Abstract
Acute kidney injury (AKI) and chronic kidney disease (CKD) are considered early and late phases of a pathologic continuum of interconnected disease states. Although changes in gene expression patterns have recently been elucidated for the transition of AKI to CKD, the epigenetic regulation of key kidney injury related genes remains poorly understood. We used multiplex RT-qPCR, ChIP-qPCR and integrative analysis to compare transcriptional and epigenetic changes at renal disease-associated genes across mouse AKI and CKD models. These studies showed that: (i) there are subsets of genes with distinct transcriptional and epigenetically profiles shared by AKI and CKD but also subsets that are specific to either the early or late stages of renal injury; (ii) differences in expression of a small number of genes is sufficient to distinguish AKI from CKD; (iii) transcription plays a key role in the upregulation of both AKI and CKD genes while post-transcriptional regulation appears to play a more significant role in decreased expression of both AKI and CKD genes; and (iv) subsets of transcriptionally upregulated genes share epigenetic similarities while downregulated genes do not. Collectively, our study suggests that identified common transcriptional and epigenetic profiles of kidney injury loci could be exploited for therapeutic targeting in AKI and CKD.
Introduction
The alarming increase in the incidence of AKI reflects multiple factors such as aging of the population, drug toxicities, and complications from procedures1. Historically, it has been taught that patients who recovered from AKI did not have adverse long-term sequelae2,3. However, more recent studies have provided evidence that not only do patients who return to baseline function after significant AKI progress toward CKD, but those with CKD are much more susceptible to AKI and thus proceed on a downward spiral2–5.
Advances in our understanding of the cellular and molecular basis of AKI and CKD are demonstrating that these two syndromes are in fact pathophysiologically interconnected1,6,7. In AKI, the initial injury to tubular epithelial cells, peritubular capillary endothelial cells, resident phagocytic cells, and pericytes triggers inflammatory responses which include the release of chemokines/cytokines and the recruitment of leukocytes8,9. Tubular cells undergo apoptosis and necrosis followed by the clearing of cell debris by phagocytic tubular epithelial cells as well as macrophages10,11. Surviving tubular epithelial cells first dedifferentiate and proliferate, and then re-differentiate back to epithelial cells, and by doing so restore normal or near-normal tubule architecture12–14. In this “adaptive” AKI repair scenario the microvascular injury and inflammation also resolve7,15. However, during the recovery phase of AKI, maladaptive repair pathways can be triggered and escalate into progressive interstitial fibrosis6,7. Here, the dedifferentiated proliferating tubule cells undergo pathologic G2/M-arrest and become senescent, remain in an epithelial-mesenchymal transition (EMT) state, and secrete several profibrotic mediators such as TGFβ16,7,16,17. The mechanistic factors that activate this trigger are largely unknown. Epigenetic regulation may play an important role in sustaining this activation. For example, aberrant promoter CpG island methylation can lead to transcriptional silencing of Rasal1 and promote renal fibrogenesis18,19.
Differential gene expression in part reflects both transcriptional and epigenetic changes. However, few studies have correlated gene expression with epigenetic changes in AKI and CKD models20–22 and no studies have been done across multiple models of acute and chronic injury. Understanding the role of epigenetic changes that are triggered during AKI and persist to drive CKD would open up potential avenues to mitigate renal injury. We used RT-qPCR and matrix chromatin immunoprecipitation (Matrix-ChIP) platforms23,24 in AKI and CKD mouse models to examine expression and epigenetic changes across a broad set of well-known kidney disease-associated genes.
Results
To investigate the relationship between epigenetic and transcriptional changes in the context of progression from AKI to CKD, we used three established mouse models of AKI: i) unilateral ischemia reperfusion (IR) at 26 h, ii) LPS at 2 h, and iii) combination of IR and LPS; and two well-characterized models of CKD: i) unilateral IR at 24–26 days and ii) unilateral ureteral obstruction (UUO) at 14 days25–29. We first assessed mRNA expression levels using RT-qPCR of a large collection of genes previously implicated in either AKI or CKD, and from these genes selected a subset for detailed epigenetic analysis by ChIP-qPCR.
AKI- and CKD-induced transcriptional changes
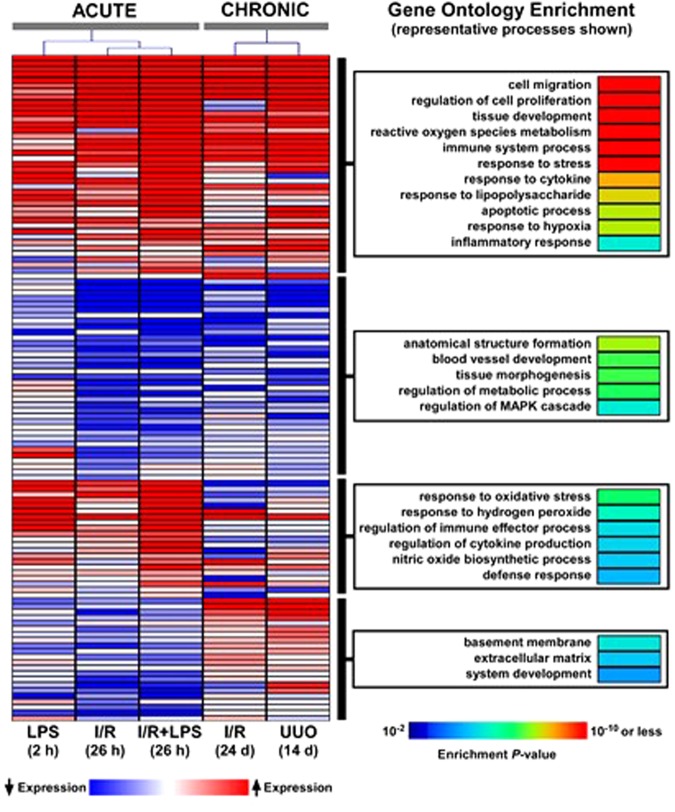
RT-qPCR25 was used to assess mRNA levels of a panel of 120 genes previously implicated in either AKI or CKD including pro-fibrotic mediators, Wnt/β-catenin pathway components, cytokines/chemokines, cell cycle, apoptosis, endothelial factors, hypoxia, components of extracellular matrix, and epigenetic mediators, (Tables S1, S3, S4 Supplement). We also included several well-studied kidney injury biomarkers. We assessed whether overall variability in gene expression across injury conditions can distinguish between the different models using correspondence analysis. We found that chronic injury transcript responses (IR 24–26 days and UUO 14 days) grouped together and clearly segregated from acute injury models (LPS 2 hrs, IR 26 hrs and I-R 26 hrs + LPS 2 hrs) (Fig. 1). In agreement with previous reports25, acute LPS and IR responses were also different from each other (Fig. 1). Analysis of these 120 candidate genes revealed four distinct expression patterns across injury models: genes that were either upregulated or downregulated in both AKI and CKD models, upregulated in AKI but not in CKD, and those that were upregulated in CKD but not in the AKI models (Figs 2 and S1). Although these 120 genes were not selected from an unbiased approach, we investigated whether the specific transcriptional patterns observed based on acute vs. chronic injury were associated with distinct functional groups using Gene Ontology (GO) analysis. Transcripts that were upregulated in both the AKI and CKD models were enriched in processes involved in cell migration and proliferation, tissue development, reactive oxygen metabolism, immune responses and other pathways known to be activated in renal injury. Transcripts that were downregulated in both AKI and CKD models were overrepresented in anatomical structure formation, blood vessel development, tissue morphogenesis and metabolic processes. The group of genes that were most highly upregulated in AKI but not in CKD included response to oxidative stress and hydrogen peroxide. Basement membrane and extracellular matrix processes topped the list of genes upregulated in CKD but not in AKI models consistent with evolving fibrogenesis as renal injury becomes more chronic. The GO analysis results are consistent with a recent transcriptome study30 and with cellular pathophysiology of AKI to CKD transition1,6–8,15,30.
Figure 1.
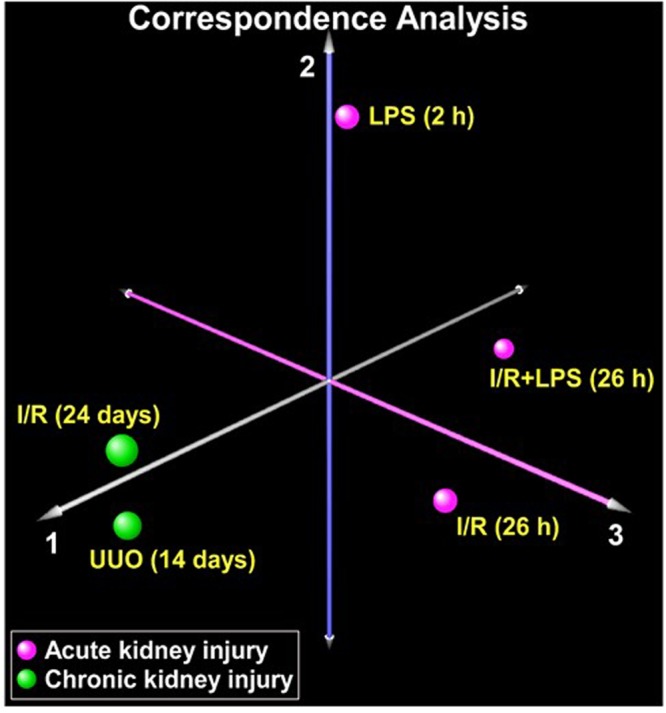
Correspondence analysis of mRNA expression variability across 120 candidate genes in acute kidney injury (AKI) and chronic kidney disease (CKD). Total RNA from injured kidneys and controls (contralateral) kidneys (n = 6 mice) was used in RT reactions with oligo-dT primers. cDNA was used in real time qPCR with gene specific primers (120 genes, Table S1, Supplement). mRNA level of a given gene in each sample was normalized to ribosomal RNA protein L32. For each experimental condition, the mean gene expression values from n = 6 mice were used for correspondence analysis. The analysis was based on assessing whether variability in gene expression across the experimental conditions distinguishes AKI models (magenta) from CKD models (green). Separation along each axis indicates differences in gene expression variability, with the first axis capturing the largest differences. As seen in the figure, AKI and CKD models segregated distinctly (axis 1), implying profound alterations in transcriptional signal between these two conditions. However, we also observed separation within the AKI models (axes 2 and 3) indicating gene expression variability between these acute renal injury conditions.
Figure 2.
Correspondence analysis of mRNA expression variability across 120 candidate genes in acute kidney injury (AKI) and chronic kidney disease (CKD). Total RNA from injured kidneys and controls (contralateral) kidneys (n = 6 mice) was used in RT reactions with oligo-dT primers. cDNA was used in real time qPCR with gene specific primers (120 genes, Table S1, Supplement). mRNA level of a given gene in each sample was normalized to ribosomal RNA protein L32. For each experimental condition, the mean gene expression values from n = 6 mice were used for correspondence analysis. The analysis was based on assessing whether variability in gene expression across the experimental conditions distinguishes AKI models (magenta) from CKD models (green). Separation along each axis indicates differences in gene expression variability, with the first axis capturing the largest differences. As seen in the figure, AKI and CKD models segregated distinctly (axis 1), implying profound alterations in transcriptional signal between these two conditions. However, we also observed separation within the AKI models (axes 2 and 3) indicating gene expression variability between these acute renal injury conditions.
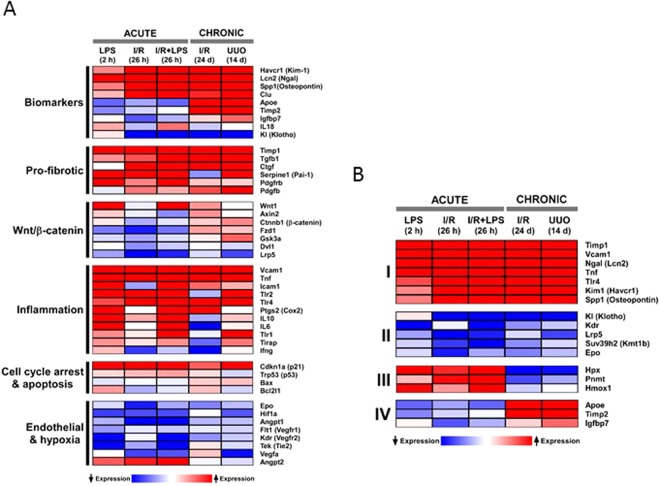
Several biomarkers of AKI have been validated and the mechanistic basis for their production are being defined31–33. Transcripts encoding AKI biomarkers including Kim-1 (Havcr1), Ngal (Lcn2), Spp1 (osteopontin) and Clu (clusterin) were increased across all AKI and CKD models (Figs 2 and 3, S1). Urinary TIMP-2 protein (tissue inhibitor of metalloproteinases 2) is a biomarker of renal injury31,34 and together with IGFBP7 (insulin-like growth factor binding protein 7) have been shown to be associated with adverse long-term outcomes in patients with AKI34,35. In contrast to Kim-1 and Ngal, Timp-2 and Igfbp734,35 were upregulated in the CKD but not in the AKI models. As serum creatinine is a poor marker of kidney function especially in children and the elderly, there is an unmet need to have additional biomarkers that define the AKI to CKD transition5. We identified several other genes that clustered with Timp-2 and Igfbp7 and were upregulated in CKD but not in AKI models, among them Apoe (apolipoprotein E) showed the largest difference between the two renal injury states (Fig. 3). Although little is known mechanistically about Apoe in renal injury, genetic variation in this gene has been linked to CKD progression36. The differences between Apoe mRNA levels in CKD compared to AKI models (also see30) suggest a potential value to explore urinary levels of APOE, together with TIMP-2, IGFBP7 and other proteins, to develop composite biomarkers of AKI-to-CKD progression.
Figure 3.
Heatmap representation of select candidate genes grouped based on their role in the pathogenesis of kidney injury. Database as in Fig. 2. (A) We chose to investigate a group of kidney injury-associated genes derived from the literature encompassing various putative roles in AKI and CKD. This analysis suggest the following. (i) mRNA levels of known biomarkers were increased in both AKI and CDK models (Kim-1, Ngal, osteopontin) while Apoe and Timp2 were highly elevated in CKD but not in AKI; (ii) several pro-fibrotic transcripts were upregulated across all AKI and CKD models; (iii) the overall magnitude of differential expression of Wnt/β-catanin pathway components was small; (iv) overall upregulation of inflammatory genes was higher in AKI than CKD; (v) except for p21 (which was upregulated across AKI and CKD) changes in cell cycle and apoptosis genes were small; (vi) generally there was downregulation of regulators of angiogenesis across AKI and CKD models; (B) Subset of genes with distinct expression patterns (see also Fig. 2) that were selected for detailed epigenetic analyses. There are shared transcriptional changes across AKI and CKD models with some exceptions (e.g. Apoe and Timp2) that could differentiate late from early injury.
Several pro-fibrotic-related transcripts were upregulated across all AKI and CKD models including Tgf-β, Ctgf, Pai-1, Pdgfβ and Pdgfr (Figs 3A and S1). This observation confirms previous reports that upregulation of these genes plays a role in the pro-fibrotic events, and our findings suggest that these effects start early in the injury process and persist.
Activated Wnt/β-catenin signaling pathway plays a key role in AKI to CKD progression, at least in part, by driving interstitial fibrosis37–39. We examined expression of several components of the Wnt pathway including Wnt1 (ligand), Fzd1 (receptor for Wnt) Ctnnb1 (β-catenin, transcription coactivator), Gsk3a (kinase), Axin2 (negative regulator), Dvl1 (DSH), and Lrp5 (co-receptor)40. The overall magnitude of differential expression of genes encoding components the Wnt/β-catenin pathway was smaller (compared to pro-fibrotic or biomarker genes) (Fig. 3A) suggesting that control of these pathways in kidney injury is predominantly at mRNA translation, protein modification and degradation steps40.
Sterile inflammatory response within the kidney is a hallmark of both AKI and CKD5,12. Several transcripts encoding known inflammatory mediators are upregulated in AKI (Tnf, Ccl2 (Mcp-1), Tlr2, Trl4, IL6, IL10, IL18, Cox2, Icam1 and Vcam1) (Fig. S1), but the expression levels of some of these genes decreased in the CKD phase (Fig. 3A). Overall, this upregulated set of genes indicates a higher inflammatory state in AKI compared to CKD.
Genes important in cell death and proliferation are induced following acute kidney injury and with the onset of CKD30. Increased expression of the cyclin-dependent kinase inhibitor p21 in AKI plays a key role in epithelial cell cycle arrest which appears to be beneficial in the acute phase41–43 but in the progression of AKI to CKD can lead to fibrosis43,44. p53 is a major regulator of cell death and its inhibition results in markedly reduced proximal tubular injury45,46. We have found that p21 mRNA expression was strongly upregulated in both the AKI and CKD models, while p53 transcript increases were small or unchanged (CKD UUO) (Figs 3A and S1). p53 regulates transcription and/or activity of several apoptosis factors including the pro-apoptopic Bax and anti-apoptopic Bcl1L2(Bcl-x). Bcl2 family of proteins have been implicated renal injury47,48. Given that changes of mRNAs encoding Bax and Bcl1L2/Bcl-x were relatively small suggest that mRNA translation and/or post-translational regulation of these factors maybe playing a more significant role in renal injury.
Tubulointerstitial hypoxia is a major contributor to the pathogenesis of AKI-to-CKD progression5,6,20,49–51. The mechanisms of capillary loss involve a host of endothelial and hypoxia-responsive factors52. The endothelial Ang-Tie-2 and VEGF-VEGFR signaling systems control angiogenesis and microvascular integrity53,54 and their downregulation (for example in sepsis) causes endothelial dysfunction and microvascular leak55. mRNA expression of several components of these signaling pathways (Angpt1, Flt1, Kdr, and Tek(Tie-2)) were downregulated across all models except for Angpt2 which was upregulated (Figs 3A and S1). Angpt1 and Angpt2 effects are typically antagonistic56 so their difference in mRNA expression is consistent with their opposing roles. The downregulation of key regulators of angiogenesis confirms the ongoing capillary dysfunction in the transition of acute to chronic kidney disease57.
Epigenetic changes in AKI and CKD
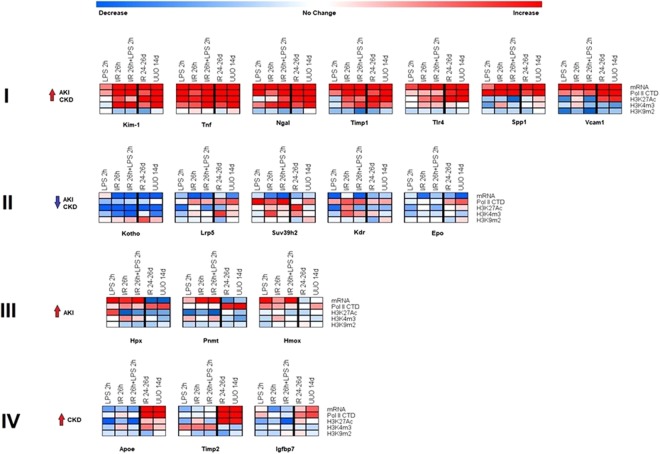
Transcript analysis identified subsets of genes that were either induced or repressed across the AKI and CKD models (Figs 2 and 3, S1). Although epigenetic changes such as DNA methylation are associated with CKD58, less is known about the correlation between histone modifications and the transcriptome during AKI and CKD. For our epigenetic studies, we selected representative candidate genes from each of the four identified expression patterns: i) upregulated in AKI and CKD models, Kim-1, Ngal, Tnf, Tlr4, Timp1 and Spp1(osteopontin); ii) downregulated in AKI and CKD, Klotho (KI), Lrp5, Kmt1b (Suv39h2), Kdr, and Epo; iii) upregulated in AKI but not in CKD, Hpx, and Pnmt, Hmox1; and iv) upregulated in CKD but not in AKI, Apoe, Timp2 and Igfbp7 (Fig. 3B). The choice of genes was driven by potential translational application in clinical settings5,34,59,60. We used the following ChIP-validated antibodies in Matrix ChIP: RNA Polymerase II CTD (Pol II CTD) as a measure of transcription levels; H3K27Ac, H3K4m1, H3K4m2, H3K4m3 permissive marks; and H3K27m3, H3K9m2, H3K9m3 repressive marks (Table S4)25,26,55,61. Across all models we found that within a given gene subset, the permissive marks H3K27Ac and H3K4m2, and the repressive H3K9m2 marks showed the most consistent changes with the transcript levels. In contrast, the permissive H3K4m1, H3K4m2 and repressive marks, H3K27m3 and H3K9m3 were less correlated with transcript levels and exhibited greater heterogeneity. We therefore have focused our discussion on H3K27Ac, H3K4m2 and H3K9m2 modifications. All ChIP data for each gene are shown in the Supplementary material (Figs S2–S20).
Genes upregulated in AKI and CKD
The upregulated transcripts (Kim-1, Ngal, Timp1, Tlr4, Tnf, Spp1, and Vcam1) were associated with higher levels of Pol II at the genes (Figs 4–6, S2–S9) suggesting that, at least in part, increased expression of these genes is transcriptionally mediated. Although all seven genes were upregulated, there were two strikingly different epigenetic subsets of genes. In one group, exemplified by Kim-1 (which also included Ngal, Timp1, Tlr4 and Tnf), upregulation of transcript and Pol II levels correlated with chromatin permissive structure as determined by increased histone acetylation and methylation marks H3K27Ac and H3K4m3, respectively (Figs 4–6, S2–S6, S9). In contrast, in the second group, there was not such correlation, i.e. transcriptional upregulation was not associated with increased H3K27Ac (Spp1) or H3K3m3 (Spp1, Vcam1) marks (Figs 4 and S2, 7, 8). All genes in this set (with the exception of Tnf) showed AKI and CKD-induced decreases in the repressive H3K9m2 modification, a change correlated with their upregulation.
Figure 4.
Integrated transcriptional and epigenetic analysis of selected sets of renal injury genes from AKI and CKD models. RT-qPCR RNA analysis. Total RNA from injured kidneys and controls kidneys (n = 6 mice for each model) was used in RT reactions with oligo-dT primers. cDNA was used in real time qPCR with gene specific primers (same as Figs 1–3). Transcript levels were normalized to ribosomal protein mRNA L32 (same as Figs 1–3) ChIP-qPCR analysis. Tissue fragments from injured and control (contralateral) kidneys (n = 6 mice for each injury model) were crosslinked and sonicated. Sheared chromatin was analyzed in Matrix ChIP-qPCR using antibodies to RNA polymerase II (Pol II) and antibodies to permissive (H3K27Ac, H3K4m3) and repressive (H3K9m2) marks. ChIP signals were normalized to input. Data represents log2 transformed ratios of means from injured kidneys over controls. Shown are genes whose expression was: (I) upregulated in both AKI and CKD; there was agreement between the increased in mRNA, Pol II and the permissive H3K27Ac levels. For most of these genes there was also increased in permissive H3K4m3 modification but decreased H3K9m3 repressive mark; (II) downregulated in both AKI and CKD;Pol II levels were either increased or in case of Klotho slightly decreased with decreased permissive but increased repressive modifications at this gene. (III) upregulated in AKI but not in CKD; Pol II levels in AKI were only moderately elevated and the epigenetic changes were small and (IV) upregulated in CKD but not in AKI; there was corresponding increase in Pol II and permissive H3K27Ac.
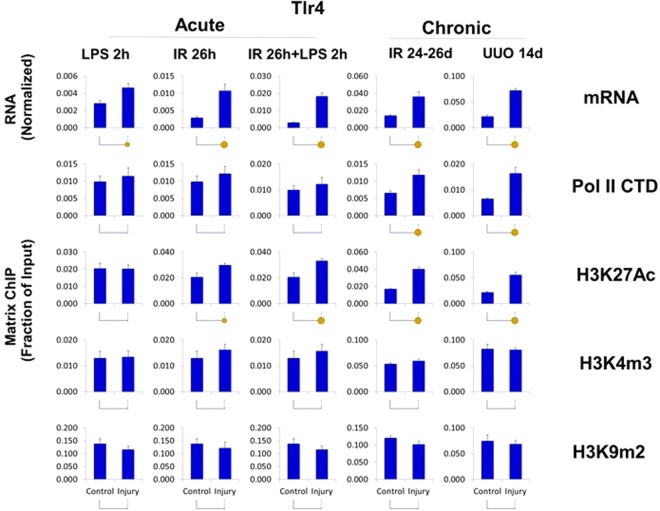
Figure 6.
Transcriptional and epigenetic analysis of Tlr4 gene in AKI and CKD models. RT-qPCR RNA and ChIP-qPCR analysis (see Fig. 4). Tlr4 transcript levels (mRNA) were normalized to L32 mRNA. Matrix ChIP-qPCR analysis of RNA polymerase II (Pol II), and permissive (H3K27Ac, H3K4m3) and repressive (H3K9m2) marks (n = 6 mice from each group). Small solid circle, p < 0.05, large circle, p < 0.01, and no circle, not statistically significant. The upregulated Tlr4 mRNA was matched by increased in Pol II levels in CKD and less so in AKI, where in the latter post-transcriptional processes maybe also playing a role.
Genes downregulated in AKI and CKD
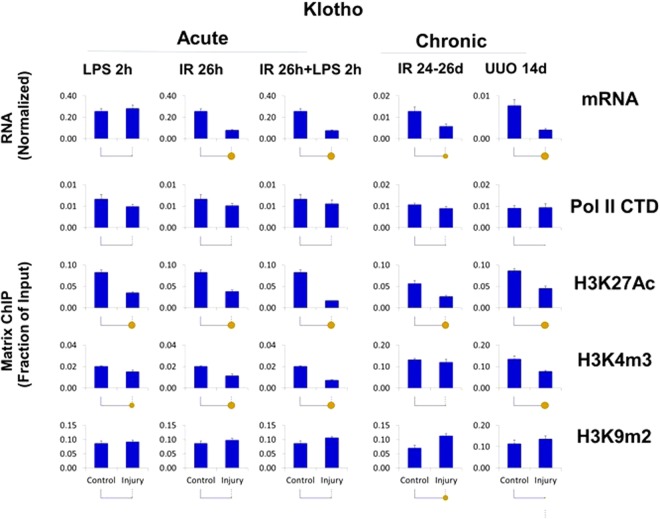
The set of mRNAs downregulated in both AKI and CKD models (Klotho, Lrp5, Suv39h2, Kdr and Epo) (Figs 4, 7 and S10–14) were not associated with matched decrease in Pol II levels suggesting that reduced message stability, rather than transcriptional suppression, play a more significant role in their lower expression. This was illustrated by Klotho (Figs 4, 7, S2 and S10), an increasingly important AKI and CKD biomarker, whose decreased levels are associated with worse renal outcomes60,62. On the one hand, decreased Klotho mRNA levels mirrored lower H3K27Ac and H3K4m3 marks while on the other there was a trend towards increased H3K9m2 levels. Other genes in this group, Lrp5 and Kdr, also exhibited decreased H3K27Ac but increased Pol II levels (Figs 4, S2, S11 and S13), again suggesting that their downregulation was meditated post-transcriptionally. In all these cases decreased transcription could also have been a contributing factor in their downregulation.
Figure 7.
Transcriptional and epigenetic analysis of Klotho (KI) gene in AKI and CKD models. RT-qPCR RNA and ChIP-qPCR analysis (see Fig. 4). Klotho transcript levels (mRNA) were normalized to L32 mRNA. Matrix ChIP-qPCR analysis RNA polymerase II (Pol II), and permissive (H3K27Ac, H3K4m3) and repressive (H3K9m2) marks (n = 6 mice from each group). Small solid circle, p < 0.05, large circle, p < 0.01, and no circle, not statistically significant. The downregulated Klotho transcripts were not matched by Pol II levels suggesting that post-transcriptional mechanisms are playing a role (e.g. splicing, message stability). Here, the epigenetic changes that correspond to mRNA levels could be playing a role.
Genes upregulated in AKI only
The common feature of genes in this set (Hpx, Pnmt and Hmox) (Figs 4, S2 and 15–S17) was the fact that the large AKI-induced increases in their transcript levels occurred with little or no change in Pol II density at these genes. Further, in both CKD models there was no increase in the mRNA levels in the injured kidney even though the Pol II levels at these genes were higher. These traits are shown for Hpx, Pnmt and Hmox in Figs S15–S17. It has previously been shown that AKI-induced Hpx expression is due, at least in part, to increased mRNA stability63. These results imply that at the time points examined epigenetic alterations are not playing a predominant role in regulating mRNA levels of these three genes in response to kidney injury. Alternatively, the epigenetic alterations occurred in a subpopulation of cells and therefore were not detectable in whole kidney samples.
Genes upregulated in CKD only
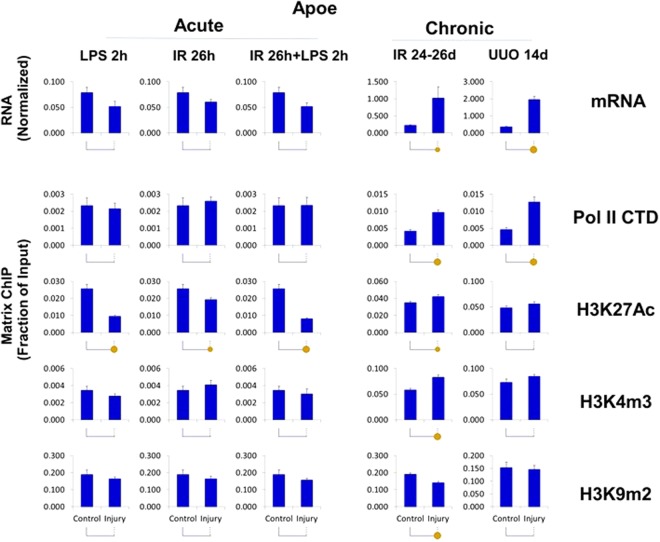
For ChIP analysis we selected three genes in this set, Apoe, Timp2 and Igfbp7. For all three genes, the CKD induced mRNA increases were matched by elevated Pol II and the permissive mark H3K27Ac levels at these loci (Figs 4 and S2). This is illustrated for Apoe in Fig. 8 (see also Figs 4, S2 and S18) and for Timp2 and Igfbp7 in Figs 4, S2 and S19–S20. The response of the Igfbp7 gene was significantly smaller than for the other two genes. Overall, these results suggest that the increased expression of this set of genes in CKD was predominantly transcriptionally mediated and that the increase in the permissive mark H3K27Ac could have contributed to this effect.
Figure 8.
Transcriptional and epigenetic analysis of Apoe gene in AKI and CKD models. RT-qPCR RNA and ChIP-qPCR analysis (see Fig. 4). Apoe transcript levels (mRNA) were normalized to L32 mRNA Matrix ChIP-qPCR analysis of RNA polymerase II (Pol II), and permissive (H3K27Ac, H3K4m3) and repressive (H3K9m2) marks (n = 6 mice from each group). Small solid circle, p < 0.05, large circle, p < 0.01, and no circle, not statistically significant. Apoe was upregulated in CKD but not AKI. The corresponding increase in Pol II and permissive marks and decreased repressive mark indicate that the upregulation is transcriptionally and epigenetically mediated.
Comparison of transcriptional and epigenetic profiles in sham, contralateral and unilaterally injured kidneys in CKD models
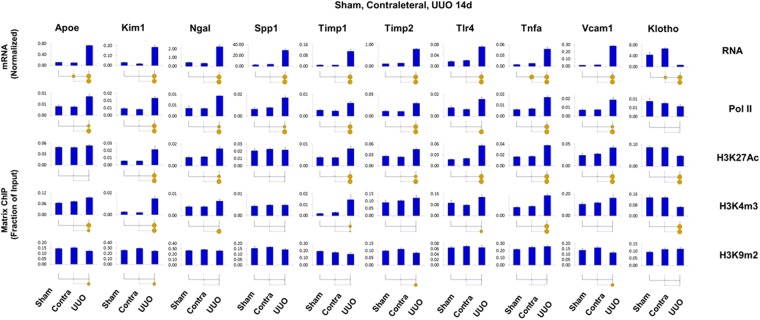
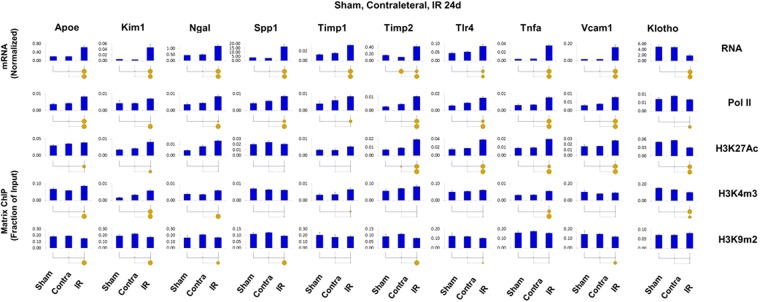
The use of contralateral kidneys in unilateral renal injury models has traditionally been used to decrease complexity and cost of experiments and to control for variability between animals. For example, the use of contralateral kidneys in unilateral AKI models has been routinely been used including epigenetic studies25,64–68. Still, in unilateral CKD models it is possible that the contralateral kidneys may undergo epigenetic changes over time so that using them as internal controls for chronic renal injury could confound data interpretation. To address this issue we carried out a series of experiments in UUO and IR CKD models where we profiled transcriptional and epigenetic renal profiles in contralateral, unilaterally injured kidneys and kidneys from sham operated mice. For these CKD studies, we chose genes whose transcripts and epigenetic modifications changed the most. This parallel analysis revealed that the differences between sham and contralateral kidneys were either small or not detectable (Figs 9 and 10) confirming above conclusions based on using contralateral kidneys as internal controls (Figs 5–8). This is in agreement with other CKD studies that showed no differences in mRNA expressions in sham and contralateral kidneys69–71, These transcriptional and epigenetic measurements demonstrate that the use of contralateral kidneys as an internal controls in unilateral CKD models is appropriate allowing to use fewer mice, expediting such studies and saving costs.
Figure 9.
Transcriptional and epigenetic analysis in sham, contralateral and UUO CKD model. Total RNA and sheared chromatin was prepared from UUO, contralateral (Contra) and sham (Sham) kidneys 14 days after surgeries. Sham UUO surgeries were performed in the same manner as the disease model surgeries but without tying the ureter. RT-qPCR RNA and ChIP-qPCR analysis (see Fig. 4). Transcript levels (mRNA) were normalized to L32 mRNA. Matrix ChIP-qPCR analysis of RNA polymerase II (Pol II), and permissive (H3K27Ac, H3K4m3) and repressive (H3K9m2) marks (n = 6 mice from each group). Small solid circle, p < 0.05, large circle, p < 0.01, and no circle, not statistically significant. The results demonstrate that transcript and epigenetic differences between contralateral and sham kidneys are either small or not detectable. Thus, the contralateral kidneys are appropriate internal controls for this chronic UUO model.
Figure 10.
Transcriptional and epigenetic analysis in sham, contralateral and IR CKD model. Total RNA and sheared chromatin was prepared from UUO, contralateral (Contra) and sham (Sham) kidneys 24 days after surgeries. Sham IR surgeries were performed in the same manner as the disease model surgeries but without clamping the vascular pedicle,. RT-qPCR RNA and ChIP-qPCR analysis (see Fig. 4). Transcript levels (mRNA) were normalized to L32 mRNA. Matrix ChIP-qPCR analysis of RNA polymerase II (Pol II), and permissive (H3K27Ac, H3K4m3) and repressive (H3K9m2) marks (n = 6 mice from each group). Small solid circle, p < 0.05, large circle, p < 0.01, and no circle, not statistically significant. The results demonstrate that transcript and epigenetic the differences between contralateral and sham kidneys are either small or not detectable. Thus, the contralateral kidneys are appropriate internal controls for this chronic IR model.
Figure 5.
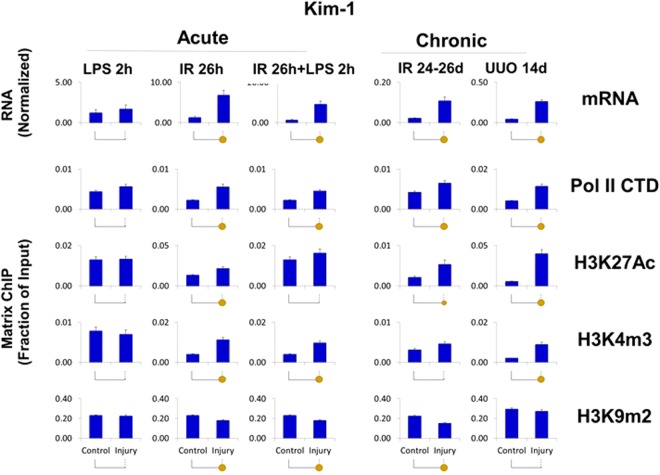
Transcriptional and epigenetic analysis of Kim-1 (Havcr1) gene in AKI and CKD models. RT-qPCR RNA and ChIP-qPCR analysis (see Fig. 4). Kim-1 transcript levels (mRNA) were normalized to L32 mRNA. Matrix ChIP-qPCR analysis of RNA polymerase II (Pol II), and permissive (H3K27Ac, H3K4m3) and repressive (H3K9m2) marks (n = 6 mice from each group) Small solid circle, p < 0.05, large circle, p < 0.01, and no circle, not statistically significant. Kim-1 transcript levels were upregulated in all model expect for LPS. mRNA levels were matched by increased in Pol II and permissive modifications and reduced repressive marks suggesting transcriptional and epigenetic control of gene expression.
Discussion
Our study is the first to simultaneously compare the transcriptional and epigenetic profiles of a panel of 120 genes previously implicated in kidney disease across acute and chronic models of renal injury (Fig. 4). We identified distinct subsets of transcription and epigenetically defined genes that are shared by AKI and CKD but also ones that are specific to either the early or late stages of renal injury. We found that expression of more than half of the genes was similarly altered in the AKI and CKD models with the remainder displaying distinct transcriptional patterns based on the temporal duration of injury (Fig. 2). Similarly, a recent unbiased RNA-seq approach also demonstrated that subsets of genes are altered at the onset and those that change expression days later persist in the progression from AKI to CKD30, suggesting that different epigenetic pathways are involved in gene expression alterations2,3.
Why some kidney injury transcriptional changes persist while others resolve or arise later in the course of renal injury is not known. We assessed transcription (Pol II levels) and histone modification profiles as one way to address this question. We found that increased expression of genes in both AKI and CKD models, is primarily transcriptionally mediated while post-transcriptional processes may play a more significant role in gene downregulation in the setting of kidney injury (e.g. Klotho, Figs 7 and S10). Although post-transcriptional regulation of message stability in renal injury has been known for more than a decade63,72,73 it is a critical area for investigation. Along these lines, it has recently been shown that renal injury-induced Klotho transcript downregulation reflects alternative splicing that leads to mRNA degradation by nonsense-mediated transcript decay74. Histone modifications play a role in alternative splicing75. Thus our results suggest that the decrease in Klotho message levels in response to renal injury could reflect, at least in part, epigenetic changes at this locus. Recognizing the key role of microRNAs (miRNAs) and other non-coding RNAs in the regulation of message stability is revealing new mechanisms of post-transcriptional processes in the pathophysiology of kidney disease progression76. Most notably, global reduction of miRNAs by conditional Dicer deletion in proximal renal tubules protects from ischemic AKI77. Thus, it seems likely that miRNAs play a key role in the AKI-to-CKD continuum. The challenge for future studies will be the identification of renal injury-induced specific miRNAs that participate in the increased degradation of transcripts relevant to AKI –to-CKD transition.
For the epigenetic studies, we focused on a set of histone marks that are among the best studied61. There was a correlation between AKI and CKD-induced changes in gene expression and histone marks for some AKI and CKD genes (e.g., Kim-1, Ngal, Timp1, Tlr4, Tnf and Klotho) (Figs 4 and S1). For genes that were upregulated in CKD only (e.g., Apoe and Timp2) there was an increase in H3K27Ac permissive modification which could have played a role in the AKI-to-CKD transition. For other genes, even those that were potently affected by renal injury (e.g. Spp1, Figs 3 and 4, S7), there were no consistent epigenetic changes. There are more than one hundred different chromatin modifications and the lack of coherent epigenetic changes may simply reflect our focus on a small group of modifications. Further, chromatin modifications have roles other than control of gene transcription including mRNA processing (e.g., splicing), maintaining nuclear architecture, priming, and inheritance61,75,78,79. We expect that further advances in high throughput epigenetic technologies will permit simultaneous profiling of many more epigenetic changes, allowing us to more comprehensively define the relationships between chromatin, transcription and post-transcriptional processes in renal injury.
This study revealed unique transcriptional and epigenetic responses to renal injury. Development of small molecules that target epigenetic enzymes and factors is one of the fastest growing therapeutic areas61,80–82. This trajectory reflects the fact that most of gene expression alterations are mediated by reversible epigenetic changes. Recent studies show that epigenetic drugs are gene selective which, likely, is dictated by unique local chromatin landscape83. Further, when used in combination epigenetic agents act synergistically which further underscores their potential to target specific groups of genes to mitigate progression of AKI to CKD61,82–84 while minimizing off target effects. For example, repletion of renal injury-induced Klotho deficiency mitigates renal injury85. Thus, it is formally possible that co-administration of selective and synergistically acting HDAC86 and lysine demethylase (KDM5B)87 inhibitors could reverse decreased H3K27Ac and H3K4m3, respectively (Fig. 7), and by doing so, restore Klotho expression and lessen or prevent chronic renal injury. Thus, this study provides a useful compendium of candidate factors for selective epigenetic interventions.
There are several limitations in our study. qPCR-based assays limited the breadth of this study to a relatively small set of genes and epigenetic modifications in five AKI and CKD models. Less biased approaches using next generation sequencing (NGS) technologies (e.g. ChIP-seq) should further advance our understanding of transcription and epigenetic basis of kidney injury. Yet, sequencing remains very costly and limit the number of ChIP-seq experiments feasible in a typical research lab. As sequencing costs come down and improvements are made in targeted sequencing, a much larger number of genes and histone marks could be screened simultaneously and over more time points. Although most of the genes we examined are primarily expressed in renal tubules, the cellular heterogeneity of the kidney could have compounded interpretation of the results. In this regard, future application of cell type enrichment, single cell and NGS technologies should facilitate transcription and epigenetic studies in enriched fractions of renal tubule, endothelial and other specific cell types isolated from injured kidneys. Finally, an important limitation of our study was using relatively few models for AKI and CKD. It is possible that other renal injury models may be associated with different transcriptional and epigenetic profiles88. Ultimately, integrative approaches using animal models of kidney disease must be scaled up to human-based studies to establish clinical relevance89.
Conclusion
Simultaneous transcriptional and epigenetic profiling across multiple models of acute and chronic kidney injury can yield meaningful insights into the pathophysiology of this complex syndrome. We found that increased transcription was mainly responsible for injury-induced gene upregulation while, post-transcriptional processes appeared to be the more predominant drivers of gene downregulation. There were distinct subsets of genes that shared overlapping epigenetic changes. Epigenetic alterations are pharmacologically reversible and an increasing numbers of small molecules are being introduced that target genome-associated epigenetic factors81,90. Further, it is increasingly being recognized that epigenetic drugs can exhibit gene selectivity opening exciting avenues to target sets of specific loci while minimizing off target effects83. This study introduces a potential paradigm for testing rationally designed epigenetic interventions.
Key points of the study
Transcriptional control plays a key role in the upregulation of both AKI and CKD genes.
Post-transcriptional control could be playing a more significant role in decreased expression of both AKI and CKD genes.
Transcriptionally upregulated genes share more epigenetic similarities than downregulated genes.
Transcription and epigenetic similarities at kidney injury related genes could be exploited to develop therapies that selectively target and correct expression of these genes during AKI and CKD.
Methods
Reagents
Bovine serum albumin (BSA), phosphate buffered saline (PBS), salmon sperm DNA, and protein A were from Sigma, and proteinase K was from Invitrogen. Formaldehyde, ethanol, NaCl, EDTA, Triton X-100, NP-40, Tris, leupeptin, PMSF, p-nitrophenyl phosphate, NaF, Na3VO4, Na2MoO4 and β-glycerophosphate were from Sigma. The antibodies were commercially available and are listed in Table S3.
Mouse models
All animal studies were performed in accordance to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Washington, Seattle Children’s Research Institute and Fred Hutchinson Cancer Research Center. Male SD-1 and C57BL/6 mice 8–10 weeks of age, 20–35 gm were used.
Acute Ischemia-Reperfusion (IR) and Lipopolysaccharide (LPS)
The experimental protocols have previously been described25,26,64,65,68. In brief, mice were anesthetized with pentobarbital and subjected to a midline abdominal incision under sterile conditions. Left renal ischemia was induced with an atraumatic microvascular clamp applied to the renal pedicle. After 30 min of unilateral renal artery occlusion, the clamp was released and reperfusion of the entire kidney was assessed visually (by loss of global cyanosis). Twenty four hours after IR injury, mice received a tail vein injection of either LPS (2 mg/kg; 0111:B4; l-2630; Sigma, St. Louis, MO; in 80 μl of saline) or saline. Two hours after injections, mice were re-anesthetized, the abdominal cavity was opened, the kidneys were harvested and rapidly frozen at −80 °C. The mice died within 30 sec due to exsanguinations. Thus, the timing of sacrifice relative to IR was 26 hrs, and 2 hrs post either LPS or saline treatment. As previously documented27, the right non-ischemic, contralateral (control) kidney recapitulates what is seen in sham operated kidneys and hence served as an internal control for IR, LPS and IR + LPS injured kidneys. Histology for IR and LPS models of AKI have previously been evaluated44,64,91.
Mouse model of chronic IR and UUO
Unilateral ureteral obstruction (UUO) and unilateral ischemia reperfusion injury was performed as previously described29. Mice were sacrificed 14 days post UUO. In brief, chronic unilateral ischemia reperfusion injury (IR) model was performed by placement of a vascular clamp on the left renal pedicle for 28 minutes while mice were kept at a constant temperature of 37 °C. Mice were sacrificed 24–26 days post IR. Sham UUO and IR surgeries were performed in the same manner as the disease model surgeries but without tying the ureter and clamping the vascular pedicle, respectively. Contralateral, Sham, UUO and IR kidneys were harvested and rapidly frozen (−80 °C). Histology for chronic UUO and IR models have previously been evaluated29,92.
RNA Extraction and cDNA Synthesis
RNA was extracted from tissue fragments using Trizol reagent as per the manufacturer’s protocol. To synthesize cDNA, 400 ng of Trizol-extracted total RNA was reverse transcribed with 200 units MMLV reverse transcriptase (Invitrogen) and oligo dT primers in 10 μl reactions in 96-well microplates. RT reactions were diluted 100-fold prior to running qPCR93. RT-qPCR primers are listed in Table. S1.
Chromatin preparation and multiplex Matrix ChIP platform
The multiplex microplate Matrix ChIP method was previously described23,24. Briefly, for ChIP assays, tissue fragments (10–20 mg) were cross-linked with formaldehyde, and chromatin was sheared using Diagenode Bioruptor. ChIP assays were done using protein A-coated 96-well polypropylene microplates as described before23. 1 μl of eluted DNA was used in 2 μl real-time PCR reactions (ABI7900HT). All PCR reactions were run in quadruplicates. Final results are expressed as fraction of input DNA24. Matrix ChIP qPCR primers to 5′ end of genes used in this study are shown in Table S2 and list of antibodies in Table S3 (Supplement).
Statistics and visualization
PCRCrunch and GraphGrid were used to acquire, store and analyze large data sets generated by microplate RT-qPCR AND Matrix ChIP-qPCR25,26. Pair-wise statistically significant differences are represented by the size of a circle for each comparison made with a small circle representing p < 0.05, a large circle indicating p < 0.01 and no circle implying non-significance. GraphGrid uses a two-tailed Student’s t-test to compute p-values26.
Correspondence analysis
Correspondence analysis, a form of multidimensional scaling similar to principal component analysis, was applied to the entire gene expression dataset to assess whether global variations in transcription distinguished acute from chronic kidney injury94. To allow easier visualization, we averaged gene expression values for each condition (n = 6 mice) to display the five experimental conditions utilized (acute: LPS 2 h, I/R 26 h, I/R + LPS 26 h; chronic: I/R 24d, UUO 14d). The first three orthogonal axes are shown in Fig. 1, each of which captures part of the overall transcriptional variability across conditions.
Heatmap and functional enrichment analysis
RT-qPCR and ChIP-qPCR values during injury conditions were normalized to their uninjured contralateral controls and were log2-transformed. Heatmaps were generated using Multiple Experiment Viewer (MeV-TM4) program on log2-transformed qPCR and ChIP-qPCR data. For each subset of genes with distinct expression pattern during AKI and CKD, we performed Gene Ontology analysis using Database for Annotation, Visualization and Integrated Discovery (DAVID, v6.8)95. Only genes with at least 1.5 fold up or down-regulation in response renal injury (AKI and/or CKD) were selected for this analysis and an FDR ≤ 0.05 was used to designate significant enrichment for a given GO category. Although the 120 candidate genes were pre-selected based on their association with kidney injury and therefore did not represent an unbiased sample, the rationale for the GO analysis was to assess whether distinct functional enrichment profiles emerged based on transcriptional patterns in acute vs. chronic renal injury.
Electronic supplementary material
Acknowledgements
This work was supported by National Institutes of Health R01 DK094934, RO1 103849, R21 GM111439, R33 CA191135 to KB. 5 K08 DK073497, 5 R03 DK083648 to DMO and RO1 DK38432 to RAZ.
Author Contributions
Conceived the study: K.B., R.S., D.M.O. Designed the experiments: K.B., R.S., D.M.O. and R.A.Z. Performed the experiments. R.S., D.M.O. and A.J. Analyzed the data: S.G., R.S., O.D. and K.B. Wrote the manuscript K.B., R.S., D.M.O., S.G., O.D.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Roya Sharifian and Daryl M. Okamura contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35943-x.
References
- 1.Ferenbach DA, Bonventre JV. Acute kidney injury and chronic kidney disease: From the laboratory to the clinic. Nephrol Ther. 2016;12(Suppl 1):S41–8. doi: 10.1016/j.nephro.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–24. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 4.Caironi P, et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N Engl J Med. 2014;370:1412–21. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 5.Basile DP, et al. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J Am Soc Nephrol. 2016;27:687–97. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26:1765–76. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferenbach, D. A. & Bonventre, J. V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol (2015). [DOI] [PMC free article] [PubMed]
- 8.Verma SK, Molitoris BA. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol. 2015;35:96–107. doi: 10.1016/j.semnephrol.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124:2355–63. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125:1620–36. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai S, et al. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med. 2016;22:183–93. doi: 10.1038/nm.4012. [DOI] [PubMed] [Google Scholar]
- 12.Humphreys BD, et al. Targeting Endogenous Repair Pathways after AKI. J Am Soc Nephrol. 2016;27:990–8. doi: 10.1681/ASN.2015030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphreys BD, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA. 2011;108:9226–31. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA. 2014;111:1527–32. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–21. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grande MT, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21:989–97. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 17.Lovisa S, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015;21:998–1009. doi: 10.1038/nm.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tampe, B. et al. Low-dose hydralazine prevents fibrosis in a murine model of acute kidney injury-to-chronic kidney disease progression. Kidney Int (2016). [DOI] [PubMed]
- 19.Tampe B, et al. Induction of Tet3-dependent Epigenetic Remodeling by Low-dose Hydralazine Attenuates Progression of Chronic Kidney Disease. EBioMedicine. 2015;2:19–36. doi: 10.1016/j.ebiom.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nangaku, M., Hirakawa, Y., Mimura, I., Inagi, R. & Tanaka, T. Epigenetic Changes in the Acute Kidney Injury-to-Chronic Kidney Disease Transition. Nephron (2017). [DOI] [PubMed]
- 21.Rodriguez-Romo, R., Berman, N., Gomez, A. & Bobadilla, N.A. Epigenetic regulation in the acute kidney injury (AKI) to chronic kidney disease transition (CKD). Nephrology (Carlton) (2015). [DOI] [PubMed]
- 22.Reddy MA, Natarajan R. Recent developments in epigenetics of acute and chronic kidney diseases. Kidney Int. 2015;88:250–61. doi: 10.1038/ki.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, et al. Microplate-based platform for combined chromatin and DNA methylation immunoprecipitation assays. BMC Mol Biol. 2011;12:49. doi: 10.1186/1471-2199-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanagin S, Nelson JD, Castner DG, Denisenko O, Bomsztyk K. Microplate-based chromatin immunoprecipitation method, Matrix ChIP: a platform to study signaling of complex genomic events. Nucleic Acids Res. 2008;36:e17. doi: 10.1093/nar/gkn001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mar D, et al. Heterogeneity of epigenetic changes at ischemia/reperfusion- and endotoxin-induced acute kidney injury genes. Kidney Int. 2015;88:734–744. doi: 10.1038/ki.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bomsztyk K, et al. Synchronous Recruitment of Epigenetic Modifiers to Endotoxin Synergistically Activated Tnf-alpha Gene in Acute Kidney Injury. PLoS One. 2013;8:e70322. doi: 10.1371/journal.pone.0070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zager RA, Johnson AC, Hanson SY, Lund S. Ischemic proximal tubular injury primes mice to endotoxin-induced TNF-alpha generation and systemic release. Am J Physiol Renal Physiol. 2005;289:F289–97. doi: 10.1152/ajprenal.00023.2005. [DOI] [PubMed] [Google Scholar]
- 28.Pennathur S, et al. The macrophage phagocytic receptor CD36 promotes fibrogenic pathways on removal of apoptotic cells during chronic kidney injury. Am J Pathol. 2015;185:2232–45. doi: 10.1016/j.ajpath.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamura DM, et al. Cysteamine modulates oxidative stress and blocks myofibroblast activity in CKD. J Am Soc Nephrol. 2014;25:43–54. doi: 10.1681/ASN.2012090962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, J. et al. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight2(2017). [DOI] [PMC free article] [PubMed]
- 31.Alge, J.L. & Arthur, J.M. Biomarkers of AKI: A Review of Mechanistic Relevance and Potential Therapeutic Implications. Clin J Am Soc Nephrol (2014). [DOI] [PMC free article] [PubMed]
- 32.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. 2014;29:1301–11. doi: 10.1093/ndt/gft510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boddu R, et al. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol Renal Physiol. 2017;313:F740–F755. doi: 10.1152/ajprenal.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyner JL, et al. Tissue Inhibitor Metalloproteinase-2 (TIMP-2)IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol. 2015;26:1747–54. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emlet DR, et al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol. 2017;312:F284–F296. doi: 10.1152/ajprenal.00271.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu CC, et al. Apolipoprotein E and progression of chronic kidney disease. JAMA. 2005;293:2892–9. doi: 10.1001/jama.293.23.2892. [DOI] [PubMed] [Google Scholar]
- 37.Xiao L, et al. Sustained Activation of Wnt/beta-Catenin Signaling Drives AKI to CKD Progression. J Am Soc Nephrol. 2016;27:1727–40. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maarouf, O.H. et al. Paracrine Wnt1 Drives Interstitial Fibrosis without Inflammation by Tubulointerstitial Cross-Talk. J Am Soc Nephrol (2015). [DOI] [PMC free article] [PubMed]
- 39.Ren S, et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl Acad Sci USA. 2013;110:1440–5. doi: 10.1073/pnas.1211179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 41.Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int. 2009;76:604–13. doi: 10.1038/ki.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishioka, S. et al. The cyclin-dependent kinase inhibitor p21 is essential for the beneficial effects of renal ischemic preconditioning on renal ischemia/reperfusion injury in mice. Kidney Int85, 871-9 (2014). [DOI] [PubMed]
- 43.Bonventre JV. Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney Int Suppl (2011) 2014;4:39–44. doi: 10.1038/kisup.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med16, 535–43, 1p following 143 (2010). [DOI] [PMC free article] [PubMed]
- 45.Fischer, M. Census and evaluation of p53 target genes. Oncogene (2017). [DOI] [PMC free article] [PubMed]
- 46.Zhang, D. et al. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol25, 2278-89 (2014). [DOI] [PMC free article] [PubMed]
- 47.Ortiz A, et al. Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure. Kidney Int. 2000;57:969–81. doi: 10.1046/j.1523-1755.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- 48.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu, J. et al. Hypoxia, HIF, and Associated Signaling Networks in Chronic Kidney Disease. Int J Mol Sci18 (2017). [DOI] [PMC free article] [PubMed]
- 50.Lemos DR, et al. Maintenance of vascular integrity by pericytes is essential for normal kidney function. Am J Physiol Renal Physiol. 2016;311:F1230–F1242. doi: 10.1152/ajprenal.00030.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1 + Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury. J Am Soc Nephrol. 2017;28:776–784. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kida Y, Tchao BN, Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol. 2014;29:333–42. doi: 10.1007/s00467-013-2430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeltsch M, Leppanen VM, Saharinen P, Alitalo K. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb Perspect Biol. 2013;5:a009183. doi: 10.1101/cshperspect.a009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saharinen P, Bry M, Alitalo K. How do angiopoietins Tie in with vascular endothelial growth factors? Curr Opin Hematol. 2010;17:198–205. doi: 10.1097/MOH.0b013e3283386673. [DOI] [PubMed] [Google Scholar]
- 55.Bomsztyk K, et al. Experimental acute lung injury induces multi-organ epigenetic modifications in key angiogenic genes implicated in sepsis-associated endothelial dysfunction. Crit Care. 2015;19:225. doi: 10.1186/s13054-015-0943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen TM, Singh H, Tahir TA, Brindle NP. Effects of angiopoietins-1 and -2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cell Signal. 2010;22:527–32. doi: 10.1016/j.cellsig.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babickova J, et al. Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 2017;91:70–85. doi: 10.1016/j.kint.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 58.Ko YA, et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol. 2013;14:R108. doi: 10.1186/gb-2013-14-10-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008;3:481–90. doi: 10.2215/CJN.03520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol. 2012;8:423–9. doi: 10.1038/nrneph.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bomsztyk K, Denisenko O. Epigenetic alterations in acute kidney injury. Semin Nephrol. 2013;33:327–340. doi: 10.1016/j.semnephrol.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drew DA, et al. Association between Soluble Klotho and Change in Kidney Function: The Health Aging and Body Composition Study. J Am Soc Nephrol. 2017;28:1859–1866. doi: 10.1681/ASN.2016080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zager RA, Johnson AC, Becker K. Renal cortical hemopexin accumulation in response to acute kidney injury. Am J Physiol Renal Physiol. 2012;303:F1460–72. doi: 10.1152/ajprenal.00426.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zager RA, Johnson AC, Becker K. Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and “end-stage” kidney disease. Am J Physiol Renal Physiol. 2011;301:F1334–45. doi: 10.1152/ajprenal.00431.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naito M, Zager RA, Bomsztyk K. BRG1 increases transcription of proinflammatory genes in renal ischemia. J Am Soc Nephrol. 2009;20:1787–96. doi: 10.1681/ASN.2009010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zager RA, Johnson AC. Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am J Physiol Renal Physiol. 2009;296:F1032–41. doi: 10.1152/ajprenal.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naito M, Bomsztyk K, Zager RA. Renal ischemia-induced cholesterol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene. Am J Pathol. 2009;174:54–62. doi: 10.2353/ajpath.2009.080602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naito M, Bomsztyk K, Zager RA. Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure. J Am Soc Nephrol. 2008;19:1321–1330. doi: 10.1681/ASN.2007121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burne-Taney MJ, Yokota N, Rabb H. Persistent renal and extrarenal immune changes after severe ischemic injury. Kidney Int. 2005;67:1002–9. doi: 10.1111/j.1523-1755.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- 70.Yoo KH, et al. Osteopontin regulates renal apoptosis and interstitial fibrosis in neonatal chronic unilateral ureteral obstruction. Kidney Int. 2006;70:1735–41. doi: 10.1038/sj.ki.5000357. [DOI] [PubMed] [Google Scholar]
- 71.de Jong MA, et al. Fibroblast growth factor 23 modifies the pharmacological effects of angiotensin receptor blockade in experimental renal fibrosis. Nephrol Dial Transplant. 2017;32:73–80. doi: 10.1093/ndt/gfw105. [DOI] [PubMed] [Google Scholar]
- 72.Ramesh G, Brian Reeves W. Cisplatin increases TNF-alpha mRNA stability in kidney proximal tubule cells. Ren Fail. 2006;28:583–92. doi: 10.1080/08860220600843839. [DOI] [PubMed] [Google Scholar]
- 73.Zager RA, Johnson AC, Naito M, Bomsztyk K. Maleate nephrotoxicity: mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol Renal Physiol. 2008;294:F187–97. doi: 10.1152/ajprenal.00434.2007. [DOI] [PubMed] [Google Scholar]
- 74.Mencke, R. et al. Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight2(2017). [DOI] [PMC free article] [PubMed]
- 75.Zhou HL, Luo G, Wise JA, Lou H. Regulation of alternative splicing by local histone modifications: potential roles for RNA-guided mechanisms. Nucleic Acids Res. 2014;42:701–13. doi: 10.1093/nar/gkt875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhatt K, Kato M, Natarajan R. Mini-review: emerging roles of microRNAs in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. 2016;310:F109–18. doi: 10.1152/ajprenal.00387.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei Q, et al. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21:756–61. doi: 10.1681/ASN.2009070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–95. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, et al. In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice. Kidney Int. 2012;82:278–91. doi: 10.1038/ki.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17:630–41. doi: 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- 81.Helin K, Minucci S. The Role of Chromatin-Associated Proteins in Cancer. Annu. Rev. Cancer Biol. 2017;1:355–77. doi: 10.1146/annurev-cancerbio-050216-034422. [DOI] [Google Scholar]
- 82.Fontecha-Barriuso, M. et al. Targeting epigenetic DNA and histone modifications to treat kidney disease. Nephrol Dial Transplant (2018). [DOI] [PubMed]
- 83.Sato T, et al. Transcriptional Selectivity of Epigenetic Therapy in Cancer. Cancer Res. 2017;77:470–481. doi: 10.1158/0008-5472.CAN-16-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Lera AR, Ganesan A. Epigenetic polypharmacology: from combination therapy to multitargeted drugs. Clin Epigenetics. 2016;8:105. doi: 10.1186/s13148-016-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu MC, et al. Recombinant alpha-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 2017;91:1104–1114. doi: 10.1016/j.kint.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ranganathan P, et al. Histone deacetylase-mediated silencing of AMWAP expression contributes to cisplatin nephrotoxicity. Kidney Int. 2016;89:317–26. doi: 10.1038/ki.2015.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tumber A, et al. Potent and Selective KDM5 Inhibitor Stops Cellular Demethylation of H3K4me3 at Transcription Start Sites and Proliferation of MM1S Myeloma Cells. Cell Chem Biol. 2017;24:371–380. doi: 10.1016/j.chembiol.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aggarwal S, Grange C, Iampietro C, Camussi G, Bussolati B. Human CD133(+) Renal Progenitor Cells Induce Erythropoietin Production and Limit Fibrosis After Acute Tubular Injury. Sci Rep. 2016;6:37270. doi: 10.1038/srep37270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agarwal A, et al. Cellular and Molecular Mechanisms of AKI. J Am Soc Nephrol. 2016;27:1288–99. doi: 10.1681/ASN.2015070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahuja N, Sharma AR, Baylin SB. Epigenetic Therapeutics: A New Weapon in the War Against Cancer. Annual Review of Medicine. 2016;67:73–89. doi: 10.1146/annurev-med-111314-035900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zahedi K, et al. The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury. Am J Physiol Cell Physiol. 2010;299:C164–74. doi: 10.1152/ajpcell.00512.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okamura DM, Lopez-Guisa JM, Koelsch K, Collins S, Eddy AA. Atherogenic scavenger receptor modulation in the tubulointerstitium in response to chronic renal injury. Am J Physiol Renal Physiol. 2007;293:F575–85. doi: 10.1152/ajprenal.00063.2007. [DOI] [PubMed] [Google Scholar]
- 93.Nelson JD, Denisenko O, Sova P, Bomsztyk K. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 2006;34:e2. doi: 10.1093/nar/gnj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fellenberg K, et al. Correspondence analysis applied to microarray data. Proc Natl Acad Sci USA. 2001;98:10781–6. doi: 10.1073/pnas.181597298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.