Abstract
Although alkaline phosphatase (ALP) correlates with cardiovascular risk in adults, there are no studies in children. We evaluated the association between serum ALP levels, calcium-phosphorus product (Ca*P) and cardiovascular risk markers in healthy children. Children aged 7.9 ± 1.4 (n = 379) were recruited in this cross-sectional study. The main outcome measures were systolic and diastolic blood pressure (SBP and DBP) and carotid intima-media thickness (cIMT). Additional assessments were body-mass index (BMI), waist circumference, homeostatic model assessment of insulin resistance (HOMA-IR) and fasting lipids, ALP, serum calcium, phosphorus and Ca*P. ALP was directly correlated with BMI (p < 0.0001), waist circumference (p < 0.0001), SBP (p < 0.0001), cIMT (p = 0.005), HOMA-IR (p < 0.0001), and fasting triglycerides (p = 0.0001). Among them, in children with Ca*P values above the median the associations were BMI (r = 0.231; p = 0.001), waist (r = 0.252; p < 0.0001), SBP (r = 0.324; p < 0.0001), cIMT (r = 0.248; p = 0.001) and HOMA-IR (r = 0.291; p < 0.0001)]. ALP independently associated with SBP (β = 0.290, p < 0.001) and cIMT (β = 0.179, p = 0.013) in children with higher Ca*P, after adjusting for confounding variables. Circulating ALP is associated with a more adverse cardiovascular profile in children with higher Ca*P. We suggest that serum ALP and Ca*P levels could contribute to the assessment of risk for cardiovascular disease in children.
Introduction
Cardiovascular disorders have become a major public health concern due to their high prevalence in the adult population, thus their prevention during childhood is crucial. Consequently, gaining insight into early processes of disease and discovering new biomarkers for early intervention may turn out to be valuable.
Alkaline phosphatase (ALP) is widely expressed, most abundantly in bone, liver and kidneys. Circulating ALP originates mostly from bone and liver in adults, and predominantly from bone from birth to adolescence1. ALP typically catalyses the removal of the phosphate group from diverse phosphate-containing molecules, among other reactions1. Physiologically, one of the main roles of ALP is to help in the mineralization of hard tissues, the process whereby hydroxyapatite is deposited in the extracellular matrix, as it supplies the required phosphorus pool2.
In adults, positive correlations of ALP with waist circumference, blood pressure and serum triglycerides have been described, although these associations have not been adjusted for BMI3. Indeed, obese adults have higher concentrations of circulating ALP4. High circulating ALP, however, has been related to cardiovascular and coronary heart disease events independently of body-mass index (BMI), systolic blood pressure (SBP) or serum triglycerides3,5. Additional studies in older adults have also supported that high ALP levels associated with an increased risk of 1.19 and 1.10 of coronary heart disease and cardiovascular mortality, respectively, after adjustment for BMI6. In adult patients with kidney disease high levels of serum ALP were also linked to increased cardiovascular-related hospitalization7. No studies, however, have been reported in children.
Both calcium and phosphorus are essential substrates involved in the mineralization process. Whereas physiological calcification occurs in hard tissues, the same process can occur pathologically in soft tissues. Serum calcium-phosphorus product (Ca*P) has been linked to vascular calcification, and thus been regarded as a risk factor for extra-skeletal calcification8. For example, an elevated serum Ca*P concentration is considered to be a risk factor for coronary artery disease in adults with metabolic syndrome9.
In this context, our aim is to study whether circulating ALP concentrations are related to cardiovascular risk markers in school-aged children, particularly in those with higher Ca*P. As a secondary aim, we study the associations of ALP with anthropometric and metabolic parameters as they might act as confounding factors in cardiovascular risk assessment.
Results
Results for anthropometric, metabolic and cardiovascular parameters are shown in Table 1 for all the studied children (n = 379) enrolled in the study, and for subgroups thereof according to the median of Ca*P. None of the variables significantly differed among low and high Ca*P subgroups, except for age, HDL cholesterol and, as expected, calcium and phosphorus levels (Table 1).
Table 1.
Anthropometric, metabolic and cardiovascular variables in the studied subjects and in subgroups according to the median of the calcium and phosphorus product (Ca*P).
| All subjects | Ca*P below the median | Ca*P above the median | |
|---|---|---|---|
| n | 379 | 189 | 190 |
| Gender (female, %) | 49.6 | 47.6 | 51.6 |
| Age (years) | 7.9 ± 1.4 | 8.1 ± 1.4 | 7.6 ± 1.5b |
| Puberty (Tanner >1, %) | 13.2 | 13.2 | 13.2 |
| BMI (kg/m2) | 19.5 ± 4.2 | 19.6 ± 4.4 | 19.3 ± 4.0 |
| BMI-SDS (z-score) | 0.67 ± 1.45 | 0.65 ± 1.46 | 0.70 ± 1.44 |
| Waist (cm) | 65 ± 12 | 65 ± 12 | 64 ± 12 |
| Fat mass (%) | 30.2 ± 9.9 | 30.7 ± 10.2 | 29.7 ± 9.4 |
| SBP (mmHg) | 106 ± 10 | 106 ± 11 | 105 ± 10 |
| DBP (mmHg) | 61 ± 8 | 61 ± 8 | 61 ± 7 |
| cIMT (mm) | 0.041 ± 0.007 | 0.041 ± 0.007 | 0.040 ± 0.007 |
| Insulin (mcU/ml) | 5.2 ± 4.7 | 5.4 ± 5.0 | 5.0 ± 4.4 |
| HOMA-IR | 1.1 ± 1.1 | 1.2 ± 1.2 | 1.1 ± 1.0 |
| HDL cholesterol (mg/dL) | 58 ± 15 | 56 ± 14 | 60 ± 15a |
| Triglycerides (mg/dL) | 61 ± 31 | 63 ± 33 | 60 ± 29 |
| Alkaline phosphatase (U/L) | 240 ± 55 | 241 ± 58 | 240 ± 52 |
| Calcium (mg/dL) | 9.9 ± 0.3 | 9.8 ± 0.3 | 10.0 ± 0.3b |
| Phosphorus (mg/dL) | 4.9 ± 0.4 | 4.6 ± 0.3 | 5.2 ± 0.3b |
| Ca*P | 49 ± 5 | 45 ± 3 | 52 ± 3b |
Data are shown as mean ± SD for quantitative variables.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; cIMT, carotid intima-media thickness; HOMA-IR, Homeostatic model assessment of insulin resistance; HDL, high-density lipoprotein; Ca*P, calcium-phosphorus product.
ap < 0.05; bp < 0.001 for differences by Student’s t-test.
As regards the whole sample of children, serum ALP levels were related to various metabolic and cardiovascular parameters. Specifically, in the whole sample of subjects, ALP showed positive correlations with BMI, BMI-SDS, waist circumference, SBP, cIMT, insulin, HOMA-IR and fasting triglycerides (Table 2); and to a lesser extent, associations were also found with age, fat fraction and DBP (Table 2).
Table 2.
Pearson correlation coefficients for alkaline phosphatase and selected variables in the studied subjects and in subgroups according to the median of calcium and phosphorus product (Ca*P).
| All subjects (n = 379) | Ca*P below the median (n = 189) | Ca*P above the median (n = 190) | ||||
|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | |
| Age (years) | 0.104 | 0.042 | 0.010 | 0.893 | 0.205 | 0.005 |
| BMI (kg/m2) | 0.186 | <0.001 | 0.148 | 0.042 | 0.231 | 0.001 |
| BMI-SDS (z-score) | 0.176 | 0.001 | 0.150 | 0.039 | 0.205 | 0.005 |
| Waist (cm) | 0.202 | <0.001 | 0.159 | 0.028 | 0.252 | <0.001 |
| Fat mass (%) | 0.136 | 0.008 | 0.123 | 0.093 | 0.151 | 0.037 |
| SBP (mmHg) | 0.214 | <0.001 | 0.125 | 0.086 | 0.324 | <0.001 |
| DBP (mmHg) | 0.110 | 0.032 | 0.103 | 0.158 | 0.119 | 0.102 |
| cIMT (mm) | 0.145 | 0.005 | 0.053 | 0.469 | 0.248 | 0.001 |
| Insulin (mcU/ml) | 0.210 | <0.001 | 0.156 | 0.032 | 0.280 | <0.001 |
| HOMA-IR | 0.211 | <0.001 | 0.150 | 0.040 | 0.291 | <0.001 |
| HDL cholesterol (mg/dL) | −0.062 | 0.226 | −0.073 | 0.318 | −0.051 | 0.485 |
| Triglycerides (mg/dL) | 0.178 | 0.001 | 0.215 | 0.003 | 0.129 | 0.075 |
| Calcium (mg/dL) | 0.094 | 0.067 | 0.084 | 0.251 | 0.125 | 0.087 |
| Phosphorus (mg/dL) | −0.036 | 0.489 | −0.004 | 0.962 | −0.098 | 0.181 |
| Ca*P | 0.000 | 0.993 | 0.045 | 0.535 | −0.030 | 0.679 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; cIMT, carotid intima-media thickness; HOMA-IR, Homeostatic model assessment of insulin resistance; HDL, high-density lipoprotein; Ca*P, calcium-phosphorus product.
When dividing the sample of children by Ca*P groups, the ALP associations with BMI, BMI-SDS, waist circumference, insulin, and HOMA-IR were independent of the Ca*P group (Table 2). Serum triglycerides positively correlated with ALP only in the lower Ca*P group (r = 0.215; p = 0.003). Within the higher Ca*P group, circulating ALP levels were specifically associated with age (r = 0.205; p = 0.005), fat mass (r = 0.151; p = 0.037), SBP (r = 0.324; p < 0.0001; Fig. 1A) and cIMT (r = 0.248; p = 0.001: Fig. 1B; Table 2).
Figure 1.
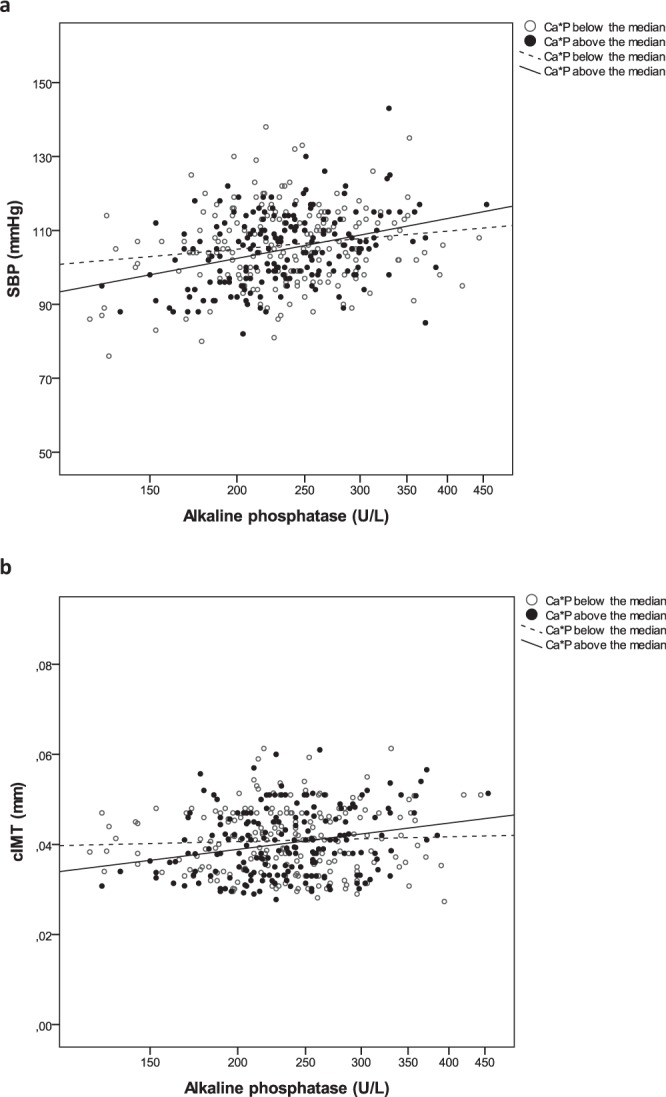
Correlation graphs between alkaline phosphatase (ALP) and cardiovascular risk markers in children. (A) Relationship between serum ALP and systolic blood pressure (SBP) in subgroups thereof according to the Ca*P median (Below the median r = 0.125, p = 0.086; above the median r = 0.324 p < 0.0001). (B) Relationship between serum ALP and carotid intima-media thickness (cIMT) in subgroups thereof according to the median of the calcium and phosphorus product (Ca*P; below the median r = 0.053, p = 0.469; above the median r = 0.248, p = 0.001).
Next, we investigated whether ALP was preferentially related to SBP and cIMT in children with higher Ca*P rather than in the lower Ca*P group. The interaction between ALP and Ca*P was significant after ANCOVA test for cIMT (p = 0.037, Table 3), confirming that the correlation between ALP and cIMT was stronger in children with higher Ca*P (r = 0.248) than in those with lower Ca*P (r = 0.053; Table 2). For SBP, the ANCOVA test did not reach statistical significance (p = 0.050, Table 3).
Table 3.
ANCOVA analysis testing the interaction of calcium and phosphorus product (Ca*P) in the association of alkaline phosphatase and SBP/cIMT (n = 379).
| SBP | cIMT | |||
|---|---|---|---|---|
| F | p-value | F | p-value | |
| Ca*P | 4.62 | 0.032 | 4.93 | 0.027 |
| Alkaline phosphatase | 19.79 | <0.001 | 9.44 | 0.002 |
| Ca*P x Alkaline phosphatase | 3.87 | 0.050 | 4.37 | 0.037 |
SBP, systolic blood pressure; cIMT, carotid intima-media thickness; Ca*P, calcium-phosphorus product.
Finally, in multivariable regression analyses adjusting for confounding variables (age, puberty, gender, BMI, HOMA-IR and triglycerides), ALP was shown to be independently associated with SBP (β = 0.290; p < 0.0001, R2 = 0.10), and with cIMT (β = 0.179; p < 0.013, R2 = 0.04; Table 4) in high Ca*P children.
Table 4.
Multivariable regression analysis of SBP and cIMT as dependent variables in the studied subjects and in subgroups thereof according to the median of calcium and phosphorus product (Ca*P).
| β | p-value | R2 | |
|---|---|---|---|
| SBP as dependent variable | |||
| All children (n = 379) | <0.001 | 0.14 | |
| BMI | 0.317 | <0.001 | 0.12 |
| Alkaline phosphatase | 0.156 | 0.002 | 0.02 |
| Ca*P below the median (n = 189) | <0.001 | 0.21 | |
| BMI | 0.396 | <0.001 | 0.20 |
| Triglycerides | 0.142 | 0.046 | 0.01 |
| Ca*P above the median (n = 190) | <0.001 | 0.12 | |
| Alkaline phosphatase | 0.295 | <0.001 | 0.10 |
| Age | 0.156 | 0.028 | 0.02 |
| cIMT as dependent variable | |||
| All children (n = 379) | <0.001 | 0.06 | |
| BMI | 0.217 | <0.001 | 0.05 |
| Alkaline phosphatase | 0.110 | 0.032 | 0.01 |
| Ca*P below the median (n = 189) | <0.001 | 0.08 | |
| BMI | 0.228 | 0.001 | 0.05 |
| Female gender | 0.199 | 0.005 | 0.03 |
| Ca*P above the median (n = 190) | <0.001 | 0.11 | |
| Age | 0.166 | 0.026 | 0.06 |
| Alkaline phosphatase | 0.180 | 0.014 | 0.04 |
| BMI | 0.150 | 0.046 | 0.01 |
Variables included in the model were the following: age, puberty, gender, BMI, SBP, HOMA-IR, triglycerides and alkaline phosphatase.
Discussion
Circulating ALP was found to relate to cardiovascular risk markers, such as SBP and cIMT, in school-aged children with higher circulating Ca*P. Serum ALP and Ca*P concentrations might thus contribute in the assessment of cardiovascular risk during childhood.
The present study appears to be the first to have related serum ALP levels to cardiovascular risk factors in children. Supporting our data, results reported in adults, showed that high ALP levels correlated with the risk of developing cardiovascular disease3,6 and total mortality5,10. Additionally, SBP and cIMT were also shown to be positively associated with serum ALP levels in hypertensive men11.
In bone, maturation of osteoblasts is coupled to the release of ALP-containing microvesicles that promote mineral deposition. Equivalent structures have been found in vascular tissue12. Metabolic imbalances, such as elevated calcium or phosphate levels, stimulate the up-regulation of several osteogenic markers in vascular smooth muscle cells, together with the secretion of the calcifying microvesicles13–16. This effect, coupled to downregulation of mineralization inhibitors in serum, eventually leads to vascular calcification15. Interestingly, ALP hydrolyses a major mineralization inhibitor, the inorganic pyrophosphate, thus regulating the propagation of mineralization17–20. Under pathological conditions, the enzyme contributes to the specific calcification of the medial layer of vasculature21, which triggers vascular changes as assessed by cIMT22. In a context of mineral homeostasis dysregulation in children, such as in renal diseases, higher serum ALP levels have been linked consistently to increased cIMT23,24. Together, these data fit with the present finding that higher ALP concentrations relate to greater cIMT in children with higher Ca*P.
Medial vascular calcification is similar to bone mineralization25 and causes concentric calcification of the blood vessel and elastinolysis26. This process has substantial cardiovascular consequences, resulting in vascular stiffening, reduced compliance and elastance, and ultimately increasing SBP and cardiac workload, and causing left ventricular cardiac hypertrophy and heart failure26–31. Thus, it seems plausible that due to early vascular calcification, SBP may be elevated. Accordingly, serum ALP levels have been associated with markers of vascular function and blood pressure in adults with and without hypertension11,32.
Ca*P levels seem to be influenced by age in our sample. Children between 7 and 10 years old may be using high levels of calcium for bone growth33, however calcium levels are rather stable during childhood34. On the contrary, phosphorus levels decrease when infants and children grow old34,35, thus affecting Ca*P levels. Therefore, our subsequent results have been adjusted for age to correct for this physiological effect. Our results did not reveal Ca*P as a cardiovascular risk factor since both groups of children showed a similar cardiovascular profile. Moreover, the role of Ca*P as a risk factor for cardiovascular diseases has been controversial36. Instead, the interaction between the Ca*P and ALP levels would indicate the outcome, thus in the high Ca*P group, ALP levels associated positively with cardiovascular risk markers (SBP and cIMT) but not in low Ca*P subgroup. This suggests the requirement of both elevated Ca*P and elevated ALP to render a poorer cardiovascular profile. As a consequence, when ALP or Ca*P were raised alone, there were no effects on SBP or cIMT. It is thus possible that high Ca*P primes children to develop vascular calcification while high ALP levels trigger the process12.
Study limitations include: (i) bone and liver isoforms of ALP were not discriminated, although, as said, most ALP derives from bone in children, (ii) the cross-sectional study design, which excluded proof of causality in the relationship between circulating ALP and either SBP or cIMT, (iii) relatively weak correlations of these clinical associations; however, taken into account that this is a population of healthy children, we believe they are notable and expect to find stronger correlations in children at higher risk for cardiovascular disease, such as those with a positive family history for this disease.
Whether the associations suggested by our results could be applied to the clinic, as part of a set of biomarkers to assess cardiovascular risk at childhood, should be confirmed in appropriate longitudinal studies. Moreover, the limitations of using ALP as a biomarker have to be explored as well, since growth spurts/puberty in children may result in highly variable ALP.
In summary, higher serum ALP levels were found to associate with a more unfavourable cardiovascular profile in children with higher Ca*P. Further studies are warranted to confirm whether circulating ALP and Ca*P levels might help in the detection of early vascular damage, and contribute to the development of paediatric strategies aimed at preventing cardiovascular disease in adulthood.
Methods
Population and ethics
A sample of school-aged children (n = 379), without a family history of cardiovascular disease (as assessed by interviewing the parents), participated in the study. Subjects were consecutively recruited among those seen in a primary care setting in Northeastern Spain [(mean age of 7.9 ± 1.4 years; mean body mass index (BMI)-standard deviation score (SDS) of 0.67 ± 1.45]. Puberty was assessed by a specifically trained nurse using Tanner criteria. Prepubertal children were those in Tanner stage I. Exclusion criteria were: major congenital anomalies; abnormal blood count; abnormal liver, kidney or thyroid functions; chronic illness or prolonged use of medication; acute illness or use of medication during the month previous to the potential enrolment. The study protocol was approved by the Institutional Review Board of Dr Josep Trueta Hospital and was carried out according with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Informed written consent was obtained from the parents. All data generated or analysed during this study are included in this published article.
Clinical assessments
Clinical examination was carried out in the morning. A calibrated scale and a Harpenden stadiometer were used to obtain weight and height measures, respectively. BMI was calculated with the following formula: weight in kg/(height in meters)2. BMI-SDS adjusted for age and sex, was computed using regional normative data37. Waist circumference at the umbilical level was measured in the supine position. Body composition was determined by bioelectric impedance (Hydra Bioimpedance Analyzer 4200, Xitron Technologies) and fat mass percentage was quantified with the formula: Fat mass = (body weight − lean mass)/*100. After a 10-minute rest, blood pressure was taken on the right arm with the child supine, using an electronic sphygmomanometer (Dinamap Pro 100, GE Healthcare).
High-resolution ultrasound measurement of carotid intima-media thickness
Carotid intima-media thickness (cIMT) was assessed by high-resolution ultrasonography (MyLabTM25, Esaote). Images were obtained using a linear 12-MHz transducer on the right side at the level of the distal common carotid artery, one centimetre away from its bifurcation. The cIMT value was computed as the average of 5 measurements. Intra-subject coefficient of variation was below 6%. None of the children included in the study exhibited visible signs of calcification as assessed by ultrasound.
Laboratory variables
Blood sampling was carried out in the morning under fasting conditions. Serum glucose was quantified by the hexokinase method. Insulin was detected by immunochemiluminiscence (Immulite 2000, Diagnostic Products). The limit of detection was 0.4 mIU/L and coefficient of variation (CV) was less than 10%. Homeostatic model assessment of insulin resistance (HOMA-IR) index was calculated according to the formula: fasting insulin (µU/mL) × fasting glucose (mg/dL)/405. Total triglycerides (TG) were quantified with glycerolphosphate oxidase (ARCHITECT, Abbott Laboratories), with detection threshold of 5 mg/dL and CV below 5%. A homogeneous method of selective detergent with accelerator (ARCHITECT, Abbott Laboratories) was used to determine HDL-cholesterol levels, with detection limit and CV of 2.5 mg/dL and inferior to 4%, respectively. ALP was quantified by colorimetrical detection of p-nitrophenyl phosphate product, with a limit of detection of 5 U/L. Inorganic phosphorus was colorimetrically assayed after reacting with ammonium molybdate in acidic medium (detection limit 0.3 mg/dL). Calcium was assessed using the chromophore 5-nitro-5′-methyl-1,2-bis(o-aminophenoxy)ethan-N,N,N’,N’-tetraacetic acid. Ca*P was calculated as the product between serum calcium and phosphorus.
Statistics
Statistical analyses were performed using SPSS version 22.0 (SPSS Inc.). The study has an 80% power to detect a significant Pearson correlation coefficient of at least 0.15 between ALP levels and cardio-metabolic parameters, accepting an alpha risk of 0.05 in a bilateral contrast (GRANMO, IMIM, version 7.12). Results are expressed as mean ± standard deviation (SD). Median Ca*P value was used to categorize subjects in subgroups with serum Ca*P above versus below the median. Non-normally distributed variables (Kolmogorov-Smirnov normality test) were mathematically transformed to improve symmetry with logarithmic and quadratic functions. Differences between Ca*P subgroups were assessed by Student t-test (continuous data) and by Chi square (categorical data). The relation between variables was tested by Pearson bivariate correlations followed by multivariable linear regression analyses. The stepwise method was used for computing the independent variables. Ca*P interaction in the association between ALP and systolic blood pressure (SBP)/cIMT was assessed by analysis of covariance (ANCOVA). Significance level was set at p < 0.05.
Acknowledgements
S.X-T is an investigator of the Sara Borrell Fund from Carlos III National Institute of Health, Spain (CD15-00162). FdZ is a Senior Investigator of the Clinical Research Fund of the Leuven University Hospital, Belgium. L.I. is a Clinical Investigator of CIBERDEM (Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders), from Carlos III National Institute of Health, Spain. J.B. is an investigator of the Miguel Servet Fund from Carlos III National Institute of Health, Spain (MS12/03239). A.L.-B. is an Investigator of the I3 Fund for Scientific Research (Ministry of Economy and Competitiveness, Spain). The study was supported by Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (ISCIII), Madrid, Spain (MS12/03239 and PI14/01625 to J.B, and PI13/01257 and PI16/01335 to A.L.-B), projects co-funded by FEDER (Fondo Europeo de Desarrollo Regional).
Author Contributions
S.X.-T. analyzed the data and wrote the draft of the manuscript, N.E. analyzed data, M.M.-C., A.P.-P., G.C.-B. and F.D.-R. acquired data, F.d.Z. and L.I. interpreted the data and participated in the critical revision of the manuscript; J.B. and A.L.-B. conceived the study, analyzed the data and critically revised the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Judit Bassols and Abel López-Bermejo jointly supervised this work.
Contributor Information
Judit Bassols, Email: jbassols@idibgi.org.
Abel López-Bermejo, Email: alopezbermejo@idibgi.org.
References
- 1.Whyte MP. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann N Y Acad Sci. 2010;1192:190–200. doi: 10.1111/j.1749-6632.2010.05387.x. [DOI] [PubMed] [Google Scholar]
- 2.Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 2010;77:4–12. doi: 10.1272/jnms.77.4. [DOI] [PubMed] [Google Scholar]
- 3.Kunutsor SK, et al. Serum Alkaline Phosphatase and Risk of Incident Cardiovascular Disease: Interrelationship with High Sensitivity C-Reactive Protein. PLoS One. 2015;10:e0132822. doi: 10.1371/journal.pone.0132822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AR, et al. Elevated serum level of human alkaline phosphatase in obesity. J Pak Med Assoc. 2015;65:1182–1185. [PubMed] [Google Scholar]
- 5.Tonelli M, et al. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation. 2009;120:1784–1792. doi: 10.1161/CIRCULATIONAHA.109.851873. [DOI] [PubMed] [Google Scholar]
- 6.Wannamethee SG, Sattar N, Papcosta O, Lennon L, Whincup PH. Alkaline phosphatase, serum phosphate, and incident cardiovascular disease and total mortality in older men. Arterioscler Thromb Vasc Biol. 2013;33:1070–1076. doi: 10.1161/ATVBAHA.112.300826. [DOI] [PubMed] [Google Scholar]
- 7.Abramowitz M, et al. Serum alkaline phosphatase and phosphate and risk of mortality and hospitalization. Clin J Am Soc Nephrol. 2010;5:1064–1071. doi: 10.2215/CJN.08621209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwak SM, et al. Dietary intake of calcium and phosphorus and serum concentration in relation to the risk of coronary artery calcification in asymptomatic adults. Arterioscler Thromb Vasc Biol. 2014;34:1763–1769. doi: 10.1161/ATVBAHA.114.303440. [DOI] [PubMed] [Google Scholar]
- 9.Kim WS, Lee DH, Youn HJ. Calcium-phosphorus product concentration is a risk factor of coronary artery disease in metabolic syndrome. Atherosclerosis. 2013;229:253–257. doi: 10.1016/j.atherosclerosis.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Li JW, Xu C, Fan Y, Wang Y, Xiao YB. Can serum levels of alkaline phosphatase and phosphate predict cardiovascular diseases and total mortality in individuals with preserved renal function? A systemic review and meta-analysis. PLoS One. 2014;9:e102276. doi: 10.1371/journal.pone.0102276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schutte R, et al. Alkaline phosphatase and arterial structure and function in hypertensive African men: the SABPA study. Int J Cardiol. 2013;167:1995–2001. doi: 10.1016/j.ijcard.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34:715–723. doi: 10.1161/ATVBAHA.113.302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Y, et al. MVsCarta: A protein database of matrix vesicles to aid understanding of biomineralization. Biosci Trends. 2015;9:190–192. doi: 10.5582/bst.2015.01061. [DOI] [PubMed] [Google Scholar]
- 14.Kapustin AN, et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109:e1–12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds JL, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004;66:2293–2299. doi: 10.1111/j.1523-1755.2004.66015.x. [DOI] [PubMed] [Google Scholar]
- 17.Harmey D, et al. Concerted regulation of inorganic pyrophosphate and osteopontin byakp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millan JL. The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int. 2013;93:299–306. doi: 10.1007/s00223-012-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prosdocimo DA, Wyler SC, Romani AM, O’Neill WC, Dubyak GR. Regulation of vascular smooth muscle cell calcification by extracellular pyrophosphate homeostasis: synergistic modulation by cyclic AMP and hyperphosphatemia. Am J Physiol Cell Physiol. 2010;298:C702–713. doi: 10.1152/ajpcell.00419.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav MC, et al. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheen CR, et al. Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res. 2015;30:824–836. doi: 10.1002/jbmr.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janda K, et al. Cardiovascular risk in chronic kidney disease patients: intima-media thickness predicts the incidence and severity of histologically assessed medial calcification in radial arteries. BMC Nephrol. 2015;16:78. doi: 10.1186/s12882-015-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gheissari A, et al. Carotid intima-media thickness in children with end-stage renal disease on dialysis. Indian J Nephrol. 2010;20:29–33. doi: 10.4103/0971-4065.62095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziolkowska H, Brzewski M, Roszkowska-Blaim M. Determinants of the intima-media thickness in children and adolescents with chronic kidney disease. Pediatr Nephrol. 2008;23:805–811. doi: 10.1007/s00467-007-0733-6. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 26.Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol. 2012;8:529–543. doi: 10.1038/nrendo.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensky NE, et al. Blood pressure and vascular calcification. Hypertension. 2010;55:990–997. doi: 10.1161/HYPERTENSIONAHA.109.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalra SS, Shanahan CM. Vascular calcification and hypertension: cause and effect. Ann Med. 2012;44(Suppl 1):S85–92. doi: 10.3109/07853890.2012.660498. [DOI] [PubMed] [Google Scholar]
- 29.Krzanowski M, et al. Relationship between aortic pulse wave velocity, selected proinflammatory cytokines, and vascular calcification parameters in peritoneal dialysis patients. J Hypertens. 2014;32:142–148. doi: 10.1097/HJH.0b013e32836569c7. [DOI] [PubMed] [Google Scholar]
- 30.McEniery CM, et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524–531. doi: 10.1161/HYPERTENSIONAHA.108.126615. [DOI] [PubMed] [Google Scholar]
- 31.Rattazzi M, Bertacco E, Puato M, Faggin E, Pauletto P. Hypertension and vascular calcification: a vicious cycle? J Hypertens. 2012;30:1885–1893. doi: 10.1097/HJH.0b013e328356c257. [DOI] [PubMed] [Google Scholar]
- 32.Cheung BM, Ong KL, Wong LY. Elevated serum alkaline phosphatase and peripheral arterial disease in the United States National Health and Nutrition Examination Survey 1999–2004. Int J Cardiol. 2009;135:156–161. doi: 10.1016/j.ijcard.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 33.New reference values for calcium. Ann Nutr Metab63, 186–192, (2013). [DOI] [PubMed]
- 34.de Kieviet W, Slaats EH, Abeling NG. Pediatric reference values for calcium, magnesium and inorganic phosphorus in serum obtained from Bhattacharya plots for data from unselected patients. J Clin Chem Clin Biochem. 1986;24:233–242. doi: 10.1515/cclm.1986.24.4.233. [DOI] [PubMed] [Google Scholar]
- 35.Ali, F. N. & Langman, C. B. In Clinical Pediatric Nephrology (eds Kanwal Kher, H. William Schnaper, & Sudesh Paul Makker) Ch. 3, 616 (Taylor & Francis Group, 2006).
- 36.O’Neill WC. The fallacy of the calcium-phosphorus product. Kidney Int. 2007;72:792–796. doi: 10.1038/sj.ki.5002412. [DOI] [PubMed] [Google Scholar]
- 37.Carrascosa Lezcano A, et al. [Spanish cross-sectional growth study 2008. Part II. Height, weight and body mass index values from birth to adulthood] An Pediatr (Barc) 2008;68:552–569. doi: 10.1157/13123287. [DOI] [PubMed] [Google Scholar]


