Abstract
Dysregulation of neuropeptides may play an important role in aging-induced impairments. In the long list of neuropeptides, pituitary adenylate cyclase–activating polypeptide (PACAP) represents a highly effective cytoprotective peptide that provides an endogenous control against a variety of tissue-damaging stimuli. PACAP has neuro- and general cytoprotective effects due to anti-apoptotic, anti-inflammatory, and antioxidant actions. As PACAP is also a part of the endogenous protective machinery, it can be hypothesized that the decreased protective effects in lack of endogenous PACAP would accelerate age-related degeneration and PACAP knockout mice would display age-related degenerative signs earlier. Recent results support this hypothesis showing that PACAP deficiency mimics aspects of age-related pathophysiological changes including increased neuronal vulnerability and systemic degeneration accompanied by increased apoptosis, oxidative stress, and inflammation. Decrease in PACAP expression has been shown in different species from invertebrates to humans. PACAP-deficient mice display numerous pathological alterations mimicking early aging, such as retinal changes, corneal keratinization and blurring, and systemic amyloidosis. In the present review, we summarize these findings and propose that PACAP deficiency could be a good model of premature aging.
Keywords: PACAP, Aging, Amyloidosis, Degeneration, Apoptosis
Introduction
Neuropeptides are common signaling molecules in the central nervous system and in the periphery involved in a wide range of physiological functions, acting as neurotransmitters, neuromodulators, or hormones. Co-existing and co-released with classical neurotransmitters, they can simultaneously modulate many different processes. Functional consequences of neuropeptides are most evident in pathophysiological processes, tissue injury, or stress situations when they promote cellular plasticity and protection against harmful stimuli. Recent human studies have revealed that dysregulation of neuropeptides may play an important role in aging-induced impairments (Ma et al. 2015; Ogren et al. 2010). In the long list of neuropeptides, pituitary adenylate cyclase–activating polypeptide (PACAP) represents a highly effective cytoprotective peptide that provides an endogenous control against a variety of tissue-damaging stimuli.
PACAP was discovered as a hypothalamic neuropeptide in 1989. In the last 30 years since its discovery, hundreds of functions of the peptide have been described and some of these effects have been emerging in therapeutic approaches of various diseases (Nakamachi et al. 2016; Rubio-Beltran et al. 2018; Werling et al. 2016). One of the most studied effects of PACAP is its protective effect due to anti-apoptotic, anti-inflammatory and antioxidant actions. As PACAP is also a part of the endogenous protective machinery, it can be hypothesized that the decreased anti-apoptotic, anti-inflammatory, and antioxidant effects in lack of endogenous PACAP would accelerate age-related degeneration and PACAP knockout mice would display age-related degenerative signs earlier. Recent results support this hypothesis showing that PACAP deficiency mimics aspects of age-related pathophysiological changes including increased neuronal vulnerability and neuronal degeneration accompanied by increased apoptosis, oxidative stress, and inflammation. In the present review, we summarize these findings and propose that PACAP deficiency could be a good model of premature aging.
PACAP and its receptors
PACAP occurs in two forms: PACAP38 and 27, with PACAP38 being dominant in vertebrates (Vaudry et al. 2009). PACAP is found in highest concentrations in the nervous system, but it is present in endocrine glands and in other peripheral tissues. The presence of two main groups of receptors (specific PAC1, and VPAC1 and VPAC2, which bind VIP with similar affinity), the currently known eight splice variants of PAC1 receptor, the transactivation of other receptors and the receptor-independent cellular uptake may explain the diverse effects of PACAP in all organs and tissues (Dickson and Finlayson 2009; Holighaus et al. 2011; Liao et al. 2018; Moody et al. 2011, 2016). PACAP acts via adenylate cyclase/protein kinase A (PKA)/mitogen-activated protein kinase (MAPK) and phospholipase C/inositol triphosphate downstream–signaling pathways, but also acts on Ca2+ release (Dickson and Finlayson 2009; Holighaus et al. 2011; Vaudry et al. 2009). The action on the signaling pathways and thus, the physiological or pharmacological effects depend on the expressed receptors, tissue/cell types, and other factors present in the environment (Vaudry et al. 2009). The expression of PACAP and the activation of PAC1/PACAP signaling also depend on a variety of factors, including developmental stage (Basille et al. 2000), balance between signaling pathways (Fukuchi et al. 2016), environmental factors (Horvath et al. 2015), physiological conditions (Kiss et al. 2007; Nemeth et al. 2006; Rudecki and Gray 2016), daily rhythm (Jozsa et al. 2001; Somogyvari-Vigh et al. 2002), and the presence of harmful stimuli (Giunta et al. 2012; Lam et al. 2012; Pettersson et al. 2014; Somogyvari-Vigh et al. 2002; Szakaly et al. 2010) and pathological conditions (Ergang et al. 2015; Feher et al. 2018; Han et al. 2014a, 2014b; Helyes et al. 2015; Sarszegi et al. 2018). PACAP’s actions are very diverse, among others, it plays important roles during the development of the nervous system and several peripheral organs (Fulop et al. 2018a; Watanabe et al. 2016), influences anxiety, stress coping and addiction-related behaviors (Iemolo et al. 2016; King et al. 2017; Kormos et al. 2016; Mai et al. 2018; Miles et al. 2018), cognitive functions (Han et al. 2014a, 2014b), feeding (Sekar et al. 2017), thermoregulation (Barrett et al. 2017; Garami et al. 2016), endocrine functions (Egri et al. 2016; Koves 2016), gastrointestinal secretion and motility (Padua et al. 2016; Reglodi et al. 2018b), urinary functions (Heppner et al. 2018), cardiac excitability (Parsons and May 2018), vascular relaxation (Ivic et al. 2017a), and tear secretion (Nakamachi et al. 2016).
PACAP has neuro- and general cytoprotective effects
PACAP is referred to as a growth factor in the nervous system (Ogata et al. 2015; Watanabe et al. 2016). Regenerative mechanisms often require reemployment of mechanisms used during early development (Waschek 2002; Ungvari et al. 2017). Indeed, PACAP is upregulated upon numerous harmful stimuli, supporting its endogenous protective effects in restorative processes (Marzagalli et al. 2016; Pettersson et al. 2014; Reglodi et al. 2018d; Somogyvari-Vigh and Reglodi 2004). Therefore, it is not surprising that both endogenous and exogenous PACAP has strong neuro- and general cytoprotective effects. PACAP is unique, since, unlike other neuropeptides, such as its structurally closest analogue, vasoactive intestinal peptide (VIP), it has a potent triple action of anti-apoptotic, anti-inflammatory, and antioxidant effect in a very broad range of tissues. This exceptional combination of PACAP’s actions is the rationale for our focus on this neuropeptide, which is evolutionarily conserved and secreted by neurons, endothelial, immune, and endocrine cells (Reglodi and Tamas 2016; Somogyvari-Vigh and Reglodi 2004; Vaudry et al. 2009). The main signaling route is likely through the protective pathways activated by the PAC1 receptor/PKA and PKC-mediated signaling, leading to a decrease in pro-apoptotic factors (e.g., caspases, cytochrome c, Bad, Bax, JNK, p38 MAPK, and apoptosis-inducing factor) and an increase in anti-apoptotic signaling molecules (e.g., Bcl-2, Bcl-xL, ERK, and 14-3-3 protein) (Brifault et al. 2016; Reglodi et al. 2018d). The accompanying effect on the anti-inflammatory pathways and antioxidative molecules help in the neuroprotective effect (Masmoudi-Kouki et al. 2011; Wada et al. 2013). In addition, PACAP stimulates the release and transactivation of other trophic factors, such as BDNF and NGF (Fukuchi et al. 2015; Moody et al. 2012; Reglodi et al. 2011). The direct effects on neurons are further supported by PACAP’s effects on glial cells, like effects on astrocytes, oligodendrocytes, and microglial cells (Delgado et al. 2003; Douiri et al. 2016; Masmoudi-Kouki et al. 2011; Vincze et al. 2011). PACAP is not only protective in neuronal and glial cells but also has highly potent general cytoprotective effects shown in various tissue types against different insults, including the kidney (Horvath et al. 2018; Sakamoto et al. 2015), intestine (Heimesaat et al. 2014; Horvath et al. 2016), bone and cartilage (Juhasz et al. 2015), Schwann cells (Castorina et al. 2008), endothelial cells (Racz et al. 2007), liver (Ji et al. 2013), skin (Kemeny et al. 2010), thymus (Zhang et al. 2012), and lungs (Onoue et al. 2004).
PACAP-deficient mice are more vulnerable to injuries
PACAP-deficient mice have helped in elucidating endogenous functions and mechanisms of PACAP. An increasing number of studies indicate many different roles played by the endogenous peptide, the lack of which leads to various disturbances. A few studies have shown that under normal circumstances, the development of most tissues in PACAP KO animals is not markedly different from wild types, only slight morphological differences can be detected (Endo et al. 2011; Farkas et al. 2017; Fulop et al. 2018a; Kovacs-Valasek et al. 2017). However, these subtle biochemical, synaptic, and ultrastructural differences may underlie the altered response to several physiological and pathological stimuli, like altered cortical axonal branching, changes in myelination process, inner ear structure, and cerebellar migration in the nervous system (Allais et al. 2007; Tamas et al. 2012; Vincze et al. 2011; Yamada et al. 2010). PACAP KO mice display several metabolic, behavioral, and inflammatory alterations (Abad and Tan 2018; Gaszner et al. 2012; Hashimoto et al. 2010; Hatanaka et al. 2008; Lajko et al. 2017; Maasz et al. 2014; Sandor et al. 2010) and have even altered intestinal bacterial flora (Heimesaat et al. 2017). Differences are especially apparent under pathological circumstances, when PACAP-deficient mice show increased sensitivity to various stressors. It has been shown that PACAP KO mice react with more severe injury in cerebral, retinal, kidney and intestinal ischemic lesions, cerebellar toxicity, and have slower nerve regeneration and impaired regeneration after spinal cord injury and stroke, further supporting PACAP’s endogenous cytoprotective role (Armstrong et al. 2008; Ferencz et al. 2010; Horvath et al. 2010; Matsumoto et al. 2016; Ohtaki et al. 2006; Szakaly et al. 2011; Tsuchikawa et al. 2012; Vaudry et al. 2005). All these results point out the important role of endogenous PACAP played in the normal development and in resistance of various tissues against stressful stimuli (Reglodi et al. 2012). This increased sensitivity is also shown by the higher mortality rate of mice lacking endogenous PACAP (Endo et al. 2011; Matsumoto et al. 2016; Reglodi et al. 2018c), leading some researchers not to use homozygous PACAP knockout mice for their studies, only heterozygous ones (Endo et al. 2011; Matsumoto et al. 2016). As PACAP is part of the endogenous protective machinery, it can be hypothesized that the decreased anti-apoptotic, anti-inflammatory, and antioxidant effects in lack of endogenous PACAP would accelerate age-related degeneration and PACAP knockout mice would display age-related degenerative signs earlier. Recent results support this hypothesis showing that PACAP deficiency mimics aspects of age-related pathophysiological changes including increased neuronal vulnerability and neuronal degeneration accompanied by increased apoptosis, oxidative stress, and inflammation. Below, we summarize these findings and propose that PACAP deficiency could be a good model of premature aging.
PACAP levels change in aging
Marked PACAP decline was described in aging rhesus macaque brains in the striatum, hippocampus, and in the temporal and parietal lobes (Han et al. 2017). In the old macaques, PACAP levels inversely correlated with advanced age, and cognitive performance in old animals showed a positive correlation with PACAP intensity (Han et al. 2017). PACAP peptide levels were reduced in the hippocampus and surrounding cortical areas in human amyloid precursor protein transgenic mice (Han et al. 2017), similarly to earlier descriptions in triple transgenic mice (Han et al. 2014b). This latter gene expression survey using three different transgenic mouse models of Alzheimer’s disease showed that PACAP was one of the three genes that were downregulated, in addition to two other trophic factors: brain-derived neurotrophic factor and insulin-like growth factor (Wu et al. 2006). These mouse models were familial Alzheimer’s knock-in mutations amyloid precursor protein and presenilin or amyloid precursor protein over-expression models (Wu et al. 2006). The decrease in PACAP expression in age-related neurodegenerative conditions were also confirmed in the human brains by several studies. In Alzheimer’s patients, decreased mRNA for PACAP was shown in the temporal lobe samples (Wu et al. 2006). The reduced PACAP expression was inversely correlated with amyloid beta and tau protein levels (Han et al. 2014b). Considering PACAP peptide levels, significant decreases were observed in brain homogenates of Alzheimer’s patients (Han et al. 2014b). Detailed analysis showed that PACAP levels were reduced in human entorhinal cortex, middle temporal and superior frontal gyri, and primary visual cortex both at the mRNA and protein levels associated with pathological hallmarks of Alzheimer’s disease: PACAP levels were reduced with higher amyloid plaque scores in the entorhinal cortex and superior temporal gyrus, but not in the primary visual cortex, a region spared in most cases of the disease. In advanced Braak stages, lower levels were detected than in moderate stages. Lower PACAP levels were measured parallel with the reduced brain tissue levels also in postmortem cisternal cerebrospinal fluid (CSF) of the same patients (Han et al. 2014a). Additionally, PACAP concentrations in patients with Alzheimer’s disease strongly correlated with functional dementia rating scores. The inverse correlation between PACAP and the pathological hallmarks of the disease imply that PACAP is not only reduced in Alzheimer’s disease but also represents the severity of this pathology (Han et al. 2014a, 2014b). These data suggest that the severely reduced neurotrophic effect of PACAP may be a contributing factor in age-related Alzheimer pathology. In a follow-up study, the same authors demonstrated that PACAP reduction was associated with mild cognitive impairment, a condition preceding Alzheimer’s disease in the same brain areas (Han et al. 2015). It was found that PACAP levels were lower not only in the superior frontal gyrus and middle temporal gyrus but also in the CSF (Han et al. 2015), although to a lesser extent than in fully developed Alzheimer’s disease. In other dementias, no such decline was observed, suggesting a disease-specific decline of PACAP. Values inversely correlated with dementia rating scores, implying a progressive decline of PACAP levels in Alzheimer-related dementias. The expression level of the PAC1 receptor shows a more complex pattern: in mild cognitive impairment, an upregulation was observed, but not in fully developed Alzheimer’s disease in the superior frontal gyrus, suggesting a transient, possibly compensatory upregulation of PAC1 receptor in mild impairment, an ability/response lost at more progressed states (Han et al. 2015). Interestingly, a marked PACAP decline was also observed in the ganglia of the pond snail important in memory formation (Pirger et al. 2014), indicating a possible evolutionary conserved link between PACAP in aging and memory loss.
A progressive decline in PACAP levels in cerebral microvessels has been shown by Tripathy et al. (2012). Released PACAP was measured along with other growth factors, like vascular endothelial growth factor and pigment epithelium-derived factor in vessels isolated from rats at 6, 12, 18, and 24 months of age. The authors found a progressive decline in PACAP levels, with highest expression at 6 months of age, in contrast to the other growth factors, where highest levels were observed at 12 months of age followed by an age-related decline (Tripathy et al. 2012). These results strongly suggest that PACAP decline may contribute to age-related cerebrovascular dysfunction associated with and aggravating several neuropathological conditions (Reglodi et al. 2016). These findings were later confirmed in cerebromicrovascular endothelial cells, which display age-related decline in PACAP mRNA expression accompanied by age-related increases in the expression of mRNAs for all three receptors (VPAC1, 2, and PAC1) (Banki et al. 2015).
A contradictory result has been described in gerbil hippocampus (Du et al. 2011). While most studies have described a decline in PACAP levels in aging, Du and coworkers found a more complex pattern in gerbil hippocampus (2011). PACAP levels decreased after birth in all hippocampal regions (hippocampus proper, dentate gyrus), at 3, 6, and 12 months of age, but later, at older ages (18 and 24 months of age), levels increased again. The exception was the mossy fiber zone, where PACAP expression was highest at 6 months of age, and a slight decrease could be observed at 24 months of age (Du et al. 2011).
Several other studies have shown changes of PACAP receptors in aging. While VPAC2 receptor expression was not changed in aged rats, VPAC1 receptor expression reduced in the cerebral cortex, hippocampus, and amygdala (Joo et al. 2005; Lee et al. 2010). Interestingly, PAC1 receptor immunoreactivity, on the other hand, has been found to be increased in specific brain areas of aged rats, where young rats had low levels of expression: neurons in the lateral septal nuclei, posterior thalamus, suprachiasmatic nucleus, and other areas in the hypothalamus, hippocampus, and mesencephalic structures like periaqueductal gray matter, nucleus of posterior commissure, and mesencephalic reticular nucleus. Furthermore, white matter oligodendrocytes were also found to have increased PAC1 receptor expression. In some areas, where young rats also show a high density of PAC1 receptor-expressing neurons (Joo et al. 2004) further increases were observed, such as in the medial habenula and anterior periventricular nucleus of the hypothalamus. Altogether, the authors found that the basic distribution pattern was unchanged, but PAC1 receptor expression increased in aged rats, which might represent a compensatory mechanism to prevent age-related decline of PACAP/PAC1-signaling pathways (Lee et al. 2010). In contrast, Kallo and coworkers found no difference in PAC1 receptor mRNA in the suprachiasmatic nucleus of aged (19–20 month-old) rats, but reduced levels of VPAC2 receptor mRNA were observed compared to young animals (Kallo et al. 2004). In the retina, aging mice did not have a difference in PAC1 receptor expression, but differences were found in PACAP knockout mice: in young knockout mice, a significant elevation of PAC1 receptor was found, while in old animals, a marked reduction was observed, possibly indicating a compensatory mechanism at an early age, which is compromised in old age (Kovacs-Valasek et al. 2017).
In a recent study, the decrease in PAC1 receptor expression was found in structures undergoing degeneration in MPTP-induced macaque model of Parkinson’s disease (Feher et al. 2018). While there was no change in the receptor expression in an area not directly linked to Parkinson’s disease (somatosensory cortex), marked reductions were observed in the striatum, external and internal pallidum, and putamen (Feher et al. 2018). The strong, specific decrease of PACAP receptor immunosignal in the basal ganglia of parkinsonian macaque monkey brains suggests that the PACAP/PAC1R system may play an important role in the development/progression of this age-related disease (Feher et al. 2018). This finding correlates with those of Chung et al. (2005) who studied the gene expression profile in the substantia nigra and ventral tegmental area in mice. They found that the expression of PACAP was significantly higher in the less vulnerable ventral tegmental area than in the primarily degenerated substantia nigra (Chung et al. 2005), possibly also accounting for the lower vulnerability of the ventral tegmental area in contrast to the substantia nigra in Parkinson’s disease.
Aging also affects the ability of PACAP to cross the blood-brain barrier (Nonaka et al. 2002). PACAP38 is known to cross the blood-brain barrier by a saturable system, showing regional differences throughout the brain (Banks 2016). Slower transport rates were observed in the whole brain, and specifically in the hippocampus, olfactory bulb, and hypothalamus of SAMP8 mice, a strain which shows accelerated aging associated with impaired learning and memory. This decreased transport is likely to contribute to the development of age-related cognitive decline and Alzheimer’s disease and can influence the delivery of therapeutic agents (Nonaka et al. 2002).
Although only sporadic studies have dealt with peripheral PACAP changes in aging, some data show that not only central but also peripheral PACAP expression is altered in aging. A marked reduction in the density of PACAP innervation in the subepithelial plexus and muscle layer of the urinary bladder have been found by Mohammed et al. (2002) in spite of the whole tissue amount remaining unchanged as measured by radioimmunoassay. Ureteric PACAP expression has also been found to decline in the distal part (Mohammed et al. 2002). The reduced PACAPergic innervation of the urinary bladder and distal ureter in old age suggests a perturbation of the urinary sensory innervation, thus the afferent limb of the voiding reflex (Mohammed et al. 2002).
Alterations in PACAP-mediated actions in aging
PACAP has diverse effects in the cardiovascular system (Parsons and May 2018). Among others, it has strong vasodilatory effects, an action shown in vessels of many different organs of diverse species (Boni et al. 2009; Edvinsson 2016; Ivic et al. 2017a) under normal and pathological conditions (Rubio-Beltran et al. 2018; Solymar et al. 2018). This effect has been shown to decrease with age, but the age-related decrease in the vasodilatory effect of PACAP seems to differentially affect central and peripheral arteries (Vamos et al. 2014). While only a slight reduction in the vasodilatory action of PACAP in lower concentration range was observed in isolated basilar arteries, a significant and substantial reduction could be detected in isolated carotid arteries in middle aged (12 month-old) and aged (30 month old) rats (Vamos et al. 2014). PACAP-induced vasomotor responses were also reduced in mice (Ivic et al. 2017b).
PACAP-induced cAMP accumulation was shown to be lower in the suprachiasmatic nucleus of aging female rats, in contrast to forskolin-induced accumulation, which was not altered (Krajnak and Lillis 2002). The authors concluded that this decreased responsiveness was either due to the decreased receptor number or to a decreased ability of PACAP to activate intracellular-signaling pathways in aging animals (Krajnak and Lillis 2002). This effect may be in the background of age-related decline in behavioral and cellular response to light and the ability of PACAP and cAMP to modulate phase-shifting effects of light.
PACAP-deficient mice—as a model of premature aging
Backup mechanisms may compensate the lack of PACAP in some, but not all, action sites. Backup mechanisms may be responsible for the lack of severe abnormalities in most organs in young age, but they might not be able to compensate PACAP deficiency under challenged conditions and aging (Maasz et al. 2014). The most obvious compensatory mechanisms would be the VIP/VPAC receptor system. Earlier studies could not confirm this hypothesis as they found no upregulation of VIP in PACAP-deficient mice (Girard et al. 2006), but VPAC-mediated backup mechanisms have been recently proven to maintain vascular responses in both young and older ages in the carotid arteries of PACAP knockout mice (Ivic et al. 2017b). Many other crosstalks and compensatory regulatory pathways have been linked to PACAP-deficiency, proven so far in a few organs, like during the development of various cells and tissues (Farkas et al. 2017; Fulop et al. 2018a; Reglodi et al. 2018a). In contrast to all other organs, a delayed aging has been described in C57BL PACAP knockout mouse testis, similarly to findings in VIP knockout mice (Lacombe et al. 2006, 2007). The testicular degeneration might be due to the production of reactive oxygen species, byproducts of steroidogenesis, a process known to be induced by PACAP along with increased spermatogenesis (Lacombe et al. 2006; Reglodi et al. 2018a).
One of the first studies showing what functional consequences PACAP deficiency has in aging was in cerebromicrovascular endothelial cells (Banki et al. 2015). The age-related decline in PACAP levels in isolated cells was accompanied by impaired capacity to form capillary-like structures and increased apoptosis. In young cerebromicrovascular endothelial cells, knockdown of autocrine PACAP expression mimicking PACAP deficiency, significantly impaired tube formation ability resulting in an aging phenotype. This shows that age-related dysregulation of endogenous PACAP-regulated pathways may contribute to cerebrovascular impairments in aging (Banki et al. 2015).
Recently, it has been shown that PACAP affects aging of various tissues of the eye. Tear secretion and corneal regeneration are compromised in aging PACAP-deficient mice (Nakamachi et al. 2016; Shioda et al. 2018). Decreased tear secretion accompanied by increased corneal keratinization was observed in aging PACAP-deficient mice, more severe in females than in males (Nakamachi et al. 2016). In young animals, no difference was found between wild type and PACAP knockout mice, but after 20 weeks of age, significant differences started to appear, with opacity in the eyes and increased corneal angiogenesis even in the heterozygous mice (Nakamachi et al. 2016). These results show that PACAP deficiency could serve as a model for dry-eye syndrome, and PACAP treatment could alleviate symptoms of this common ophthalmic disease. Another recent study has described accelerated retinal aging in mice lacking endogenous PACAP (Kovacs-Valasek et al. 2017). In young animals, no difference could be found in the basic retinal morphology, but marked changes could be observed in aging similarly to earlier findings in ischemia-induced retinopathy (Szabadfi et al. 2012). In retinas of aged KO animals, horizontal and rod bipolar cell sprouting was observed into the photoreceptor layer and a decreased ganglionic cell number was detected. Muller glial cells displayed an increased glial fibrillary acidic protein expression, typical in increased retinal damage (Werling et al. 2016). Several further differences were found in the activation of MAPK-signaling pathways (Kovacs-Valasek et al. 2017). These results clearly indicate that lack of endogenous PACAP accelerates retinal aging.
Our most recent results show that PACAP-deficient mice exhibit a type of systemic amyloidosis that appears more generalized, more severe, more advanced, and appears in more individuals than in wild-type mice (Reglodi et al. 2018c). We have systematically described organ-specific appearance of amyloid deposits and found most severe and early deposits in the gastrointestinal tract, kidney, liver, spleen, skin, thyroid, and trachea, with apolipoprotein-AIV being the dominating amyloid protein in the deposits. Central and peripheral nervous system was devoid of these amyloid deposits. Tissue damages were also accompanied by functional disturbances, including increased serum creatinine levels indicative of kidney malfunction. The systemic severe amyloidosis may also explain the observation that PACAP-deficient mice have increased spontaneous mortality (Reglodi et al. 2018c). In spite of no apparent inflammatory histological changes or blood cell counts and serum parameters, a disturbed cytokine profile was found, possibly creating a pro-inflammatory milieu that favors deposition of amyloid in different organs (Reglodi et al. 2018c).
Another possible factor contributing to the increased vulnerability and accelerated aging can be the diminished antioxidant potential in PACAP deficiency. PACAP itself possesses very weak or no antioxidant effects (Ohtaki et al. 2010; Reglodi et al. 2004; Solymar et al. 2018). However, several studies have shown that PACAP has indirect antioxidant capacity by decreasing oxidative stress and its consequences as well as by inducing the expression of antioxidant molecules (Douiri et al. 2016; Ferencz et al. 2009; Masmoudi-Kouki et al. 2011), an action compromised in PACAP-deficient mice (Ferencz et al. 2010; Laszlo et al. 2015; Szakaly et al. 2011; Vaudry et al. 2005). One study has evaluated the antioxidant status of PACAP-deficient mice in aging (Ohtaki et al. 2010). The authors found that while there was no difference in the plasma oxidative metabolite concentration and the antioxidative potential between wild type and PACAP KO mice at young age, there was a significant decrease in the antioxidant potential accompanied by an increase in oxidative metabolites in aging PACAP KO mice compared to wild types. This phenomenon was also present in heterozygous mice, although to a lesser extent than in homozygous animals. These results show that the ability of the animals fighting against oxidative stress is severely damaged in the lack of PACAP at older ages, probably contributing to several pathological conditions and the increased vulnerability of these mice (Ohtaki et al. 2010). A proteomic analysis from brain samples of PACAP KO mice has also revealed several differences in proteins involved in metabolic processes, energy homeostasis, and structural integrity (Maasz et al. 2014; Rivnyak et al. 2018). These results suggest that the disturbed energy balance and metabolic status can be partially compensated under unchallenged circumstances, but not under pathological conditions and aging.
Reversal of age-related pathophysiological changes by PACAP
The protective effects of PACAP in models of neurodegenerative diseases have been widely documented and its effects in models of Parkinson’s, Huntington’s, and Alzheimer’s diseases are known (Cabezas-Llobet et al. 2018; Reglodi et al. 2011, 2017; Yang et al. 2015). However, in spite of these diseases characteristically appearing in older ages, most studies have been performed in young animals, which can yield misleading extrapolations to older age groups (Tamas et al. 2005, 2006). In a unilateral 6-OHDA-induced model of Parkinson’s disease, the protective efficacy of PACAP has been compared in young and old animals (Reglodi et al. 2006). It was found that although to a lesser extent, PACAP was still able to ameliorate hypokinetic and asymmetrical behavioral symptoms in old animals and could also reduce dopaminergic cell loss in the substantia nigra, indicating that the neuroprotective effects of PACAP were still present in older ages.
Direct evidence for PACAP reversing age-related conditions is still very limited. Interestingly, one of the first results came from an invertebrate model of aging (Pirger et al. 2014). As PACAP is a remarkably well-conserved neuropeptide, a lot of its actions are also present in lower animals, including invertebrates (Kiss and Pirger 2013; Krajcs et al. 2015; Lugo et al. 2013; Mertens et al. 2007; Ng et al. 2012). These include actions that are important in aging processes, like apoptosis (Pirger et al. 2008), regeneration (Varhalmi et al. 2008), immune (Somogyi et al. 2009), and cognitive (Pirger et al. 2010) functions. A recent study has described that PACAP is able to decrease the deteriorating effects of rotenone, a neurotoxin inducing parkinsonian effects in mollusks (Maasz et al. 2017). PACAP treatment could decrease mortality, attenuate the hypokinetic effects of rotenone treatment, and also reverse the dopamine loss (Maasz et al. 2017). A study more directly related to aging has found that PACAP is able to reverse age-related cognitive decline in the pond snail (Pirger et al. 2014). Exogenous PACAP treatment is known to be both necessary and instructive for memory formation after reward conditioning in the pond snail (Pirger et al. 2010). In aging snails, where endogenous PACAP levels were found to be lower and memory is declining, exogenous PACAP treatment could boost memory formation indicating that PACAP is able to attenuate age-related memory loss (Pirger et al. 2014). There is strong data suggesting that in these model organisms, the anti-geronic effects of PACAP are synergistic with/similar to that of IGF-1, the receptor signaling, and anti-apoptotic effects of which require PAC1 receptor transactivation (Delcourt et al. 2007). These findings support the concept that although depending on different upstream signaling pathways, the CNS effects of IGF-1 and PACAP converge into common transcriptional cascades. In light of these findings, IGF-1 deficiency and PACAP-deficiency share similarities not only during development but also during age-related processes (Ashpole et al. 2017; Bennis et al. 2017; Fang et al. 2017; Fulop et al. 2018b; Podlutsky et al. 2017; Sonntag et al. 2013; Tarantini et al. 2017).
Regarding the above-described age-related decline of PACAP in cerebromicrovascular endothelial cells associated with impaired angiogenic capacity, it has been shown that this impaired angiogenesis can be reversed by PACAP. Overexpression of the peptide in aged cells resulted in a young phenotype in tube-formation capacity and caspase activity, and the same results were obtained by exogenous PACAP treatment (Banki et al. 2015).
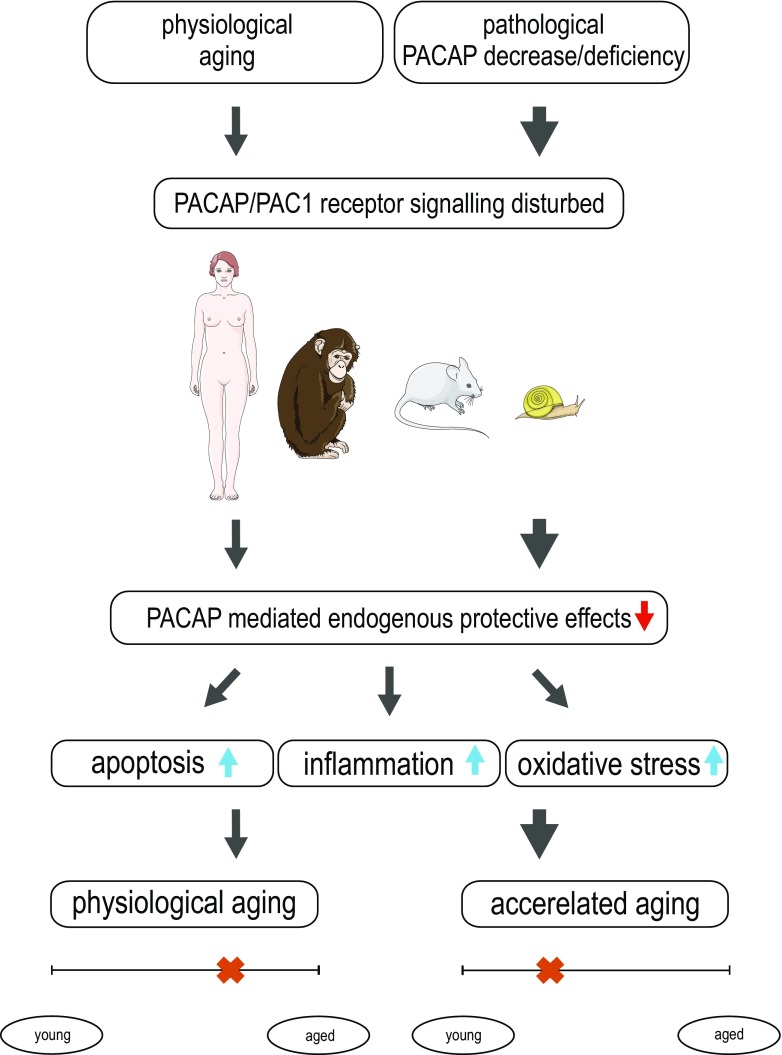
In summary, our review described findings associated with the cytoprotective peptide PACAP in aging. It is proposed that several age-related symptoms can correspond to decline in PACAP signaling. The decreased protective effects in lack of endogenous PACAP accelerate age-related degeneration and PACAP knockout mice display age-related degenerative signs earlier than wild-type animals. Thus, PACAP deficiency mimics aspects of age-related pathophysiological changes including increased neuronal vulnerability and systemic degeneration accompanied by increased apoptosis, oxidative stress, and inflammation, and we suggest that PACAP deficiency could be a good model of premature aging (proposed concept summarized in Fig. 1).
Fig. 1.
Summary of our proposal that PACAP deficiency mimics aspects of age-related pathophysiological changes by increased apoptosis, inflammation, and oxidative stress. It is proposed that several age-related symptoms can correspond to the decline in PACAP signaling. The decreased protective effects in lack of endogenous PACAP accelerate age-related degeneration and PACAP knockout mice display age-related degenerative signs earlier than wild-type animals (marked by a cross on the timeline)
Funding information
This study received financial support from NAP2017-1.2.1-NKP-2017-00002; GINOP-2.3.2-15-2016-00050 “PEPSYS”, MTA-TKI14016; NKFIH K119759, FK129190, Bolyai Scholarship, PTE-AOK KA-2017-15, EFOP-3.6.3-VEKOP-16-2017-00009, EFOP-3.6.1.-16-2016-00004 Comprehensive Development for Implementing Smart Specialization Strategies at the University of Pécs; New Excellence Program, UNKP-16-4 and 18-2, TAMOP 4.2.4.A/2-11-1-2012-0001, EFOP-3.6.3-VEKOP-16-15 2017-00008, “The role of neuro-inflammation in neurodegeneration: from molecules to clinics.” Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the 20765-3/2018/FEKUTSTRAT.
References
- Abad C, Tan Y (2018) Immunomodulatory roles of PACAP and VIP: lessons from knockout mice. J Mol Neurosci. 10.1007/s12031-018-1150-y [DOI] [PubMed]
- Allais A, Burel D, Isaac ER, Gray SL, Basille M, Ravni A, Sherwood NM, Vaudry H, Gonzalez BJ. Altered cerebellar development in mice lacking pituitary adenylate cyclase-activating polypeptide. Eur J Neurosci. 2007;25:2604–2618. doi: 10.1111/j.1460-9568.2007.05535.x. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Nobuta H, Waschek JA. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151:63–73. doi: 10.1016/j.neuroscience.2007.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, Csiszar A, Ungvari Z. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2015;70:665–674. doi: 10.1093/gerona/glu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA (2016) Transport of pituitary adenylate cyclase activating polypeptide across the blood-brain barriers: consequences for disease states and therapeutic effects. In: Reglodi and Tamas (ed) Pituitary adenylate cyclase activating polypeptide-PACAP, Current Topics in Neurotoxicity 11. Springer Nature, New York, pp 815–832
- Barrett KT, Daubenspeck JA, Wilson RJA. Pituitary adenylate cyclase activating polypeptide (PACAP) drives cardiorespiratory responses to heat stress in neonatal mice. Am J Physiol Regul Integr Comp Physiol. 2017;313:R385–R394. doi: 10.1152/ajpregu.00118.2017. [DOI] [PubMed] [Google Scholar]
- Basille M, Vaudry D, Coulouarn Y, Jegou S, Lihrmann I, Fournier A, Vaudry H, Gonzalez B. Comparative distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) binding sites and PACAP receptor mRNAs in the rat brain during development. J Comp Neurol. 2000;425:495–509. doi: 10.1002/1096-9861(20001002)425:4<495::AID-CNE3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience. 2017;39:51–59. doi: 10.1007/s11357-017-9957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni LJ, Ploug KB, Olesen I, Jansen-Olesen I, Gupta S. The in vivo effect of VIP, PACAP-38 and PACAP-27 and mRNA expression of their receptors in rat middle meningeal artery. Cephalalgia. 2009;29:837–847. doi: 10.1111/j.1468-2982.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- Brifault C, Vaudry D, Wurtz O (2016) The neuropeptide PACAP, a potent disease modifier candidate for brain stroke treatment. In: Reglodi and Tamas (ed) Pituitary adenylate cyclase activating polypeptide-PACAP, Current Topics in Neurotoxicity 11. Springer Nature, New York, pp 583–606
- Cabezas-Llobet N, Vidal-Sancho L, Masana M, Fournier A, Alberch J, Vaudry D, Xifró X (2018) Pituitary adenylate cyclase-activating polypeptide (PACAP) enhances hippocampal synaptic plasticity and improves memory performance in Huntington’s disease. Mol Neurobiol. 10.1007/s12035-018-0972-5 [DOI] [PubMed]
- Castorina A, Tiralongo A, Giunta S, Carnazza ML, Rasi G, D’Agata V. PACAP and VIP prevent apoptosis in schwannoma cells. Brain Res. 2008;1241:29–35. doi: 10.1016/j.brainres.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcourt N, Thouvenot E, Chanrion B, Galéotti N, Jouin P, Bockaert J, Marin P. PACAP type I receptor transactivation is essential for IGF-1 receptor signaling and antiapoptotic activity in neurons. EMBO J. 2007;26:1542–1551. doi: 10.1038/sj.emboj.7601608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the production of inflammatory mediators by activated microglia. J Leukoc Biol. 2003;73:155–164. doi: 10.1189/jlb.0702372. [DOI] [PubMed] [Google Scholar]
- Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther. 2009;121:294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Douiri S, Bahdoudi S, Hamdi Y, Cubì R, Basille M, Fournier A, Vaudry H, Tonon MC, Amri M, Vaudry D, Masmoudi-Kouki O. Involvement of endogenous antioxidant systems in the protective activity of pituitary adenylate cyclase-activating polypeptide against hydrogen peroxide-induced oxidative damages in cultured rat astrocytes. J Neurochem. 2016;137:913–930. doi: 10.1111/jnc.13614. [DOI] [PubMed] [Google Scholar]
- Du P, Lee CH, Choi JH, Yoo KY, Lee YL, Kang IJ, Hwang IK, Kim JD, Won MH. Pituitary adenylate cyclase-activating polypeptide-immunoreactive cells in the ageing gerbil hippocampus. Anat Histol Embryol. 2011;40:389–396. doi: 10.1111/j.1439-0264.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L (2016) Pituitary adenylate cyclase activating polypeptide (PACAP) in maigraine pathophysiology. In: Reglodi and Tamas (ed) Pituitary adenylate cyclase activating polypeptide-PACAP, Current Topics in Neurotoxicity 11. Springer Nature, New York, pp 609–615
- Egri P, Fekete C, Denes A, Reglodi D, Hashimoto H, Fulop BD, Gereben B. Pituitary adenylate cyclase-activating polypeptide (PACAP) regulates the hypothalamo-pituitary-thyroid (HPT) axis via type 2 deiodinase in male mice. Endocrinology. 2016;157:2356–2366. doi: 10.1210/en.2016-1043. [DOI] [PubMed] [Google Scholar]
- Endo K, Nakamachi T, Seki T, Kagami N, Wada Y, Nakamura K, Kishimoto K, Hori M, Tsuchikawa D, Shinntani N, Hashimoto H, Baba A, Koide R, Shioda S. Neuroprotective effect of PACAP against NMDA-induced retinal damage in the mouse. J Mol Neurosci. 2011;43:22–29. doi: 10.1007/s12031-010-9434-x. [DOI] [PubMed] [Google Scholar]
- Ergang P, Vodička M, Soták M, Klusoňová P, Behuliak M, Řeháková L, Zach P, Pácha J. Differential impact of stress on hypothalamic-pituitary-adrenal axis: gene expression changes in Lewis and Fisher rats. Psychoneuroendocrinology. 2015;53:49–59. doi: 10.1016/j.psyneuen.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Fang Y, McFadden S, Darcy J, Hill CM, Huber JA, Verhulst S, Kopchick JJ, Miller RA, Sun LY, Bartke A. Differential effects of early-life nutrient restriction in long-lived GHR-KO and normal mice. Geroscience. 2017;39:347–356. doi: 10.1007/s11357-017-9978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas J, Sandor B, Tamas A, Kiss P, Hashimoto H, Nagy AD, Fulop BD, Juhasz T, Manavalan S, Reglodi D. Early neurobehavioral development of mice lacking endogenous PACAP. J Mol Neurosci. 2017;61:468–478. doi: 10.1007/s12031-017-0887-z. [DOI] [PubMed] [Google Scholar]
- Feher M, Gaszner B, Tamas A, Gil-Martinez AL, Fernandez-Villalba E, Herrero MT, Reglodi D. Alteration of the PAC1 receptor expression in the basal ganglia of MPTP-induced parkinsonian macaque monkeys. Neurotox Res. 2018;33:702–715. doi: 10.1007/s12640-017-9841-7. [DOI] [PubMed] [Google Scholar]
- Ferencz A, Kiss P, Weber G, Helyes Z, Shintani N, Baba A, Reglodi D. Comparison of intestinal warm ischemic injury in PACAP knockout and wild-type mice. J Mol Neurosci. 2010;42:435–442. doi: 10.1007/s12031-010-9357-6. [DOI] [PubMed] [Google Scholar]
- Ferencz A, Racz B, Tamas A, Reglodi D, Lubics A, Nemeth J, Nedvig K, Kalmar-Nagy K, Horvath OP, Weber G, Roth E. Influence of PACAP on oxidative stress and tissue injury following small-bowel autotransplantation. J Mol Neurosci. 2009;37:168–176. doi: 10.1007/s12031-008-9132-0. [DOI] [PubMed] [Google Scholar]
- Fukuchi M, Kuwana Y, Tabuchi A, Tsuda M. Balance between cAMP and Ca(2+) signals regulates expression levels of pituitary adenylate cyclase-activating polypeptide gene in neurons. Genes Cells. 2016;21:921–929. doi: 10.1111/gtc.12393. [DOI] [PubMed] [Google Scholar]
- Fukuchi M, Tabuchi A, Kuwana Y, Watanabe S, Inoue M, Takasaki I, Izumi H, Tanaka A, Inoue R, Mori H, Komatsu H, Takemori H, Okuno H, Bito H, Tsuda M. Neuromodulatory effect of Gαs- or Gαq-coupled G-protein-coupled receptor on NMDA receptor selectively activates the NMDA receptor/Ca2+/calcineurin/cAMP response element-binding protein-regulated transcriptional coactivator 1 pathway to effectively induce brain-derived neurotrophic factor expression in neurons. J Neurosci. 2015;35:5606–5624. doi: 10.1523/JNEUROSCI.3650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop BD, Sandor B, Szentleleky E, Karanyicz E, Reglodi D, Gaszner B, Zakany R, Hashimoto H, Juhasz T, Tamas A (2018a) Altered notch signaling in developing molar teeth of pituitary adenylate cyclase activating polypeptide (PACAP)-deficient mice. J Mol Neurosci. 10.1007/s12031-018-1146-7 [DOI] [PubMed]
- Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel Ares MN, Toth P, Yabluchanskiy A, Conley SM, Ballabh P, Martinez-Lemus LA, Ungvari Z, Csiszar A (2018b) IGF-1 deficiency promotes pathological remodeling of cerebral arteries: a potential mechanism contributing to the pathogenesis of Intracerebral hemorrhages in aging. J Gerontol A Biol Sci Med Sci. 10.1093/gerona/gly144 [DOI] [PMC free article] [PubMed]
- Garami A, Pakai E, Rumbus Z, Solymar M (2016) The role of PACAP in the regulation of body temperature. In: Reglodi and Tamas (ed) Pituitary adenylate cyclase activating polypeptide-PACAP, Current Topics in Neurotoxicity 11. Springer Nature, New York, pp 239–257
- Gaszner B, Kormos V, Kozicz T, Hashimoto H, Reglodi D, Helyes Z. The behavioral phenotype of pituitary adenylate cyclase activating polypeptide deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger Westphal nucleus, ventral lateral septum and dorsal raphe nucleus. Neuroscience. 2012;202:283–299. doi: 10.1016/j.neuroscience.2011.11.046. [DOI] [PubMed] [Google Scholar]
- Girard BA, Lelievre V, Braas KM, Razinia T, Vizzard MA, Ioffe Y, El Meskini R, Ronnett GV, Waschek JA, May V. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 2006;99:499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- Giunta S, Castorina A, Bucolo C, Magro G, Drago F, D'Agata V. Early changes in pituitary adenylate cyclase-activating peptide, vasoactive intestinal peptide and related receptors expression in retina of streptozotocin-induced diabetic rats. Peptides. 2012;37:32–39. doi: 10.1016/j.peptides.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Han P, Caselli RJ, Baxter L, Serrano G, Yin J, Beach TG, Reiman EM, Shi J. Association of pituitary adenylate cyclase-activating polypeptide with cognitive decline in mild cognitive impairment due to Alzheimer disease. JAMA Neurol. 2015;72:333–339. doi: 10.1001/jamaneurol.2014.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Liang W, Baxter LC, Yin J, Tang Z, Beach TG, Caselli RJ, Reiman EM, Shi J. Pituitary adenylate cyclase-activating polypeptide is reduced in Alzheimer disease. Neurology. 2014;82:1724–1728. doi: 10.1212/WNL.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Nielsen M, Song M, Yin J, Permenter MR, Vogt JA, Engle JR, Dugger BN, Beach TG, Barnes CA, Shi J (2017) The impact of aging on brain pituitary adenylate cyclase activating polypeptide, pathology and cognition in nice and rhesus macaques. Front Aging Neurosci 9(180). 10.3389/fnagi.2017.00180 [DOI] [PMC free article] [PubMed]
- Han P, Tang Z, Yin J, Maalouf M, Beach TG, Reiman EM, Shi J. Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity. Neurobiol Aging. 2014;35:2064–2071. doi: 10.1016/j.neurobiolaging.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Hashimoto H, Shintani N, Ohi K, Hori H, Saitoh O, Kosuga A, Tatsumi M, Iwata N, Ozaki N, Kamijima K, Baba A, Takeda M, Kunugi H. Possible association between the pituitary adenylate cyclase activating polypeptide (PACAP) gene and major depression. Neurosci Lett. 2010;468:300–302. doi: 10.1016/j.neulet.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Hatanaka M, Tanida M, Shintani N, Isojima Y, Kawaguchi C, Hashimoto H, Kakuda M, Haba R, Nagai K, Baba A. Lack of light-induced elevation of renal sympathetic nerve activity and plasma corticosterone levels in PACAP-deficient mice. Neurosci Lett. 2008;444:153–156. doi: 10.1016/j.neulet.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Heimesaat MM, Dunay IR, Bölke S, Fischer A, Grundmann U, Alutis M, Kühl AA, Tamas A, Toth G, Dunay MP, Göbel UB, Reglődi D, Bereswill S. Pituitary adenylate cyclase activating polypeptide ameliorates experimental acute ileitis and extra-intestinal sequelae. PLoS One. 2014;9:e108389. doi: 10.1371/journal.pone.0108389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimesaat MM, Reifenberger G, Vicena V, Illes A, Horvath G, Tamas A, Fulop BD, Bereswill S, Reglodi D. Intestinal microbiota changes in mice lacking pituitary adenylate cyclase activating polypeptide (PACAP) – bifidobacteria make the difference. Eur J Microbiol Immunol (Bp) 2017;7:187–199. doi: 10.1556/1886.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyes Z, Kun J, Dobrosi N, Sandor K, Nemeth J, Perkecz A, Pinter E, Szabadfi K, Gaszner B, Tekus V, Szolcsanyi J, Steinhoff M, Hashimoto H, Reglodi D. Biro T (2015) pituitary adenylate cyclase activating polypeptide (PACAP) is up-regulated in murine skin inflammation and mediates transient receptor potential vanilloid-induced neurogenic edema. J Invest Dermatol. 2015;135:2209–2218. doi: 10.1038/jid.2015.156. [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Hennig GW, Nelson MT, May V, Vizzard MA (2018) PACAP38-mediated bladder afferent nerve activity hyperexcitability and Ca2+ activity in urothelial cells from mice. J Mol Neurosci. 10.1007/s12031-018-1119-x [DOI] [PMC free article] [PubMed]
- Holighaus Y, Mustafa T, Eiden LE. PAC1hop, null and hip receptors mediate differential signaling through cyclic AMP and calcium leading to splice variant-specific gene induction in neural cells. Peptides. 2011;32:1647–1655. doi: 10.1016/j.peptides.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath G, Illes A, Heimesaat MM, Bardosi A, Bardosi S, Tamas A, Fulop BD, Opper B, Nemeth J, Ferencz A, Reglodi D (2016) Protective intestinal effects of pituitary adenylate cyclase activating polypeptide. In: Reglodi and Tamas (ed) Pituitary adenylate cyclase activating polypeptide-PACAP, Current Topics in Neurotoxicity 11. Springer Nature, New York, pp 271–288
- Horvath G, Kiss P, Nemeth J, Lelesz B, Tamas A, Reglodi D. Environmental enrichment increases PACAP levels in the CNS of adult rats. Neuro Endocrinol Lett. 2015;36:143–147. [PubMed] [Google Scholar]
- Horvath G, Mark L, Brubel R, Szakaly P, Racz B, Kiss P, Tamas A, Helyes Z, Lubics A, Hashimoto H, Baba A, Shintani N, Furjes G, Nemeth J, Reglodi D. Mice deficient in pituitary adenylate cyclase activating polypeptide display increased sensitivity to renal oxidative stress in vitro. Neurosci Lett. 2010;469:70–74. doi: 10.1016/j.neulet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- Horvath G, Reglodi D, Czetany P, Illes A, Reman G, Fekete A, Toth G, Laszlo E, Opper B (2018) Effects of pituitary adenylate cyclase activating polypeptide in human proximal tubule cells against gentamicin toxicity. Int J Pept Res Ther. 10.1007/s10989-017-9666-5
- Iemolo A, Seiglie M, Blasio A, Cottone P, Sabino V. Pituitary adenylate cyclase-activating polypeptide (PACAP) in the central nucleus of the amygdala induces anxiety via melanocortin receptors. Psychopharmacology. 2016;233:3269–3277. doi: 10.1007/s00213-016-4366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivic I, Fulop BD, Juhasz T, Reglodi D, Toth G, Hashimoto H, Tamas A, Koller A. Backup mechanism maintains PACAP/VIP-induced arterial relaxations in PACAP-deficient mice. J Vasc Res. 2017;54:180–192. doi: 10.1159/000457798. [DOI] [PubMed] [Google Scholar]
- Ivic I, Solymar M, Fulop BD, Hashimoto H, Toth G, Tamas A, Koller A, Reglodi D. Aging-induced modulation of pituitary adenylate cyclase-activating peptide- and vasoactive intestinal peptide-induced vasomotor responses in the arteries of mice. J Vasc Res. 2017;54:359–366. doi: 10.1159/000481781. [DOI] [PubMed] [Google Scholar]
- Ji H, Zhang Y, Shen XD, Gao F, Huang CY, Abad C, Busuttil RW, Waschek JA, Kupiec-Weglinski JW. Neuropeptide PACAP in mouse liver ischemia and reperfusion injury: immunomodulation by the cAMP-PKA pathway. Hepatology. 2013;57:1225–1237. doi: 10.1002/hep.25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Lim HC, Lee KH, Cha CI. Reduced immunoreactivities of a vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptor (VPAC1 receptor) in the cerebral cortex, hippocampal region, and amygdala of aged rats. Brain Res. 2005;1064:166–172. doi: 10.1016/j.brainres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Jozsa R, Somogyvari-Vigh A, Reglodi D, Hollosy T, Arimura A. Distribution and daily variations of PACAP in the chicken brain. Peptides. 2001;22:1371–1377. doi: 10.1016/S0196-9781(01)00477-6. [DOI] [PubMed] [Google Scholar]
- Juhasz T, Szentleleky E, Cs SS, Takacs R, Dobrosi N, Engler M, Tamas A, Reglodi D, Zakany R. Pituitary adenylate cyclase activating polypeptide (PACAP) pathway is induced by mechanical load and reduces the activity of hedghog signaling in chondrogenic micromass cell cultures. Int J Mol Sci. 2015;16:17344–17367. doi: 10.3390/ijms160817344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallo I, Kalamatianos T, Piggins HD, Coen CW. Ageing and the diurnal expression of mRNAs for vasoactive intestinal peptide and for the VPAC2 and PAC1 receptors in the suprachiasmatic nucleus of male rats. J Neuroendocrinol. 2004;16:758–766. doi: 10.1111/j.1365-2826.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- Kemeny A, Reglodi D, Cseharovszky R, Hashimoto H, Baba A, Szolcsanyi J, Helyes Z. Pituitary adenylate cyclase activating deficiency enhances oxazolone-induced allergic contact dermatitis in mice. J Mol Neurosci. 2010;42:443–449. doi: 10.1007/s12031-010-9368-3. [DOI] [PubMed] [Google Scholar]
- King SB, Lezak KR, O'Reilly M, Toufexis DJ, Falls WA, Braas K, May V, Hammack SE. The effects of prior stress on anxiety-like responding to intra-BNST pituitary adenylate cyclase activating polypeptide in male and female rats. Neuropsychopharmacology. 2017;42:1679–1687. doi: 10.1038/npp.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Pirger Z. Multifunctional role of PACAP-like peptides in molluscs. Protein Pept Lett. 2013;20:628–635. doi: 10.2174/0929866511320060003. [DOI] [PubMed] [Google Scholar]
- Kiss P, Reglodi D, Tamas A, Lubics A, Lengvari I, Jozsa R, Somogyvari-Vigh A, Szilvassy Z, Nemeth J. Changes of PACAP levels in the brain show gender differences following short-term water and food deprivation. Gen Comp Endocrinol. 2007;152:225–230. doi: 10.1016/j.ygcen.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Kormos V, Gaspar L, Kovacs LA, Farkas J, Gaszner T, Csernus V, Balogh A, Hashimoto H, Reglodi D, Helyes Z, Gaszner B (2016) Reduced response to chronic mild stress in PACAP mutant mice is associated with blunted FosB expression in limbic forebrain and brainstem centers. Neuroscience 330:335–358. Get rights and content 10.1016/j.neuroscience.2016.06.004 [DOI] [PubMed]
- Kovacs-Valasek A, Szabadfi K, Denes V, Szalontai B, Tamas A, Kiss P, Szabo A, Gy S, Reglodi D, Gabriel R. Accelerated retinal aging in PACAP KO mice. Neuroscience. 2017;348:1–10. doi: 10.1016/j.neuroscience.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Koves K (2016) Presence and role of PACAP in endocrine glands of mammals. In: Reglodi and Tamas (ed) Pituitary adenylate cyclase activating polypeptide-PACAP, Current Topics in Neurotoxicity 11. Springer Nature, New York, pp 161–178
- Krajcs N, Hernadi L, Zs P, Reglodi D, Toth G, Kiss T. PACAP modulates acetylcholine-elicited contractions at nicotinic neuromuscular contacts of the land snail. J Mol Neurosci. 2015;57:492–500. doi: 10.1007/s12031-015-0605-7. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Lillis TO Aging alters light- and PACAP-induced cAMP accumulation in the suprachiasmatic nucleus of female rats. Brain Res. 2002;950:297–303. doi: 10.1016/S0006-8993(02)03075-5. [DOI] [PubMed] [Google Scholar]
- Lacombe A, Lelievre V, Roselli CE, Muller JM, Waschek JA, Vilain E. Lack of vasoactive intestinal peptide reduces testosterone levels and reproductive aging in mouse testis. J Endocrinol. 2007;194:153–160. doi: 10.1677/JOE-07-0102. [DOI] [PubMed] [Google Scholar]
- Lacombe A, Lelievre V, Roselli CE, Salameh W, Lue YH, Lawson G, Muller JM, Waschek JA, Vilain E. Delayed testicular aging in pituitary adenylate cyclase-activating peptide (PACAP) null mice. Proc Natl Acad Sci U S A. 2006;103:3793–3798. doi: 10.1073/pnas.0505827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajko A, Meggyes M, Fulop BD, Gede N, Reglodi D, Szereday L. Comparative analysis of decidual and peripheral immune cells and immune-checkpoint molecules during pregnancy in wild-type and PACAP-deficient mice. Am J Reprod Immunol. 2017;9:e13035. doi: 10.1111/aji.13035. [DOI] [PubMed] [Google Scholar]
- Lam SY, Liu Y, Liong EC, Tipoe GL, Fung ML. Upregulation of pituitary adenylate cyclase activating polypeptide and its receptor expression in the rat carotid body in chronic and intermittent hypoxia. Adv Exp Med Biol. 2012;758:301–306. doi: 10.1007/978-94-007-4584-1_41. [DOI] [PubMed] [Google Scholar]
- Laszlo E, Varga A, Kovacs K, Jancso G, Kiss P, Tamas A, Szakaly P, Fulop B, Reglodi D. Ischemia/reperfusion-induced kidney injury in heterozygous PACAP deficient mice. Transplant Proc. 2015;47:2210–2215. doi: 10.1016/j.transproceed.2015.07.027. [DOI] [PubMed] [Google Scholar]
- Lee JC, Cho YJ, Kim J, Kim N, Kang BG, Cha CI, Joo KM. Region-specific changes in the immunoreactivity of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC2, and PAC1 receptor) in the aged rat brains. Brain Res. 2010;1351:32–40. doi: 10.1016/j.brainres.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Liao C, May V, Li J (2018) PAC1 receptors: shapeshifters in motion. J Mol Neurosci. 10.1007/s12031-018-1132-0 [DOI] [PMC free article] [PubMed]
- Lugo JM, Carpio Y, Morales R, Rodríguez-Ramos T, Ramos L, Estrada MP. First report of the pituitary adenylate cyclase activating polypeptide (PACAP) in crustaceans: conservation of its functions as growth promoting factor and immunomodulator in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2013;35:1788–1796. doi: 10.1016/j.fsi.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Ma BQ, Zhang M, Ba L. Plasma pituitary adenylate cyclase-activating polypeptide concentrations and mortality after acute spontaneous basal ganglia hemorrhage. Clin Chim Acta. 2015;439:102–106. doi: 10.1016/j.cca.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Maasz G, Zs P, Reglodi D, Petrovics D, Schmidt J, Kiss P, Rivnyak A, Hashimoto H, Avar P, Jambor E, Tamas A, Gaszner B, Mark L. Comparative protein composition of the brains of PACAP deficient mice using mass spectrometry based proteomic analysis. J Mol Neurosci. 2014;54:310–319. doi: 10.1007/s12031-014-0264-0. [DOI] [PubMed] [Google Scholar]
- Maasz G, Zrinyi Z, Reglodi D, Petrovics D, Rivnyak A, Kiss T, Jungling A, Tamas A, Pirger Z. Pituitary adenylate cyclase-activating polypeptide (PACAP) has neuroprotective function in dopamine-based neurodegeneration developed in rat and snail parkinsonian models. Dis Model Mech. 2017;10:127–139. doi: 10.1242/dmm.027185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai HN, Chung YH, Shin EJ, Sharma N, Jeong JH, Jang CG, Saito K, Nabeshima T, Reglodi D, Kim HC. IL-6 knockout mice are protected from cocaine-induced kindling behaviors; possible involvement of JAK2/STAT3 and PACAP signaling. Food Chem Toxicol. 2018;116:249–263. doi: 10.1016/j.fct.2018.04.031. [DOI] [PubMed] [Google Scholar]
- Marzagalli R, Leggio GM, Bucolo C, Pricoco E, Keay KA, Cardile V, Castorina S, Salomone S, Drago F, Castorina A. Genetic blockade of the dopamine D3 receptor enhances hippocampal expression of PACAP and receptors and alters their cortical distribution. Neuroscience. 2016;316:279–295. doi: 10.1016/j.neuroscience.2015.12.034. [DOI] [PubMed] [Google Scholar]
- Masmoudi-Kouki O, Douiri S, Hamdi Y, Kaddour H, Bahdoudi S, Vaudry D, Basille M, Leprince J, Fournier A, Vaudry H, Tonon MC, Amri M. Pituitary adenylate cyclase-activating polypeptide protects astroglial cells against oxidative stress-induced apoptosis. J Neurochem. 2011;117:403–411. doi: 10.1111/j.1471-4159.2011.07185.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Nakamachi T, Watanabe J, Sugiyama K, Ohtaki H, Murai N, Sasaki S, Xu Z, Hashimoto H, Seki T, Miyazaki A, Shioda S. Pituitary adenylate cyclase-activating polypeptide (PACAP) is involved in adult mouse hippocampal neurogenesis after stroke. J Mol Neurosci. 2016;59:270–279. doi: 10.1007/s12031-016-0731-x. [DOI] [PubMed] [Google Scholar]
- Mertens I, Husson SJ, Janssen T, Lindemans M, Schoofs L. PACAP and PDF signaling in the regulation of mammalian and insect circadian rhythms. Peptides. 2007;28:1775–1783. doi: 10.1016/j.peptides.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Miles OW, May V, Hammack SE (2018) Pituitary adenylate cyclase-activating peptide (PACAP) signaling and the dark side of addiction. J Mol Neurosci. 10.1007/s12031-018-1147-6 [DOI] [PMC free article] [PubMed]
- Mohammed H, Hannibal J, Fahrenkrug J, Santer R. Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: effects of age. Urol Res. 2002;30:248–255. doi: 10.1007/s00240-002-0261-6. [DOI] [PubMed] [Google Scholar]
- Moody TW, Ito T, Osefo N, Jensen RT. VIP and PACAP: recent insights into their functions/roles in physiology and disease from molecular and genetic studies. Curr Opin Endocrinol Diabetes Obes. 2011;18:61–67. doi: 10.1097/MED.0b013e328342568a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TW, Nuche-Berenguer B, Jensen RT. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr Opin Endocrinol Diabetes Obes. 2016;23:38–47. doi: 10.1097/MED.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TW, Osefo N, Nuche-Berenguer B, Ridnour L, Wink D, Jensen RT. Pituitary adenylate cyclase-activating polypeptide causes tyrosine phosphorylation of the epidermal growth factor receptor in lung cancer cells. J Pharmacol Exp Ther. 2012;341:873–381. doi: 10.1124/jpet.111.190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamachi T, Ohtaki H, Seki T, Yofu S, Kagami N, Hashimoto H, Shintani N, Baba A, Mark L, Lanekoff I, Kiss P, Farkas J, Reglodi D, Shioda S. PACAP suppresses dry eye signs by stimulating tear secretion. Nat Commun. 2016;7:12034. doi: 10.1038/ncomms12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth J, Tamas A, Jozsa R, Horvath JE, Jakab B, Lengvari I, Arimura A, Lubics A, Reglódi D. Changes in PACAP levels in the central nervous system after ovariectomy and castration. Ann N Y Acad Sci. 2006;1070:468–473. doi: 10.1196/annals.1317.063. [DOI] [PubMed] [Google Scholar]
- Ng SY, Chow BK, Kasamatsu J, Kasahara M, Lee LT. Agnathan VIP, PACAP and their receptors: ancestral origins of today’s highly diversified forms. PLoS One. 2012;7:e44691. doi: 10.1371/journal.pone.0044691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka N, Banks WA, Mizushima H, Shioda S, Morley JE. Regional differences in PACAP transport across the blood-brain barrier in mice: a possible influence of strain, amyloid beta protein, and age. Peptides. 2002;23:2197–2202. doi: 10.1016/S0196-9781(02)00248-6. [DOI] [PubMed] [Google Scholar]
- Ogata K, Shintani N, Hayata-Takano A, Kamo T, Higashi S, Seiriki K, Momosaki H, Vaudry D, Vaudry H, Galas L, Kasai A, Nagayasu K, Nakazawa T, Hashimoto R, Ago Y, Matsuda T, Baba A, Hashimoto H. PACAP enhances axon outgrowth in cultured hippocampal neurons to a comparable extent as BDNF. PLoS One. 2015;10:e0120526. doi: 10.1371/journal.pone.0120526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren SO, Kuteeva E, Elvander-Tottie E, Hökfelt T. Neuropeptides in learning and memory processes with focus on galanin. Eur J Pharmacol. 2010;626:9–17. doi: 10.1016/j.ejphar.2009.09.070. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, Yofu S, Hashimoto H, Shintani N, Baba A, Kopf M, Iwakura Y, Matsuda K, Arimura A, Shioda S. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, Satoh A, Nakamachi T, Yofu S, Dohi K, Mori H, Ohara K, Miyamoto K, Hashimoto H, Shintani N, Baba A, Matsunaga M, Shioda S. Regulation of oxidative stress by pituitary adenylate cyclase-activating polypeptide (PACAP) mediated by PACAP receptor. J Mol Neurosci. 2010;42:397–403. doi: 10.1007/s12031-010-9350-0. [DOI] [PubMed] [Google Scholar]
- Onoue S, Ohmori Y, Endo K, Yamada S, Kimura R, Yajima T. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide attenuate the cigarette smoke extract-induced apoptotic death of rat alveolar L2 cells. Eur J Biochem. 2004;271:1757–1767. doi: 10.1111/j.1432-1033.2004.04086.x. [DOI] [PubMed] [Google Scholar]
- Padua D, Vu JP, Germano PM, Pisegna JR. The role of neuropeptides in mouse models of colitis. J Mol Neurosci. 2016;59:203–210. doi: 10.1007/s12031-015-0688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RL, May V (2018) PACAP-induced PAC1 receptor internalization and recruitment of endosomal signaling regulate cardiac neuron excitability. J Mol Neurosci. 10.1007/s12031-018-1127-x [DOI] [PMC free article] [PubMed]
- Pettersson LM, Geremia NM, Ying Z, Verge VM. Injury-associated PACAP expression in rat sensory and motor neurons is induced by endogenous BDNF. PLoS One. 2014;9:e100730. doi: 10.1371/journal.pone.0100730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirger Z, Laszlo Z, Kemenes I, Toth G, Reglodi D, Kemenes G. A homologue of vertebrate adenylate cyclase activating polypeptide is both necessary and instructive for the rapid formation of associative memory in an invertebrate. J Neurosci. 2010;30:13766–13773. doi: 10.1523/JNEUROSCI.2577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirger Z, Naskar S, Laszlo Z, Gy K, Reglodi D, Kemenes I. Reversal of age related learning deficiency by the vertebrate pituitary adenylate cyclase activating polypeptide (PACAP) and insulin-like growth factor-1 (IGF-1) in a novel invertebrate model of aging: the pond snail (Lymnaea stagnalis) J Gerontol A Biol Sci Med Sci. 2014;69:1331–1338. doi: 10.1093/gerona/glu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirger Z, Nemeth J, Hiripi L, Toth G, Kiss P, Lubics A, Tamas A, Hernadi L, Kiss T, Reglodi D. PACAP has anti-apoptotic effect in the salivary gland of an invertebrate species, Helix pomatia. J Mol Neurosci. 2008;36:105–114. doi: 10.1007/s12031-008-9070-x. [DOI] [PubMed] [Google Scholar]
- Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 2017;39:147–160. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Gasz B, Borsiczky B, Gallyas F, Jr, Tamas A, Jozsa R, Lubics A, Kiss P, Roth E, Ferencz A, Toth G, Hegyi O, Wittmann I, Lengvari I, Somogyvari-Vigh A, Reglodi D. Protective effects of pituitary adenylate cyclase activating polypeptide in endothelial cells against oxidative stress-induced apoptosis. Gen Comp Endocrinol. 2007;153:115–123. doi: 10.1016/j.ygcen.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Cseh S, Somoskoi B, Fulop BD, Szentleleky E, Szegeczki V, Kovacs A, Varga A, Kiss P, Hashimoto H, Tamas A, Bardosi A, Manavalan S, Bako E, Zakany R, Juhasz T. Disturbed spermatogenic signaling in pituitary adenylate cyclase activating polypeptide deficient mice. Reproduction. 2018;155:129–139. doi: 10.1530/REP-17-0470. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Zs F, Tamás A, Lubics A, Szeberényi J, Alexy T, Tóth K, Zs M, Borsiczky B, Rőth E, Szalontay L, Lengvári I. Effects of PACAP on in vitro and in vivo neuronal cell death, platelet aggregation, and production of reactive oxygen radicals. Regul Pept. 2004;123:51–59. doi: 10.1016/j.regpep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Helyes Zs, Nemeth J, Vass RA, Tamas A (2016) PACAP as a potential biomarker: alterations of PACAP levels in human physiological and pathological conditions. In: Reglodi and Tamas (ed) Pituitary adenylate cyclase activating polypeptide-PACAP, Current Topics in Neurotoxicity 11. Springer Nature, New York, pp 815–832
- Reglodi D, Illes A, Opper B, Schafer E, Tamas A, Nemeth J, Horvath G (2018b) Presence and effects of pituitary adenylate cyclase activating polypeptide under physiological and pathological conditions in the stomach. Front Endocrinol (Lausanne) 9(90). 10.3389/fendo.2018.00090 [DOI] [PMC free article] [PubMed]
- Reglodi D, Jungling A, Longuespée R, Kriegsmann J, Casadonte R, Kriegsmann M, Juhasz T, Bardosi A, Tamas A, Fulop BD, Kovacs K, Zs N, Sparks J, Miseta A, Mazzucchelli G, Hashimoto H, Bardosi A. Accelerated pre-senile systemic amyloidosis in PACAP knockout mice – a protective role of PACAP in age-related degenerative processes. J Pathol. 2018;245:478–490. doi: 10.1002/path.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Kiss P, Lubics A, Tamas A. Review of the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des. 2011;17:962–972. doi: 10.2174/138161211795589355. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Kiss P, Szabadfi K, Atlasz T, Gabriel R, Horvath G, Szakaly P, Sandor B, Lubics A, Laszlo E, Farkas J, Matkovits A, Brubel R, Hashimoto H, Ferencz A, Vincze A, Helyes Z, Welke L, Lakatos A, Tamas A. PACAP is an endogenous protective factor-insights from PACAP-deficient mice. J Mol Neurosci. 2012;48:482–492. doi: 10.1007/s12031-012-9762-0. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Renaud J, Tamas A, Tizabi Y, Socías B, Del-Bel E, Raisman-Vozari R. Novel tactics for neuroprotection in Parkinson’s disease: role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol. 2017;155:120–148. doi: 10.1016/j.pneurobio.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A. Pituitary adenylate cyclase activating polypeptide – PACAP. New York: Springer Nature; 2016. [Google Scholar]
- Reglodi D, Tamas A, Jungling A, Vaczy A, Rivnyak A, Fulop BD, Szabo E, Lubics A, Atlasz T. Protective effects of pituitary adenylate cyclase activating polypeptide against neurotoxic agents. Neurotoxicology. 2018;66:185–194. doi: 10.1016/j.neuro.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A, Lengvari I, Toth G, Szalontay L, Lubics A. Comparative study on the effects of PACAP in young, aging, and castrated males in a rat model of Parkinson’s disease. Ann N Y Acad Sci. 2006;1070:518–524. doi: 10.1196/annals.1317.072. [DOI] [PubMed] [Google Scholar]
- Rivnyak A, Kiss P, Tamas A, Balogh D, Reglodi D. Review on PACAP-induced transcriptomic and proteomic changes in neuronal development and repair. Int J Mol Sci. 2018;19:E1020. doi: 10.3390/ijms19041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Beltrán E, Correnti E, Deen M, Kamm K, Kelderman T, Papetti L, Vigneri S, Maassen Van Den Brink A, Edvinsson L, European Headache Federation School of Advanced Studies (EHF-SAS) PACAP38 and PAC1 receptor blockade: a new target for headache? J Headache Pain. 2018;19:64. doi: 10.1186/s10194-018-0893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudecki AP, Gray SL. PACAP in the defense of energy homeostasis. Trends Endocrinol Metab. 2016;27:620–632. doi: 10.1016/j.tem.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Kuno K, Takemoto M, He P, Ishikawa T, Onishi S, Ishibashi R, Okabe E, Shoji M, Hattori A, Yamaga M, Kobayashi K, Kawamura H, Tokuyama H, Maezawa Y, Yokote K. Pituitary adenylate cyclase-activating polypeptide protects glomerular podocytes from inflammatory injuries. J Diabetes Res. 2015;2015:727152. doi: 10.1155/2015/727152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor K, Kormos V, Botz B, Imreh A, Bolcskei K, Gaszner B, Markovics A, Szolcsanyi J, Shintani N, Hashimoto H, Baba A, Reglodi D, Helyes Z. Impaired nocifensive behaviours and mechanical hyperalgesia, but enhanced thermal allodynia in pituitary adenylate cyclase activating polypeptide deficient mice. Neuropeptides. 2010;44:363–371. doi: 10.1016/j.npep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Sarszegi Z, Szabo D, Gaszner B, Konyi A, Reglodi D, Nemeth J, Lelesz B, Polgar B, Jungling A, Tamas A (2018) Examination of pituitary adenylate cyclase activating polypeptide (PACAP) as a potential biomarker in heart failure patients. J Mol Neurosci. 10.1007/s12031-017-1025-7 [DOI] [PubMed]
- Sekar R, Wang L, Chow BK (2017) Central control of feeding behavior by the secretin, PACAP, and glucagon family of peptides. Front Endocrinol (Lausanne) 8(18). 10.3389/fendo.2017.00018 [DOI] [PMC free article] [PubMed]
- Shioda S, Takenoya F, Hirabayashi T, Wada N, Seki T, Nonaka N, Nakamachi T (2018) Effects of PACAP on dry eye symptoms, and possible use for therapeutic application. J Mol Neurosci. 10.1007/s12031-018-1087-1 [DOI] [PubMed]
- Solymar M, Ivic I, Balasko M, Fulop BD, Toth G, Tamas A, Gy R, Koller A, Reglodi D. Pituitary adenylate cyclase activating polypeptide (PACAP) ameliorates vascular dysfunction induced by hyperglycemia. Diab Vasc Dis Res. 2018;15:277–285. doi: 10.1177/1479164118757922. [DOI] [PubMed] [Google Scholar]
- Somogyi I, Boros A, Engelmann P, Nemeth J, Lubics A, Tamas A, Kiss P, Reglodi D, Pollak E, Molnar L. Pituitary adenylate cyclase activating polypeptide (PACAP)-like compounds could modulate the activity of coelomocytes in earthworm. Ann N Y Acad Sci. 2009;1163:521–523. doi: 10.1111/j.1749-6632.2009.04431.x. [DOI] [PubMed] [Google Scholar]
- Somogyvari-Vigh A, Jozsa R, Reglodi D, Hollósy T, Meggyesi R, Lengvari I, Arimura A. Influence of pinealectomy on levels of PACAP and cAMP in the chicken brain. Regul Pept. 2002;109:9–13. doi: 10.1016/S0167-0115(02)00164-7. [DOI] [PubMed] [Google Scholar]
- Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des. 2004;10:2861–2889. doi: 10.2174/1381612043383548. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z (2013) Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci 5(27). 10.3389/fnagi.2013.00027 [DOI] [PMC free article] [PubMed]
- Szabadfi K, Atlasz T, Kiss P, Danyadi B, Tamas A, Helyes Z, Hashimoto H, Shintani N, Baba A, Toth G, Gabriel R, Reglodi D. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) are more susceptible to retinal ischemic injury in vivo. Neurotox Res. 2012;21:41–48. doi: 10.1007/s12640-011-9254-y. [DOI] [PubMed] [Google Scholar]
- Szakaly P, Horvath G, Kiss P, Laszlo E, Farkas J, Furjes G, Nemeth J, Reglodi D. Changes in pituitary adenylate cyclase-activating polypeptide following renal ischemia-reperfusion in rats. Transplant Proc. 2010;42:2283–2286. doi: 10.1016/j.transproceed.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Szakaly P, Laszlo E, Kovacs K, Racz B, Horvath G, Ferencz A, Lubics A, Kiss P, Tamas A, Brubel R, Opper B, Baba A, Hashimoto H, Farkas J, Matkovits A, Magyarlaki T, Zs H, Reglodi D. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) show increased susceptibility to in vivo renal ischemia/reperfusion injury. Neuropeptides. 2011;45:113–121. doi: 10.1016/j.npep.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Tamas A, Lubics A, Lengvari I, Reglodi D. Effects of age, gender, and gonadectomy on neurochemistry and behavior in animal models of Parkinson’s disease. Endocrine. 2006;29:275–287. doi: 10.1385/ENDO:29:2:275. [DOI] [PubMed] [Google Scholar]
- Tamas A, Lubics A, Szalontay L, Lengvari I, Reglodi D. Age- and gender differences in behavioral and morphological outcome after 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Behav Brain Res. 2005;158:221–229. doi: 10.1016/j.bbr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Tamas A, Szabadfi K, Nemeth A, Fulop B, Kiss P, Atlasz T, Gabriel R, Hashimoto H, Baba A, Shintani N, Helyes Z, Reglodi D. Comparative examination of inner ear in wild type and pituitary adenylate cyclase activating polypeptide (PACAP) deficient mice. Neurotox Res. 2012;21:435–444. doi: 10.1007/s12640-011-9298-z. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D, Sanchez A, Yin X, Martinez J, Grammas P. Age-related decrease in cerebrovascular-derived neuroprotective proteins: effect of acetaminophen. Microvasc Res. 2012;8400:278–285. doi: 10.1016/j.mvr.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchikawa D, Nakamachi T, Tsuchida M, Wada Y, Hori M, Farkas J, Yoshikawa A, Kagami N, Imai N, Shintani N, Hashimoto H, Atsumi T, Shioda S. Neuroprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on spinal cord injury. J Mol Neurosci. 2012;48:508–517. doi: 10.1007/s12031-012-9817-2. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Fülöp GA, Kiss T, Csiszar A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. Geroscience. 2017;39:491–498. doi: 10.1007/s11357-017-9995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamos Z, Ivic I, Cseplo P, Toth G, Tamas A, Reglodi D, Koller A. Pituitary adenylate cyclase activating polypeptide (PACAP) induces relaxations of peripheral and cerebral arteries, which are impaired differently by aging. J Mol Neurosc. 2014;54:535–542. doi: 10.1007/s12031-014-0349-9. [DOI] [PubMed] [Google Scholar]
- Varhalmi E, Somogyi I, Kiszler G, Nemeth J, Reglodi D, Lubics A, Kiss P, Tamas A, Pollak E, Molnar L. Expression of PACAP-like compounds during the caudal regeneration of the earthworm Eisenia fetida. J Mol Neurosci. 2008;36:166–174. doi: 10.1007/s12031-008-9125-z. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Hamelink C, Damadzic R, Eskay RL, Gonzalez B, Eiden LE. Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides. 2005;26:2518–2524. doi: 10.1016/j.peptides.2005.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincze A, Reglodi D, Zs H, Hashimoto H, Shintani H, Abraham H. Role of pituitary adenylate cyclase activating polypeptide (PACAP) in myelination of the rodent brain: lessons from PACAP-deficient mice. Int J Dev Neurosci. 2011;29:923–935. doi: 10.1016/j.ijdevneu.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Wada Y, Nakamachi T, Endo K, Seki T, Ohtaki H, Tsuchikawa D, Hori M, Tsuchida M, Yoshikawa A, Matkovits A, Kagami N, Imai N, Fujisaka S, Usui I, Tobe K, Koide R, Takahashi H, Shioda S. PACAP attenuates NMDA-induced retinal damage in association with modulation of the microglia/macrophage status into an acquired deactivation subtype. J Mol Neurosci. 2013;51:493–502. doi: 10.1007/s12031-013-0017-5. [DOI] [PubMed] [Google Scholar]
- Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Seki T, Shioda S (2016) PACAP and neural development. In: Reglodi and Tamas (ed) Pituitary adenylate cyclase activating polypeptide-PACAP, Current Topics in Neurotoxicity 11. Springer Nature, New York, pp 65–82
- Werling D, Reglodi D, Banks WA, Salameh TS, Kovacs K, Kvarik T, Vaczy A, Kovacs L, Mayer F, Danyadi B, Lokos E, Tamas A, Toth G, Zs B, Tamas A, Atlasz T. Ocular delivery of PACAP1-27 protects the retina from ischemic damage in rodents. Invest Ophthalmol Vis Sci. 2016;57:6683–6691. doi: 10.1167/iovs.16-20630. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Ciallella JR, Flood DG, O'Kane TM, Bozyczko-Coyne D, Savage MJ. Comparative analysis of cortical gene expression in mouse models of Alzheimer’s disease. Neurobiol Aging. 2006;27:377–386. doi: 10.1016/j.neurobiolaging.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Yamada K, Matsuzaki S, Hattori T, Kuwahara R, Taniguchi M, Hashimoto H, Shintani N, Baba A, Kumamoto N, Yamada K, Yoshikawa T, Katayama T, Tohyama M. Increased stathmin1 expression in the dentate gyrus of mice causes abnormal axonal arborizations. PLoS One. 2010;5:e8596. doi: 10.1371/journal.pone.0008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Jiang X, Ji R, Meng L, Liu F, Chen X, Xin Y. Therapeutic potential of PACAP for neurodegenerative diseases. Cell Mol Biol Lett. 2015;20:265–278. doi: 10.1515/cmble-2015-0008. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yu R, Liu X, Guo X, Zeng Z. The expression of PAC1 increases in the degenerative thymus and low dose PACAP protects female mice from cyclophosphamide induced thymus atrophy. Peptides. 2012;38:337–343. doi: 10.1016/j.peptides.2012.09.009. [DOI] [PubMed] [Google Scholar]