Abstract
Background
Patients with nonobstructive coronary artery disease (CAD) have worse outcomes compared with those without CAD; however, few studies have compared the intermediate- and long-term impact of CAD severity as a function of patient sex.
Methods
We evaluated 5-year and long-term all-cause mortality of women and men undergoing elective coronary angiography at a single center by degree of CAD: no CAD (1%−24% stenosis), nonobstructive CAD (25%−69% epicardial stenosis or 25%−49% left main stenosis), or obstructive CAD (epicardial stenosis ≥70% or left main stenosis ≥50%), both overall and after adjusting for baseline clinical risk factors using Cox proportional-hazards models.
Results
Between January 1986 and July 2010, 8,766 women and 11,638 men underwent angiography and were followed for a median of 9.2 years. The majority (67%) of women had no CAD or nonobstructive CAD, whereas the majority of men had obstructive CAD (56%, P < .001). In both sexes, increasing CAD was associated with increased 5-year risk of mortality. Risk-adjusted hazard ratios (vs no CAD) for women were 1.36 (95% CI, 1.16–1.60) and 1.86 (1.61–2.16) for nonobstructive and obstructive CAD, respectively; corresponding hazard ratios for men were 1.24 (1.06–1.45) and 1.38 (1.20–1.59). After risk adjustment, 5-year mortality risk was higher in men than in women at all levels of CAD severity. The relationships between severity of CAD and mortality risk during long-term follow-up in women and men were similar to the 5-year relationships above.
Conclusions
Although women undergoing elective catheterization have less severe CAD than men, nonobstructive CAD is prevalent in both sexes and carries a worse prognosis than no CAD. These data suggest a need for further investigation to establish optimal therapies for this at-risk group of patients with nonobstructive CAD. (Am Heart J 2018;206:51–60.)
Nonobstructive coronary artery disease (CAD) has been variably defined but generally refers to a finding on invasive angiography or computed tomographic angiography (CTA) in which the epicardial coronary vessels have some degree of visible atherosclerosis but less than would be considered necessary to obstruct blood flow. Whereas those with nonobstructive CAD have traditionally been reassured as being at reduced risk of myocardial infarction (MI), recent studies have established their prognosis to be significantly worse than those without any disease.1 Some studies have suggested that the impact of nonobstructive CAD on prognosis may vary by patient sex, with women having worse outcomes than their male counterparts.2–4
To date, there has been limited information on the intermediate- and long-term prognostic outcomes of non-obstructive CAD in women and men. Using the Duke Databank for Cardiovascular Diseases (DDCD), a large, well-characterized registry of all patients who underwent elective diagnostic cardiac catheterization at Duke University Medical Center between 1986 and 2010, we specifically examined1 the prevalence of nonobstructive CAD in women and men,2 the association of disease severity and unadjusted and adjusted 5-year and long-term outcomes in women and men, and3 whether there were significant sex differences in intermediate- and long-term prognosis after accounting for both clinical factors and disease severity.
Methods
Study population
Data were obtained from the DDCD on patients who underwent coronary angiography from January 1986 through July 2010 and were at least 18 years of age. Patients were excluded if the primary indication for catheterization was acute coronary syndrome; if the patient had an acute MI less than 6 weeks prior; if the primary or secondary indication for catheterization did not include ischemic heart disease; if the primary indication for or final conclusion of the catheterization was severe valvular disease and/or congenital heart disease; if the patient had a history of cardiac transplant, prior percutaneous coronary intervention, or prior coronary artery bypass graft surgery; or if the angiographic data were incomplete. Only the first qualifying diagnostic catheterization for each patient was included in the analysis.
Baseline clinical variables for each patient were stored in the DDCD using methods previously described.5 CAD severity was visually graded as percentage of luminal stenosis by the operator at the time of angiography. The primary definition of CAD severity was classified into 3 levels according to the following hierarchy: obstructive CAD if maximum epicardial vessel stenosis was ≥70% or left main vessel stenosis was ≥50%; nonobstructive CAD if maximum epicardial vessel stenosis was 25%−69% or left main stenosis was 25%−49%; or no CAD if maximum epicardial vessel or left main stenosis was 1%−24%. It is important to note that those with “no CAD” might actually have residual atheroma that is significant but is masked by coronary artery remodeling.6 A more accurate term would be “no angiographic evidence of CAD,” but for ease of reading we will refer to this group as “no CAD.”
We also defined CAD severity according to number of vessels involved. If left main stenosis of 25%−49% was present and all 3 epicardial vessels had <25% stenosis, or if the left main had <25% stenosis and 1 epicardial vessel had 25%−69% stenosis, then the patient was considered to have 1-vessel nonobstructive CAD. If left main stenosis of 25%−49% was present and 1 epicardial vessel had 25%−69% stenosis, or if the left main had <25% stenosis and 2 epicardial vessels had 25%−69% stenosis, then the patient was considered to have 2-vessel nonobstructive disease. If left main stenosis of 25%−49% was present and 2 epicardial vessels had 25%−69% stenosis, or if the left main had <25% stenosis and all 3 epicardial vessels had 25%−69% stenosis, then the patient was considered to have 3-vessel nonobstructive disease. For obstructive disease, having left main stenosis <50% and single epicardial vessel stenosis ≥70% was defined as 1-vessel obstructive disease. Having left main stenosis <50% and stenosis ≥70% in 2 epicardial vessels was defined as 2-vessel obstructive disease. Finally, having left main stenosis ≥50% and/or stenosis ≥70% in all 3 epicardial vessels was defined as 3-vessel obstructive disease.
For sensitivity analyses, an alternative definition of CAD severity was ≥50% stenosis of epicardial vessels or left main for obstructive CAD, 25%−49% stenosis of epicardial vessels or left main for nonobstructive CAD, and <25% stenosis for no CAD.
The primary end point was time from first qualifying catheterization to all-cause death up to 5 years, as this is a time frame that is generally clinically relevant. Mortality data were ascertained from annual follow-up surveys, Duke health records, and annual queries of the National Death Index through December 31, 2014, for patients on follow-up protocol (patients with obstructive CAD), and from Duke health records and a search of the Social Security Death Master File obtained in October 2011 for patients not on follow-up protocol.7 To ensure that similar follow-up was evaluated in patients across the spectrum of CAD severity, we censored vital status follow-up on October 1, 2011. Patients without a death reported before October 1, 2011, were assumed to be alive on that date, except for patients for whom the Social Security Death Master File indicated a death before October 1, 2011, but for whom DDCD records indicated the patient was alive after the death date. For these patients, vital status follow-up was censored at the date of their last DDCD contact if earlier than October 1, 2011. The secondary end point was all-cause mortality using all follow-up data collected through October 1, 2011, to evaluate whether long-term relationships were similar to those censored at 5 years.
To examine sex-based differences in process of care measures, early revascularization among patients with obstructive CAD was included as another secondary end point and was defined as percutaneous coronary intervention and/or coronary arterial bypass graft within 30 days of catheterization.
Statistical analysis
A summary of demographic and clinical characteristics of the analysis cohort was stratified by sex. Continuous characteristics were summarized with median and inter-quartile range; categorical variables were summarized with frequency count and percentage. Characteristics were compared between men and women using a χ2 statistic for categorical variables and a Wilcoxon rank sum statistic for continuous variables. Cumulative event rates were estimated by the Kaplan-Meier method censoring at last date known alive/last follow-up, and cumulative event rate curves were plotted for the primary definition of CAD severity stratified by sex.
The relationship between CAD severity (using the primary definition, the definition based on number of vessels, and the sensitivity analysis definition), sex, and survival was analyzed using Cox proportional-hazards regression. A base model included main effects and the interaction between CAD severity and sex. An adjusted model included the following baseline covariates in addition to the base model terms: age (continuous), body mass index (continuous), race (white, black, Native American, other), history of MI, history of diabetes, history of hypertension, history of hyperlipidemia, history of smoking, history of peripheral vascular disease, history of congestive heart failure, binary indicator of more than 1 noncardiovascular Charlson comorbidity, history of chronic obstructive pulmonary disease, year of catheterization (5-level categorical variable: 1986–1990, 1991–1995, 1996–2000, 2001–2005, 2006–2010), and renal disease (defined as chronic renal insufficiency, creatinine >3 mg/dL, history of kidney transplant, or use of hemodialysis). The 2 continuous variables were modeled allowing for a flexible (natural cubic spline) relationship. Covariates for inclusion in the adjusted model were prespecified prior to analysis. Overall comparison between men and women was evaluated from a Cox model including only sex, from a Cox model including sex and stratifying by CAD severity, and from a Cox model including sex and adjustment covariates and stratifying by CAD severity. Visual inspection of the cumulative event rate curves was used to assess for substantial violations of the proportional hazards assumption for sex and CAD severity.
Hazard ratios (HRs) were computed for the Cox proportional-hazards regression model by maximizing the partial likelihood using the Efron method for handling tied event times. HRs for men versus women at each level of CAD severity, and the HR for CAD severity (reference = no CAD) by sex were estimated. In addition, HRs for all 6 combinations of sex by CAD severity were estimated with women with no CAD as the reference group. Both unadjusted (base model) and adjusted estimates were obtained. Wald χ2 statistics were calculated to test for significance at a level of .05, and Wald 95% confidence limits were presented.
Logistic regression was used to examine sex differences in revascularization within 30 days among patients with obstructive CAD. The model included the risk factors listed above as adjustment variables. Maximum likelihood estimates of odds ratios and 95% CIs were calculated.
By construction, the analysis data set did not include any observations with missingness in sex or CAD severity. All covariates included in the Cox proportional hazards model had no missingness with the exception of race (missingness = 2.2%). Missingness in this variable was considered to be completely at random, and a complete case analysis was performed. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
This study was funded by the Duke Clinical Research Institute. Ms Brucker received funding support from the National Institutes of Health (5T32HL079896). There was no other extramural funding to support this project. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
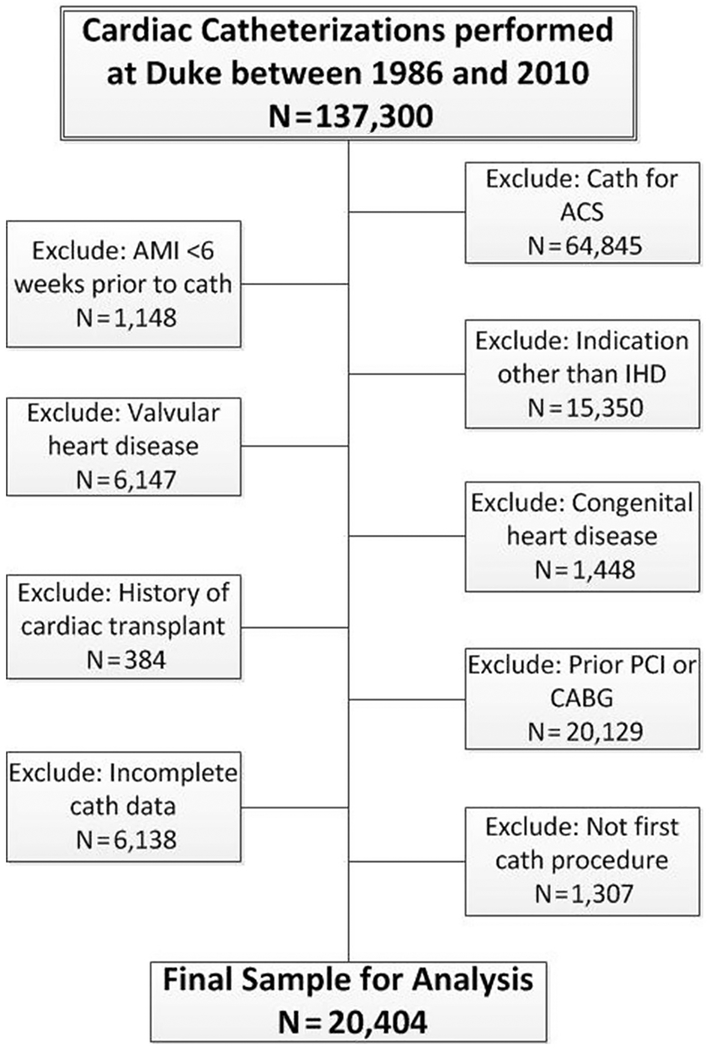
Between January 1986 and July 2010, 137,300 coronary angiogram procedures were performed at Duke University Medical Center: 51,287 (37%) in women and 86,013 (63%) in men. Of these, 20,404 procedures (16%), of which 8,766 (43%) were in women and 11,638 (57%) in men, met our inclusion criteria (Figure 1). Vital status follow-up was complete through October 1, 2011, except in 43 (0.2%) patients. Patients were followed for a median of 9.2 years (minimum 0 year, maximum 25.7 years).
Figure 1.
Flowchart of patient selection. ACS, acute coronary syndrome; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; cath, catheterization; IHD, ischemic heart disease; PCI, percutaneous coronary intervention.
Baseline characteristics of the patients by sex are outlined in Table I. Women were more likely to be older (median 60 vs 59 years, P < .001) and nonwhite (28% vs 19%, P < .001), to have diabetes (25% vs 21%, P < .001), and to be hypertensive (62% vs 56%, P < .001) compared with men. On the other hand, men were more likely to smoke (62% vs 38%, P < .001) and to have a history of prior MI (15% vs 8%, P < .001). Women and men were similarly likely to have noncardiac comorbidities, as determined by the Charlson index (noncardiac Charlson index >1: 12% for both, P = .665). Baseline characteristics of the patients by CAD severity are outlined in Supplemental Table I.
Table I.
Baseline characteristics of the study population by sex
| N = 20,404 | n = 11,638 | n = 8766 | ||
|---|---|---|---|---|
| Demographics | ||||
| Age, y, median (IQR) | 60 (51.0–68.0) | 59 (50.0–67.0) | 60 (51.0–69.0) | <.001 |
| Race, n (%) | <.001 | |||
| White | 15,369 (77.0) | 9195 (80.6) | 6174 (72.1) | |
| Black | 3900 (19.5) | 1828 (16.0) | 2072 (24.2) | |
| Native American | 385 (1.9) | 199 (1.7) | 186 (2.2) | |
| Other | 306 (1.5) | 180 (1.6) | 126 (1.5) | |
| Medical history, n (%) | ||||
| Diabetes | 4647 (22.8) | 2434 (20.9) | 2213 (25.2) | <.001 |
| Hypertension | 11,897 (58.3) | 6482 (55.7) | 5415 (61.8) | <.001 |
| Hyperlipidemia | 9475 (46.4) | 5328 (45.8) | 4147 (47.3) | .030 |
| Smoking history | 10,528 (51.6) | 7201 (61.9) | 3327 (38.0) | <.001 |
| History of MI | 2409 (11.8) | 1726 (14.8) | 683 (7.8) | <.001 |
| History of cerebrovascular disease | 1662 (8.1) | 911 (7.8) | 751 (8.6) | .056 |
| History of PVD | 1693 (8.3) | 1042 (9.0) | 651 (7.4) | <.001 |
| History of CHF | 4897 (24.6) | 2539 (22.3) | 2358 (27.6) | <.001 |
| History of COPD | 1095 (5.4) | 634 (5.4) | 461 (5.3) | .554 |
| Noncardiac Charlson index >1 | 2472 (12.1) | 1400 (12.0) | 1072 (12.2) | .665 |
| Renal disease | 345 (1.7) | 196 (1.7) | 149 (1.7) | .932 |
| Presenting characteristics | ||||
| Chest pain in last 6 wk, n (%) | 14,949 (73.4) | 8140 (70.0) | 6809 (77.8) | <.001 |
| Systolic BP, mm Hg, median (IQR) | 140 (124.0–157.0) | 139 (124.0–154.0) | 140 (125.0–160.0) | <.001 |
| Diastolic BP, mm Hg, median (IQR) | 80 (70.0–89.0) | 80 (72.0–90.0) | 80 (70.0–86.0) | <.001 |
| Pulse, beat/min, median (IQR) | 72 (63.0–80.0) | 70 (61.0–80.0) | 73 (65.0–83.0) | <.001 |
| BMI, kg/m2, median (IQR) | 27.7 (24.5–31.6) | 27.5 (24.8–30.9) | 27.9 (24.0–33.0) | <.001 |
| eGFR,* mL/min/1.73 m2, median (IQR) | 75.4 (59.9–90.6) | 76.8 (61.9–91.2) | 73.4 (57.2–89.9) | <.001 |
PVD, peripheral vascular disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; BP, blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Calculated using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
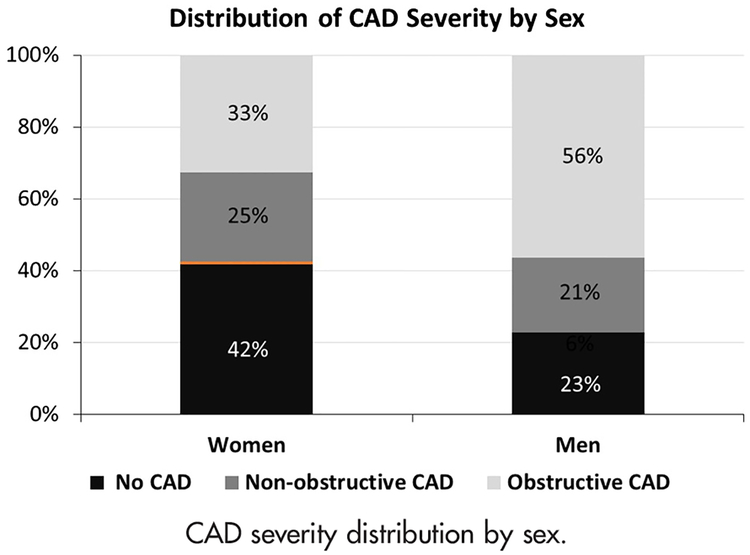
The distribution of CAD severity found on angiography was significantly different between women and men (overall P < .001; Figure 2): a larger proportion of women had no CAD (42% vs 23%) and nonobstructive CAD (25% vs 21%) compared with men, and a smaller proportion had obstructive CAD (33% vs 56%) (all P < .001). Whereas women more frequently had 1-vessel nonobstructive CAD than men (12% vs 9%, P < .0001), similar proportions of women and men had 2- and 3-vessel nonobstructive disease (Supplemental Table II). In contrast, men more frequently had 1-, 2-, and 3-vessel obstructive CAD than women.
Figure 2.
CAD severity distribution by sex.
Of the 2,858 women and 6,531 men who underwent angiography and had obstructive CAD, 1,538 (54%) women and 3,782 (58%) men underwent revascularization within 30 days of the catheterization (P = .0002). After adjustment for possible confounding factors, men with obstructive CAD remained more likely to undergo revascularization compared with women (OR, 1.19; 95% CI, 1.08–1.31).
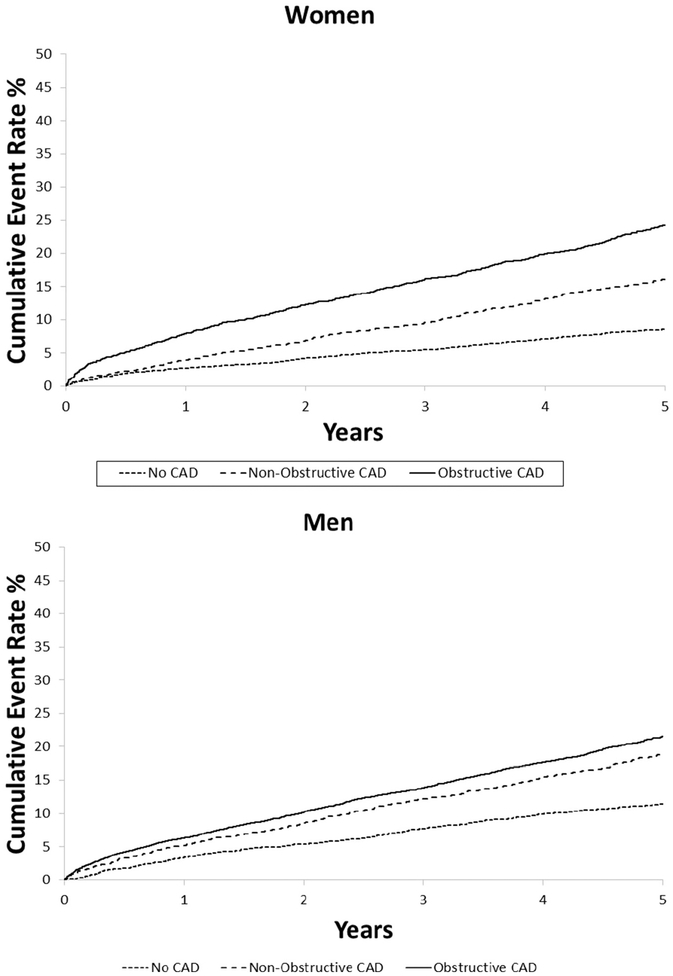
Within 5 years after catheterization, 3,409 deaths were reported (2092 in men, 1317 in women). In both women and men, increasing levels of CAD severity were associated with increasing 5-year incidence of mortality, both for the primary definition of CAD severity (Figure 3) and when number of diseased vessels was taken into account (Figure 4). After adjustment for risk factors, likelihood of mortality during follow-up increased with rising CAD severity in both women and men (Table II). Of note, the relationship between CAD severity and mortality was modified by sex (interaction P < .001).
Figure 3.
Five-year cumulative incidence of mortality by CAD severity level within each sex.
Figure 4.
Five-year cumulative incidence of mortality by number of diseased vessels within each sex. V, vessel; Non-obs, nonobstructive; Obs, obstructive.
Table II.
Association between CAD severity (reference = sex-specific “no CAD”) and 5-year mortality within each sex
| HR (95% CI) | P value | HR (95% CI) | P value | |||
|---|---|---|---|---|---|---|
| Women | ||||||
| No CAD | 3699 (42.2) | 8.6 (7.7–9.6) | Ref | Ref | ||
| Nonobstructive | 2209 (25.2) | 16.1 (14.6–17.8) | 1.93 (1.65–2.25) | <.0001 | 1.36 (1.16–1.60) | .0002 |
| Obstructive | 2858 (32.6) | 24.2 (22.7–25.9) | 3.08 (2.69–3.53) | <.0001 | 1.86 (1.61–2.16) | <.0001 |
| Men | ||||||
| No CAD | 2661 (22.9) | 11.5 (10.3–12.8) | Ref | Ref | ||
| Nonobstructive | 2446 (21.0) | 19.0 (17.4–20.6) | 1.71 (1.48–1.98) | <.0001 | 1.24 (1.06–1.45) | .0067 |
| Obstructive | 6531 (56.1) | 21.5 (20.5–22.5) | 1.98 (1.74–2.24) | <.0001 | 1.38 (1.20–1.59) | <.0001 |
KM, Kaplan-Meier.
Adjusted model includes the following covariates: age, race, BMI, prior MI, diabetes, hypertension, hyperlipidemia, peripheral vascular disease, congestive heart failure, chronic obstructive pulmonary disease, renal disease, smoking history, noncardiac Charlson comorbidity index >1, and year of cath. Interaction between CAD severity and sex was significant in both the unadjusted and adjusted models (interaction P < .001 for both).
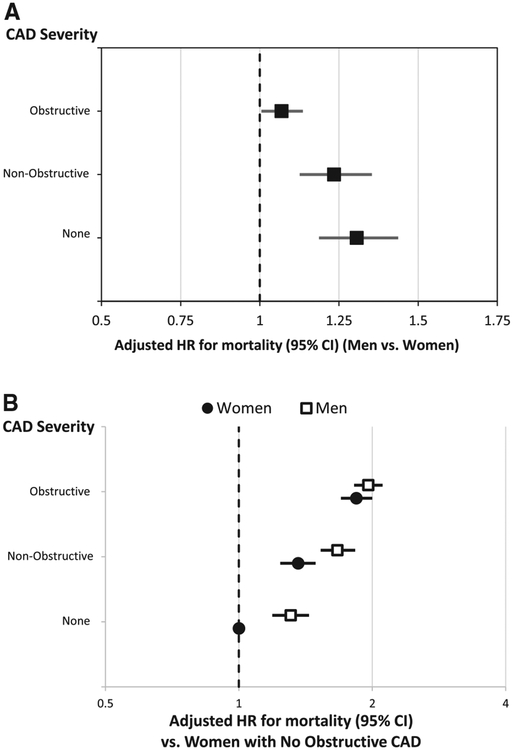
Compared with women, men had higher 5-year mortality rates before adjustment for clinical factors in those with no CAD and nonobstructive CAD, but a lower likelihood of mortality during follow-up in those with obstructive CAD (Table III). However, after adjustment, men had worse outcomes compared with women at all levels of CAD (no CAD adjusted HR, 1.56 [95% CI, 1.32–1.85], P < .0001; nonobstructive CAD adjusted HR, 1.43 [95% CI, 1.23–1.65], P < .0001; obstructive CAD adjusted HR 1.16 [95% CI, 1.05–1.28], P = .002) (Figure 5, A). Five-year mortality HRs of men and women with each degree of CAD compared with women with no CAD are shown in Figure 5, B.
Table III.
Comparison of men versus women (reference = women at each level of CAD) of 5-year mortality at each level of CAD severity
| HR for M vs F (95% CI) | P value | HR for M vs F (95% CI) | P value | |
|---|---|---|---|---|
| Overall,† stratified by CAD severity | 1.03 (0.96–1.10) | .4951 | 1.29 (1.20–1.40) | <.0001 |
| No CAD | 1.35 (1.15–1.59) | .0002 | 1.56 (1.32–1.85) | <.0001 |
| Nonobstructive | 1.20 (1.04–1.38) | .0113 | 1.43 (1.23–1.65) | <.0001 |
| Obstructive | 0.87 (0.79–0.95) | .0030 | 1.16 (1.05–1.28) | .0020 |
Adjusted model includes the following covariates: age, race, BMI, prior MI, diabetes, hypertension, hyperlipidemia, peripheral vascular disease, congestive heart failure, chronic obstructive pulmonary disease, renal disease, smoking history, noncardiac Charlson comorbidity index >1, and year of cath. Interaction between CAD severity and sex was significant in both the unadjusted and adjusted models (interaction P < .01 for both).
Overall sex HRs are estimated by fitting the unadjusted Cox model stratified by CAD severity and the adjusted Cox model stratified by CAD severity.
Figure 5.
Adjusted comparison of 5-year mortality between men and women at each level of CAD severity. A, Comparison of men and women at each level of CAD severity. B, Comparison of men and women at each level of CAD severity to women with no CAD.
Sensitivity analysis with the alternative definition of CAD severity, using the threshold of 50% stenosis for obstructive CAD, showed similar results to the primary analysis above, except that 5-year mortality risk in men with nonobstructive CAD was not significantly different from those with no CAD after adjustment for risk factors (Supplemental Table III, Supplemental Figure 1).
For the secondary end point of long-term mortality using all follow-up (median 9.2 years), 9,247 deaths were reported (5,474 in men, 3,773 in women). Similar to the primary end point of all-cause mortality censored at 5 years of follow-up, increasing levels of CAD severity were associated with increased cumulative incidence of mortality during long-term follow-up (data not shown). After adjustment, likelihood of long-term mortality during follow-up increased with rising CAD severity in both women (nonobstructive CAD adjusted HR, 1.34 [95% CI,1.22–1.47], reference is no CAD, P < .0001; obstructive CAD adjusted HR, 1.81 [95% CI, 1.66–1.96, P < .0001) and men (nonobstructive CAD adjusted HR, 1.27 [95% CI,1.15–1.40], reference is no CAD, P < .0001; obstructive CAD adjusted HR, 1.48 [95% CI, 1.36–1.61], P < .0001) (Supplemental Table IV). Similar to the primary end point of mortality with 5-year follow-up, during longer follow-up, men had worse outcomes compared with women at all levels of CAD severity (Supplemental Figure 2, A). Long-term mortality HRs of men and women with each degree of CAD compared with women with no CAD are shown in Supplemental Figure 2, B.
Discussion
There is a growing appreciation for the high prevalence of short-term clinical risks associated with nonobstructive CAD,8 but there has been limited investigation into medium- to long-term risks and whether this applies similarly to women and men. Our study in >11,000 patients undergoing elective angiography at Duke University Medical Center over a 14-year period revealed that nonobstructive CAD (25%−69% epicardial vessel stenosis or 25%−49% left main stenosis) was common in both women and men. We also found that, relative to those without disease, having nonobstructive CAD was associated with higher intermediate- and long-term mortality risk in both men and women. Lastly, prognosis was worse among men at all severities of CAD compared with women. These results persisted using several different definitions of CAD severity and for mortality outcomes through 5 years after first qualifying catheterization as well as longer term.
Our finding that the prevalence of nonobstructive CAD is relatively similar between the sexes is consistent with recent data on the topic, although we were able to provide longer-term follow-up. For example, examination of a mixed group of symptomatic and asymptomatic patients in the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter registry revealed that approximately 30% of both women and men had nonobstructive CAD, defined as 1%−49% luminal stenosis as assessed by coronary CTA.9 Similarly, in 11,223 patients referred for angiography in Eastern Denmark, a significantly higher proportion of women than men had normal coronary arteries on catheterization (48% vs 19%, respectively), but a similar proportion had nonobstructive CAD (17% vs 14%, respectively), defined as 1%−49% stenosis of an epicardial vessel.10
Our data on the clinical outcomes of patients with varying degrees of CAD underscore the prognostic importance of nonobstructive CAD for both men and women. A gradient of risk associated with increasing severity of CAD is clearly seen in both sexes in our cohort. This extends the findings of previous studies that found similar patterns over shorter, medium-term follow-up periods of 1–7.5 years.1,10–12 Even among those with nonobstructive CAD, a gradient of risk exists such that increased number of vessels involved is associated with increased mortality. On the other hand, the relative differences in risk between women and men associated with nonobstructive CAD are less clear in the literature. Shaw et al examined 4-year survival in a sample (N = 1,127) of patients who underwent CTA and found that the presence of nonobstructive CAD was a predictor of mortality in women but not in men.2 Similarly, the authors of a study of patients undergoing angiography in British Columbia, Canada, found that women with nonobstructive CAD (1%−49% epicardial vessel stenosis) were 2.43 times more likely than men with the same anatomy to experience a major adverse cardiac event (MACE) at 1 year.3 However, by 3 years, this difference became nonsignificant. In contrast, 2 examinations of patients in the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter registry found that women with nonobstructive CAD (1%−49% epicardial vessel stenosis) had similar MACE and all-cause mortality outcomes as men with similar disease severity over a mean of 2.3 years of follow-up.13,14 Similarly, Jespersen et al, in their analysis of patients who underwent angiography in Eastern Denmark between 1996 and 2009, showed that men and women without obstructive CAD had similar risks of MACE and all-cause mortality over 7.5 years.10 Our study extends these findings by observing that, over a substantially longer follow-up period (median 9.2 years, maximal 25.7 years), women with nonobstructive disease (25%−69% epicardial vessel stenosis or 25%−49% left main stenosis) have a lower mortality compared with men. In addition, there appears to be a greater disparity in outcomes in women between those with obstructive and nonobstructive disease than in men, in whom the outcomes in those with obstructive and nonobstructive disease are more similar. This may be because women with obstructive disease were less likely to undergo revascularization compared with men with obstructive disease. Interestingly, however, women with obstructive disease still had better outcomes than men with obstructive disease. Furthermore, there is an excess relative risk for men compared with women with obstructive CAD (HR 1.16 [1.05–1.28]); however, this difference in risk between men and women is smaller than in those with nonobstructive CAD (HR 1.43 [1.23–1.65]) or no CAD (HR 1.56 [1.32–1.85]). Thus, there is a significant interaction between CAD severity and sex.
The differences in results between our study and the previous studies may be due to the longer follow-up period and larger sample size that was available for our examination. Another possible explanation could be that stress testing was used less frequently in our older cohort compared with some of the more contemporary angiography studies; thus, the newer cohorts may have a higher proportion of patients with functional ischemia who are sent to catheterization. Because functional ischemia due to nonobstructive disease or microvascular angina is more common in women than men15 and is associated with better prognosis than those with ischemia due to obstructive disease,16 this could have contributed to the improved outcomes in women observed in our study, whereas newer angiography studies did not find this difference. Another possible explanation is the fact that we used coronary angiography rather than CTA. Indeed, CTA-based registries have often been shown to have low mortality rates, which may decrease their power to detect mortality differences between groups.13,17,18 Our data also provide valuable long-term information about the expected outcomes of men and women across the spectrum of CAD, from normal coronary arteries to obstructive CAD. In contrast to several previous studies that showed no difference in short-term outcomes between men and women with normal coronary arteries,2,10,13,14 we observed that women with normal coronary arteries on angiography were 64% as likely to die within 5 years from catheterization compared with their male counterparts after adjusting for risk factors. We noted worse 5-year and long-term outcomes in men with obstructive disease, which also differ from some of the previous literature in this area. Specifically, Shaw et al found that women with at least 2-vessel obstructive CAD had worse outcomes than men with the same severity of CAD,2 and Min et al showed that among patients with 3-vessel and/or left main disease, women had higher mortality compared with men.13
Our study has several strengths, including the enrollment of men and women concurrently, using consecutive index catheterizations, and very long follow-up. In addition, the similar findings using multiple definitions of CAD severity add to the robustness of our findings. Our study also has several caveats that should be taken into account. First, this was a single-center study that only included individuals who were selected to receive a catheterization, and therefore, the conclusions may not be fully generalizable. Second, our analyses may suffer from residual confounding, although we took all standard clinical factors into account. Third, because those in the “no CAD” group may actually have atheroma that is masked on angiography by coronary artery remodeling, the benefit of truly having no CAD may be underestimated. Fourth, the National Death Index, which was used to identify mortality in those with obstructive disease, is more sensitive in correctly identifying death than is the Social Security Death Master File, which was used in patients without obstructive disease.19,20 This could have introduced a source of bias in the ascertainment of outcomes. Fifth, medical therapy has changed dramatically over the course of the study, and our analyses, although adjusted for year of index procedure, do not necessarily fully account for this. It is reassuring, however, that the 5-year mortality analyses produce similar results to the long-term analyses. In addition, we cannot assess whether differences by sex in the prescription of optimal medical therapy contributed to outcome differences by sex. Sixth, the grading system for CAD severity within the DDCD changed slightly during the study period. Although our CAD severity groups can be used with both the old and the new grading system, this change may have influenced provider behavior such that some patients might have been classified differently if they presented before versus after the change in grading system. To some degree, our adjustment for year of index catheterization might account for this. Finally, we had only all-cause mortality data available to us; it is possible that analyses with cause-specific mortality and other cardiovascular outcomes may have yielded different results.
Conclusion
Although women undergoing elective catheterization generally have less severe CAD than men, nonobstructive CAD is prevalent in both sexes and carries a worse prognosis than no CAD. Furthermore, the long-term prognosis of nonobstructive and obstructive CAD is worse in men compared with women. The difference in relative risk between men and women is greater for nonobstructive CAD than it is for obstructive CAD. These data indicate that nonobstructive CAD carries a significant long-term risk in both sexes, suggesting a need for further investigation to establish optimal therapies for this at-risk group.
Supplementary Material
Acknowledgements
We thank Michael Mackenzie for his statistical programming efforts on this project.
Funding
This study was funded by the Duke Clinical Research Institute. Ms Brucker receives funding support from the National Institutes of Health (5T32HL079896).
Disclosures
Dr Peterson has received grants from Janssen and Eli Lilly, and personal fees from AstraZeneca, Bayer, Janssen, and Sanofi. Dr Douglas reports receiving grant support from HeartFlow and service on a data and safety monitoring board for GE HealthCare. The other authors report no potential conflicts of interest.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahj.2018.09.014.
References
- 1.Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312:1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw LJ, Min JK, Narula J, et al. Sex differences in mortality associated with computed tomographic angiographic measurements of obstructive and nonobstructive coronary artery disease: an exploratory analysis. Circ Cardiovasc Imaging 2010;3:473–81. [DOI] [PubMed] [Google Scholar]
- 3.Sedlak TL, Lee M, Izadnegahdar M, et al. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J 2013;166:38–44. [DOI] [PubMed] [Google Scholar]
- 4.Pepine CJ, Ferdinand KC, Shaw LJ, et al. Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol 2015;66:1918–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris PJ, Lee KL, Harrell FE Jr, et al. Outcome in medically treated coronary artery disease. Ischemic events: nonfatal infarction and death. Circulation 1980;62:718–26. [DOI] [PubMed] [Google Scholar]
- 6.Inaba S, Mintz GS, Shimizu T, et al. Compensatory enlargement of the left main coronary artery: insights from the PROSPECT study. Coron Artery Dis 2014;25:98–103. [DOI] [PubMed] [Google Scholar]
- 7.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol 1990;131:160–8. [DOI] [PubMed] [Google Scholar]
- 8.Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;47:S21–9. [DOI] [PubMed] [Google Scholar]
- 9.Schulman-Marcus J, Hartaigh BO, Gransar H, et al. Sex-specific associations between coronary artery plaque extent and risk of major adverse cardiovascular events: the CONFIRM Long-Term Registry. JACC Cardiovasc Imaging 2016;9:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–44. [DOI] [PubMed] [Google Scholar]
- 11.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston N, Schenck-Gustafsson K, Lagerqvist B. Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur Heart J 2011;32:1331–6. [DOI] [PubMed] [Google Scholar]
- 13.Min JK, Dunning A, Lin FY, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58: 849–60. [DOI] [PubMed] [Google Scholar]
- 14.Leipsic J, Taylor CM, Gransar H, et al. Sex-based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology 2014;273: 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuruvilla S, Kramer CM. Coronary microvascular dysfunction in women: an overview of diagnostic strategies. Expert Rev Cardiovasc Ther 2013;11:1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J 2013;166:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veltman CE, de Graaf FR, Schuijf JD, et al. Prognostic value of coronary vessel dominance in relation to significant coronary artery disease determined with non-invasive computed tomography coronary angiography. Eur Heart J 2012;33:1367–77. [DOI] [PubMed] [Google Scholar]
- 18.Ostrom MP, Gopal A, Ahmadi N, et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol 2008;52:1335–43. [DOI] [PubMed] [Google Scholar]
- 19.Kraut A, Chan E, Landrigan PJ. The costs of searching for deaths: National Death Index vs Social Security Administration. Am J Public Health 1992;82:760–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology 2001;12:259–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.