Abstract
Immune genes show remarkable levels of adaptive variation shaped by pathogen-mediated selection. Compared to humans, however, population polymorphism in animals has been understudied. To provide an insight into immunogenetic diversity in birds, we sequenced complete protein-coding regions of all Toll-like receptor (TLR) genes with direct orthology between mammals and birds (TLR3, TLR4, TLR5 and TLR7) in 110 domestic chickens from 25 breeds and compared their variability with a corresponding human dataset. Chicken TLRs (chTLRs) exhibit on average nine-times higher nucleotide diversity than human TLRs (hTLRs). Increased potentially functional non-synonymous variability is found in chTLR ligand-binding ectodomains. While we identified seven sites in chTLRs under positive selection and found evidence for convergence between alleles, no selection or convergence was detected in hTLRs. Up to six-times more alleles were identified in fowl (70 chTLR4 alleles vs. 11 hTLR4 alleles). In chTLRs, high numbers of alleles are shared between the breeds and the allelic frequencies are more equal than in hTLRs. These differences may have an important impact on infectious disease resistance and host-parasite co-evolution. Though adaptation through high genetic variation is typical for acquired immunity (e.g. MHC), our results show striking levels of intraspecific polymorphism also in poultry innate immune receptors.
Introduction
Domestic chickens and humans have a lot in common. Given their joint history, the domestic chicken is now an abundant and widespread species around the world. Both humans and chickens now serve as basic research model species representing genome references for birds and mammals. Yet domestic chicken breeds remain rarely studied from the perspective of evolutionary immunology. This is despite Darwin himself pointed out that variation in domestic fowl populations provides an excellent system for investigating evolution through natural and artificial selection1. Selection is particularly strong at shaping host-pathogen interactions, where different genes can be selected for genetic diversity. In the light of these facts, it is surprising how little attention has been paid to immunologically-relevant genetic variation in the highly diversified yet phenotypically standardised breeds of domestic fowl2–5.
Unlike the many laboratory and commercial lines more typically studied6,7, many domestic chicken breeds exhibit high genetic and phenotypic variation8–10. Several hundreds of breeds are now recognised worldwide, many of which are only locally distributed, having been maintained as stable phenotypic forms for centuries11. These traditional fowl breeds were originally domesticated from free-living red junglefowl subspecies, with possible admixture of other Gallus species, on multiple occasions in different regions of Asia12. This, together with distinct trade-driven migration routes and selection for different human needs in different environments, may have diversified the breeds phenotypically as regards pathogen resistance.
Previous studies suggest that the chicken genome is approximately two-times richer in exon polymorphism than the human genome13,14. From an evolutionary perspective, variation in innate immune receptor genes, which form a direct molecular interface between pathogens and their hosts, is particularly appealing since major evolutionary adaptations among polymorphic variants can be predicted15. Toll-like receptors (TLRs) act as innate immunity sensors responsible for detection of invading pathogen ligands during early phases of an infection16. TLRs are type I transmembrane proteins present either on the cell surface or in the intracellular compartments. They typically consist of a pathogen-recognition horseshoe-shaped ectodomain, a short segment spanning the membrane and an intracellular toll/interleukin-1 receptor (TIR) signalling domain17.
TLRs are encoded by a multigene family which is only partially conserved across vertebrates, e.g. people and chickens have similar numbers of TLR genes18, but only four functionally distinct TLRs show direct orthology between both species19: endosomal viral-dsRNA-sensing TLR320,21; TLR4 detecting bacterial lipopolysaccharide (LPS) and various other pathogen-derived and host-derived compounds on cell surfaces22,23; cell-surface-based bacterial-flagellin-sensing TLR524,25; and endosomal viral-ssRNA-sensing TLR726. The other TLRs may be duplicated (e.g. chicken TLR1 and TLR227), pseudogenised (chicken TLR826) or unique in either of the species (e.g. human TLR928; or chicken TLR1529 and TLR2130). Although human TLR7 and TLR8 are closely related, they slightly differ in their natural ligand preferences31–34.
Although usually unable to avoid expression of TLR ligands, pathogens in many cases have succeeded in evolving structural modifications that impair recognition by TLRs35. Co-evolution with pathogens can then select for diversification in TLR alleles through specific adaptations to ligand variants15. Accordingly, most parts of the TLR molecule remain highly conservative due to purifying selection, while other parts, such as the ligand-binding regions, exhibit striking variability, both at the interspecific and intraspecific levels36–40. This variation could affect disease resistance41,42.
In this study, we compare genetic variability and evolutionary patterns in TLR3, TLR4, TLR5 and TLR7 in humans (the only other species with large-sample intraspecific TLR diversity data publicly available), represented by 25 world-wide populations, and domestic chickens, represented by 25 traditional breeds. Information on sequence variation in these receptors is used to show differences in levels of potentially functional variation and the number of sites under positive selection between humans and domestic chickens. Furthermore, we also compare data on allele frequencies and allele sharing. Besides this, we examined the patterns of TLR variation with respect to a neutral mitochondrial marker and linked population structure in chicken TLRs (chTLR) to neutral population structure based on 19 microsatellites (allowing us comparison with previously published evidence for chicken breeds). In doing so, this study provides a pioneering insight into understanding the remarkable levels of variation in non-human immunogenetics when investigated outside traditional commercial and inbred models.
Results
Comparison of genetic variation in human and chicken TLRs
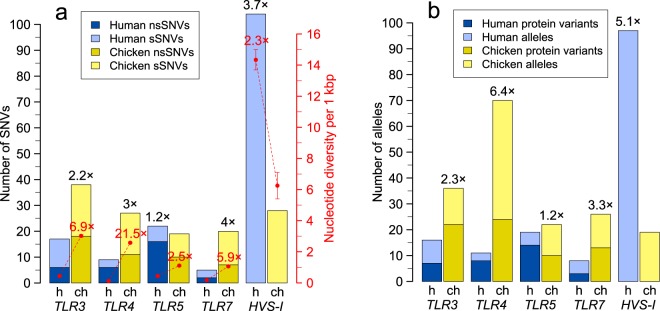
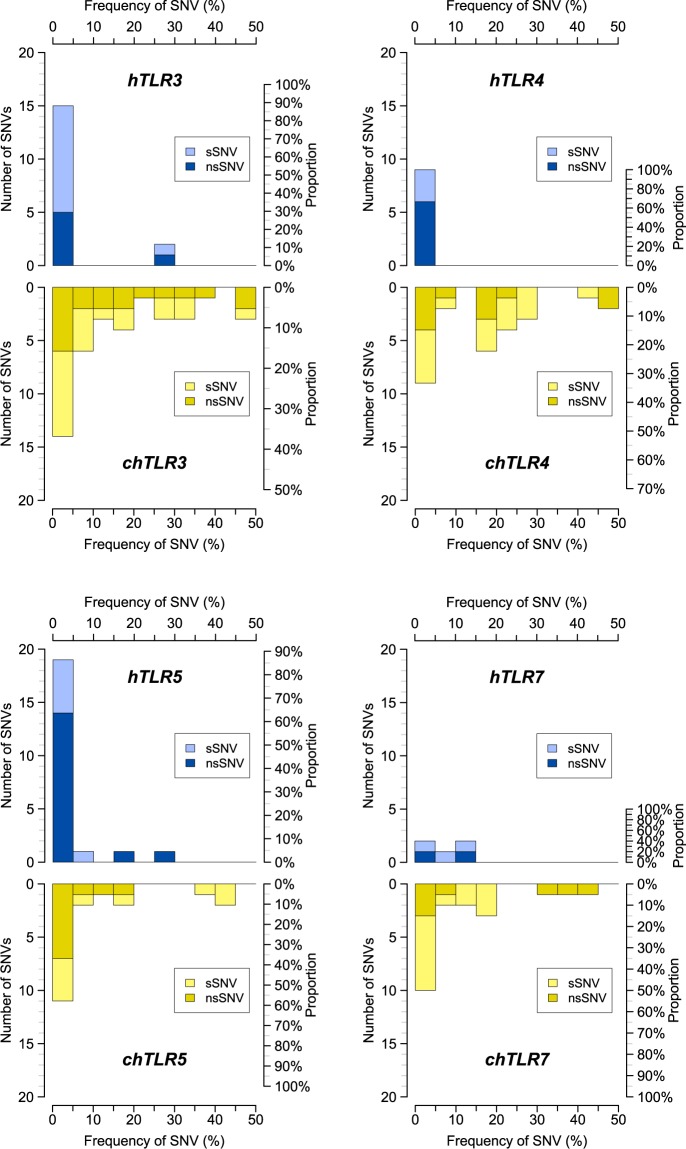
We sequenced complete protein-coding DNA sequences (CDSs) for all TLRs with direct orthologues between mammals and birds (TLR3, TLR4, TLR5 and TLR7) in 110 chickens (25 breeds) to compare variability with a corresponding set of 110 randomly-selected humans sampled from 25 populations around the world as part of the 1000 Genomes Project43. For a complete description of SNVs detected in chicken TLRs (chTLR), including their frequencies, see Supplementary Table S1. In most chTLRs (TLR3, TLR4 and TLR7) we observed between 2.2× and 4× more single nucleotide variants (SNVs) than in human TLRs (hTLRs), with 17 SNVs in hTLR3 vs 38 SNVs in chTLR3; 9 SNVs in hTLR4 vs 27 SNVs in chTLR4; and 5 SNVs in hTLR7 vs 20 SNVs in chTLR7 (Fig. 1a, Table 1). Only in TLR5 did we detect slightly more SNVs in hTLR5 (22 SNVs) than in chTLR5 (19 SNVs). Interestingly, 86% of the hTLR5 SNVs were very rare variants with frequencies below 5%, while only 58% of the SNVs were below 5% frequency in chTLR5 (Fig. 2). This is reflected in the nucleotide diversity (π), which was 2.5× higher in chTLR5 than hTLR5 (Fig. 1a, Table 1). While the frequency of SNVs in all hTLR genes was highly skewed, those found in chTLRs had a more equal variant representation and more SNVs of medium frequencies. This was also true for non-synonymous single nucleotide variants (nsSNVs) potentially affecting TLR structure and function (Fig. 2). The higher number of SNVs and more equal variant frequency meant that chTLRs exhibited nucleotide diversity up to 21.5× higher than hTLRs (Table 1). Unlike hTLRs, we found no sequences with internal STOP codons in chTLRs. In contrast to TLRs, part of the mitochondrial control region previously used as a neutral marker of variability in humans (hypervariable segment I; HVS-I) harboured 3.7× more SNVs in humans than chickens (Fig. 1a). Our results do not appear to be affected by the particular selection of the 110 individuals in the chicken or human populations. In chickens, we tested the effect of variation in the numbers of individuals across the breeds on those breeds where we have at least 6 animals per breed represented in the data set (six breeds: Araucana AR, Brahma BH, Czech Golden Pencilled CZ, La Flèche LF, Rosecomb Bantam RO, and Sebright SE). The results show that the average chicken nucleotide diversity does not importantly change with the number of individuals used per breed (2, 4 or 6): chTLR3 π110 = 0.00302, π(2 ind × 6 br) = 0.00300, π(4 ind × 6 br) = 0.00296, π(6 ind × 6 br) = 0.00301; chTLR4 π110 = 0.00258, π(2 ind × 6 br) = 0.00241, π(4 ind × 6 br) = 0.00250, π(6 ind × 6 br) = 0.00244; chTLR5 π110 = 0.00111, π(2 ind × 6 br) =0.00122, π(4 ind × 6 br) = 0.00114, π(6 ind × 6 br) = 0.00117; chTLR7 π110 = 0.00106, π(2 ind × 6 br) = 0.00099, π(4 ind × 6 br) =0.00100, π(6 ind × 6 br) = 0.00096. Also increasing the human dataset to the full sample of 2504 people represented in the 1000 Genomes Project did not importantly alter our estimates of human TLR nucleotide diversity: hTLR3 π110 = 0.00044 vs. π2504 = 0.00042, hTLR4 π110 = 0.00012 vs. π2504 = 0.00016, hTLR5 π110 = 0.00044 vs. π2504 = 0.00045, and hTLR7 π110 = 0.00018 vs. π2504 = 0.00019.
Figure 1.
Genetic variability in human and chicken Toll-like receptors (TLRs) and hypervariable segment I (HVS-I) of the mitochondrial DNA. (a) Comparison of numbers of single nucleotide variants (SNVs; left axis) and nucleotide diversity (right axis; red) found in human (h; blue) and chicken (ch; yellow) TLRs and HVS-I. Synonymous variants are shown in light colours and non-synonymous variants in dark colours. (b) Comparison of numbers of alleles and protein variants of TLRs found in human (h; blue) and chicken (ch; yellow) populations and number of alleles found in HVS-I. Total numbers of TLR protein variants are highlighted in dark colours, while numbers of alleles are represented by the total height of each bar (combination of light and dark colours). Numbers above the bars and points indicate the fold differences between values for humans and chickens. Error bars denote standard deviations (in TLR genes the bars are too short to be visible).
Table 1.
Diversity statistics of human and chicken TLRs.
| Gene | Sp | L (bp) | SNVs | nsSNVs | k | π ± SD | A | PV | Hd ± SD |
|---|---|---|---|---|---|---|---|---|---|
| TLR3 | h | 2715 | 17 | 6 | 1.187 | 0.00044 ± 0.00002 | 16 | 7 | 0.759 ± 0.016 |
| ch | 2691 | 38 | 18 | 8.120 | 0.00302 ± 0.00008 | 36 | 22 | 0.903 ± 0.011 | |
| TLR4 | h | 2520 | 9 | 6 | 0.310 | 0.00012 ± 0.00002 | 11 | 8 | 0.244 ± 0.038 |
| ch | 2532 | 27 | 11 | 6.540 | 0.00258 ± 0.00005 | 70 | 24 | 0.947 ± 0.010 | |
| TLR5 | h | 2577 | 22 | 16 | 1.143 | 0.00044 ± 0.00003 | 19 | 14 | 0.709 ± 0.025 |
| ch | 2586 | 19 | 10 | 2.866 | 0.00111 ± 0.00004 | 22 | 10 | 0.854 ± 0.013 | |
| TLR7 | h | 3150 | 5 | 2 | 0.566 | 0.00018 ± 0.00002 | 8 | 3 | 0.481 ± 0.037 |
| ch | 3180 | 20 | 7 | 3.363 | 0.00106 ± 0.00005 | 26 | 13 | 0.849 ± 0.017 |
Sp: species (h: human, ch: chicken); L (bp): sequence length in base pairs; SNVs: number of single nucleotide variants; nsSNVs: number of non-synonymous single nucleotide variants; k: average number of nucleotide differences between two sequences; π: nucleotide diversity (average number of nucleotide differences per site between two sequences); SD: standard deviation; A: number of alleles; PV: number of protein variants; Hd: haplotype diversity.
Figure 2.
Comparison of minor variant frequencies in human and chicken TLR single nucleotide variants (SNVs). Human TLRs (hTLRs) are highlighted in blue and chicken TLRs (chTLRs) in yellow, while allele frequencies of equivalent TLRs are shown specularly. Synonymous variants are shown in light colours and non-synonymous variants in dark colours. The left axis shows number of SNVs, while the right axis indicates the proportion of SNVs on a relative scale.
Location and physicochemical properties of the coding variants
While nsSNVs were more evenly distributed across genes in hTLRs, almost all nsSNVs (with three exceptions, two in chTLR3 and one in chTLR7) in chTLRs were located in the ligand-binding ectodomains (see Supplementary Fig. S1 and Supplementary Table S1). Based on 3D model measurements, thirteen chicken amino acid substitutions neighboured the predicted functional sites of TLRs with topological proximities of less than 10 Å (Fig. 3, Supplementary Table S1). Twelve nsSNVs found in chTLR3, six in chTLR4, six in chTLR5 and two in chTLR7 resulted in important differences in residue physicochemical properties, and hence may substantially influence the resultant ligand-binding features of the receptors (Fig. 3, Supplementary Table S1). Four amino acid substitutions in chTLR3, one in chTLR4, two in chTLR5 and one in chTLR7 were predicted by the PROVEAN analysis (score lower than the cut-off of −2.5) to affect the biological functions of the receptors (Fig. 3, Supplementary Tables S1 and S2). All of them were located in the ligand-binding ectodomain, but only three lied close to the predicted functional sites (<10 Å). Five of these non-conservative substitutions were consistent with the above-mentioned radical substitutions changing the physicochemical properties of the molecules. This was different than in hTLRs, where out of the six non-conservative substitutions detected by PROVEAN (one in hTLR3, one in hTLR4 and five in hTLR5), only three were located in the ectodomain, while the other three lied in the signalling TIR domain. Two of these substitutions were STOP codons (one in hTLR4 and one in hTLR5), three radically changed the residuum properties and only one was conservative (Supplementary Table S2).
Figure 3.
Projection of amino acid substitutions onto 3D models of chicken TLR ectodomains. Radical alternations are shown in red, conservative in blue and functionally important sites known from mammalian68,90,91 or fish92 studies are highlighted in black. Substitutions closer than 10 Å to functional sites are highlighted by a rectangle, non-conservative sites (PROVEAN) are indicated with yellow and positively selected sites (IFEL, FUBAR) with green arrows.
Detection of positive selection acting on TLRs
In total, we identified five sites in chTLR4 (A26V, R261K, K343R, Y383H and Q611R) and two in chTLR7 (I121V and S447G) that were under significant positive selection (IFEL: p < 0.05; FUBAR: posterior probability > 0.95; Supplementary Table S3). Two sites in chTLR4 (Y383H and Q611R) and the two sites in chTLR7 were recognised based on the two independent statistical approaches. In addition, we found three sites in chTLR3 (S17F, S399R, K513R), one site in chTLR4 (S23C) and two sites in chTLR5 (S409T and A619E) where positive selection was detected with only marginal non-significance (IFEL: 0.05 < p < 0.1; FUBAR: posterior probability 0.90 < p < 0.95). In contrast, we identified only two putatively positively selected sites in hTLRs (hTLR4: T399I; hTLR5: I644F), both being marginally non-significant in the tests. All positively selected sites in both species were located in the ligand-binding ectodomains, with the exception of I644F in hTLR5 which lay in the transmembrane domain (Fig. 3). None of the positively selected sites was consistent with the non-conservative sites with putatively dramatic functional effects detected by the PROVEAN analysis (Supplementary Table S2 and S3).
Allelic variability in TLRs and its distribution across chicken breeds
We identified 1.2× to 6.4× higher levels of TLR haplotype diversity (Hd) in chickens than in humans (Fig. 1b, Table 1; for a complete list of chTLR alleles and corresponding protein variants see Supplementary Table S4). Only in hTLR7 (site 665), chTLR3 (sites 495 and 1781), chTLR4 (site 903) and chTLR5 (site 2001) was there significant evidence for recombination between alleles (p < 0.01; confirmed by both SBP and GARD methods). Species-specific difference in allele number had a clear impact on the shape of the haplotype networks, with networks constructed from hTLR sequences exhibiting star-like patterns and networks constructed from chTLR sequences showing far more complex patterns (Supplementary Fig. S2). Haplotype diversity ranged from 0.244 to 0.759 in hTLRs, being in all cases lower than in chTLRs (Hd range 0.849–0.947; Table 1). The variation of individual human populations is generally low over all the receptors (Fig. S2). Similar to the nucleotide diversity, also in the case of haplotype diversity our results do not appear to be affected by the particular selection of the 110 individuals in the chicken or human population. In chickens, the effect of variation in the numbers of individuals across the breeds (the same six breeds as above) on the haplotype diversity was minor: chTLR3 Hd110 = 0.903, Hd(2 ind × 6 br) = 0.877, Hd(4 ind × 6 br) = 0.892, Hd(6 ind × 6 br) = 0.885; chTLR4 Hd110 = 0.947, Hd(2 ind × 6 br) = 0.913, Hd(4 ind × 6 br) = 0.941, Hd(6 ind × 6 br) = 0.942; chTLR5 Hd110 = 0.854, Hd(2 ind × 6 br) = 0.855, Hd(4 ind × 6 br) = 0.864, Hd(6 ind × 6 br) = 0.863; chTLR7 Hd110 = 0.849, Hd(2 ind × 6 br) = 0.855, Hd(4 ind × 6 br) = 0.825, Hd(6 ind × 6 br) = 0.824, and increasing the human dataset to the full sample of 2504 people represented in the 1000 Genomes Project did not importantly alter our estimate of the human TLR haplotype diversity: hTLR3 Hd110 = 0.759 vs. Hd2504 = 0.747, hTLR4 Hd110 = 0.244 vs. Hd2504 = 0.289, hTLR5 Hd110 = 0.709 vs. Hd2504 = 0.711, and hTLR7 Hd110 = 0.481 vs. Hd2504 = 0.481.
In chTLRs, many alleles were shared between chicken breeds (Supplementary Fig. S2 and S3 and Supplementary Table S4). Despite this allele sharing, distinguishable population structure was revealed based on both neutral loci (19 microsatellites) and TLRs alleles (Supplementary Fig. S4). Unlike microsatellites, however, variation in the allelic frequency of chTLRs did not allow detailed resolution of breed identity with increasing number of dividing groups (K).
Convergent evolution in chTLRs
We detected signals of convergent evolution in chTLR alleles, giving origin to six protein variants in chTLR4 and two in chTLR7 (Fig. 4, Supplementary Table S5). In contrast, no signs of convergence were identified in human TLRs or any other chicken TLRs.
Figure 4.
Convergent evolution in chicken TLR4 and TLR7, as indicated by identical protein variants originating from unrelated alleles. Alleles encoding the same protein variant are marked in the haplotype network with the same colour. Distinct clusters of convergent alleles are numbered, highlighted with circles and marked with A or B. (For distances between clusters see Supplementary Table S5).
Discussion
Immune-related genes involved in host defence are remarkable for their high levels of genetic polymorphism. Although typically investigated in genes of the vertebrate Major Histocompatibility Complex (MHC), innate immune receptors may also harbour striking levels of interspecific as well as intraspecific evolutionarily adaptive variation. Our results indicate that while this pattern remains elusive in human innate immune genes it can be clearly documented in domestic fowl. Since their domestication from wild red junglefowl (Gallus gallus) at least 4000 years ago44,45, chickens have evolved in a shared environment with humans46, coming into contact with joint microbial challenges including viral (e.g. avian influenza viruses47) or bacterial infections (e.g. Salmonella48, Campylobacter49; Helicobacter50,51 and others52). However, probably due to differences in their ancestral populations and distinct modes of recent selection, the two species are differentially equipped to face these threats. Therefore, chickens and humans may respond very differently to the same set of pathogens that infect both species (e.g. when infected with gastrointestinal pathogens53). Here we show that chicken TLR (chTLR) genes from population sets of comparable sizes exhibit up to 20× higher nucleotide diversity in up to 6× more alleles than human TLRs (hTLRs). As an example, Georgel et al. detected only four TLR4 alleles in a French human population54, compared with the 70 TLR4 alleles now detected in European chickens.
Previous genomic research has shown that, in general, the chicken genome is about 2× richer in exon polymorphism than the human genome13,14. Interestingly, we found even higher levels of genetic variation in chicken TLRs, with up to 4× more single nucleotide variants (SNVs) in chTLRs than hTLRs. The levels of human TLR nucleotide diversity indicated in our study (0.00012–0.00044) are fully comparable to those previously reported by Barreiro et al. (0.00027–0.00071)55. While the nucleotide diversity we report for chTLRs (0.00106–0.00302) is about 30× lower than that in the variable region of the human (0.024–0.071)56, chicken (0.0371–0.0400)57 and red junglefowl MHC (0.068–0.101)58, it is still far higher than either the gene average59 or levels typical for non-coding regions in humans60. Thus, though the distinct patterns of TLR variation observed in humans and chickens partially reflect the general features of their genomes, the contrast is higher than expected. This is further supported by the contrasting trend in mitochondrial HVS-I variation, which is almost 4× richer in human SNVs. Interestingly, it was in this same HVS-I region that the lower genetic variation of humans compared to other primates was first documented61; yet here we observe higher variation in humans than chickens. Our results show that while inter-breed differences (not tested in this study) change with increasing sample size within the breeds, the above-mentioned difference between species described in our dataset holds true even with changing sample sizes used for the analysis. This importantly supports the plausibility of our conclusions. However, we do not claim that the difference we found between human and chicken TLRs represents a general difference between birds and mammals. In wild house mouse TLR4 the levels of nucleotide diversity were previously found intermediate to the values detected in this study for humans and chickens62.
TLR5, with 22 human SNVs and 19 chicken SNVs, appears to break the general trend outlined above. In order to fully comprehend TLR5 variability, however, it is important to consider SNV frequency. Since 86% of hTLR5 variability comprises very rare variants, final nucleotide diversity (π) in TLR5 is actually more than 2× higher in chickens than humans. This pattern suggests weaker negative selection in hTLR5 compared to other hTLRs. In agreement with this hypothesis, STOP-codon-encoding substitutions have been identified in hTLR563 and some avian species64. We found no chTLR5 pseudogenes, suggesting a conservative essential function in chickens.
Only around 60% of chicken protein-coding genes are known to possess a single human orthologue65. There are several TLRs with orthologues missing or with several paralogues66. Therefore, to provide a biologically meaningful human-chicken comparison, the TLR genes investigated in this study were selected to represent direct orthologues with confirmed and conserved ligand-specificity. In chickens, most nsSNVs were located in regions encoding the ectodomains, suggesting a potential significance for TLR ligand-binding specificity. We recorded 26 non-synonymous (ns) SNVs that have an important impact on residue physicochemical properties, and 13 amino acid substitutions topologically neighbouring receptor functional sites. Of these, five amino acid substitutions (V68D, S89F, Q92K and A646S in chTLR3 and G413S in chTLR5) belong to both these groups and represent candidate sites putatively altering ligand specificity. Furthermore, the S89F variation in TLR3 was identified as a non-conservative substitution by the PROVEAN analysis.
The functional significance of some nsSNVs in chickens is further supported by the results of selection analysis. In contrast to hTLRs, we identified significant positive selection acting on the ligand-binding ectodomains in chTLR4 and chTLR7. While the functional significance of the putative positively selected TLR7 positions remains unclear, there are several candidate sites in TLR4 with high support for a functional effect. In particular, sites 261 and 343 are topological neighbours to the predicted MD-2-dimmerisation and LPS-binding residues67,68. Site 343 has previously been identified as under positive selection at the interspecific level in Galloanserae40, with positive selection at neighbouring sites also being reported in other avian and mammalian taxa39,69,70. Positions 383 and 611 in chTLR4, which harbour non-conservative substitutions that have been recognised as positively selected using several independent approaches (despite lying out of the predicted functional sites), also appear to be relevant. Sites adjacent to position 611 have also been reported as under selection in other vertebrates39,69. Finally, residues 383 and 611 have been identified as responsible for differences in salmonellosis resistance in chickens42.
Ongoing positive selection, therefore, appears stronger in chickens than humans, as supported by the distinct SNV frequency and allelic variability patterns in chTLRs and hTLRs. Since both SNVs and alleles show highly skewed frequency distributions in hTLRs, while those in chTLRs exhibit more equal SNV and allele representation, we assume that negative selection on TLRs is stronger in humans, while chTLRs appear to be under stronger balancing selection. From this perspective, chTLRs are closer in their co-evolution with pathogens to MHC than hTLRs71. Additional support for this view can be gained from the star-like hTLR haplotype network patterns (typical for post-selective-sweep populations). In contrast, the complex chTLR net-like patterns are indicative of either recombination or convergence. Correspondingly, haplotype diversity is low in hTLRs, while the levels in chTLRs resemble those of MHC57. Despite the generally higher recombination rates reported for the chicken genome65, our evidence of only four recombination breakpoints in 154 TLR alleles does not suggest that high levels of chTLR allelic variability were gained through recombination. Hence, convergent evolution may have been more important in chTLR evolution, especially in TLR4.
Of the total number of 154 chTLR alleles in four genes, 100 did not occur in more than one breed. Most of these 100 were low frequency alleles. In contrast, alleles that are more frequent are largely shared between breeds, which may have resulted from ancestral polymorphism maintained across selective breeding events or from inter-breed gene flow. Nevertheless, population structure is distinguishable, with both neutral microsatellites and TLRs suggesting differentiation in the breeds. This confirms previous reports of differentiation in chicken breeds using microsatellites8, though the shared polymorphism in chTLRs seems to be higher than in neutral markers. Apparently, some of the investigated chicken breeds possess high genetic diversity that could not be reliably fully captured with the limited sample used in this study. Therefore, this study has no ambition to compare the breeds one to another. Yet, recognising this phenomenon of inter-breed variation in immunogenetic variability, our study opens the way to further targeted research in larger population datasets.
The emergence of the domestic chicken was associated with inter(sub)specific hybridisation44,72,73 increasing their genetic diversity, similarly to humans74. Through their recent evolutionary history, both humans and chickens have been selected for survival under similar novel pathogenic environments. Unlike humans, that have been shown to exhibit relatively low levels of genetic variation61, chickens may be more diversified given their heterogeneous origin and artificial selection for diversity based on human cultural and economic needs. Taken together, our study documents that the broad spectrum of geographically distinct, locally-adapted chicken populations maintains tremendous immunogenetic variation in TLRs. Shaped by natural selection, the diversity shown by chicken TLRs far exceeds that in human TLRs, providing also an insight into the genetic basis of breed-specific variation in resistance to various infectious diseases75. While the data from chickens and humans cannot be directly generalised to birds and mammals, our results highlight that there are important interspecific differences in the levels of putatively functional genetic polymorphism in the innate immune receptors across vertebrates.
Methods
Genetic samples
Genetic samples of 110 chickens representing 25 randomly chosen traditional domestic breeds were provided by hobby breeders from the Czech Republic, Slovakia, Germany, France and Italy. The breeds selected were reported to have their origin in the Indian subcontinent, southeast Asia, Europe and South America76,77. Although the relatedness of these breeds is unknown and the pedigree information is missing, the breed-flock variation secured sampling of unrelated individuals. For each breed, 1–14 tissue (blood, feather or muscle) samples (average n = 4.4) were collected and stored in 96% ethanol at −20 °C. After analysis, all samples were deposited in the Genetic Bank of the Department of Zoology, Charles University, Prague (GRbio Institution Code: ZCU). For detailed information on the samples used see Supplementary Dataset 1. This research was carried out in accordance with Czech legislation (Act No 246/1992 Coll., on the protection of animals against cruelty) and approved by the Ethical Committee of the Faculty of Science, Charles University (Reference no. 34712/2010–30).
Molecular analysis of sequence variation in selected chicken genes
For sequencing of complete protein-coding DNA sequences (CDSs) of four chicken TLRs (chTLRs; 2691 bp of chTLR3, 2532 bp of chTLR4, 2586 bp of chTLR5 and 3180 bp of chTLR7) and 1–521 bp of hypervariable segment I (HVS-I; reference GenBank ID NC_001323.1) of the mitochondrial DNA control region describing the main chicken mitochondrial haplogroups44 we used Sanger sequencing that provides longer reads than most NGS techniques. Alleles were identified using PHASE78 and verified by cloning. Unique chTLR allele sequences were deposited in GenBank (IDs listed in Supplementary Table S4). For detailed protocols see Supplementary Methods online.
Sequence variation in selected human genes
Variability of human TLRs (hTLRs) and HVS-I was assessed based on sequence data available through the 1000 Genomes Project Phase 343, downloaded using the Data Slicer Tool. We extracted all hTLR variable sites in CDSs for all individuals. Due to the necessity of including two alleles of hTLR7, which is localised on the X chromosome in humans, only females were pre-filtered. A subset of 110 individuals from 25 populations was then randomly selected in R v. 3.2.179. As with chickens, the population sample size varied around an average of n = 4.4 individuals per population. For detailed information on the human sequences used see Supplementary Methods online.
Genetic diversity measurements in sequence data
Nucleotide diversity (π), average number of nucleotide differences (k) and haplotype diversity (Hd) for all selected TLRs and HVS-I were calculated in DnaSP v. 5.10.0178. Haplotype networks for all TLRs were constructed in Network v. 4.6.1.2. (Fluxus Technology) using a median-joining algorithm80. Additional properties, such as breed/population and TLR protein variant, were visualised in Network publisher v. 2.0.0.1 (Fluxus Technology). Alignments were screened for recombination breakpoints using the SBP (Single Breakpoint) and GARD (Genetic Algorithm for Recombination Detection) tools, available on the Datamonkey server81.
Prediction of the effects of amino acid substitutions on protein function
PROVEAN (Protein Variation Effect Analyzer) v 1.182 was used to predict the functional effect of the amino acid substitutions detected in the TLRs in our data set. The algorithm blasted and used between 181 and 265 homologous sequences from the NCBI NR protein database to predict the level of conservation of the individual sites in the particular TLRs. As a threshold for the effect on biological function we took the default value of delta alignment score of −2.5.
Selection analysis
Positive selection was tested using tools available on the Datamonkey server83. Individual sites under diversifying and purifying selection were detected using the codon-based maximum likelihood methods, FUBAR84 and IFEL. Results with P-values < 0.05 (IFEL) or posterior probabilities > 95% (FUBAR) were considered significant.
Protein structures
In order to ascertain the precise location of the variation in proteins, we adopted the approach previously described by Vinkler et al.40. Briefly, amino acid sequences coding the ligand-binding ectodomains of chTLRs were used to generate 3D models in the I-TASSER on-line tool85. Since proteolytic cleavage between leucine rich repeat 14 and 15 in the TLR7 endosomal domain has been shown to be essential for murine activation86 and human87 TLR7, we modelled the two parts of TLR7 separately. Models with highest C-scores were selected for analysis. Functional sites reported in the literature were visualised together with the variable sites, the distances between them being measured using the PyMOL Molecular Graphics System v. 1.5 (Schrödinger, LLC). The accuracy of the 3D models was checked using the ModFOLD Model Quality Assessment Server v. 4.088. All structures achieved high levels of confidence at P < 0.05 and Global model quality scores > 0.384. Details on amino acid classification adopted are provided in Supplementary Methods online.
Analysis of chicken population structure
The sample set of 110 chickens were genotyped for 19 unlinked microsatellite markers. Genetic population structure for both chTLR alleles and microsatellites was determined based on the Bayesian clustering approach implemented in STRUCTURE v. 2.3.489. For detailed description of our approach see Supplementary Methods online.
Electronic supplementary material
Acknowledgements
We are grateful to all breeders who kindly contributed to this study with samples from their birds. Namely we would like to express our gratitude to the Poultry committee of the Czech Breeders’ Association, to Dr. Hyláková from the Vyškov Zoological Garden, Czech Republic, and to Dr. Inga Tiemann from the Poultry Research Center, Bruno-Dürigen-Institut, Germany for their collaboration. We also wish to thank Iva Martincová for her help with animal handling, to Zdeňka Csibreiová for help with laboratory tasks, to Hana Velová for valuable comments on early versions of the manuscript, and to Dr. Kevin Roche for English language correction. This work was supported by the Czech Science Foundation (Project No. P502/12/P179), Charles University (Project No. PRIMUS/17/SCI/12 and Research Centre No. 204069), Charles University Grant Agency (Project No. 504214), Ministry of Education, Youth and Sports of the Czech Republic (Project No. INTER-COST LTC18060) and through Institutional Research Support (SVV 260 434 / 2018).
Author Contributions
M.V. designed the research, Z.Ś., A.Š., L.B., A.B. and A.F. conducted the study and Z.Ś. and P.M. analysed the data. Z.Ś. and M.V. wrote the main manuscript text, Z.Ś. prepared all figures and all authors reviewed the manuscript.
Data Availability
Unique chTLR allele sequences were deposited in GenBank (GenBank IDs KU235138- KU235484).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36226-1.
References
- 1.Darwin, C. The Variation of Animals and Plants Under Domestication. (Murray J., 1868). [PMC free article] [PubMed]
- 2.Huang YQ, et al. Haplotypic variation and characteristics across the toll-like receptor 3 locus in chickens. Anim. Genet. 2012;43:343–347. doi: 10.1111/j.1365-2052.2011.02244.x. [DOI] [PubMed] [Google Scholar]
- 3.Ruan W, Wu Y, Zheng SJ. Different genetic patterns in avian Toll-like receptor (TLR)5 genes. Mol. Biol. Rep. 2012;39:3419–3426. doi: 10.1007/s11033-011-1113-7. [DOI] [PubMed] [Google Scholar]
- 4.Ruan W, An J, Wu Y. Polymorphisms of Chicken TLR3 and 7 in Different Breeds. PLoS ONE. 2015;10:e0119967. doi: 10.1371/journal.pone.0119967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruan WK, Wu YH, An J, Zheng SJ. Polymorphisms of chicken Toll-like receptors 4, 15, and 21 in different breeds. Poult. Sci. 2012;91:2512–2516. doi: 10.3382/ps.2012-02319. [DOI] [PubMed] [Google Scholar]
- 6.Hillel J, et al. Biodiversity of 52 chicken populations assessed by microsatellite typing of DNA pools. Genet. Sel. Evol. GSE. 2003;35:533–557. doi: 10.1186/1297-9686-35-6-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muir WM, et al. Genome-wide assessment of worldwide chicken SNP genetic diversity indicates significant absence of rare alleles in commercial breeds. Proc. Natl. Acad. Sci. 2008;105:17312–17317. doi: 10.1073/pnas.0806569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyimo CM, et al. Global diversity and genetic contributions of chicken populations from African, Asian and European regions. Anim. Genet. 2014;45:836–848. doi: 10.1111/age.12230. [DOI] [PubMed] [Google Scholar]
- 9.Lyimo CM, et al. Maternal genealogical patterns of chicken breeds sampled in Europe. Anim. Genet. 2015;46:447–451. doi: 10.1111/age.12304. [DOI] [PubMed] [Google Scholar]
- 10.Weigend S, Romanov M. n. Current strategies for the assessment and evaluation of genetic diversity in chicken resources. Worlds Poult. Sci. J. 2001;57:275–288. doi: 10.1079/WPS20010020. [DOI] [Google Scholar]
- 11.Delany, M. E. Genetic diversity and conservation of poultry. In Poultry genetics, breeding, and biotechnology (eds. Muir, W. M. & Aggrey, S. E.) 257–281 (CABI Pub, 2003).
- 12.Groeneveld LF, et al. Genetic diversity in farm animals – a review. Anim. Genet. 2010;41:6–31. doi: 10.1111/j.1365-2052.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- 13.Sachidanandam R, et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409:928–933. doi: 10.1038/35057149. [DOI] [PubMed] [Google Scholar]
- 14.Wong GK-S, et al. A genetic variation map for chicken with 2.8 million single-nucleotide polymorphisms. Nature. 2004;432:717–722. doi: 10.1038/nature03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinkler M, Albrecht T. The question waiting to be asked: Innate immunity receptors in the perspective of zoological research. Folia Zool. 2009;58:15–28. [Google Scholar]
- 16.Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 17.Kang, J. Y. & Lee, J.-O. Structural Biology of the Toll-Like Receptor Family. In Annual Review of Biochemistry, Vol. 80 (eds. Kornberg, R. D., Raetz, C. R. H., Rothman, J. E. & Thorner, J. W.) 80, 917–941 (Annual Reviews, 2011). [DOI] [PubMed]
- 18.Velová, H., Gutowska-Ding, M. W., Burt, D. W. & Vinkler, M. Toll-like receptor evolution in birds: gene duplication, pseudogenisation and diversifying selection. Mol. Biol. Evol. 39, 2170–2184 (2018). [DOI] [PMC free article] [PubMed]
- 19.Cormican P, et al. The avian Toll-Like receptor pathway–subtle differences amidst general conformity. Dev. Comp. Immunol. 2009;33:967–973. doi: 10.1016/j.dci.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Karpala AJ, Lowenthal JW, Bean AG. Activation of the TLR3 pathway regulates IFNβ production in chickens. Dev. Comp. Immunol. 2008;32:435–444. doi: 10.1016/j.dci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz H, et al. Chicken toll-like receptor 3 recognizes its cognate ligand when ectopically expressed in human cells. J. Interferon Cytokine Res. 2007;27:97–101. doi: 10.1089/jir.2006.0098. [DOI] [PubMed] [Google Scholar]
- 22.Keestra AM, Putten JPM. van. Unique Properties of the Chicken TLR4/MD-2 Complex: Selective Lipopolysaccharide Activation of the MyD88-Dependent Pathway. J. Immunol. 2008;181:4354–4362. doi: 10.4049/jimmunol.181.6.4354. [DOI] [PubMed] [Google Scholar]
- 23.Kogut MH, et al. Expression and function of Toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 2005;29:791–807. doi: 10.1016/j.dci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal M, et al. Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar typhimurium. Infect. Immun. 2005;73:2344–2350. doi: 10.1128/IAI.73.4.2344-2350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keestra AM, de Zoete MR, van Aubel RAMH, van Putten JPM. Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol. Immunol. 2008;45:1298–1307. doi: 10.1016/j.molimm.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Philbin VJ, et al. Identification and characterization of a functional, alternatively spliced Toll-like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology. 2005;114:507–521. doi: 10.1111/j.1365-2567.2005.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temperley ND, Berlin S, Paton IR, Griffin DK, Burt DW. Evolution of the chicken Toll-like receptor gene family: A story of gene gain and gene loss. BMC Genomics. 2008;9:62. doi: 10.1186/1471-2164-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz A, Shen S, Adelson DL, Xavier S, Zhu JJ. Identification and sequence analysis of chicken Toll-like receptors. Immunogenetics. 2005;56:743–753. doi: 10.1007/s00251-004-0740-8. [DOI] [PubMed] [Google Scholar]
- 29.Higgs R, et al. Induction of a novel chicken Toll-like receptor following Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2006;74:1692–1698. doi: 10.1128/IAI.74.3.1692-1698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roach JC, et al. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. USA. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanji H, Ohto U, Shibata T, Miyake K, Shimizu T. Structural reorganization of the Toll-like receptor 8 dimer induced by agonistic ligands. Science. 2013;339:1426–1429. doi: 10.1126/science.1229159. [DOI] [PubMed] [Google Scholar]
- 32.Tanji H, et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 2015;22:109–115. doi: 10.1038/nsmb.2943. [DOI] [PubMed] [Google Scholar]
- 33.Shibata T, et al. Guanosine and its modified derivatives are endogenous ligands for TLR7. Int. Immunol. 2016;28:211–222. doi: 10.1093/intimm/dxv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, et al. Structural Analysis Reveals that Toll-like Receptor 7 Is a Dual Receptor for Guanosine and Single-Stranded RNA. Immunity. 2016;45:737–748. doi: 10.1016/j.immuni.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Reddick LE, Alto NM. Bacteria Fighting Back: How Pathogens Target and Subvert the Host Innate Immune System. Mol. Cell. 2014;54:321–328. doi: 10.1016/j.molcel.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alcaide M, Edwards SV. Molecular Evolution of the Toll-Like Receptor Multigene Family in Birds. Mol. Biol. Evol. 2011;28:1703–1715. doi: 10.1093/molbev/msq351. [DOI] [PubMed] [Google Scholar]
- 37.Downing T, Lloyd AT, O’Farrelly C, Bradley DG. The Differential Evolutionary Dynamics of Avian Cytokine and TLR Gene Classes. J. Immunol. 2010;184:6993–7000. doi: 10.4049/jimmunol.0903092. [DOI] [PubMed] [Google Scholar]
- 38.Smirnova I, Poltorak A, Chan EK, McBride C, Beutler B. Phylogenetic variation and polymorphism at the Toll-like receptor 4 locus (TLR4) Genome Biol. 2000;1:research002. doi: 10.1186/gb-2000-1-1-research002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinkler M, Bryjová A, Albrecht T, Bryja J. Identification of the first Toll-like receptor gene in passerine birds: TLR4 orthologue in zebra finch (Taeniopygia guttata) Tissue Antigens. 2009;74:32–41. doi: 10.1111/j.1399-0039.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- 40.Vinkler M, Bainová H, Bryja J. Protein evolution of Toll-like receptors 4, 5 and 7 within Galloanserae birds. Genet. Sel. Evol. 2014;46:72. doi: 10.1186/s12711-014-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beaumont C, et al. Effect of two candidate genes on the Salmonella carrier state in fowl. Poult. Sci. 2003;82:721–726. doi: 10.1093/ps/82.5.721. [DOI] [PubMed] [Google Scholar]
- 42.Leveque G, et al. Allelic Variation in TLR4 Is Linked to Susceptibility to Salmonella enterica Serovar Typhimurium Infection in Chickens. Infect. Immun. 2003;71:1116–1124. doi: 10.1128/IAI.71.3.1116-1124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature526, 68–74 (2015). [DOI] [PMC free article] [PubMed]
- 44.Miao Y-W, et al. Chicken domestication: an updated perspective based on mitochondrial genomes. Heredity. 2013;110:277–282. doi: 10.1038/hdy.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tixier-Boichard M, Bed’hom B, Rognon X. Chicken domestication: from archeology to genomics. C. R. Biol. 2011;334:197–204. doi: 10.1016/j.crvi.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Larson G, Fuller DQ. The Evolution of Animal Domestication. Annu. Rev. Ecol. Evol. Syst. 2014;45:115–136. doi: 10.1146/annurev-ecolsys-110512-135813. [DOI] [Google Scholar]
- 47.Van Reeth K. Avian and swine influenza viruses: our current understanding of the zoonotic risk. Vet. Res. 2007;38:243–260. doi: 10.1051/vetres:2006062. [DOI] [PubMed] [Google Scholar]
- 48.Guard-Petter J. The chicken, the egg and Salmonella enteritidis. Environ. Microbiol. 2001;3:421–430. doi: 10.1046/j.1462-2920.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- 49.Hermans D, et al. Poultry as a Host for the Zoonotic Pathogen Campylobacter jejuni. Vector-Borne Zoonotic Dis. 2012;12:89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- 50.Stanley J, et al. Helicobacter pullorum sp. nov.-genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiol. Read. Engl. 1994;140(Pt 12):3441–3449. doi: 10.1099/13500872-140-12-3441. [DOI] [PubMed] [Google Scholar]
- 51.Javed S, Gul F, Javed K, Bokhari H. Helicobacter pullorum: An Emerging Zoonotic Pathogen. Front. Microbiol. 2017;8:604. doi: 10.3389/fmicb.2017.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hampson DJ. The Spirochete Brachyspira pilosicoli, Enteric Pathogen of Animals and Humans. Clin. Microbiol. Rev. 2018;31(UNSP):e00087–17. doi: 10.1128/CMR.00087-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrow PA. The paratyphoid salmonellae. Rev. Sci. Tech. Int. Off. Epizoot. 2000;19:351–375. doi: 10.20506/rst.19.2.1225. [DOI] [PubMed] [Google Scholar]
- 54.Georgel, P., Macquin, C. & Bahram, S. The Heterogeneous Allelic Repertoire of Human Toll-Like Receptor (TLR) Genes. PLoS ONE4, (2009). [DOI] [PMC free article] [PubMed]
- 55.Barreiro, L. B. et al. Evolutionary Dynamics of Human Toll-Like Receptors and Their Different Contributions to Host Defense. PLoS Genet. 5, (2009). [DOI] [PMC free article] [PubMed]
- 56.Buhler S, Sanchez-Mazas A. HLA DNA Sequence Variation among Human Populations: Molecular Signatures of Demographic and Selective Events. PLOS ONE. 2011;6:e14643. doi: 10.1371/journal.pone.0014643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, et al. Sequence variations of the MHC class I gene exon 2 and exon 3 between infected and uninfected chickens challenged with Marek’s disease virus. Infect. Genet. Evol. 2014;21:103–109. doi: 10.1016/j.meegid.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 58.Worley K, et al. Single locus typing of MHC class I and class II B loci in a population of red jungle fowl. Immunogenetics. 2008;60:233–247. doi: 10.1007/s00251-008-0288-0. [DOI] [PubMed] [Google Scholar]
- 59.Li WH, Sadler LA. Low nucleotide diversity in man. Genetics. 1991;129:513–523. doi: 10.1093/genetics/129.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Z, Yu N, Fu Y-X, Li W-H. Nucleotide Variation and Haplotype Diversity in a 10-kb Noncoding Region in Three Continental Human Populations. Genetics. 2006;174:399–409. doi: 10.1534/genetics.106.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gagneux P, et al. Mitochondrial sequences show diverse evolutionary histories of African hominoids. Proc. Natl. Acad. Sci. 1999;96:5077–5082. doi: 10.1073/pnas.96.9.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fornuskova A, Bryja J, Vinkler M, Macholán M, Piálek J. Contrasting patterns of polymorphism and selection in bacterial-sensing toll-like receptor 4 in two house mouse subspecies. Ecol. Evol. 2014;4:2931–2944. doi: 10.1002/ece3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wlasiuk G, Khan S, Switzer WM, Nachman MW. A History of Recurrent Positive Selection at the Toll-Like Receptor 5 in Primates. Mol. Biol. Evol. 2009;26:937–949. doi: 10.1093/molbev/msp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bainová H, et al. First evidence of independent pseudogenization of Toll-like receptor 5 in passerine birds. Dev. Comp. Immunol. 2014;45:151–155. doi: 10.1016/j.dci.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Hillier LW, et al. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- 66.Boyd A, Philbin VJ, Smith AL. Conserved and distinct aspects of the avian Toll-like receptor (TLR) system: implications for transmission and control of bird-borne zoonoses. Biochem. Soc. Trans. 2007;35:1504–1507. doi: 10.1042/BST0351504. [DOI] [PubMed] [Google Scholar]
- 67.Ohto U, Fukase K, Miyake K, Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc. Natl. Acad. Sci. USA. 2012;109:7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park BS, et al. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 69.Areal H, Abrantes J, Esteves PJ. Signatures of positive selection in Toll-like receptor (TLR) genes in mammals. BMC Evol. Biol. 2011;11:368. doi: 10.1186/1471-2148-11-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grueber, C. E., Wallis, G. P. & Jamieson, I. G. Episodic Positive Selection in the Evolution of Avian Toll-Like Receptor Innate Immunity Genes. PLoS ONE9, (2014). [DOI] [PMC free article] [PubMed]
- 71.Key FM, Teixeira JC, de Filippo C, Andrés AM. Advantageous diversity maintained by balancing selection in humans. Curr. Opin. Genet. Dev. 2014;29:45–51. doi: 10.1016/j.gde.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Eriksson J, et al. Identification of the Yellow Skin Gene Reveals a Hybrid Origin of the Domestic Chicken. PLOS Genet. 2008;4:e1000010. doi: 10.1371/journal.pgen.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y-P, et al. Multiple maternal origins of chickens: out of the Asian jungles. Mol. Phylogenet. Evol. 2006;38:12–19. doi: 10.1016/j.ympev.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 74.Dannemann M, Andrés AM, Kelso J. Introgression of Neandertal- and Denisovan-like Haplotypes Contributes to Adaptive Variation in Human Toll-like Receptors. Am. J. Hum. Genet. 2016;98:22–33. doi: 10.1016/j.ajhg.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson KL, Brewer MT, Rasmussen MA, Carlson SA. Identification of heritage chicken breeds with diminished susceptibility to intestinal colonization by multiple antibiotic-resistant Salmonella spp. Livest. Sci. 2015;182:34–37. doi: 10.1016/j.livsci.2015.10.018. [DOI] [Google Scholar]
- 76.Pavel, I. & Tuláček, F. Vzorník plemen drůbeže (Catalogue of poultry breeds). (Český svaz chovatelů 2006).
- 77.Scrivener, D. Poultry Breeds and Management: An Introductory Guide. (Crowood Press, Limited, 2008).
- 78.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinforma. Oxf. Engl. 2009;25:1451–2. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 79.R Core Team. A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna). Available at: https://www.r-project.org/. (2015).
- 80.Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 81.Pond SLK, Posada D, Gravenor MB, Woelk CH, Frost SDW. Automated Phylogenetic Detection of Recombination Using a Genetic Algorithm. Mol. Biol. Evol. 2006;23:1891–1901. doi: 10.1093/molbev/msl051. [DOI] [PubMed] [Google Scholar]
- 82.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the Functional Effect of Amino Acid Substitutions and Indels. PLOS ONE. 2012;7:e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pond SLK, Frost SDW. Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinforma. Oxf. Engl. 2005;21:2531–3. doi: 10.1093/bioinformatics/bti320. [DOI] [PubMed] [Google Scholar]
- 84.Murrell B, et al. FUBAR: A Fast, Unconstrained Bayesian AppRoximation for Inferring Selection. Mol. Biol. Evol. 2013;30:1196–1205. doi: 10.1093/molbev/mst030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang J, Zhang Y. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 2015;43:W174–181. doi: 10.1093/nar/gkv342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanno A, et al. Essential role for Toll-like receptor 7 (TLR7)-unique cysteines in an intramolecular disulfide bond, proteolytic cleavage and RNA sensing. Int. Immunol. 2013;25:413–422. doi: 10.1093/intimm/dxt007. [DOI] [PubMed] [Google Scholar]
- 87.Hipp MM, et al. The Processed Amino-Terminal Fragment of Human TLR7 Acts as a Chaperone To Direct Human TLR7 into Endosomes. J. Immunol. 2015;194:5417–5425. doi: 10.4049/jimmunol.1402703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGuffin LJ, Buenavista MT, Roche DB. The ModFOLD4 server for the quality assessment of 3D protein models. Nucleic Acids Res. 2013;41:W368–W372. doi: 10.1093/nar/gkt294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pritchard JK, Stephens M, Donnelly P. Inference of Population Structure Using Multilocus Genotype Data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu L, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei T, et al. Homology modeling of human Toll-like receptors TLR7, 8, and 9 ligand-binding domains. Protein Sci. 2009;18:1684–1691. doi: 10.1002/pro.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoon S, et al. Structural Basis of TLR5-Flagellin Recognition and Signaling. Science. 2012;335:859–864. doi: 10.1126/science.1215584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Unique chTLR allele sequences were deposited in GenBank (GenBank IDs KU235138- KU235484).