Abstract
Objective:
A respiratory function monitor (RFM) may improve positive pressure ventilation (PPV) technique, but many providers do not use RFM data appropriately during delivery room resuscitation. We sought to use eye tracking technology to identify RFM parameters that neonatal providers view most commonly during simulated PPV.
Design:
Mixed methods study. Neonatal providers performed RFM-guided PPV on a neonatal manikin while wearing eye-tracking glasses to quantify visual attention on displayed RFM parameters (i.e., exhaled tidal volume, flow, leak). Participants subsequently provided qualitative feedback on the eye-tracking glasses.
Setting:
Level 3 academic neonatal intensive care unit
Participants:
Twenty neonatal resuscitation providers
Main Outcome Measures:
Visual attention: overall gaze sample percentage; total gaze duration, visit count and average visit duration for each displayed RFM parameter. Qualitative feedback: willingness to wear eye-tracking glasses during clinical resuscitation.
Results:
Twenty providers participated in this study. The mean gaze sample captured was 93% (standard deviation 4%). Exhaled tidal volume waveform was the RFM parameter with the highest total gaze duration (median 23%, interquartile [IQ] range 13–51%), highest visit count (median 5.17 per 10 seconds, IQ range 2.82–6.16) and longest visit duration (median 0.48 seconds, IQ range 0.38 – 0.81 seconds). All participants were willing to wear the glasses during clinical resuscitation
Conclusion:
Wearable eye tracking technology is feasible to identify gaze fixation on the RFM display and is well accepted by providers. Neonatal providers look at exhaled tidal volume more than any other RFM parameter. Future applications of eye tracking technology include use during clinical resuscitation.
Introduction
Positive pressure ventilation (PPV) is the cornerstone of neonatal resuscitation (1) but is technically challenging in preterm infants after birth. Mask leak and airway obstruction are common during PPV and lead to inadequate (too small) inflations. Conversely, many providers deliver excessive inflations, which contribute to acute lung injury.(2) There are few tools in the delivery room to identify these impediments, and neonatal providers’ subjective assessment of PPV is poor. (2–5)
A respiratory function monitor (RFM) uses an in-line flow sensor between the gas flow and facemask to calculate and display data on delivered inflations during PPV. Typical parameters displayed on an RFM include flow, tidal volumes, and pressure; these are displayed in both numeric and waveform format. Using a RFM improves the quality of PPV performed in a simulated environment.(6)
While the RFM improves PPV technique in simulation, little is known about how providers use the RFM to guide clinical neonatal resuscitation.(6–8) In an audit of clinical resuscitations with a visible RFM, the majority of providers reported that they did not use RFM data to inform their resuscitative efforts.(9) Other investigators found that many providers incorrectly interpret the presented RFM data.(10) One potential reason for this is that the standard RFM display is complex, with up to seven numeric values and four waveforms presented. In addition, it is possible that providers do not use the RFM because of the added cognitive burden during a high stress clinical resuscitation. A key knowledge gap is that we do not know which displayed RFM parameters neonatal providers use most when performing PPV.
Wearable eye tracking glasses provide the ability to ascertain and classify the wearer’s focus of visual attention.(11–13) Eye tracking has been used in other medical fields to assess differences in gaze behavior between trainee and expert surgeons, (13, 14) and to evaluate and quantify expertise in radiologists performing ultrasound-guided regional anesthesia.(15) Eye tracking may also have application in the delivery room setting to identify how providers visually attend to the available monitors during neonatal resuscitation. To date, the only published delivery room eye tracking study was a small pilot (n=6) that did not focus on the RFM.(16)
The objective of this simulation study was to assess the feasibility of wearable eye tracking technology to identify neonatal providers’ visual attention patterns on the RFM display when performing simulated PPV. Our second objective was to determine neonatal providers’ assessment of the acceptability of wearable eye tracking glasses in a clinical setting.
Methods
Study design and population
This was a prospective observational study conducted at the Hospital of Pennsylvania, an academic level 3 neonatal intensive care unit (17) between April 1, 2016 and December 31, 2016. Twenty neonatal providers who were trained in the Neonatal Resuscitation Program and routinely perform PPV for preterm infants during delivery room resuscitation volunteered to participate. We excluded providers who require corrective glasses and do not wear contact lenses or are unable see within a two feet visible range without glasses. At the time of this study, the RFM was not routinely used during clinical resuscitation in our hospital. The University of Pennsylvania Institutional Review Board approved this study. The study team provided study information packets and obtained verbal consent from all participants.
Equipment
Manikin:
A Laerdal Resusci baby manikin (Laerdal, Stavanger, Norway) was modified by removing the lung and stomach bags and positioning a test lung within the chest cavity. The test lung was connected to the manikin’s mouth with an endotracheal tube, and the system was confirmed to be leak-free using the RFM.
RFM and respiratory equipment:
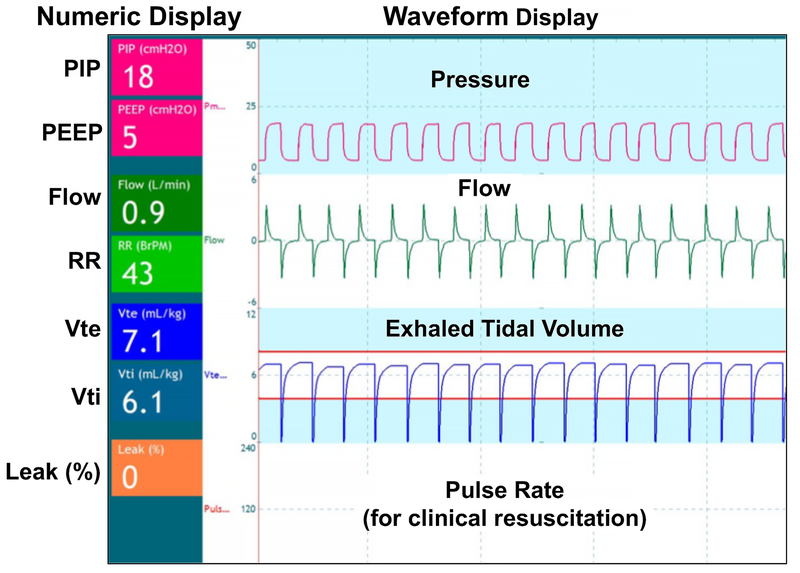
RFM measurements were made with the New Life Box Respiratory Function Monitor (Advanced Life Diagnostics, Weener, Germany). The New Life Box uses an in-line sensor (Avea VarFlex™ Flow Transducer: CareFusion, Yorba Linda, CA), which placed in line between the T-piece infant resuscitator (Neopuff: Fisher & Paykel Healthcare, Auckland, New Zealand) and facemask (Vital Signs Size 2 infant face mask, CareFusion, Yorba Linda, CA). The sensor is a pneumotachometer that detects pressure before and after the orifice in order to measure gas flow to and away from the manikin. The RFM integrates these signals to display the following parameters: flow, peak inspiratory pressure and positive end-expiratory pressure, inspiratory and expiratory tidal volume, mask leak, and respiratory rate. These are displayed using both waveform and numeric format (Figure 1). The initial Neopuff settings were peak inspiratory pressure of 20 cm H2O, positive end expiratory pressure of 5 cm H2O, using a flow rate of 8–10 L/min.
Figure 1:
NewLife Box Respiratory Function Monitor Display (reproduced by permission, NewLifeBox® Neo-RSD).
Numeric data displayed on the left side of the screen include: PIP (peak inspiratory pressure), PEEP (positive end expiratory pressure), flow, RR (respiratory rate), Vte (exhaled tidal volume in mL/kg), Vti (inspiratory tidal volume in mL/kg), and mask leak (%). The numerical data are constantly refreshed and represent real-time values of each respiratory parameter at a specific point in time.
Waveform data displayed include pressure, flow, exhaled tidal volume, and pulse rate (using streaming pulse oximeter data during clinical resuscitation). The 2 horizontal orange lines in the Vte waveform region indicate 4mL/kg and 8mL/kg.
Eye tracking glasses:
We used the Tobii Pro Glasses 2 (Tobii Technology, Stockholm, Sweden, www.tobii.com), a light (45 grams) and non-obstructive headset (Supplemental Figure 1). An outward-facing scene camera captures the provider’s visual field and a microphone records audio. Near infrared lights illuminate the pupils, and two cameras aimed at each eye capture the pupil diameter and movements at 50 Hz. The eye tracker measures characteristics of the user’s eye and integrates them with an internal physiological 3D eye model to calculate the gaze data. Before each simulation, we ensured the participant’s eyes were centered in each frame of the glasses and followed the manufacturer’s calibration procedure. This process typically takes less than 30 seconds.
Procedure
The manikin was placed on a flat surface in front of the participants. The RFM was located directly behind the manikin in the providers’ line of sight (Supplemental Figure 2). A study member read a standard script orienting the subjects to the RFM and provided visual aids with fixed screen shots of RFM displays of typical scenarios (ie: mask leak, airway obstruction). Participants had unlimited time to ask questions about the RFM display and interpretation but did not practice PPV using the RFM prior to starting the recording the session. Participants then performed PPV for two minutes using the RFM to target exhaled tidal volume between 4–8mL/kg, ventilation rate of 40–60/min, and mask leak <30%. During PPV, the participants could adjust their mask position and direct a study member to alter the pressure settings on the Neopuff based on their interpretation of the displayed RFM data.
Immediately after the simulation, the participants reviewed their recording with the study team in a semi-structured think aloud interview. The goals of the think aloud interview were to confirm the eye tracking recording accurately reflected the participant’s focus of visual attention, to determine the providers’ thought process during the simulation, and to assess the participants’ ability to correctly interpret and use the data presented on the RFM. In addition, we requested feedback on the eye tracking glasses and asked providers if they would be willing to wear the eye tracking glasses during clinical resuscitation. Verbal comments made during the simulation and interview were transcribed and coded for reference during data processing.
Data processing
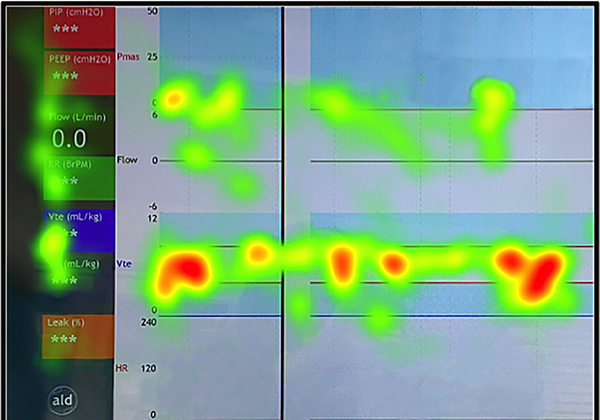
We imported the eye tracking recordings into Tobii Analyzer Pro and Tobii Pro Lab software (Tobii Technology, Stockholm, Sweden, www.tobii.com). The gaze data were automatically mapped onto an uploaded image of the RFM screen through the Tobii Real World Mapping process.(18) A member of the study team reviewed the accuracy of the locations of the gaze points placed by the automated Real World Mapping. Heat maps, graphical representation of the gaze data, (18) were generated for each participant to qualitatively demonstrate primary areas of visual attention (Figure 2).
Figure 2:
Example heat map. Heat maps are a graphical representation of the mapped gaze data, where colors indicate the density of gaze fixations (density increases from green-yellow-orange-red). Here the heat map demonstrates the summary of all gaze points on the respiratory function monitor (RFM) recorded during the simulation. These are mapped onto an exported image of the RFM screen. The highest density of gaze fixations, indicated by the red color of the gaze point clusters, are seen overlying the exhaled tidal volume waveform region.
An area of interest (AOI) is a sub-region of an image that is defined in the Tobii software. Gaze metrics are then calculated for each specified AOI. The RFM screen displays seven numeric values on the left and four central waveforms. We created 11 areas of interest to represent these regions on an image of the RFM display. We also defined AOIs to represent the manikin and Neopuff.
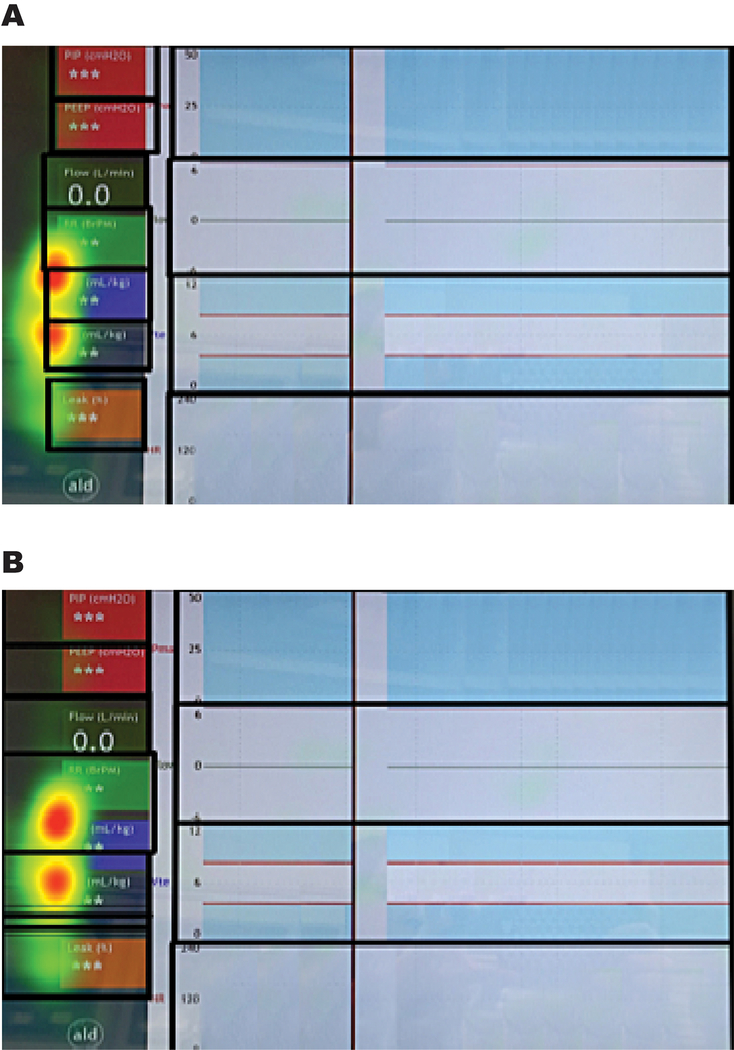
Even following successful calibration of the eye tracking glasses, it is possible to have a small but systematic offset between the actual and measured gaze location.(19). To account for individual offsets in eye tracking recordings, (19) we reviewed the videos, the verbalizations from the think aloud interviews, and the heat maps to customize the AOI borders for each participant (Figure 3). The final processed data was exported using the raw gaze filter.(20)
Figure 3.
Systematic gaze offset. This heat map from a study participant demonstrates a systematic gaze offset. Areas of high gaze density are mapped to the left and downwards of the numeric respiratory parameters located on the left side of the respiratory function monitor (RFM) display.
In figure 3A the original borders of the areas of interest (thick black borders) follow the natural rectangular boundaries of the displayed regions on the RFM screen, but these borders intersect the high gaze density regions displayed in the heat maps. In figure 3B the borders of the areas of interest are adjusted to accommodate the offset seen in the heat map.
Visual parameter outcomes
We assessed the gaze sample percentage across the entire recording. We measured the following visual attention parameters: total gaze duration, visit count, and average visit duration (Table 1). Not all recordings were exactly the same duration; therefore, absolute visit count was transformed into a rate per ten seconds.
Table 1:
Visual attention parameters
| Parameter | Description | Interpretation | Unit |
|---|---|---|---|
| Total Gaze Duration | Cumulative time each participant gazed on an area of interest, divided by the cumulative duration of gaze samples * | Where am I looking the most? | Percentage |
| Visit Count | Number of visits within an area of interest per 10 seconds | How often am I looking at an area? | Rate |
| Average Visit Duration | Average time spent in an area of interest on a single visit | When I do look at an area, how much time am I spending in that area? | Seconds |
| Gaze sample percentage | Number of eye tracking gaze points identified, divided by the theoretical maximum** | How much of my gaze data is truly captured? | Percentage |
The cumulative duration of gaze samples accounts for gaze sample percentage. i.e. if the entire recording lasted 100 seconds, but the gaze sample percentage was only 90%, the cumulative duration of gaze samples was 90 seconds
The sampling frequency is 50Hz, so the theoretical maximum is 50 samples per second. If the software adequately identifies all 50-gaze samples within each minute, the value of gaze sample percentage would be 100%. Blinking usually causes 5–10% data loss.
Data analysis
Using Stata version 14.0 (Statacorp, College Station, TX), we generated summary statistics for provider demographics and the visual attention parameters (total gaze duration, average visit duration, and visit count).
Eye tracking glasses cannot pick up gaze points in the periphery and therefore differential use of peripheral vision (based on height) could impact the gaze sample percentage. (21) Similarly, contact lenses could potentially affect the quality of data acquisition. We assessed the association between height and gaze sample percentage using Spearman’s correlation and the association between contact lenses and gaze sample percentage using Wilcoxon rank sum test. A p-value <0.05 was considered statistically significant.
Results
Study participants had a mean age of 33 years; most (65%) were neonatal fellows (Table 2). No providers had previous experience using a RFM to guide clinical resuscitation prior to participating in the study. Eye tracking glasses captured a mean of 93% (standard deviation 4%) of gaze samples. Gaze sample percentage was not significantly correlated with participant height (p=0.25), and there was no significant association between contact lens use and gaze sample percentage (p=0.57)
Table 2:
Demographic characteristics of participants
| Characteristic | Participants (n=20) |
|---|---|
| Age (years), mean (SD) [range] | 33.1 (2.23) |
| Female | 13 (65%) |
| Height (cm), mean (SD) | 170 (8.55) |
| Role | |
| Attending neonatologist | 5 |
| Neonatal fellow | 13 |
| Neonatal nurse practitioner | 2 |
| Clinical Experience (years), mean (SD) | 6 (2.09) |
| Visual aids | |
| Yes, contact lenses | 9 |
| Yes, glasses removed | 2 |
| No need for visual aids | 9 |
Abbreviations: cm= centimeters, SD= standard deviation
The exhaled tidal volume waveform AOI had the highest gaze duration, visit count, and average visit duration. The flow waveform, numeric respiratory rate, and numeric leak AOIs were also commonly viewed (Table 3). Cumulatively, providers looked at waveform parameters for a median of 33% of the simulation and numeric values for median of 20% of the simulation. The median total gaze duration was 6% (intraquartile range [IQR] 1–15%) on the manikin and 0% (IQR 0–1%) on the Neopuff.
Table 3:
Visual attention measurements for displayed parameters on the respiratory function monitor
| Area of interest | Total Gaze Duration, median (IQR) | Visit Count (rate per 10 s), median (IQR) | Average Visit Duration (s), median (IQR) |
|---|---|---|---|
| Exhaled tidal volume (waveform) | 23% (13% – 51%) | 5.17 (2.82 – 6.16) | 0.48 (0.38 – 0.81) |
| Flow (waveform) | 9% (5% – 22%) | 3.22 (2.11 – 5.05) | 0.29 (0.18 – 0.53) |
| Respiratory rate (number) | 5% (2% – 7%) | 2.52 (1.34 – 3.85) | 0.18 (0.12 – 0.28) |
| Leak (number) | 5% (1% – 11%) | 1.92 (0.46 – 3.82) | 0.27 (0.21 – 0.46) |
| Exhaled tidal volume (number) | 3% (1% − 8%) | 2.02 (1.01– 4.63) | 0.17 (0.09 – 0.25) |
| Inspiratory tidal volume (number) | 3% (1% – 6%) | 2.48 (0.59 – 4.50) | 0.12 (0.07 – 0.23) |
| Positive end expiratory pressure (number) | 2% (1% – 4%) | 1.13 (0.39 – 2.32) | 0.15 (0.12 – 0.21) |
| Flow (number) | 2% (1% – 4%) | 1.48 (1.11 – 3.38) | 0.14 (0.08 – 0.18) |
| Pulse rate (waveform) | 1% (0% – 1%) | 1.61 (0.62 – 2.61) | 0.05 (0.03 – 0.11) |
| Pressure (waveform) | 0% (0% – 3%) | 0.70 (0.40 – 1.50) | 0.07 (0.04 – 1.16) |
| Peak inspiratory pressure (number) | 0% (0% – 2%) | 0.28 (0.14 – 1.45) | 0.11 (0.05 – 0.23) |
Abbreviations: IQR= interquartile range; s= seconds
In the think aloud interview, participants qualitatively confirmed that the visual representation captured by the eye tracking glasses corresponded with their focus of cognitive attention during the simulation. Four subjects (20%) mentioned negative comments about the eye tracking glasses, such as discomfort wearing the glasses or concern with dropping the eye tracking equipment. Despite these reactions, all four of these participants were still willing to wear the glasses, and all 20 participants (100%) agreed they would be willing to wear eye-tracking equipment during clinical resuscitation in a code leader role.
Discussion
We used a novel application of wearable eye tracking technology to identify where neonatal providers focused their visual attention on a RFM display during simulated PPV. We found that resuscitators gazed for the longest duration and frequency upon displayed waveforms, particularly exhaled tidal volume waveform. Participants evaluated the eye tracking glasses positively and reported their willingness to wear them in a clinical setting.
Although respiratory function monitoring is an active line of investigation in delivery room research,(6–10) little is known about how providers use the RFM clinically. In audit of preterm resuscitation, Schilleman et al. found that few providers used the RFM data to guide PPV. (9) Conversely, it is possible that the RFM might distract providers from noticing and attending to other essential audiovisual cues.(22) Improving the usability of RFM displays could address both of these potential barriers. In this simulation study, we identified four respiratory parameters that were most commonly used by neonatal providers during PPV. These results could be applied to refining future design of RFMs to better display essential data. In addition, these findings could also inform training programs to help providers use and interpret RFM data during resuscitation.
While this study provides preliminary findings regarding the salient regions of a RFM display, visual attention patterns likely differ during clinical resuscitation.(23) We acknowledge that additional areas of interest, such as the infant and other providers, may compete with the RFM and other physiological monitors during clinical resuscitation. The configuration of equipment and personnel in the delivery room could also impact the usability of the RFM clinically. In addition, added stress and cognitive burden during delivery room resuscitation may alter providers’ visual attention patterns.(24) Law et al. reported that visual attention on the infant differed between resuscitations based on the level of resuscitation interventions required.(16) As that study only reported on 5 subjects, it is unknown if these differences result from the increased cognitive burden of advanced resuscitation or simply represent inter-provider variation. Ultimately, clinical studies are needed to assess whether and how providers use the RFM during delivery room resuscitation.
One limitation of eye tracking technology is that visual attention does not necessarily indicate cognitive focus. Providers’ eyes may have been fixated on a particular part of the RFM display while they were mentally processing separate information from other audio-visual cues. We addressed this by inviting participants to review their eye-tracking recording during the think-aloud interview; participants confirmed that their focus of cognitive attention corresponded with the recorded eye tracking gaze points. Another limitation is that automated gaze mapping with the Tobii software may have resulted in some lost mapped gaze points. Finally, we acknowledge that providers’ behavior and visual attention patterns may have been influenced by the targeted coaching they received during their orientation to the RFM.
Study strengths include creation of individual AOIs instead of standard AOIs for all participants to address systematic offsets in gaze data, consistent with other eye tracking studies. (19) In addition, we conducted think aloud interviews to confirm the accuracy of eye tracking data and assess whether the visual attention represented their focus of cognitive attention.
Conclusions:
Wearable eye tracking technology is a feasible method to identify regional gaze fixation on the RFM display and is acceptable to neonatal resuscitation providers. Resuscitators gazed at the waveform data, particularly that of exhaled tidal volume, longer and more frequently than the numeric values on the screen. These findings may hold relevance for designing RFM displays and training providers to use the RFM. Future applications of eye tracking technology include defining visual attention patterns on the RFM during clinical resuscitation.
Supplementary Material
What is already known on this topic:
A respiratory function monitor (RFM) may improve the quality of positive pressure ventilation (PPV) performed by neonatal resuscitation providers.
Many providers do not use the displayed RFM data appropriately to guide PPV during clinical delivery room resuscitation.
The RFM parameters that are most useful to neonatal providers have not been defined.
What this study adds:
Wearable eye tracking technology provides a feasible method to identify regional gaze fixation on the RFM display and is well accepted by neonatal providers.
Providers spend more time looking at exhaled tidal volume waveform data than any other RFM parameter to guide PPV.
Acknowledgements
We would like to thank our colleagues who volunteered to participate in this study. Dr. Foglia is supported by a NICHD Career Development Award, K23HD084727.
List of abbreviations
- AOI
Area of interest
- IQR
Interquartile range
- PPV
Positive pressure ventilation
- RFM
Respiratory function monitor
Footnotes
Conflicts of Interest
The authors disclose no conflicts of interest. Dr. te Pas helped to design the NewLife Box monitor; he does not have any financial stake in the company that manufactures this monitor.
References
- 1.te Pas AB, Davis PG, Hooper SB, Morley CJ. From liquid to air: breathing after birth. J Pediatr. 2008;152(5):607–11. [DOI] [PubMed] [Google Scholar]
- 2.Schmolzer GM, Kamlin OC, O’Donnell CP, Dawson JA, Morley CJ, Davis PG. Assessment of tidal volume and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F393–7. [DOI] [PubMed] [Google Scholar]
- 3.Schmolzer GM, Dawson JA, Kamlin CO, O’Donnell CP, Morley CJ, Davis PG. Airway obstruction and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed. 2011;96(4):F254–7. [DOI] [PubMed] [Google Scholar]
- 4.Poulton DA, Schmolzer GM, Morley CJ, Davis PG. Assessment of chest rise during mask ventilation of preterm infants in the delivery room. Resuscitation. 2011;82(2):175–9. [DOI] [PubMed] [Google Scholar]
- 5.van Vonderen JJ, van Zanten HA, Schilleman K, Hooper SB, Kitchen MJ, Witlox RS, et al. Cardiorespiratory Monitoring during Neonatal Resuscitation for Direct Feedback and Audit. Front Pediatr. 2016;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood FE, Morley CJ, Dawson JA, Davis PG. A respiratory function monitor improves mask ventilation. Arch Dis Child Fetal Neonatal Ed. 2008;93(5):F380–1. [DOI] [PubMed] [Google Scholar]
- 7.Schmolzer GM, Morley CJ, Wong C, Dawson JA, Kamlin CO, Donath SM, et al. Respiratory function monitor guidance of mask ventilation in the delivery room: a feasibility study. J Pediatr. 2012;160(3):377–81.e2. [DOI] [PubMed] [Google Scholar]
- 8.Schmolzer GM, Kamlin OC, Dawson JA, te Pas AB, Morley CJ, Davis PG. Respiratory monitoring of neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed. 2010;95(4):F295–303. [DOI] [PubMed] [Google Scholar]
- 9.Schilleman K, Siew ML, Lopriore E, Morley CJ, Walther FJ, Te Pas AB. Auditing resuscitation of preterm infants at birth by recording video and physiological parameters. Resuscitation. 2012;83(9):1135–9. [DOI] [PubMed] [Google Scholar]
- 10.Milner A, Murthy V, Bhat P, Fox G, Campbell ME, Milner AD, et al. Evaluation of respiratory function monitoring at the resuscitation of prematurely born infants. Eur J Pediatr. 2015;174(2):205–8. [DOI] [PubMed] [Google Scholar]
- 11.Duchowski AT. A breadth-first survey of eye-tracking applications. Behav Res Methods Instrum Comput. 2002;34(4):455–70. [DOI] [PubMed] [Google Scholar]
- 12.Hermens F, Flin R, Ahmed I. Eye movements in surgery: A literature review. Journal of Eye Movement Research. 2013;6(4). [Google Scholar]
- 13.Tien T, Pucher PH, Sodergren MH, Sriskandarajah K, Yang GZ, Darzi A. Differences in gaze behaviour of expert and junior surgeons performing open inguinal hernia repair. Surg Endosc. 2015;29(2):405–13. [DOI] [PubMed] [Google Scholar]
- 14.Tien T, Pucher PH, Sodergren MH, Sriskandarajah K, Yang GZ, Darzi A. Eye tracking for skills assessment and training: a systematic review. J Surg Res. 2014;191(1):169–78. [DOI] [PubMed] [Google Scholar]
- 15.Harrison TK, Kim TE, Kou A, Shum C, Mariano ER, Howard SK. Feasibility of eye-tracking technology to quantify expertise in ultrasound-guided regional anesthesia. J Anesth. 2016;30(3):530–3. [DOI] [PubMed] [Google Scholar]
- 16.Law BHY, Cheung PY, Wagner M, van Os S, Zheng B, Schmolzer G. Analysis of neonatal resuscitation using eye tracking: a pilot study. Arch Dis Child Fetal Neonatal Ed. 2017. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics. 2012;130(3):587–97. [DOI] [PubMed] [Google Scholar]
- 18.Fong A, Hoffman D, Ratwani RM. Making Sense of Mobile Eye-Tracking Data in the Real-World. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. 2016;60(1):1569–73. [Google Scholar]
- 19.Weibel N, Fouse A, Emmenegger C, Kimmich S, Hutchins E. Let’s look at the cockpit: exploring mobile eye-tracking for observational research on the flight deck Proceedings of the Symposium on Eye Tracking Research and Applications; Santa Barbara, California: 2168573: ACM; 2012. p. 107–14. [Google Scholar]
- 20.Tobii Pro Lab User Manual 2017. [Available from: https://www.tobiipro.com/siteassets/tobii-pro/user-manuals/tobii-pro-lab-user-manual.pdf/?v=1.58.]
- 21.Andersen NE, Dahmani L, Konishi K, Bohbot VD. Eye tracking, strategies, and sex differences in virtual navigation. Neurobiol Learn Mem. 2012;97(1):81–9. [DOI] [PubMed] [Google Scholar]
- 22.Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2015;132(16 Suppl 1):S204–41. [DOI] [PubMed] [Google Scholar]
- 23.Ericsson KA. An expert-performance perspective of research on medical expertise: the study of clinical performance. Med Educ. 2007;41(12):1124–30. [DOI] [PubMed] [Google Scholar]
- 24.Tomizawa Y, Aoki H, Suzuki S, Matayoshi T, Yozu R. Eye-tracking analysis of skilled performance in clinical extracorporeal circulation. J Artif Organs. 2012;15(2):146–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.