Abstract
Multifocal cerebral microhemorrhages (CMHs, also known as “cerebral microbleeds”), which are associated with rupture of small intracerebral vessels, have been recognized as an important cause for cognitive decline in older adults. Although recent studies demonstrate that CMHs are highly prevalent in patients 65 and older, many aspects of the pathogenesis and clinical significance of CMHs remain obscure. In this longitudinal observational study, a case of a 77-year-old man with multifocal CMHs is described, in whom the rupture of intracerebral vessels could be linked to repeatedly performing extended Valsalva maneuvers. This patient was initially seen with acute aphasia after performing a prolonged Valsalva maneuver during underwater swimming. T2-weighted magnetic resonance imaging revealed a left acute frontal intracerebral hemorrhage (ICH) with multiple CMHs. The aphasia was resolved and no cognitive impairment was present. Two years later, he developed unsteadiness and confusion after performing two prolonged Valsalva maneuvers during underwater swimming separated by about 12 days. Repeat brain imaging revealed an acute right and a subacute left ICH, with a marked interval increase in the number of CMHs. The patient also exhibited manifest memory loss after the second admission and was diagnosed with dementia. These observations suggest that prolonged Valsalva maneuver is potentially a common precipitating cause of both CMHs and symptomatic ICHs. The Valsalva maneuver both increases the systolic arterial pressure and gives rise to a venous pressure wave transmitted to the brain in the absence of the competent antireflux jugular vein valves. This pressure increase is superimposed on existing hypertension and/or increases in blood pressure due to exercise and increased venous return due to immersion of the body in water. We advocate that further studies are needed to distinguish between CMHs with arterial and venous origins and their potential to lead to ICH induced by Valsalva maneuver as well as to determine whether these lesions have a predilection for a particular location.
Keywords: Vascular contributors to cognitive impairment and dementia, VCID, Vascular cognitive impairment, VCI, Vascular aging, Cerebrovascular, Cerebromicrovascular, Stroke, Transient ischemic attack
Introduction
In recent years, continued advances in MRI technology have allowed for the increased detection of small chronic intracerebral hemorrhages (< 5 to 10 mm in diameter) termed cerebral microhemorrhages (CMHs; also described as microbleeds) (Ungvari et al., 2017a). CMHs appear as small, round, or oval hypointense lesions corresponding to focal hemosiderin depositions surrounded by essentially normal brain tissue, which can be best detected using T2*-weighted gradient-recall echo (T2*-GRE) MRI sequences and are typically not seen on CT. Because paramagnetic hemosiderin remains present for a long time at the location of previous bleedings, these imaging technologies enable the sensitive assessment of cumulative CMH burden.
There is growing evidence that CMHs are clinically not silent and contribute significantly to cognitive decline(Ungvari et al., 2017a). It has now also become clear that CMHs frequently occur in combination with larger intracerebral hemorrhages (ICHs) suggesting shared pathomechanisms(Ungvari et al., 2017a). Despite recent advances in our understanding the cellular and molecular mechanisms that promote microvascular fragility in the aging(Ungvari et al., 2017a), several critical aspects of the pathogenesis of CMHs remain elusive.
In this longitudinal observational study, a case of a 77-year-old man with multifocal CMHs is described, in whom the rupture of intracerebral vessels could be linked to repeatedly performing prolonged Valsalva maneuvers during underwater swimming. This unique clinical observation allowed us to formulate a novel hypothesis considering the potential causal role of the Valsalva maneuver and, in particular, the impact of increased venous pressure in the pathophysiology of CMHs.
Case presentation
A 77-year-old right-handed man was admitted to the hospital with complaints of acute onset of difficulty finding words. This developed immediately after performing a prolonged Valsalva maneuver as part of an extended underwater swimming where he remained submerged for more than half of the length of an Olympic-size pool. He has been an avid swimmer throughout most of his adult life, and he described the underwater swimming described above as likely the longest in terms of length and duration. Past medical history included gout, aortic stenosis, and chronic atrial fibrillation. Although a prior diagnosis of hypertension was recorded, he was never on antihypertensive medications and managed to control his blood pressure with diet and exercise. His medications included allopurinol and warfarin. Initial examination found only decreased word production with naming errors and mild paraphasia. Magnetic resonance imaging of the brain revealed an acute left frontal intracerebral hemorrhage (ICH) measuring approximately 5 cc with an additional 22 small areas of signal loss on gradient echo T2-weighted images in both hemispheres consistent with CMHs (Fig. 1). The vast majority of the CMHs (18/22) were in a lobar distribution (temporal, parietal, and occipital) with the remaining 4 in the basal ganglia and cerebellum. Laboratory data, including complete blood count, hepatic and renal function tests, electrolytes, lipid profile, urine analysis, fasting glucose, C-reactive protein, ANA, ESR, and uric acid were normal. Initial INR (international normalized ratio) was 1.8, and it was rapidly reversed with subsequent INR at 0.9. Of note, prior INR was never above 2.5. Additional investigations include echocardiogram and ultrasound of carotid arteries, which failed to reveal any significant pathology. His language improved during the admission, and at the time of discharge, he was very close to baseline. Cognitive screening performed with the Montreal cognitive assessment (MoCA) was 26 at the time of hospital discharge, with minimal impact noted from his language deficit. He was discharged with a diagnosis of intracerebral hemorrhage and amyloid angiopathy. After extensive review of risks and benefits with the medical team, he was not placed on anticoagulation or antiplatelet therapy. At 2 months post-discharge, outpatient clinic evaluation found complete recovery of language occurred within 2 months, and repeat MoCA at the time was 28. He had no new neurological sign or symptoms and remained fully independent. He also resumed his active lifestyle, including swimming, although he did not perform any underwater swimming similar to the length and duration that preceded his initial admission.
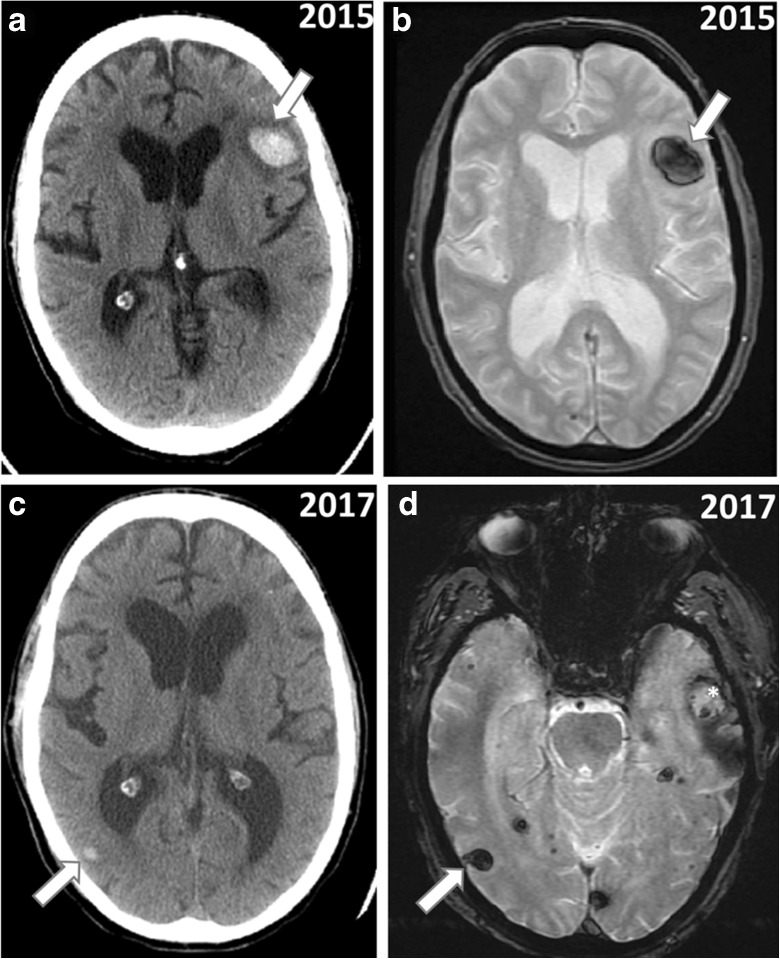
Fig. 1.
Performing a prolonged Valsalva maneuver during extended underwater swimming induced intracerebral hemorrhages on two separate occasions. CT (A) versus T2*-GRE MRI (B) showing left hemispheric hemorrhage (arrow) at the time of initial admission in 2015 after first Valsalva maneuver-related incident during underwater swimming. CT (C) versus T2*-GRE MRI (D) showing right hemispheric acute bleed (arrow) and left hemispheric subacute bleed (star) at the time of second admission in 2017 after two Valsalva maneuvers performed during underwater swimming separated by about 2 weeks. Note that the CT and MRI images have different imaging angles. MR images were obtained on a 1.5 T field strength scanner (GE medical systems)
Two years later, he presented again to the hospital complaining of sudden onset of unsteadiness and confusion, after performing two separate prolonged Valsalva maneuvers during underwater swimming of similar length and duration to the one that preceded his initial admission, separated by about 12 days. Both instances were witnessed by his wife, and she indicated that he developed difficulty finding words immediately after the first maneuver, yet declined to seek medical evaluation. As he developed confusion and unsteadiness following the second maneuver, 12 days later, he finally agreed to undergo medical evaluation. On examination, his vital signs were normal, including blood pressure. He had mild paraphasia and mildly impaired repetition and comprehension, but no other focal deficits were noted. Repeat brain MRI (Fig. 1) revealed an acute right parietal ICH (3.8 cc) and subacute left temporal ICH (4.6 cc), with a marked increase in the number of CMHs from 22 to 35. Most of the CMHs developed between the two scans were in a lobar distribution, but we noted also two additional deep CMHs. The patient also exhibited manifest memory loss after the second admission, with a repeat MoCA score of 19, and was diagnosed with dementia. Repeat laboratory tests, including INR and additional investigations including electroencephalogram, were normal. He remained stable over the next 6 months after discharge, with improvement noted in his language but no change in his cognitive score. He has since quit swimming.
We consider the left frontal and temporal lesions responsible for the language deficits noted on both admissions, while the confusion and confusion and overt memory loss reaching the severity of dementia are most probably related to the bilateral hemispheric ICHs and very large number of CMHs in a predominantly lobar distribution.
Discussion
A unique case of a patient is reported, who developed multiple acute ICHs and significant increase in the number of CMHs during the performance of Valsalva maneuvers on repeated occasions, which resulted in clinically significant cognitive decline reaching the severity of dementia. The swimming style of our patient involved performing regular underwater swimming, which would include Valsalva maneuvers. However, we noted a very close temporal relationship between the performing unusually prolonged Valsalva maneuvers and the acutely symptomatic hemorrhages (Fig. 2). Below, we discuss the similarities between the pathologies of CMHs and larger ICHs, highlight the synergistic role of aging and elevated blood pressure in the pathogenesis of CMHs, discuss the contributions of CMHs in cognitive decline, and present a mechanistic hypothesis explaining the role of the Valsalva maneuver in the generation of CMHs.
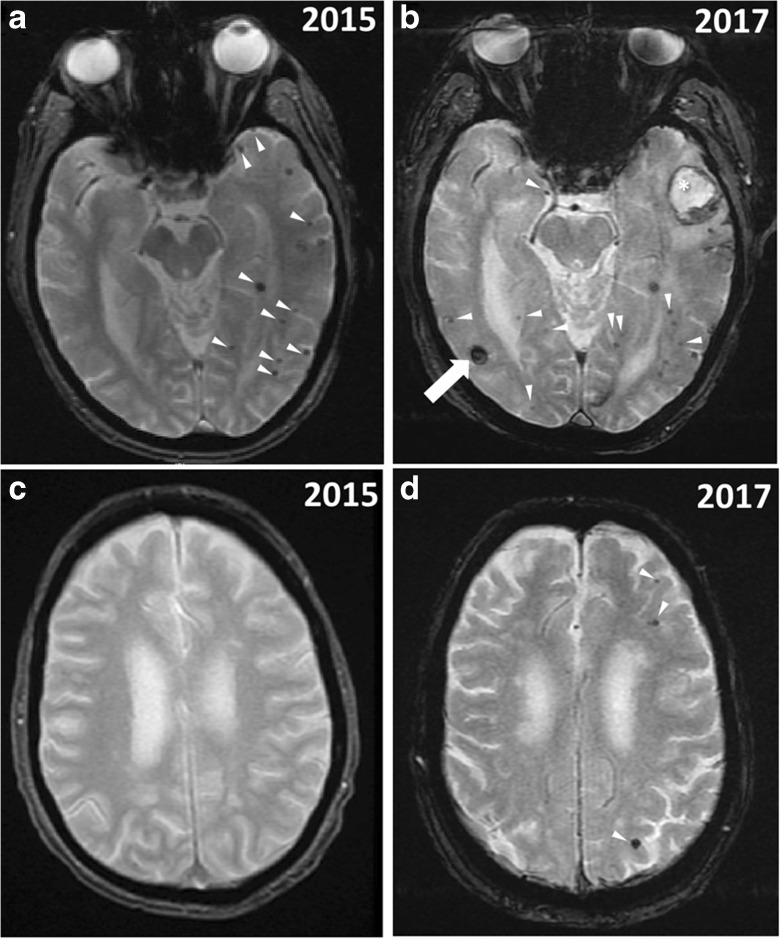
Fig. 2.
Increased number of cerebral microhemorrhages visible on T2*-GRE MRI sequences after performance of extended Valsalva maneuvers during prolonged underwater swimming. A, C Cerebral microhemorrhages (please see white arrowheads) at the time of initial admission in 2015 after first Valsalva maneuver-related incident during underwater swimming. B, D Increased number of cerebral microhemorrhages (white arrowheads indicate new CMHs) at the time of second admission in 2017 after two Valsalva maneuvers performed during underwater swimming separated by about 2 weeks. Note also the right hemispheric acute bleed (arrow) and left hemispheric subacute bleed (star) in panel B. A significant memory loss was noted after the second Valsalva-related incident. At the time of the hospital admission in 2017, the patient had a MoCA score of 19/30 and was diagnosed with dementia. MRI images were obtained on a 1.5 T field strength scanner (GE medical systems)
Association of CMHs and larger strokes
Clinical studies demonstrate that the presence of CMHs predicts subsequent ICHs in elderly patients(Tsushima et al., 2003), or in patients with ischemic stroke (Fan et al., 2003; Nighoghossian et al., 2002). It is significant that the patient presented here developed simultaneously larger ICHs and multiple CMHs in response to the same challenges. These associations suggest that similar cellular and molecular mechanisms promote vascular fragility at different levels of the cerebral circulation leading both to CMHs and larger ICHs.
Risk factors for CMHs
The patient presented here was at significant risk for CMHs. There is strong evidence that age is the most significant independent risk factor for CMHs (Ungvari et al., 2017a; Chai et al., 2016; Jeerakathil et al., 2004; Caunca et al., 2016). Prevalence of CMHs is low in younger adults and significantly increases with advanced age (Poels et al., 2010; Romero et al., 2014). The current evidence shows that the prevalence of CMHs is between 24 and 56% in elderly patients (Poels et al., 2010; Hilal et al., 2014; Poels et al., 2011; Yang et al., 2015; Takashima et al., 2011; Lee et al., 2004; Wiegman et al., 2014). However, due to rapid development and increased availability of advanced imaging methods, this figure is expected to significantly increase in the foreseeable future(Werring, 2011). Approximately half of the patients present with multiple CMHs (Hilal et al., 2014), similar to the patient presented here.
High blood pressure is another important risk factor for CMHs (Jeerakathil et al., 2004; Romero et al., 2014; Cordonnier et al., 2007; Roob et al., 1999; Sveinbjornsdottir et al., 2008; Vernooij et al., 2008). The critical role of the synergistic interaction of aging and high blood pressure in the pathogenesis of CMHs is suggested by the observations that hypertension almost exclusively promotes CMHs in older adults and rodent models of aging (Ungvari et al., 2017a; Tarantini et al., 2017a; Toth et al., 2015).
Cerebral amyloid angiopathy and Alzheimer’s disease (AD) are also important risk factors for CMHs (Ungvari et al., 2017a; Yates et al., 2011; Pettersen et al., 2008; Yates et al., 2014; Benedictus et al., 2013). AD patients often exhibit multiple CMHs with a cortical/subcortical localization (Yates et al., 2011; Ni et al., 2015; Gorelick et al., 2011), which are thought to exacerbate cognitive decline (Pettersen et al., 2008; Goos et al., 2009). In AD patients, cerebral amyloid angiopathy (CAA), which is manifested as deposition of amyloid β protein and other protein aggregates in intracerebral vessels, exhibits a similar distribution profile (Vinters & Gilbert, 1983; Biffi & Greenberg, 2011), and it is likely that majority of CMHs in AD patients develop in vessels affected by CAA. This concept is supported by the observation that animal models of CAA also exhibit multifocal CMHs (Fisher et al., 2011). Importantly, CAA can also be present in the brain of older adults even in the absence of manifest dementia (prevalence of severe CAA is > 21% in individuals aged > 85 (Lancet, 2001)), suggesting that amyloid deposition in the wall of microvessels may also play a significant role in microvascular fragility and the pathogenesis of CMHs in older patients not diagnosed with AD. There is also strong evidence supporting a critical role of CAA in the pathogenesis of larger ICHs as well (Biffi & Greenberg, 2011).
Molecular mechanisms contributing to the increased microvascular fragility associated with aging
Studies in preclinical animal models have recently revealed important cellular and molecular mechanisms responsible for the dramatic age-related increases in the susceptibility of older adults to CMHs (Ungvari et al., 2017a; Tarantini et al., 2017a; Toth et al., 2015). Age-related changes in extracellular matrix homeostasis and consequential changes in to the tensile strength of the vascular wall are critical factors in the increased propensity for development of CMHs in aging(Ungvari et al., 2017a). Studies in laboratory mice suggest that a critical mechanisms by which aging facilitates development of CMHs involve exacerbation of hypertension-induced oxidative stress (by upregulation of NADPH oxidases and mitochondrial production of reactive oxygen species) and redox-sensitive activation of matrix metalloproteinases (MMPs), which degrade collagen and elastin and other components of the basal lamina and extracellular matrix, in the cerebral vasculature(Toth et al., 2015; Springo et al., 2015a). Amyloid deposition can also significantly weaken the wall of the cerebral vessels, compromising vascular resilience to high pressure, leading to both CMHs and lobar ICHs (Biffi & Greenberg, 2011).
Age-related functional alterations that increase the likelihood of CMHs in older adults
Aging is associated with increased aortic stiffness, which compromises the Windkessel function increasing systolic pressure and pressure pulsatility in older adults (Diaz-Otero et al., 2016; Phan et al., 2016). There is strong evidence linking cerebromicrovascular injury and development of CMHs to the transmission of pulsatile high pressure waves into the thin-walled distal portion of the brain microcirculation in older individuals. Excessive backward propagation of the reflected arterial pressure waves from the periphery to the brain may augment pressure pulsatility transmitted to the brain microcirculation in older adults. The available data suggest that age-related dysfunction of cerebral autoregulatory mechanisms, including impaired myogenic constriction and functional maladaptation of proximal resistance arteries to hypertension (Springo et al., 2015b), allows the pressure waves penetrate the cerebral circulation without significant attenuation and reaches the fragile cerebral microvessels, increasing the likelihood of pressure-induced CMHs.
Role of CMHs in cognitive decline
The Montreal cognitive assessment (MoCA) is a sensitive screening instrument recommended to identify patients with vascular cognitive impairment. The MoCA assesses several cognitive domains, including visuospatial abilities, multiple aspects of executive functions, short-term memory recall, phonemic fluency, verbal abstraction, attention, concentration, working memory, language, and orientation. In our case, we noted a clear temporal association between interval development of CMHs, and also ICHs, and cognitive impairment. This observation supports the need for larger population-based cross-sectional studies showing that patients with CMHs have higher incidence of cognitive dysfunction (Chai et al., 2016; Hilal et al., 2014; Wu et al., 2014; van Norden et al., 2011; Poels et al., 2012; Werring et al., 2004; Werring et al., 2010; Yakushiji et al., 2015; Yakushiji, 2016), followed by longitudinal studies examining the impact of additional CMHs on cognitive status. Evidence from the literature suggests that that increased CMH burden predicts the severity of cognitive dysfunction (Wu et al., 2014). The behavioral/cognitive consequences of CMHs evidently depend on their locations(van Norden et al., 2011). The patient presented here had CMHs with a lobar distribution involving temporal, parietal, and occipital areas with additional presence of several deep, non-lobar, CMHs. The results of a recent meta-analysis demonstrate that CMHs in the deep brain regions, lobar regions, basal ganglia, and thalamus are associated with significant cognitive decline(Wu et al., 2014). CMHs located in the temporal lobe associate with memory and attention impairment, whereas frontal lobe CMHs were reported to result in impairment of memory, concept shifting, psychomotor speed, and attention(van Norden et al., 2011). CMHs in the deep and lobar regions were shown to associate with attention/executive and fluency domains(Valenti et al., 2016). There are several limitations to establishing direct cognitive associations according to the location of CMHs in the patient presented here, including the co-occurrence of larger ICHs and the higher educational level of the patient that may affect his performance on various tasks of the MoCA. The pathomechanisms by which CMHs negatively impact cognitive function likely involve focal brain damage and consequential disruption of neuronal and astrocytic communication in the neighboring cerebral regions(Heringa et al., 2014). CMHs also likely promote focal inflammatory changes and blood brain barrier disruption as well as microglia activation(Rosidi et al., 2011).
Potential role of Valsalva maneuver in the pathogenesis of arteriolar CMHs
In the patient presented here, we hypothesize that development of CMHs as well as larger ICHs was causally linked to the repeated performance of Valsalva maneuvers during underwater swimming. The Valsalva maneuver (defined as a forced expiratory blow against a closed glottis) that the patient performed likely lasted well over 20 s. The Valsalva maneuver triggers a typical sequence of complex hemodynamic events, resulting in a significant transient arterial pressure increase that can reach ~ 200 mmHg (Lee et al., 1954; Kroeker & Wood, 1956; Monge Garcia et al., 2009; Reyes et al., 1967). Immersion in water also exposed the patient’s body to a pressure gradient, which likely resulted in a redistribution of blood and consequential increases in cardiac output contributing to the increased blood pressure. In addition, the patient was exercising, which further elevated blood pressure. The Valsalva maneuver also has documented effects on the autoregulation of cerebral blood flow (Tiecks et al., 1995). The available evidence suggests that increased systolic blood pressure wave during the Valsalva maneuver reaches the brain and imposes an unduly burden upon the distal, vulnerable portion on the cerebral microcirculation, especially in older patients with compromised autoregulatory mechanisms. On the basis of studies performed in preclinical animal models of hypertension-induced CMHs (Ungvari et al., 2017a; Tarantini et al., 2017a; Toth et al., 2015), we hypothesize that increased penetration of high pressure to the cerebral microcirculation combined with the increased fragility of the cerebral microvessels contributed significantly to the genesis of CMHs in the patient presented here. In that regard, it would be highly informative to assess cerebral autoregulation and pressure pulsatility in this patient in subsequent studies.
The Valsalva maneuver is fairly common in many other everyday activities that involve moderate exertion, including weight lifting, blowing air into inflatable devices or musical instruments (e.g., oboe), intense coughing, vomiting, nose blowing, and strain during defecation or sexual intercourse. Depending on the levels of expiratory strain, intrathoracic pressure in these conditions may increase well over > 150–200 mmHg (Sharpey-Schafer, 1953), which is then transmitted to the circulation and reaches the brain. It is likely that when elderly patients’ microvascular pressure exceeds the threshold for structural injury in weakened cerebral microvessels, CMHs ensue.
Potential role of Valsalva maneuver in the pathogenesis of venous CMHs?
During Valsalva maneuver, the increased intrathoracic pressure is transmitted to the venous circulation, resulting in significant increases in central venous pressure(Wysoki et al., 2001). As a consequence, a retrograde venous pressure wave may also reach the vulnerable cerebral microcirculation contributing to the pathogenesis of CMHs (Ungvari et al., 2017a). In the internal jugular veins, valves are present, which have a significant role in the prevention of venous reflux and the penetration of backward venous pressure waves into the cerebral venous system during the Valsalva maneuver (Chung et al., 2014; Zivadinov, 2013; Zivadinov & Chung, 2013; Fisher et al., 1982). However, in aged individuals, the internal jugular vein valves are often incompetent enabling retrograde transmission of increased venous pressure. Although the role of CMHs of venous origin is not well understood in the brain, there is strong evidence that retinal hemorrhages of venous origin can be generated by Valsalva maneuvers (Al-Mujaini & Montana, 2008; Chapman-Davies & Lazarevic, 2002). Our observations warrant further studies to establish the link between jugular vein valve insufficiency and CMH incidence in older individuals.
Perspectives
In conclusion, this report illustrates that performing a Valsalva maneuver may promote the development of CMHs (Fig. 3) as well as trigger the emergence of larger, acutely symptomatic, ICHs. Our findings are in agreement with the results of large cross-sectional studies that increased CMH burden predicts cognitive decline. Despite recent advances in detection of CMHs and in our understanding of their diagnostic and prognostic values, a number of key questions related to their pathogenesis remain to be clarified. Future studies both in humans and in animal models should characterize what percentage of CMHs occurs in the arterioles, in the capillaries, or in the venules and determine how this pattern is affected by Valsalva maneuver. Longitudinal studies on individuals who are frequently exposed to increased intrathoracic pressure due to forced expiration (e.g., professional bass and woodwind instrument players) would be quite informative in that regard. Preclinical studies should provide additional proof-of-concept that increases in central venous pressure indeed exacerbate microvascular injury and increase CMH burden. There is increasing evidence that aging promotes critical functional and structural alterations in the cerebral arterial circulation (Toth et al., 2015; Springo et al., 2015a; Springo et al., 2015b; Castillo-Carranza et al., 2017; Toth et al., 2017; Toth et al., 2013; Tucsek et al., 2014a; Tucsek et al., 2014b; Csiszar et al., 2017; Tarantini et al., 2017b; Tarantini et al., 2017c; Tucsek et al., 2017; Ungvari et al., 2017b; Ungvari et al., 2017c). Further studies are warranted to better understand the pro-fragility effects of aging in the venous circulation and capillaries as well. The role of novel cellular mechanisms potentially involved in age-related vascular pathologies and increased microvascular fragility, including neuroendocrine changes (Tarantini et al., 2017a; Tarantini et al., 2016a; Tarantini et al., 2016b), dysregulation of the extracellular matrix (Meschiari et al., 2017), and non-conventional mechanisms of microvascular inflammation (e.g., persistent infection of the vascular cells with cytomegalovirus (Aiello et al., 2017; Jackson et al., 2017; Leng et al., 2017; Nikolich-Zugich & van Lier, 2017; Souquette et al., 2017)), should be explored. Studies on novel preclinical animal models of aging (Ashpole et al., 2017; Fang et al., 2017; Podlutsky et al., 2017; An et al., 2017; Bennis et al., 2017; Deepa et al., 2017; Urfer et al., 2017) to understand pathogenesis of CMHs should also be encouraged. In the clinical practice in addition to the importance of blood pressure control, lifestyle interventions limiting temporal blood pressure surges should be emphasized for prevention of CMHs. Patients at risk should be advised to avoid Valsalva maneuvers (e.g., no underwater swimming and weight lifting, promotion of the consumption of stool-loosening foods, and reconsidering exercise practices).
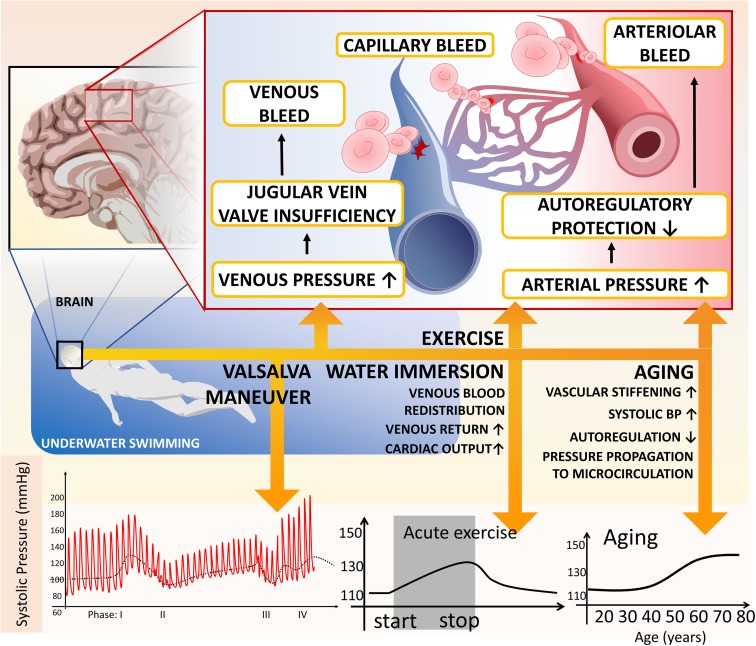
Fig. 3.
Model depicting the mechanisms by which Valsalva maneuvers performed during underwater swimming may promote the development of CMHs in older adults. The Valsalva maneuver increases arterial pressure, which is transmitted to the vulnerable downstream portion of the cerebral microcirculation due to impaired autoregulatory protection and stiffening of the conduit arteries in older adults. In addition, both acute exercise and immersion in water (by causing redistribution of blood and consequential increases in cardiac output) contribute to the increased blood pressure. Age-related MMP activation, oxidative stress, and amyloid deposition in the vascular wall increase microvascular fragility predisposing older individuals to the development of CMHs. The Valsalva maneuver also significantly increases venous pressure, which is transmitted to the thin-walled cerebral venules due to the age-related dysfunction of jugular vein valves, promoting CMHs of venular and/or capillary origin
Funding information
This work was supported by grants from the American Heart Association (ST); the Oklahoma Center for the Advancement of Science and Technology (to AC, AY, and ZU); the National Center for Complementary and Alternative Medicine (R01-AT006526 to ZU); the National Institute on Aging (R01-AG055395, R01-AG047879, and R01-AG038747); the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS100782 and R01-NS056218); a Pilot Grant from the Stephenson Cancer Center funded by the National Cancer Institute Cancer Center Support Grant P30CA225520 awarded to the University of Oklahoma Stephenson Cancer Center; the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (U54GM104938 to AY); the Presbyterian Health Foundation (to ZU, AC, AY, and CP); the European Union-funded grants EFOP-3.6.1-16-2016-00008, 20765-3/2018/FEKUTSTRAT, EFOP-3.6.2.-16-2017-00008, GINOP-2.3.2-15-2016-00048, and GINOP-2.3.3-15-2016-00032; the National Research, Development and Innovation Office (NKFI-FK123798); and the Hungarian Academy of Sciences (Bolyai Research Scholarship BO/00634/15 to PT). The authors acknowledge the support from the NIA-funded Geroscience Training Program in Oklahoma (T32AG052363).
Compliance with ethical standards
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Aiello AE, Chiu YL, Frasca D. How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? Geroscience. 2017;39:261–271. doi: 10.1007/s11357-017-9983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mujaini AS, Montana CC. Valsalva retinopathy in pregnancy: a case report. J Med Case Rep. 2008;2:101. doi: 10.1186/1752-1947-2-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JY, Quarles EK, Mekvanich S, Kang A, Liu A, Santos D, Miller RA, Rabinovitch PS, Cox TC, Kaeberlein M. Rapamycin treatment attenuates age-associated periodontitis in mice. Geroscience. 2017;39:457–463. doi: 10.1007/s11357-017-9994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedictus MR, Goos JD, Binnewijzend MA, Muller M, Barkhof F, Scheltens P, Prins ND, van der Flier WM. Specific risk factors for microbleeds and white matter hyperintensities in Alzheimer’s disease. Neurobiol Aging. 2013;34:2488–2494. doi: 10.1016/j.neurobiolaging.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience. 2017;39:51–59. doi: 10.1007/s11357-017-9957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011;7:1–9. doi: 10.3988/jcn.2011.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Carranza DL, Nilson AN, Van Skike CE, Jahrling JB, Patel K, Garach P, Gerson JE, Sengupta U, Abisambra J, Nelson P, Troncoso J, Ungvari Z, Galvan V, Kayed R. Cerebral microvascular accumulation of tau oligomers in Alzheimer’s disease and related tauopathies. Aging Dis. 2017;8:257–266. doi: 10.14336/AD.2017.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caunca MR, Del Brutto V, Gardener H, Shah N, Dequatre-Ponchelle N, Cheung YK, Elkind MS, Brown TR, Cordonnier C, Sacco RL, Wright CB (2016) Cerebral microbleeds, vascular risk factors, and magnetic resonance imaging markers: the northern Manhattan study. J Am Heart Assoc 5 [DOI] [PMC free article] [PubMed]
- Chai C, Wang Z, Fan L, Zhang M, Chu Z, Zuo C, Liu L, Mark Haacke E, Guo W, Shen W, Xia S. Increased number and distribution of cerebral microbleeds is a risk factor for cognitive dysfunction in hemodialysis patients: a longitudinal study. Medicine (Baltimore) 2016;95:e2974. doi: 10.1097/MD.0000000000002974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman-Davies A, Lazarevic A. Valsalva maculopathy. Clin Exp Optom. 2002;85:42–45. doi: 10.1111/j.1444-0938.2002.tb03071.x. [DOI] [PubMed] [Google Scholar]
- Chung CP, Beggs C, Wang PN, Bergsland N, Shepherd S, Cheng CY, Ramasamy DP, Dwyer MG, Hu HH, Zivadinov R. Jugular venous reflux and white matter abnormalities in Alzheimer’s disease: a pilot study. J Alzheimers Dis. 2014;39:601–609. doi: 10.3233/JAD-131112. [DOI] [PubMed] [Google Scholar]
- Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017;39:359–372. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience. 2017;39:187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol. 2016;310:H365–H375. doi: 10.1152/ajpheart.00562.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YH, Zhang L, Lam WW, Mok VC, Wong KS. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke. 2003;34:2459–2462. doi: 10.1161/01.STR.0000090841.90286.81. [DOI] [PubMed] [Google Scholar]
- Fang Y, McFadden S, Darcy J, Hill CM, Huber JA, Verhulst S, Kopchick JJ, Miller RA, Sun LY, Bartke A. Differential effects of early-life nutrient restriction in long-lived GHR-KO and normal mice. Geroscience. 2017;39:347–356. doi: 10.1007/s11357-017-9978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J, Vaghaiwalla F, Tsitlik J, Levin H, Brinker J, Weisfeldt M, Yin F. Determinants and clinical significance of jugular venous valve competence. Circulation. 1982;65:188–196. doi: 10.1161/01.CIR.65.1.188. [DOI] [PubMed] [Google Scholar]
- Fisher M, Vasilevko V, Passos GF, Ventura C, Quiring D, Cribbs DH. Therapeutic modulation of cerebral microhemorrhage in a mouse model of cerebral amyloid angiopathy. Stroke. 2011;42:3300–3303. doi: 10.1161/STROKEAHA.111.626655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goos JD, Kester MI, Barkhof F, Klein M, Blankenstein MA, Scheltens P, van der Flier WM. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke. 2009;40:3455–3460. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringa SM, Reijmer YD, Leemans A, Koek HL, Kappelle LJ, Biessels GJ. Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease. J Alzheimers Dis. 2014;38:211–221. doi: 10.3233/JAD-130542. [DOI] [PubMed] [Google Scholar]
- Hilal S, Saini M, Tan CS, Catindig JA, Koay WI, Niessen WJ, Vrooman HA, Wong TY, Chen C, Ikram MK, Venketasubramanian N. Cerebral microbleeds and cognition: the epidemiology of dementia in Singapore study. Alzheimer Dis Assoc Disord. 2014;28:106–112. doi: 10.1097/WAD.0000000000000015. [DOI] [PubMed] [Google Scholar]
- Jackson SE, Redeker A, Arens R, van Baarle D, van den Berg SPH, Benedict CA, Cicin-Sain L, Hill AB, Wills MR. CMV immune evasion and manipulation of the immune system with aging. Geroscience. 2017;39:273–291. doi: 10.1007/s11357-017-9986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham study. Stroke. 2004;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- Kroeker EJ, Wood EH. Beat-to-beat alterations in relationship of simultaneously recorded central and peripheral arterial pressure pulses during Valsalva maneuver and prolonged expiration in man. J Appl Physiol. 1956;8:483–494. doi: 10.1152/jappl.1956.8.5.483. [DOI] [PubMed] [Google Scholar]
- De Lee GJ, Matthews MB and Sharpey-Schafer EP. The effect of the Valsalva manoeuver on the systemic and pulmonary arterial pressure in man. Br Heart J 1954;16:311–316 [DOI] [PMC free article] [PubMed]
- Lee SH, Bae HJ, Ko SB, Kim H, Yoon BW, Roh JK. Comparative analysis of the spatial distribution and severity of cerebral microbleeds and old lacunes. J Neurol Neurosurg Psychiatry. 2004;75:423–427. doi: 10.1136/jnnp.2003.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Kamil J, Purdy JG, Lemmermann NA, Reddehase MJ, Goodrum FD. Recent advances in CMV tropism, latency, and diagnosis during aging. Geroscience. 2017;39:251–259. doi: 10.1007/s11357-017-9985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience. 2017;39:7–18. doi: 10.1007/s11357-017-9959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge Garcia MI, Gil Cano A, Diaz Monrove JC. Arterial pressure changes during the Valsalva maneuver to predict fluid responsiveness in spontaneously breathing patients. Intensive Care Med. 2009;35:77–84. doi: 10.1007/s00134-008-1295-1. [DOI] [PubMed] [Google Scholar]
- Neuropathology Group. Medical Research Council Cognitive F and Aging S. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 2001;357:169–175 [DOI] [PubMed]
- Ni J, Auriel E, Martinez-Ramirez S, Keil B, Reed AK, Fotiadis P, Gurol EM, Greenberg SM, Viswanathan A. Cortical localization of microbleeds in cerebral amyloid angiopathy: an ultra high-field 7T MRI study. J Alzheimers Dis. 2015;43:1325–1330. doi: 10.3233/JAD-140864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nighoghossian N, Hermier M, Adeleine P, Blanc-Lasserre K, Derex L, Honnorat J, Philippeau F, Dugor JF, Froment JC, Trouillas P. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2*-weighted brain MRI study. Stroke. 2002;33:735–742. doi: 10.1161/hs0302.104615. [DOI] [PubMed] [Google Scholar]
- Nikolich-Zugich J, van Lier RAW. Cytomegalovirus (CMV) research in immune senescence comes of age: overview of the 6th International Workshop on CMV and Immunosenescence. Geroscience. 2017;39:245–249. doi: 10.1007/s11357-017-9984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norden AG, van den Berg HA, de Laat KF, Gons RA, van Dijk EJ, de Leeuw FE. Frontal and temporal microbleeds are related to cognitive function: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke. 2011;42:3382–3386. doi: 10.1161/STROKEAHA.111.629634. [DOI] [PubMed] [Google Scholar]
- Pettersen JA, Sathiyamoorthy G, Gao FQ, Szilagyi G, Nadkarni NK, St George-Hyslop P, Rogaeva E, Black SE. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- Phan TS, Li JK, Segers P, Chirinos JA (2016) Misinterpretation of the determinants of elevated forward wave amplitude inflates the role of the proximal aorta. J Am Heart Assoc 5 [DOI] [PMC free article] [PubMed]
- Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 2017;39:147–160. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–S106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, Vernooij MW. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–661. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
- Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM and Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology 2012;78:326–333 [DOI] [PubMed]
- Reyes AJ, Dubra JE, Mastrascusi MC, Nin C and De Bayarres MA. Arterial blood pressure response to the Valsalva maneuver in normal persons and hypertensive patients. Arq Bras Cardiol 1967;20:101–103 [PubMed]
- Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, Kase CS, Wolf PA, Seshadri S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. 2014;45:1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52:991–994. doi: 10.1212/WNL.52.5.991. [DOI] [PubMed] [Google Scholar]
- Rosidi NL, Zhou J, Pattanaik S, Wang P, Jin W, Brophy M, Olbricht WL, Nishimura N, Schaffer CB. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PLoS One. 2011;6:e26612. doi: 10.1371/journal.pone.0026612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpey-Schafer EP. The mechanism of syncope after coughing. Br Med J. 1953;2:860–863. doi: 10.1136/bmj.2.4841.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquette A, Frere J, Smithey M, Sauce D, Thomas PG. A constant companion: immune recognition and response to cytomegalovirus with aging and implications for immune fitness. Geroscience. 2017;39:293–303. doi: 10.1007/s11357-017-9982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–1359. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab. 2015;35:527–530. doi: 10.1038/jcbfm.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, Lopez OL, van Buchem MA, Jonsson PV, Gudnason V, Launer LJ. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Mori T, Hashimoto M, Kinukawa N, Uchino A, Yuzuriha T, Yao H. Clinical correlating factors and cognitive function in community-dwelling healthy subjects with cerebral microbleeds. J Stroke Cerebrovasc Dis. 2011;20:105–110. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38:273–289. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, Csiszar A. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. Age (Dordr) 2016;38:239–258. doi: 10.1007/s11357-016-9943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zolei-Szenasi D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fulop GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O'Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiecks FP, Lam AM, Matta BF, Strebel S, Douville C, Newell DW. Effects of the Valsalva maneuver on cerebral circulation in healthy adults. A transcranial Doppler study. Stroke. 1995;26:1386–1392. doi: 10.1161/01.STR.26.8.1386. [DOI] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. AJNR Am J Neuroradiol. 2003;24:88–96. [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fulop G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience. 2017;39:385–406. doi: 10.1007/s11357-017-9981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Fulop GA, Kiss T, Csiszar A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. Geroscience. 2017;39:491–498. doi: 10.1007/s11357-017-9995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DE, Kaeberlein M. Asymptomatic heart valve dysfunction in healthy middle-aged companion dogs and its implications for cardiac aging. Geroscience. 2017;39:43–50. doi: 10.1007/s11357-016-9956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti R, Del Bene A, Poggesi A, Ginestroni A, Salvadori E, Pracucci G, Ciolli L, Marini S, Nannucci S, Pasi M, Pescini F, Diciotti S, Orlandi G, Cosottini M, Chiti A, Mascalchi M, Bonuccelli U, Inzitari D, Pantoni L. Cerebral microbleeds in patients with mild cognitive impairment and small vessel disease: the Vascular Mild Cognitive Impairment (VMCI)-Tuscany study. J Neurol Sci. 2016;368:195–202. doi: 10.1016/j.jns.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke. 1983;14:924–928. doi: 10.1161/01.STR.14.6.924. [DOI] [PubMed] [Google Scholar]
- Werring DJ. Cerebral microbleeds. New York: Cambridge University Press; 2011. [Google Scholar]
- Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, Brown MM, Jager HR. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265–2275. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299:131–135. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Wiegman AF, Meier IB, Schupf N, Manly JJ, Guzman VA, Narkhede A, Stern Y, Martinez-Ramirez S, Viswanathan A, Luchsinger JA, Greenberg SM, Mayeux R, Brickman AM. Cerebral microbleeds in a multiethnic elderly community: demographic and clinical correlates. J Neurol Sci. 2014;345:125–130. doi: 10.1016/j.jns.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Feng C, Zhao Y, Jin AP, Fang M, Liu X. A meta-analysis of association between cerebral microbleeds and cognitive impairment. Med Sci Monit. 2014;20:2189–2198. doi: 10.12659/MSM.891004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysoki MG, Covey A, Pollak J, Rosenblatt M, Aruny J, Denbow N. Evaluation of various maneuvers for prevention of air embolism during central venous catheter placement. J Vasc Interv Radiol. 2001;12:764–766. doi: 10.1016/S1051-0443(07)61451-1. [DOI] [PubMed] [Google Scholar]
- Yakushiji Y and Werring DJ. Cerebrovascular disease: lobar cerebral microbleeds signal early cognitive impairment. Nat Rev Neurol 2016;12:680–682 [DOI] [PubMed]
- Yakushiji Y, Noguchi T, Charidimou A, Eriguchi M, Nishihara M, Hara M, Nanri Y, Horikawa E, Nishiyama M, Werring DJ, Hara H. Basal ganglia cerebral microbleeds and global cognitive function: the Kashima Scan Study. J Stroke Cerebrovasc Dis. 2015;24:431–439. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Yang Q, Yang Y, Li C, Li J, Liu X, Wang A, Zhao J, Wang M, Zeng X, Fan D. Quantitative assessment and correlation analysis of cerebral microbleed distribution and leukoaraiosis in stroke outpatients. Neurol Res. 2015;37:403–409. doi: 10.1179/1743132815Y.0000000027. [DOI] [PubMed] [Google Scholar]
- Yates PA, Sirisriro R, Villemagne VL, Farquharson S, Masters CL, Rowe CC. Cerebral microhemorrhage and brain beta-amyloid in aging and Alzheimer disease. Neurology. 2011;77:48–54. doi: 10.1212/WNL.0b013e318221ad36. [DOI] [PubMed] [Google Scholar]
- Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, Rowe CC. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014;82:1266–1273. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R. Is there a link between the extracranial venous system and central nervous system pathology? BMC Med. 2013;11:259. doi: 10.1186/1741-7015-11-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R, Chung CP. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med. 2013;11:260. doi: 10.1186/1741-7015-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]