7.Coviello C, Keunen K, Kersbergen KJ, et al. Effects of early nutrition and growth on brain volumes, white matter microstructure and neurodevelopmental outcome in preterm newborns. Pediatr Res 2017. October 25 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Lapillonne A, Griffin IJ. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr 2013;162(3 Suppl 1):S7-16. [DOI] [PubMed] [Google Scholar]
- 9.Stephens BE, Walden RV, Gargus RA, et al. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics 2009;123:1337-43. [DOI] [PubMed] [Google Scholar]
- 10.dit Trolli SE, Kermorvant-Duchemin E, Huon C, Bremond-Gignac D, Lapillone A. Early lipid supply and neurological development at one year in very low birth weight (VLBW) preterm infants. Early Hum Dev 2012;88(Suppl11):S25-9. [DOI] [PubMed] [Google Scholar]
- 11.Gnigler M, Neubauer V, Griesmaier E, Zotter S, Kager K, Kiechl-Kohlendorfer U. Very preterm children are at increased risk of reduced processing speed at 5 years of age, predicted by typical complications of prematurity and prenatal smoking. Acta Paediatr 2015. March;104(3):e124-9. [DOI] [PubMed] [Google Scholar]
- 12.Mulder H, Pitchford NJ, Marlow N. Processing speed mediates executive function difficulties in very preterm children in middle childhood. J Int Neuropsychol Soc 2011. May;17(3)445-54. [DOI] [PubMed] [Google Scholar]
- 13.Mulder H, Pitchford NJ, Marlow N. Processing speed and working memory underlie academic attainment in very preterm children. Arch Dis Child Fetal Neonatal Ed 2010. July;95(4):F267-72. [DOI] [PubMed] [Google Scholar]
- 14.McCullough DL. Visual evoked potentials in infants In: de Haan M, ed. Infant EEG and Event-related Potentials. London, UK: Psychology Press, 2007:41-76. [Google Scholar]
- 15.Parkinson JRC, Hyde MJ, Gale C, Santhakumaran, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics 2013;131:e1240-63. [DOI] [PubMed] [Google Scholar]
- 16.Pfister KM, Ramel SE. Optimizing growth and neurocognitive development while minimizing metabolic risk in preterm infants. Curr Pediatr Rep 2014. December;2(4):269-75. [Google Scholar]
- 17.Belfort MB, Gillman MW, Buka SL, Casey PH, McCormick MC. Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J Pediatr 2013;163(6):1564-9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casey PH, Bradley RH, Whiteside-Mansell L, Barrett K, Gossett JM, Simpson PM. Evolution of obesity in a low birth weight cohort. J Perinatol 2012;32:91–6. [DOI] [PubMed] [Google Scholar]
- 19.Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatrics 2003;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roggero P, Gianni ML, Amato O, et al. Evaluation of air-displacement plethysmography for body composition assessment in preterm infants. Pediatr Res 2012;72:316–20. [DOI] [PubMed] [Google Scholar]
- 21.Foman SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 eyars. Am J Clin Nutr 1982;35(5 Suppl):1169-75. [DOI] [PubMed] [Google Scholar]
- 22.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res 2003;53:486–92. [DOI] [PubMed] [Google Scholar]
- 23.Sainz RD, Urlando A. Evaluation of a new pediatric air-displacement plethysmograph for body-composition assessment by means of chemical analysis of bovine tissue phantoms. Am J Clin Nutr 2003;77:364–70. [DOI] [PubMed] [Google Scholar]
- 24.Yao M, Nommsen-Rivers L, Dewey K, Urlando A. Preliminary evaluation of a new pediatric air displacement plethysmograph for body composition assessment in infants. Acta Diabetol 2003;40 Suppl 1:S55–8. [DOI] [PubMed] [Google Scholar]
- 25.Ma G, Yao M, Liu Y, et al. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr 2004;79:653–60. [DOI] [PubMed] [Google Scholar]
- 26.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr 2001. January;138(1):92-100. [DOI] [PubMed] [Google Scholar]
- 27.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology 2013. March;80(11 Suppl 3):S54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeBoer T, Scott LS, Nelson CA. Event-related potentials in developmental populations In: Handey T, ed. Methodological Handbook for Research Using Event-related Potentials. The MIT Press; 2005:263-297 [Google Scholar]
- 29.American Academy of Pediatrics, Committee on Nutrition Nutritional needs of preterm infants In: Kleinman R, ed. Pediatric Nutrition Handbook, 5th edn. Elk Grove Village, IL: American Academy of Pediatrics, 2004:23–55. [Google Scholar]
- 30.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009. August;124(2):717-28. [DOI] [PubMed] [Google Scholar]
- 31.Skullerud K. Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, Alzheimer changes, and cerebral atherosclerosis. Acta Neurol Scand 1985;102: Suppl c:1–94. [PubMed] [Google Scholar]
- 32.Forbes GB. Relation of lean body mass to height in children and adolescents. Pediatr Res 1972;6:32–7. [DOI] [PubMed] [Google Scholar]
- 33.Fuglestad A, Rao R, Georgieff M. The role of nutrition in cognitive development In: Nelson CA, Luciana L, eds. Handbook of Developmental Cognitive Neuroscience. 2nd edn. Cambridge, MA: MIT Press, 2008:623–42. [Google Scholar]
- 34.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 2000;284(11):1417–24. [DOI] [PubMed] [Google Scholar]
- 35.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 2008;153(2):170–5. [DOI] [PubMed] [Google Scholar]
- 36.Hansen-Pupp I, Hovel H, Lofqvist C, et al. Circulatory insulin like growth factor-I and brain volumes in relation to neurodevelopmental outcomes in very preterm infants. Pediatr Res 2013;74(5):564–9. [DOI] [PubMed] [Google Scholar]
- 37.Lee KH, Calikoglu AS, Ye P, D’Ercole AJ. Insulin-like growth factor-I (IGF-I) ameliorates and IGF binding protein-1 (IGFBP-1) exacerbates the effects of undernutrition on brain growth during early postnatal life: studies in IGF-I and IGFBP-1 transgenic mice. Pediatr Res 1999;45(3):331–6. [DOI] [PubMed] [Google Scholar]
- 38.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 2012;59:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerkhof GF, Breukhoven PE, Leunissen RWJ, Willemsen RH, Hokken-Koelega ACS. Does preterm birth influence cardiovascular risk in early adulthood? J Pediatr 2012;161:390–6. [DOI] [PubMed] [Google Scholar]
- 40.Tinnion R, Gillone J, Cheetham T, Embleton N. Preterm birth and subsequent insulin sensitivity: a systematic review. Arch Dis Child 2014;99:362–8. [DOI] [PubMed] [Google Scholar]
- 41.Scheurer JM, Zhang L, Gray HL, Weir K, Demerath EW, Ramel SE. Body composition trajectories from infancy to preschool in children born preterm versus full-term. J Pediatr Gastroenterol Nutr 2017. June;64(6):e147-53.</References> [DOI] [PubMed] [Google Scholar]