Abstract
Background:
In clinical practice, calcifications seen on CT studies within the large brain arteries are often referred as a surrogate marker for cholesterol-mediated atherosclerosis. However, limited data exists to support the association between calcification and atherosclerosis. In this study, we examined if intracranial arterial calcifications were associated with cholesterol-mediated intracranial large artery atherosclerosis (ILAA) within the arteries of the Circle of Willis in an autopsy based sample.
Methods:
We carried out a cross-sectional analysis of histopathological characteristics of brain large arteries obtained from autopsy cases. Brain large arteries were examined for evidences of calcifications, which were rated as macroscopic (coalescent) and microscopic (scattered). In addition to calcification, we also obtained measurement of the arterial wall and the presence of ILAA and non-atherosclerotic arterial fibrosis. We built hierarchical models adjusted for demographic and vascular risk factors to assess the relationship between calcification and ILAA.
Results:
In univariate analysis, the presence of any arterial calcifications was associated with cerebral infarcts (29% v. 14%, p<0.01). Multi-variate analysis revealed that among all calcifications, coalescent calcifications were not associated with ILAA. In contrast, scattered calcifications were associated with ILAA (p<0.001), decreased lumen diameter (−1.87 +/− 0.41 mm p≤0.001) and increased luminal stenosis (0.03 +/− 0.01% p≤0.006). These findings were independent of age, sex or other vascular risk factors.
Conclusions:
This study demonstrates that coalescent calcifications in brain large arteries, although associated with morbidity, are not synonymous with cholesterol driven ILAA. Understanding the precise pathological components of cerebrovascular disease, including non-atherosclerotic arterial calcifications, will help develop individualized therapies beyond amelioration of traditional risk factors such as hyperlipidemia.
INTRODUCTION
Strokes are a major burden of disease worldwide. In the United States, strokes are the 5th leading cause of death, with approximately 800,000 cases each year, of which a majority are ischemic1. Intracranial large artery atherosclerosis (ILAA) is a major contributor to ischemic stroke, with increased risk among Black and Hispanic populations2. The mainstay of therapy is identification of ILAA using non-invasive neuroimaging, and mediation of various risk factors such as hyperlipidemia and diabetes. Currently, the presence of ILAA is presumed by the presence of focal luminal narrowing on lumen-based imaging such as brain CTA or MRA or by identifying the presence of arterial calcifications on brain CT.3 However, not all brain arterial stenosis are atherosclerotic, as in cases of fibromuscular dysplasia or large artery vasculitis,4, 5. Further, many atherosclerotic plaques have only modest degrees of luminal stenosis.6 There is also non-atherosclerotic effects from aging, including degenerative changes consisting of elastin loss, concentric intima thickening with relative outward arterial remodeling.7
A significant focus has been placed on ILAA plaques and their vulnerability, which is influenced by large lipid-rich necrotic cores and thin fibrous caps inferred to make these plaques more prone to rupture and to produce distal artery-to-artery emboli. 8, 9. However, less is known about the influence of arterial calcifications on cerebrovascular pathophysiology. Currently, calcifications seen on CT, are commonly taken to be a surrogate marker for ILAA and are presumed to be atherosclerotic. However, arterial calcifications are not always associated with cholesterol deposition or with atheromas,10, 11 -- both core aspects of atherosclerosis defined pathologically.12 It remains uncertain if the presence of calcifications in the brain large arteries maybe used as proof of ILAA and thus be managed similarly to cholesterol-mediated atherosclerosis.
In this study, we investigated the histopathological relationship between brain large artery calcifications and the corresponding vascular pathology. In particular, we examined for pathological differences between large calcifications, which are more likely to be detected radiographically (“coalescent”), and small, microscopic, calcifications, which are likely below the limits of radiographic resolution (“scattered”) and thus likely to be missed on image-based studies. Our aim was to test the hypothesis that large calcifications in the brain large arteries are not reliably associated with ILAA, defined here as a cholesterol-mediated process as evidenced by lipid deposition in the arterial wall.
METHOD:
Data and cases for this study were obtained from the Brain Arterial Remodeling Study (BARS). The origin of the autopsy cases and the methods used at each of the donor brain banks/tissue collections have been previously described 6, 13, 14. Briefly, autopsy cases were examined to remove the Circle of Willis with all available brain large arteries. As part of the routine neuropathological assessment, each brain was examined for areas of ischemic infarction. Individuals with history of atrial fibrillation, endocarditis at death, mechanical valves, cardiac clots or large cortical infarcts in multiple arterial territories were considered to have cardio-embolic infarcts. Infarcts in subcortical locations or single arterial territories without cardioembolic sources were considered to have non-cardioembolic infarcts. Demographic information and vascular risk factors (ie, hypertension, diabetes) were collected from chart review, self-reported by prospective donors while alive or by post-mortem family member(s) interview, or inferred based on medication list. For the purposes of this study, we excluded cases with known HIV or AIDS. The data that support the findings of this study are available from the corresponding author upon reasonable request. Each brain collection was approved by the respective institutional review board and consents were obtained accordingly.
Brain large arteries from the Circle of Willis were systematically extracted and analyzed as previously described.13, 15 Briefly, 6-micron thick cross-sections were obtained from the proximal and distal segments of each paraffin-embedded artery, stained with H&E, elastic van Gieson (for elastin), Masson trichrome (for collagen), and Congo red (for amyloid. Each stained slide was digitized using Olympus Soft Imaging Solutions software running a high-speed, high resolution Olympus VS110 virtual slide scanning system (Olympus America Inc, Center Valley, PA) with 10x magnification and scale =0.643 um/pixel. The morphometric features of each artery were measured in the digital images with previously described methods 15. First, the arterial layers were segmented by pre-defined color intensity-based thresholds and adjusted for deformations that typically occur during the fixation and preparation processes. Each arterial segment was examined for luminal stenosis, defined as the actual lumen area divided by the area encircled by the internal elastic lamina 16 . The degree of elastin, collagen and amyloid content of each artery was quantified by average pixel staining intensity via the Visiopharm Integrator System (Hoersholm, Denmark). Because the overall intensity varied slightly by batches of staining, we controlled for the overall background intensity. Arteries were considered to have high staining intensity for their respective markers if the background-adjusted mean average intensity was in the upper quartile of the respective distribution.
Procedures for brain arterial categorical phenotype assessments: Histological examination was performed for evidence of ILAA (defined strictly by the presence of an atheroma), arterial fibrosis (i.e. non-atherosclerotic fibrosis, defined as arterial wall degeneration with no evidence atheroma) and presence of any calcification. Non-atherosclerotic fibrosis represents a similar phenotype as the fibrocalcific plaque used by the revised AHA classification of arterial pathology, 17 but in this case because calcification was the outcome of interest, we rated it independently of whether calcification was present or not. Arteries with any calcification were subsequently rated as coalescent (or macrocalcification) or scattered (or microcalcification) by visual assessments (Figure 1). The idea being this subdivision is that there exist precedence that calcification may differ in their role of plaque vulnerability,18 and creating a binary variable based on macroscopic appearance would make this classification simple and reliable. In general, large, macroscopic calcifications were considered coalescent while small, microscopic calcifications were considered scattered. To determine inter-rater reliability, 100 arteries were separately by a senior neuropathologist (JamG) and a Vascular Neurologist (JosG). The inter-reader reliability for any calcifications was κ=0.79, scattered calcification κ=0.73, and coalescent calcification κ=0.95.
Figure 1: Examples of brain arterial calcifications.
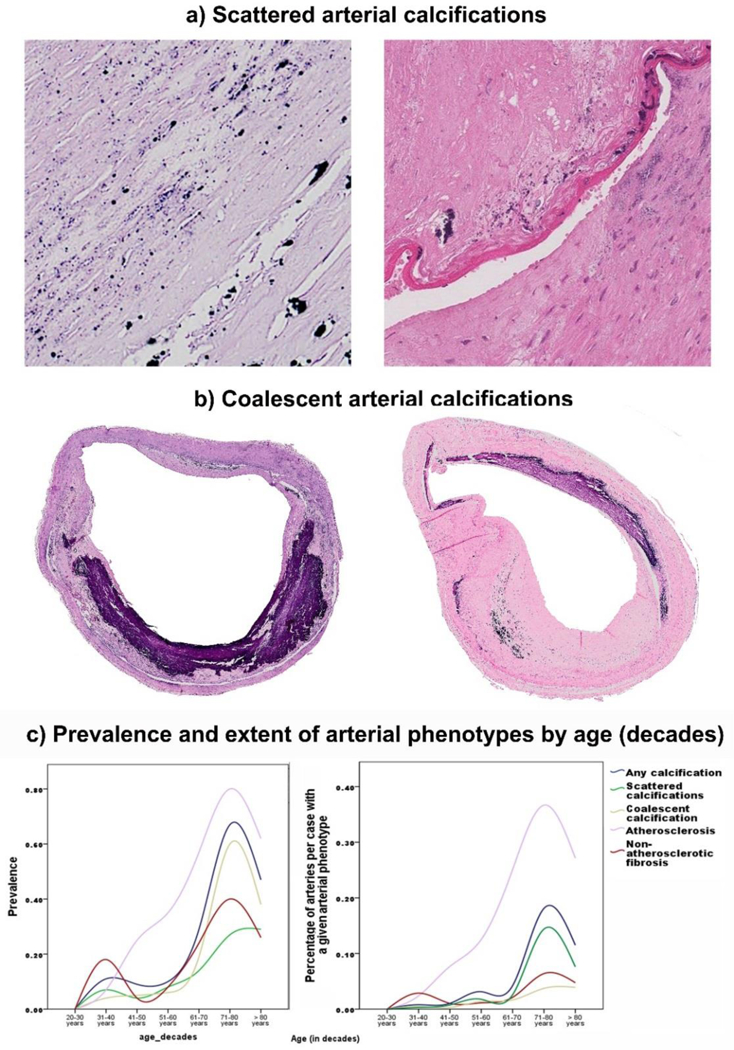
Cross sectional examples of Scattered (a) and Coalescent (b) arterial calcifications stained with H&E. By visual assessment, small, microscopic calcifications were considered scattered while large, macroscopic calcifications were considered coalescent. Prevalence and distribution of atherosclerosis, non-atherosclerotic fibrosis, and calcifications subtype by age of study sample (panel c).
Statistical analysis:
Descriptive statistics were reported with percentage or mean ± standard deviations in the entire sample and stratified by calcification status. We used chi-squared or student’s T-test to assess for univariate statistical differences by calcification status. We then built multi-adjusted, multilevel generalized linear models with a binomial link, progressively adjusting for demographics, vascular risk factors and arterial phenotype and quantitative measures. The three dependent variables of interest were any calcification, and then separated as coalescent versus scattered. If a given artery had both phenotypes, we included that artery in each model. The P value was adjusted by the number of multiple comparisons to define statistical significance (P=0.05/number of comparisons by outcome). The analysis was carried out with SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS:
Demographic Characteristics
The sample included 211 autopsy cases with a mean age of 57 ± 18 years. The sample had a higher percentage of men (60%) and the majority were non-Hispanic whites (73%). In total, 45 subjects (21%) were identified with having any type of calcifications in their brain large arteries. These individuals tended to be older, have higher rates of hypertension, dyslipidemia, and coronary artery disease (CAD). The vascular risk factors of both groups are shown in Table 1.
Table 1:
Demographic characteristics of the sample used in this study
| Entire sample (N=211) |
Stratified by calcification status | |||
|---|---|---|---|---|
| No calcification (N=166) |
Calcification present (N= 45) |
P value | ||
|
Demographic Information | ||||
| Age (in years, mean ± SD, median, IQR) |
57 ± 18, 53, 44–69 |
53 ± 16, 50, 43- 59 |
72 ± 18, 73, 56–87 |
≤ 0.001 |
| Sex M:F (Male sex %) | 127:84 (60) | 103:63 (62) | 24:21 (53) | 0.28 |
| Non-Hispanic white (N,%) | 155:56 (73) | 123:43 (74) | 32:13 (71) | 0.68 |
|
Vascular Risk Factors | ||||
| HTN (N,%) | 86 (41) | 58 (35) | 28 (62) | ≤ 0.001 |
| Diabetes (N,%) | 37 (18) | 28 (17) | 9 (20) | 0.62 |
| Dyslipidemia (N,%) | 47 (22) | 26 (16) | 21 (47) | ≤ 0.001 |
| Statins use N(%)* | 15 (16) | 7(10) | 8(35) | 0.005 |
| Smoking (N,%) | 106 (50) | 87 (52) | 19 (42) | 0.22 |
| CAD (N,%) | 71 (34) | 50 (30) | 21 (47) | 0.04 |
Abbreviations: IQR, interquartile range; N, number; HTN, Hypertension; CAD, Coronary Artery Disease
Data available only in 93 (44%) of the cases
Pathological Examination
On neuropathological assessment, 36 subjects (17%) had evidence of cerebral infarcts of which 16 (8%) were believed to be non- cardioembolic. Of note, presence of any type of calcifications were associated with ischemic infarcts (Table 2). Histological examination of these brain large arteries revealed 78 subjects (37%) with evidence of cholesterol-driven atherosclerosis and 31 subjects (15%) with non-atherosclerotic fibrosis. Only 10% of cases had evidence of both ILAA and fibrosis. Both ILAA and fibrosis were associated with presence of calcifications.
Table 2:
Pathological Findings in this study
| Entire sample (N=211) |
Stratified by calcification status | |||||
|---|---|---|---|---|---|---|
| No calcification (N=166) |
Any calcification (N=45) | P value |
||||
| Scattered (N= 12) |
Coalescent (N= 20) |
Mixed (N= 13) |
||||
|
Gross Pathological Findings | ||||||
| Any infarct (N,%) |
36 (17) | 23 (64) | 2 (6) | 6 (16) | 5 (14) | 0.05 |
| Non-embolic infarcts (N,%) |
16 (8) | 8 (50) | 1 (6) | 4 (25) | 3 (19) | 0.01 |
| ILAA-related infarct (N,%) |
4 (2) | 2 (50) | 0 | 2 (50) | 0 | 0.05 |
| Lacunar infarct (N,%) |
12 (6) | 6 (50) | 1 (8) | 2 (17) | 3 (25) | 0.02 |
|
Histological Findings | ||||||
| Atherosclerosis (N,%) |
78 (37) | 43 (55) | 6 (8) | 17 (21) | 12 (15) | 0.001 |
| Non- Atherosclerotic fibrosis |
31(15) | 9 (29) | 7 (23) | 7 (23) | 8 (25) | 0.001 |
Abbreviations: ILAA, intracranial large artery atherosclerosis.
We evaluated 1699 brain arterial segments (on average 8–9 arteries per subject), out of which 91 had any calcification (5.4%). Some subjects may have had one or more arteries with calcifications (Examples shown in the supplemental data). Among arteries with calcifications, 94.5 % were localized in the intima (54% through the intima and 40% exclusively or largely localized in the IEL with or without minor spilling into the media), 2.2% in the media, 2.2% in the adventitia and 1.1% had a relative equal distribution of calcifications in intima and adventitia. Calcifications near or in the IEL had often a nodular appearance and commonly involved exclusively the IEL (as shown in various examples found in the supplemental data). Internal elastic lamina calcifications were more likely to have a coalescent appearance compared with calcifications in the luminal aspect of the intima (43% vs. 24%, P=0.03) and occasionally seem to arise from aan artery with no or minimal intimal thickening.
Association between arterial calcifications and study variables
In multivariate analysis adjusting for demographic and vascular risk factors, all arterial calcifications were more common with older age (Figure 1) and in larger brain arteries (Table 3). Other associations varied by calcification phenotype. Coalescent calcifications were associated with a greater change in inter-adventitial diameter. However, these large calcifications were not associated with cholesterol-driven ILAA. Conversely, scattered arterial calcifications were associated with both ILAA and non-atherosclerotic fibrosis. Further adjustment for statins use did not change these associations.
Table 3:
Association between calcification subtype and study variables.
| All Calcifications | Coalescent Calcifications |
Scattered Calcifications |
|
|---|---|---|---|
| Beta coefficient ± SE, P value | |||
| Age (in years) | 0.03 ± 0.01, 0.009** |
0.02 ± 0.01, 0.16 |
0.03 ± 0.01, 0.003** |
| Male sex | −0.02 ± 0.35, 0.96 |
0.10 ± 0.35, 0.76 |
0.18 ± 0.39, 0.65 |
| Nonwhite race | 0.53 ± 0.40, 0.19 |
0.38 ± 0.45, 0.40 |
0.15 ± 0.43, 0.73 |
| Interadventitial diameter (per mm) |
0.90 ± 0.18, ≤0.001** |
1.17 ± 0.32, ≤0.001** |
0.45 ± 0.11, ≤0.001** |
| Hypertension | 0.50 ± 0.41, 0.229 |
0.35 ± 0.45, 0.43 |
0.48 ± 0.45, 0.28 |
| Diabetes | −1.27 ± 0.36 ≤0.001** |
−0.56 ± 0.37 0.13 |
−0.95 ± 0.46 0.04* |
| Dyslipidemia | −0.06 ± 0.38, 0.87 |
0.06 ± 0.40, 0.89 |
−0.08 ± 0.42, 0.85 |
| Smoking | 0.33 ± 0.32, 0.31 |
0.03 ± 0.39, 0.93 |
0.34 ± 0.33, 0.30 |
| Atherosclerosis | 1.59 ± 0.32 ≤0.001** |
0.55 ± 0.53 ≤0.29 |
1.96 ± 0.36 ≤0.001** |
| Fibrosis | 2.72 ± 0.39 ≤0.001** |
2.37 ± 0.58 ≤0.001** |
2.23 ± 0.39 ≤0.001** |
Analytic note:
Adjusted for age, sex, ethnicity, interadventitial diameter, arterial type (anterior or posterior circulation), Country of Origin (USA or International), presence of atherosclerosis and non-atherosclerotic fibrosis
P<0.05
p≤0.025 (Bonferroni correction 0.05/2)
Association between Arterial Calcifications and pathological remodeling:
In multivariate analysis adjusting for multiple factors (see above), the presence of large coalescent calcifications was only associated with concentric intimal thickening but no other measures of pathological vascular remodeling or degenerative changes (Table 4). Conversely, small scattered calcifications were significantly associated with pathological vascular remodeling, such as smaller lumen diameter and lumen-to-wall ratio, as well as increased media thickness and luminal stenosis.
Table 4:
Association between brain arterial calcifications and pathological remodeling
| All Calcifications | Coalescent Calcifications |
Scattered Calcifications |
|
|---|---|---|---|
| Beta coefficient ± SE, P value | |||
| Lumen diameter (per mm) |
−2.47 ± 0.52, ≤0.001** |
−0.93 ± 0.75, 0.22 | −1.87 ± 0.41, ≤0.001** |
| Media thickness (per micron) |
0.05 ± 0.002, 0.04** | 0.002 ± 0.003, 0.55 | 0.007 ± 0.003, 0.006** |
| Lumen-to-wall ratio | −0.08± 0.03, 0.002** | −0.06 ± 0.03, 0.05* | −0.07 ± 0.02, 0.003** |
| Luminal stenosis (%) |
0.03 ± 0.01, 0.02** | 0.01 ± 0.02, 0.53 | 0.03 ± 0.01, 0.006** |
| Increased arterial collagen |
−0.91 ± 0.60, 0.13 | 0.39 ± 0.85, 0.64 | −1.19 ± 0.75, 0.11 |
| Decreased elastin content |
0.48 ± 0.44, 0.27 | 0.46 ± 0.63, 0.46 | 0.42 ± 0.37, 0.24 |
| Large artery amyloidosis |
0.60 ± 0.43, 0.16 | 1.45 ± 0.73, 0.05* | 0.24 ± 0.41, 0.55 |
| Concentric intima thickening |
0.39 ± 0.28, 0.16 | 1.11 ± 0.44, 0.01** | −0.57 ± 0.40, 0.16 |
Analytic Note: Adjusted for age, sex, ethnicity, interadventitial diameter, arterial type (anterior or posterior circulation), Country of Origin (USA or International), presence of atherosclerosis and non-atherosclerotic fibrosis. All associations adjusted for this model except for concentric intimal thickening, which excluded atherosclerosis/fibrosis due to collinearity with these two factors.
p<0.05
p<0.025 (Bonferroni correction 0.05/2)
DISCUSSION
In this study, we demonstrate the pathological heterogeneity between large coalescent calcifications and scattered small calcifications within the brain large arteries (as shown in figure 1 and in the supplemental data). In particular, coalescent calcifications were not associated with cholesterol-driven ILAA while scattered calcifications were. Only scattered calcifications were associated with pathological vascular remodeling such as more severe stenosis and reduced lumen diameter – factors traditionally associated with ILAA on imaging. These findings were independent of vascular risk factors such as hypertension, diabetes, dyslipidemia or smoking. These findings support the hypothesis that large arterial calcifications, which we believe will be more likely to be detected clinically on brain CT, are not synonymous with ILAA (one example shown in Figure 2). The prevalence of calcifications in the arteries in our sample is higher than reported in other cohorts with systemic analysis of the circle of Willis (5.4% versus 3% of all arteries assessed).19 This discrepancy may relate to the difference in vascular risk factors and slightly higher proportion of men in our study. Additionally, we did not decalcified our specimen before fixation and embedding, which may have facilitate identification of calcium deposits in our sample.
Figure 2: Example of the correlation between calcification type and radiographic appearance.
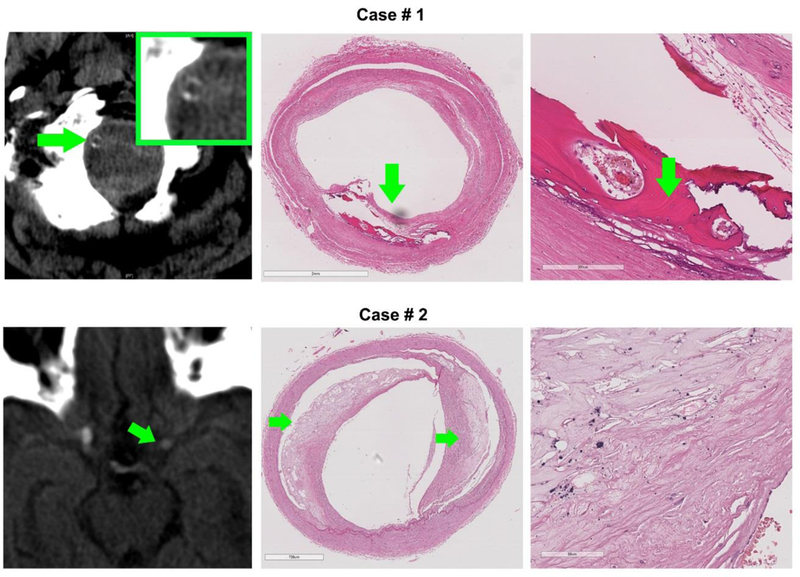
Case 1, top row- A calcified vertebral artery seen on CT radiography revealed an area of extensive, coalescent ossification (green arrow) in addition to accompanying scattered calcifications (appear black in H&E) and concentric intima thickening with no cholesterol crystals. (Stains: H&E). Case 2, bottom row – The most proximal portion of the left middle cerebral artery appears hyperdense on CT radiography (left column, green arrow). Histopathological analysis of the artery showed evidence of two atheroma (middle column, green arrows) with a relatively thin fibrous cap. With further magnification, scattered calcifications (appear black in H&E) are appreciated in the periphery of the atheroma.
The relationship between coronary arterial calcifications, CAD, and mortality is well established in the cardiac literature 20, 21 . Fewer studies have examined the relationship between arterial calcifications and clinically silent cerebrovascular disease, and in particular with ischemic stroke subtypes. Calcifications in both intracranial and extracranial arteries are associated with non-cardioembolic ischemic strokes22. Among stroke patients, intracranial arterial calcifications have been shown to be associated with future recurrent strokes and mortality23. In our study, presence of any calcifications in the brain large arteries was associated with CAD and ischemic infarcts. When adjusted for age and risk factors, scattered calcifications were still associated with CAD but the association attenuated for ischemic infarcts. Taken together, these results indicate that although vascular calcifications may not be taken as evidence of cholesterol-driven arterial pathology, their presence suggest increased arterial aging and may be a negative marker of overall cardiovascular health.
Arterial calcifications may also influence the risk factors for various stroke mechanisms. Prior research has demonstrated an association between arterial calcifications and lacunar infarcts, a phenomenon not seen with non-lacunar infarcts 24. In contrast, symptomatic carotid plaques have a lower degrees of calcifications when compared to extracranial plaques of similar size, implying that calcifications may play a role in stabilizing vulnerable ILAA plaques25 at the expense of increasing arterial stenosis. Unlike strokes from ILAA, lacunar strokes are attributed to small arterial disease or branch occlusive disease. Given these arteries’ small caliber, they are particularly sensitive to the increasing sheer stresses seen from pathological remodeling associated with brain large arteries calcifications as seen in our study.
Individuals with calcifications were substantially older and more likely to have medical co-morbidities, such as hypertension and hyperlipidemia. These associations are likely the downstream consequence of chronic inflammatory pathways causing intimal changes26. Inflammatory cytokines, such as nuclear factor-kB, are responsible for inflammatory changes in endothelial cells and migration of inflammatory cells into the vessel wall 27, as well as calcification of smooth muscle tissue11via RANKL activation. Additionally, studies have shown these inflammatory pathways are accelerated in the presence of diabetes and chronic kidney disease28. In our study, calcifications were negatively associated with diabetes after adjusting for atherosclerosis, however. This finding suggest that the association between diabetes and calcification is at least partially mediated by the diabetes role in atherosclerosis. Another possibility is that our study was underpowered to detect an association between diabetes and calcification due to a relatively low prevalence of calcifications in cases with diabetes (only 9 cases had both diabetes and calcification).
The strengths of this study include the careful histopathological description of brain arteries and the morphological composition of vascular lesions. To our knowledge, this is the first study examining the histological relationship between arterial calcifications and cerebrovascular arterial pathology. By examining the actual vasculature, we were able to characterize the exact components of vessel disease, a distinct advantage over studies relying on image-based surrogates of pathology. This approach highlighted the pathological significance of scattered calcification which maybe missed on image-based studies. Additionally, non-Hispanic blacks and Hispanics were well represented in our sample, which is important given the different rates of ILAA in these communities.
The result of this study should be contextualized in the light of its strengths and limitations. Foremost, as a retrospective analysis of autopsy-based data, our conclusions are limited to associations and cannot infer causality. As such, we cannot determine to what degree these pathological findings in the cerebral vasculature contributed to ischemic strokes nor how these changes contributed to morbidity or mortality. It may be possible that calcifications versus atheromatous plaques contribute differently to ischemic strokes, with different implications for severity. Such potential discrepancies warrants further research into atherosclerotic morphology and its relationship to strokes. Additionally, we were not able to obtain every single artery of the Circle of Willis in all cases, and we were not able to evaluate for extracranial carotid disease, limiting the interpretation of stroke etiology in non-cardioembolic infarcts. Furthermore, there was limited information on use of statin therapy, with only 93 subjects with available data on ante-mortem medications. Although there was a higher proportion of subjects with calcifications on statins, adjusting for their use did not change any associations. Vascular risk factors were obtained in some cases by self-report or inference, a potential source of bias or error. Finally, as our data was derived from autopsy specimens, an inherent selection bias may limit generalizability.
In summary, we confirmed the hypothesis that brain large artery coalescent calcifications are not associated with ILAA. Smaller, scattered brain large artery calcification are associated with ILAA, and by virtue of their size, maybe missed on imaging based studies. Pathologically, scattered arterial calcifications were most significantly associated with measures of both inward and outward vascular remodeling. This study adds to the growing body of literature highlighting the heterogeneous composition of arterial plaques and their clinical relevance. Future studies can use immunological staining to look for specific drivers of these vascular lesions, such as specific proteins or proteases. Identifying and understanding specific pathological components of cerebrovascular disease is an important step towards developing individualized therapies beyond just amelioration of traditional risk factors such as hyperlipidemia and diabetes.
Supplementary Material
Highlights.
-
-
Examination of the histopathological relationship between intracranial calcifications and atherosclerosis in large brain arteries.
-
-
Large intracranial arterial calcifications are not synonymous with cholesterol driven atherosclerosis in large brain arteries.
-
-
Microscopic calcifications are associated with increased intra-arterial luminal stenosis.
-
-
Non-atherosclerotic arterial calcifications are associated with increased morbidity
Acknowledgments
Funding:
-
-
AHA 13CRP14800040 (PI Jose Gutierrez)
-
-
NIH R01MH64168 (PI Andrew Dwork)
-
-
NIH R25MH080663 and U24MH100931 (PI Susan Morgello)
-
-
NIH P50AG08702 (PI Scott Small)
-
-
NIH N271201300028C (Deborah Mash)
-
-
Vital Projects Fund
Disclosures: Dr Elkind received compensation for providing consultative services for Biogen IDEC, Biotelemetry/Cardionet, BMS-Pfizer Partnership, Boehringer-Ingelheim, Daiichi-Sankyo, and Janssen Pharmaceuticals; received research support from diaDexus, Inc, and the National Institutes of Health (NIH)/National Institute of Neurological Disease and Stroke (NINDS); has given expert legal opinions on behalf of Merck/Organon (NuvaRing and stroke litigation); and served on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. He received royalties from UpToDate for chapters related to stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors report no conflicts. All Authors have approved this final article.
REFERENCES:
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation 2017;135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and hispanics: The northern manhattan study. Circulation 2005;111:1327–1331 [DOI] [PubMed] [Google Scholar]
- 3.Pikija S, Magdic J, Knific A. Are arterial calcifications a marker of remodeling in vertebrobasilar territory? Stroke 2014;45:874–876 [DOI] [PubMed] [Google Scholar]
- 4.Plouin PF, Baguet JP, Thony F, Ormezzano O, Azarine A, Silhol F, et al. High prevalence of multiple arterial bed lesions in patients with fibromuscular dysplasia: The arcadia registry (assessment of renal and cervical artery dysplasia). Hypertension 2017;70:652–658 [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez J, Katan M, Elkind MS. Collagen, vascular and infectious diseases. In: Grotta JC, Albers GW, Broderick JP, Kasner SE, Lo EH, Sacco RL, et al. , eds. Stroke: Pathophysiology, diagnosis, and management Elsevier Health Sciences; 2015:619. [Google Scholar]
- 6.Gutierrez J, Elkind MS, Virmani R, Goldman J, Honig L, Morgello S, et al. A pathological perspective on the natural history of cerebral atherosclerosis. Int J Stroke 2015;10:1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez J, Honig L, Elkind MS, Mohr JP, Goldman J, Dwork AJ, et al. Brain arterial aging and its relationship to alzheimer dementia. Neurology 2016;86:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part i. Circulation 2003;108:1664–1672 [DOI] [PubMed] [Google Scholar]
- 9.Ohman EM. Chronic stable angina. N Engl J Med 2016;375:293. [DOI] [PubMed] [Google Scholar]
- 10.Fisher C, Gore I, Okabe N, White P. Calcification of the carotid siphon. Circulation 1965;32:538–548 [DOI] [PubMed] [Google Scholar]
- 11.Virmani R, Joner M, Sakakura K. Recent highlights of atvb: Calcification. Arterioscler Thromb Vasc Biol 2014;34:1329–1332 [DOI] [PubMed] [Google Scholar]
- 12.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr., et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Arterioscler Thromb Vasc Biol 1995;15:1512–1531 [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez J, Rosoklija G, Murray J, Chon C, Elkind MS, Goldman J, et al. A quantitative perspective to the study of brain arterial remodeling of donors with and without hiv in the brain arterial remodeling study (bars). Front Physiol 2014;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez J, Goldman J, Honig LS, Elkind MS, Morgello S, Marshall RS. Determinants of cerebrovascular remodeling: Do large brain arteries accommodate stenosis? Atherosclerosis 2014;235:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez J, Elkind MSV, Petito C, Chung DY, Dwork AJ, Marshall RS. The contribution of hiv infection to intracranial arterial remodeling: A pilot study. Neuropathology 2013;33:256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371–1375 [DOI] [PubMed] [Google Scholar]
- 17.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262–1275 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Osborne MT, Tung B, Li M, Li Y. Imaging cardiovascular calcification. Journal of the American Heart Association 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denswil NP, van der Wal AC, Ritz K, de Boer OJ, Aronica E, Troost D, et al. Atherosclerosis in the circle of willis: Spatial differences in composition and in distribution of plaques. Atherosclerosis 2016;251:78–84 [DOI] [PubMed] [Google Scholar]
- 20.Rifkin DE, Ix JH, Wassel CL, Criqui MH, Allison MA. Renal artery calcification and mortality among clinically asymptomatic adults. J Am Coll Cardiol 2012;60:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860–1870 [DOI] [PubMed] [Google Scholar]
- 22.van Dijk AC, Fonville S, Zadi T, van Hattem AM, Saiedie G, Koudstaal PJ, et al. Association between arterial calcifications and nonlacunar and lacunar ischemic strokes. Stroke 2014;45:728–733 [DOI] [PubMed] [Google Scholar]
- 23.Bugnicourt JM, Leclercq C, Chillon JM, Diouf M, Deramond H, Canaple S, et al. Presence of intracranial artery calcification is associated with mortality and vascular events in patients with ischemic stroke after hospital discharge: A cohort study. Stroke 2011;42:3447–3453 [DOI] [PubMed] [Google Scholar]
- 24.Bos D, Ikram MA, Elias-Smale SE, Krestin GP, Hofman A, Witteman JC, et al. Calcification in major vessel beds relates to vascular brain disease. Arterioscler Thromb Vasc Biol 2011;31:2331–2337 [DOI] [PubMed] [Google Scholar]
- 25.Kwee RM. Systematic review on the association between calcification in carotid plaques and clinical ischemic symptoms. J Vasc Surg 2010;51:1015–1025 [DOI] [PubMed] [Google Scholar]
- 26.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol 2014;34:715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brasier AR. The nuclear factor-kappab-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res 2010;86:211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shemesh J, Tenenbaum A, Fisman EZ, Koren-Morag N, Grossman E. Coronary calcium in patients with and without diabetes: First manifestation of acute or chronic coronary events is characterized by different calcification patterns. Cardiovasc Diabetol 2013;12:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


