Abstract
Onychomycosis (OM) is a common nail disease. Although controversial, vascular diseases are considered independent predictors of OM and vice versa. Sequential pattern mining (SPM) has not been previously used for statistical analysis in dermatology, but it is an efficient method for identifying frequent association rules in multiple sequential data sets. The aim of our study was to identify the relationship between OM and vascular diseases in the real world through a population-based study using SPM. We obtained population-based data recorded from 2002 to 2013 by the Health Insurance Research and Assessment Agency. Cases of vascular-related disease and OM were identified using the diagnostic codes of the International Classification of Diseases 10th Revision, version 2010. SPM measures were based on comorbidity and duration values. We estimated 3-year risk for progression from OM to vascular disease and vice versa using logistic regression. Patients with varicose veins and peripheral vascular disease had higher OM comorbidity (comorbidity: 1.26% and 0.69%, respectively) than did those with other vascular diseases. Patients diagnosed with varicose veins and peripheral vascular disease were diagnosed with OM after 25.50 and 55.10 days, respectively, which was a shorter duration than that observed for other diseases. Patients with OM were at higher risk for peripheral vascular disease (adjusted odds ratio (aOR) 1.199 [95% confidence interval (CI) 1.151–1.249]) and varicose veins (aOR 1.150 [95% CI 1.063–1.245]). Patients with peripheral vascular disease (aOR 1.128 [95% CI 1.081–1.177]) were at higher risk for OM, while patients with varicose veins had no significant risk for OM. Careful consideration of varicose veins or peripheral vascular disease is required for proper management of comorbidities in patients with OM.
Introduction
Onychomycosis (OM) is a common condition, accounting for up to half of all reported nail diseases1,2. The estimated prevalence of OM in the general population ranges from 2 to 11%3. OM is associated with increasing age, gender, chronic diseases, smoking, immune dysfunction, and vascular diseases3–5. Among these risk factors, vascular diseases such as chronic vascular insufficiency and peripheral arterial disease are considered independent predictors of OM4–6. Conversely, OM could be considered a warning sign of cardiovascular disease1. However, according to one study, the prevalence of venous insufficiency was increased in patients with OM, but the prevalence of peripheral arterial disease was not increased7. The association of vascular disease with OM remains unclear; thus, large-scale studies of their relationship are needed.
Sequential pattern mining (SPM), among other association rule mining methods, is an efficient method for finding frequent association rules in multiple sequential data sets8,9. SPM is based on analyzing transaction histories at the point of sale in the retail industry, but it is also useful for identifying signs of complications when multiple diseases are present in a patient’s medical records.
The aim of our study was to identify the relationship between OM and vascular disease in the real world through a population-based study using SPM.
Methods
Data source
We obtained population-based data recorded from January 1, 2002 to December 31, 2013 by the Health Insurance Research and Assessment Agency (HIRA), which is affiliated with the National Health Insurance Corporation (NHIC) of Korea. The HIRA database contains comprehensive health-related information about the population of Korea and comprises an eligibility database, health examination database, medical care institution database, and medical treatment database. The entire Korean population is covered by the NHIC. In 2013, 97.2% (n = 49,989,620) of the population was covered by the NHIC database, and the remaining 2.8% (n = 1,458,871) of the population was covered by the medical aid system10. In addition, the HIRA provides a standardized, stratified one million-person sample population (n = 1,116,789) from the NHIC database.
Our study data were extracted from the National Health Insurance Corporation (NHIC) claim database. All data were anonymized and provided to the researcher except for the patient’s personal information. This study was approved by the Institutional Review Board of the Korean NHIC (no. NHIS-2017-1-002). The study design was approved by the Ethics Committee of Seoul St. Mary’s Hospital, the Catholic University of Korea (approval no. KC16EISE0923) and followed all relevant principles of the Declaration of Helsinki. Informed consent was not necessary due to the nature of our study.
Identification of vascular disease and OM
We used the diagnostic codes of the International Classification of Diseases 10th Revision (ICD-10), version 2010. Patients with OM were defined as those who were diagnosed with B35.1 ICD-10 code. Patients with vascular-related disease were identified by vascular disease-related ICD-10 codes (primary hypertension [I10], angina pectoris [I20], cerebral infarction [I63], other peripheral vascular diseases [I73], varicose veins [I83], and hemorrhoids [I84]). To investigate the incidence of vascular diseases newly occurring in OM and vice versa, we excluded patients who had been diagnosed with vascular disease codes or OM at least once in 2002 to 2003.
SPM
We used SPM to examine relationships between diseases caused by time differences, and SPM measures were based on confidence and duration values. Confidence was defined as the conditional probability of a sequential pattern9, and duration was the average occurrence time of the sequential pattern. In this analysis, gender, ICD-10, age, and date were used as variables to identify the sequential pattern of the disease using statistical software SAS Enterprise Miner version 13.2 (SAS Institute, Cary, NC, USA).
Statistical analysis
To determine whether OM occurred more frequently in patients with each vascular disease and whether each vascular disease occurred in patients with OM, we established a cohort of patients diagnosed from 2010 to 2013. We excluded patients who were diagnosed with OM or each vascular disease before 2010. We compared the logistic regression results with the occurrence of each vascular disease in patients with new OM and the occurrence of each vascular disease in the control group. Gender and age adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were calculated using multivariable logistic regression modeling. All data were analyzed using SAS Enterprise Miner version 13.2 (SAS Institute, Cary, NC, USA). A value of P < 0.05 was considered statistically significant.
Results
Table 1 shows the frequencies of patients with OM, hypertension, angina pectoris, cerebral infarction, peripheral vascular disease, varicose veins, and hemorrhoids by gender and age. Based on the results for each code, the total number of male patients was 88,818, and the total number of female patients was 77,548. The number of patients aged 20 to 64 years was 117,781 (70.80%), the number of patients older than 65 years was 46,094 (27.71%), and the number of patients aged 0 to 19 years was 2,491 (1.50%). Hypertension showed the highest frequency (55,514), followed by OM (47,275) and hemorrhoids (22,672).
Table 1.
Frequencies of patients with diagnostic codes for onychomycosis, primary hypertension, angina pectoris, cerebral infarction, other peripheral vascular diseases, varicose vein and hemorrhoids by gender and age.
| Disease | Gender | Frequency | Age | Frequency | Total |
|---|---|---|---|---|---|
| Onychomycosis | Male | 25,423 | 0~19 | 464 | 47,275 |
| 20~64 | 20,396 | ||||
| Over 65 | 4,563 | ||||
| Female | 21,852 | 0~19 | 791 | ||
| 20~64 | 17,901 | ||||
| Over 65 | 3,160 | ||||
| Primary Hypertension | Male | 29,177 | 0~19 | 43 | 55,514 |
| 20~64 | 18,014 | ||||
| Over 65 | 11,120 | ||||
| Female | 26,337 | 0~19 | 156 | ||
| 20~64 | 19,879 | ||||
| Over 65 | 6,302 | ||||
| Angina pectoris | Male | 8,046 | 0~19 | 44 | 16,222 |
| 20~64 | 4,745 | ||||
| Over 65 | 3,257 | ||||
| Female | 8,176 | 0~19 | 69 | ||
| 20~64 | 5,666 | ||||
| Over 65 | 2,441 | ||||
| Cerebral infarction | Male | 8,047 | 0~19 | 14 | 15,544 |
| 20~64 | 2,736 | ||||
| Over 65 | 5,297 | ||||
| Female | 7,497 | 0~19 | 15 | ||
| 20~64 | 3,505 | ||||
| Over 65 | 3,977 | ||||
| Other peripheral vascular diseases | Male | 4,942 | 0~19 | 47 | 7,713 |
| 20~64 | 3,055 | ||||
| Over 65 | 1,840 | ||||
| Female | 2,771 | 0~19 | 44 | ||
| 20~64 | 1552 | ||||
| Over 65 | 1,175 | ||||
| Varicose veins | Male | 915 | 0~19 | 6 | 1,426 |
| 20~64 | 785 | ||||
| Over 65 | 124 | ||||
| Female | 511 | 0~19 | 6 | ||
| 20~64 | 377 | ||||
| Over 65 | 128 | ||||
| Hemorrhoids | Male | 12,268 | 0~19 | 379 | 22,672 |
| 20~64 | 10,557 | ||||
| Over 65 | 1,332 | ||||
| Female | 10,404 | 0~19 | 413 | ||
| 20~64 | 8,613 | ||||
| Over 65 | 1,378 |
Figure 1 shows sequence patterns between diseases and comorbidities, as well as the durations extracted through SPM. Parentheses indicate the data for each disease. The comorbidity association rule hypertension ⇒ OM indicates the percentage of patients with OM among patients with hypertension11. The duration refers to the sequence (average number of days) between diseases. A comorbidity of 1.67% for the association of OM ⇒ hypertension was the highest value of all association rules, followed by values of 0.61%, 0.27%, 0.16%, 0.13% and 0.12% for the association rules of hemorrhoids, angina, cerebral infarction, varicose veins, and peripheral vascular disease, respectively. The duration 131.27 days for the association rule of OM ⇒ varicose veins was the shortest duration, followed by 180.91, 192.64, 208.24, 220.36, and 347.53 days for the association rules of angina, cerebral infarction, peripheral vascular disease, hemorrhoids, and hypertension, respectively. A comorbidity of 1.26% for the association rule of varicose veins ⇒ OM was the highest value for all association rules, followed by 0.92%, 0.84%, 0.71%, 0.69% and 0.48% for the associations of hypertension, hemorrhoids, angina, peripheral vascular disease and cerebral infarction, respectively. The duration of 25.50 days for the association rule of varicose veins ⇒ OM was the shortest duration, followed by 55.10, 108.80, 133.66, 169.53, and 173.31 days for the association rules of peripheral vascular disease, angina, hemorrhoids, cerebral infarction, and hypertension, respectively.
Figure 1.
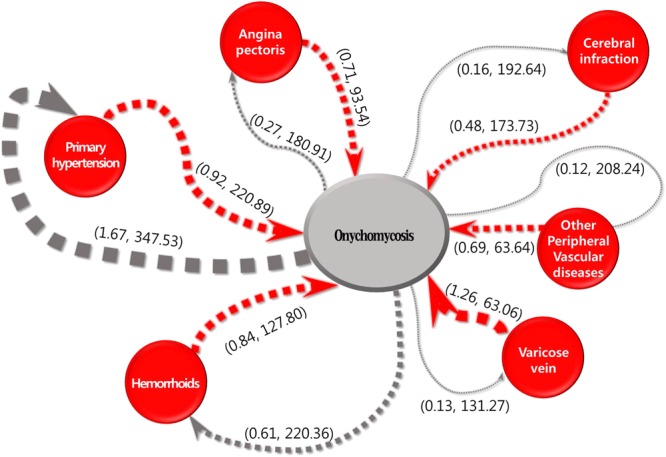
Confidences and onset durations for sequence patterns between onychomycosis and primary hypertension, angina pectoris, cerebral infarction, other peripheral vascular diseases, varicose veins and hemorrhoids. The first number in parentheses indicates the confidence (%), and the second number indicates onset duration (days).
Figure 2 illustrates rules and their confidences and durations based on gender. For males (Fig. 2a), a comorbidity of 1.93% for the association rule of OM ⇒ hypertension was the highest value. The duration of 150.67 days for the association rule of OM ⇒ cerebral infarction was the shortest duration, followed by 166.38, 250.93, 258.46, 287.84, and 367.90 days for the association rules of varicose veins, hemorrhoids, angina, peripheral vascular disease, and hypertension, respectively. The comorbidity of 1.96% for the association rule of varicose veins ⇒ OM was the greatest value. The duration of 24.00 days for the association rule of varicose veins ⇒ OM was shortest duration, followed by 59.52, 123.79, 153.93, 155.43, and 198.07 days for the association rules of peripheral vascular disease, angina, hemorrhoids, cerebral infarction, and hypertension, respectively.
Figure 2.
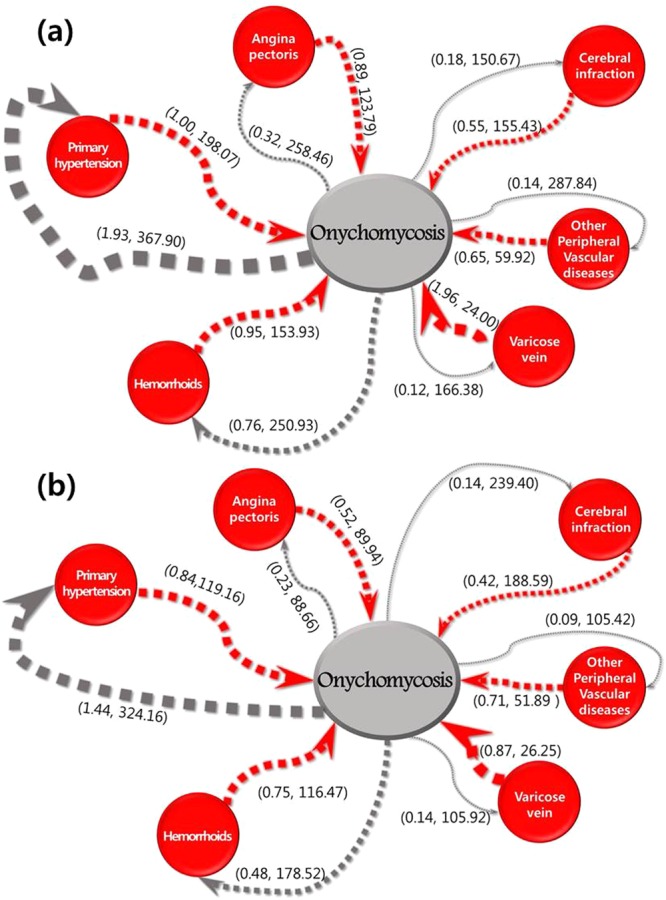
Confidences and onset durations for sequence patterns between onychomycosis and each vascular disease in (a) males and (b) females. The first number in parentheses indicates the confidence (%), and the second number indicates onset duration (days).
Among females (Fig. 2b), the comorbidity of 1.44% for the association rule of OM ⇒ hypertension was the highest value. The duration of 88.66 days for the association rule of OM ⇒ angina was the shortest duration. The comorbidity of 0.87% for the association rule of varicose veins ⇒ OM was the highest value. The duration of 26.25 days for the association rule of varicose veins ⇒ OM was shortest duration, followed by 51.89, 89.94, 116.47, 119.16 and 188.59 days for the association rules of peripheral vascular disease, angina, hemorrhoids, hypertension and cerebral infarction, respectively.
Table 2 shows the aORs and CI for comorbidities. Patients with OM were at higher risk for peripheral vascular disease (aOR 1.199 [95% CI 1.151–1.249]) and varicose veins (OR 1.150 [95% CI 1.063–1.245]). The risks of hypertension and hemorrhoids decreased by 7.4% (95% CI 0.865–0.959) and 8.4% (95% CI 0.882–0.852), respectively. No significant differences were observed in the risk of angina and cerebral infarction in patients with OM.
Table 2.
Risk of onychomycosis for each vascular disease and vice versa.
| 3-year risk of each vascular disease in patients with OM | OR (95% CI) | P | 3-year risk of OM in patients with vascular disease | OR (95% CI) | P | ||
|---|---|---|---|---|---|---|---|
| Hypertension | |||||||
| Controls | 0.076% (62,276/818,743) | Reference | Controls | 0.061% (49,120/802,664) | Reference | ||
| OM | 0.071% (3,694/52,137) | 0.926 (0.895–0.959) | <0.0001 | Hypertension | 0.069% (4,481/65 405) | 1.128 (1.093–1.165) | <0.0001 |
| Angina pectoris | |||||||
| Controls | 0.024% (21,793/909,004) | Reference | Controls | 0.07% (66,039/944,945) | Reference | ||
| OM | 0.025% (1,643/67,124) | 1.021 (0.971–1.075) | 0.412 | Angina pectoris | 0.072% (1,642/22,834) | 1.031 (0.980–1.085) | 0.236 |
| Cerebral infarction | |||||||
| Controls | 0.015% (13,710/912,852) | Reference | Controls | 0.071% (68,031/956,751) | Reference | ||
| OM | 0.014% (958/68,709) | 0.927 (0.868–0.991) | 0.25 | Cerebral infarction | 0.063% (890/14,204) | 0.873 (0.816–0.935) | <0.0001 |
| Other peripheral vascular disease | |||||||
| Controls | 0.033% (29,871/907,277) | Reference | Controls | 0.069% (64,451/936,629) | Reference | ||
| OM | 0.039% (2,587/65,978) | 1.199 (1.151–1.249) | <0.0001 | Peripheral vascular disease | 0.077% (2,417/31,417) | 1.128 (1.081–1.177) | <0.0001 |
| Varicose veins | |||||||
| Controls | 0.008% (7,747/919,540) | Reference | Controls | 0.072% (69,313/969,680) | Reference | ||
| OM | 0.01% (675/69,750) | 1.150 (1.063–1.245) | 0.001 | Varicose veins | 0.068% (552/8,079) | 0.953 (0.873–1.039) | 0.273 |
| Hemorrhoids | |||||||
| Controls | 0.048% (43,964/909,631) | Reference | Controls | 0.07% (64,529/926,999) | Reference | ||
| OM | 0.045% (2,950/66,335) | 0.916 (0.882–0.952) | <0.0001 | Hemorrhoids | 0.059% (2,672/45,601) | 0.832 (0.799–0.866) | <0.0001 |
Patients with hypertension (OR 1.128 [95% CI 1.093–1.165]) and peripheral vascular disease (OR 1.128 [95% CI 1.081–1.177]) were at higher risk for OM. The risk of OM was decreased by 12.7% (95% CI 0.816–0.935) in patients with cerebral infarction and 16.8% (95% CI 0.799–0.866) in patients with hemorrhoids. No significant differences were observed in the risk of OM in patients with angina and those with varicose veins.
Discussion
We investigated the comorbidity between OM and vascular diseases using SPM. We also investigated the onset duration from OM to each vascular disease and vice versa. In our study, patients with OM were at significantly increased risk of varicose veins and peripheral vascular disease. Furthermore, the onset duration of OM in patients with varicose veins or peripheral vascular disease was relatively short compared to that of other vascular diseases.
The comorbidity of OM was highest (1.26%) in patients with varicose veins. The percentage of new onset OM in patients with vascular disease and vice versa was defined as confidence. Since the value of the “confidence” parameter is synonymous with “comorbidity” in epidemiology, we also regarded confidence as comorbidity9. Because chronic venous insufficiency including varicose veins can lead to toenail OM, our results are consistent with those of previous studies6,7,12. In previous studies, 36.1% and 35.7% of chronic venous insufficiency patients had OM5,6. However, these previous studies were cross-sectional and relatively small (36 and 42 patients). Since our study used population-based standard samples (n = 1,116,789) and individuals with previously diagnosed vascular disease or OM were excluded, the comorbidity of OM was different from that in previous studies. Our results can be interpreted as the probability of each vascular disease occurring in patients with OM.
Because SPM was performed to estimate the disease probability, logistic regression was used to investigate the 3-year risk of OM for each vascular disease and vice versa. Among patients with OM, the risk of varicose veins was increased by 15% compared with that among controls. However, the OM risk among patients with varicose veins was not significantly different from that among controls. These results may be attributed to the diagnosis of OM before the diagnosis of varicose veins. Kulac, et al.6. suggested that OM could be an early finding in some patients with chronic venous insufficiency, consistent with our results.
A duration of 25.5 days was required for patients with varicose veins to develop OM. Progressive microangiopathic changes in nail capillaries are thought to contribute to the occurrence of OM6. According to Mosmiller, et al.13, lymphocyte counts were significantly lower in patients with severe chronic venous insufficiency than in those with mild insufficiency; moreover, the total WBC count was not significantly different between the two groups. Karahan et al. also reported similar data14. Lower lymphocyte counts can decrease cellular immunity, and immunosuppression and decreased cellular immunity are well-known predisposing factors for OM15. Therefore, OM occurs more rapidly in chronic venous sufficiency than in other vascular diseases.
Peripheral vascular disease (ICD-10 code I73) includes artery spasms, intermittent claudication and Raynaud syndrome and is synonymous with peripheral artery disease16. In our study, peripheral vascular disease exhibited 0.69% comorbidity with OM, which was lower than that of varicose veins. This difference in comorbidity with varicose veins may be due to the intensive, intermittent insufficiency of peripheral vascular disease. By contrast, varicose vein or chronic venous insufficiency is sustained. Peripheral vascular diseases, except for asymptomatic peripheral vascular disease, are more likely to be treated before microangiopathic changes in nail capillaries induce OM. The time to onset of OM was 55.1 days in patients with peripheral vascular disease, and more time was required for OM than for varicose veins to develop. Once microangiopathic changes have occurred in nail capillaries, OM manifests in patients with peripheral vascular disease as varicose veins. The onset duration of OM in patients with peripheral vascular disease is longer than that in patients with varicose veins because microangiopathic changes in peripheral vascular disease occur more intermittently than those in varicose veins.
The correlation between OM and peripheral vascular disease is controversial1,7. In our study, compared with controls, patients with OM showed a 19.9% increased risk of peripheral vascular disease. Patients with peripheral vascular disease also exhibited a 12.8% greater risk of OM than did controls. However, in patients with OM, the probability of varicose veins was 0.13%, and the probability of peripheral vascular disease was only 0.12%. In contrast to Fukunaga et al.1, concluding that OM is a warning sign for peripheral vascular disease or varicose veins is difficult.
For hypertension or cerebral infarction, the significant changes in OM risk appear to be related to the frequency of hospital visits. In our study, the risk of OM was increased by 12.8% in patients with hypertension and decreased by 12.7% in patients with cerebral infarction. We could not find a report showing that OM was associated with hypertension or cerebral infarction3,4,15. Patients with hypertension visit the clinic regularly and have many opportunities to consult with their physician about their condition. Therefore, the diagnosis rate of OM among patients with hypertension is increased. However, the rate of OM in patients with cerebral infarction is decreased because they have more difficultly visiting their physician than do patients with other diseases. Further research on the risk of OM in patients with hypertension or cerebral infarction is needed.
The risk of hemorrhoids in patients with OM and vice versa was significantly decreased in our study. These results may have been observed because the epidemiologic spectra of the two diseases are different. Hemorrhoids are more frequent in higher socioeconomic status groups, whereas OM is more frequent in lower socioeconomic status groups17,18. Diabetes mellitus is a well-known risk factor for OM, but it does not increase the risk of hemorrhoids17,19. We suggest that the risk of comorbidity of these two diseases is decreased because the patient spectrum is different.
In our study, men took more time to visit the hospital due to OM than did women. Although OM is more prevalent in men than in women, men visit the hospital later than do women. The difference between the incidence of OM in men and women might be a reflection of the degree to which individuals are concerned about the appearance of their nails20. Because complications can occur when OM is left untreated in patients with diabetes or chronic diseases, physicians should be more concerned about the presence of OM in male patients.
This study has several limitations. First, because data were collected from the NHIC claims database, other potentially relevant data, including smoking history, family history and exercise history, were unavailable. Therefore, we focused on varicose veins and peripheral vascular disease, which were considered independent predictors of OM. Second, this study was a retrospective cohort study. Another limitation was the possibility of underestimation of diagnoses. Underestimation could occur when the symptoms of each disease were mild and because patients might not visit their physicians. Nevertheless, the strength of this study is the inclusion of data with standardized, stratified information from a one million-person sample population.
In conclusion, patients with OM patients were at increased risk for peripheral vascular disease and varicose veins; additionally, patients with peripheral vascular disease were at increased risk for OM. Careful consideration of varicose veins or peripheral vascular disease is required for the proper management of comorbidities in patients with OM.
Acknowledgements
This work was supported by a Paul-Janssen grant from the Korean Dermatologic Association (2017). This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (Nos. NRF-2015R1C1A2A01054767 and No.2018R1D1A1B07044100).
Author Contributions
Chul Hwan Bang, MD; Hyun Ji Lee, MD; and Ji Hyun Lee, MD, PhD wrote the main manuscript text. Jae Woong Yoon and Suk Jun Lee, PhD, prepared figures and tables. Jun Young Lee, MD, PhD, and Young Min Park, MD, PhD, supervised this work. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chul Hwan Bang and Jae Woong Yoon contributed equally
Suk Jun Lee and Ji Hyun Lee jointly supervised this work.
Contributor Information
Suk Jun Lee, Email: sjlee@kw.ac.kr.
Ji Hyun Lee, Email: ejee@catholic.ac.kr.
References
- 1.Fukunaga A, et al. Onychomycosis as a warning sign for peripheral arterial disease. Acta Derm Venereol. 2013;93:747–748. doi: 10.2340/00015555-1576. [DOI] [PubMed] [Google Scholar]
- 2.Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014;28:1480–1491. doi: 10.1111/jdv.12323. [DOI] [PubMed] [Google Scholar]
- 3.Sigurgeirsson B, Steingrimsson O. Risk factors associated with onychomycosis. J Eur Acad Dermatol Venereol. 2004;18:48–51. doi: 10.1111/j.1468-3083.2004.00851.x. [DOI] [PubMed] [Google Scholar]
- 4.Gupta AK, et al. The epidemiology of onychomycosis: possible role of smoking and peripheral arterial disease. J Eur Acad Dermatol Venereol. 2000;14:466–469. doi: 10.1046/j.1468-3083.2000.00124.x. [DOI] [PubMed] [Google Scholar]
- 5.Shemer A, Nathansohn N, Kaplan B, Trau H. Toenail abnormalities and onychomycosis in chronic venous insufficiency of the legs: should we treat? J Eur Acad Dermatol Venereol. 2008;22:279–282. doi: 10.1111/j.1468-3083.2007.02401.x. [DOI] [PubMed] [Google Scholar]
- 6.Kulac M, et al. Venous insufficiency in patients with toenail onychomycosis. J Ultrasound Med. 2005;24:1085–1089. doi: 10.7863/jum.2005.24.8.1085. [DOI] [PubMed] [Google Scholar]
- 7.Ozkan F, et al. Frequency of peripheral arterial disease and venous insufficiency in toenail onychomycosis. J Dermatol. 2013;40:107–110. doi: 10.1111/1346-8138.12020. [DOI] [PubMed] [Google Scholar]
- 8.Harms SK, Deogun JS. Sequential association rule mining with time lags. Journal of Intelligent Information Systems. 2004;22:7–22. doi: 10.1023/a:1025824629047. [DOI] [Google Scholar]
- 9.Tai YM, Chiu HW. Comorbidity study of ADHD: applying association rule mining (ARM) to National Health Insurance Database of Taiwan. Int J Med Inform. 2009;78:e75–83. doi: 10.1016/j.ijmedinf.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Song SO, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal R, Imielinski T, Swami A. Database mining: a performance perspective. IEEE Transactions on Knowledge and Data Engineering. 1993;5:914–925. doi: 10.1109/69.250074. [DOI] [Google Scholar]
- 12.Saez de Ocariz MM, Arenas R, Ranero-Juarez GA, Farrera-Esponda F, Monroy-Ramos E. Frequency of toenail onychomycosis in patients with cutaneous manifestations of chronic venous insufficiency. Int J Dermatol. 2001;40:18–25. doi: 10.1046/j.1365-4362.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- 13.Mosmiller LT, Steele KN, Shrader CD, Petrone AB. Evaluation of inflammatory cell biomarkers in chronic venous insufficiency. Phlebology. 2017;32:634–640. doi: 10.1177/0268355517701806. [DOI] [PubMed] [Google Scholar]
- 14.Karahan O, et al. Simple blood tests as predictive markers of disease severity and clinical condition in patients with venous insufficiency. Blood Coagul Fibrinolysis. 2016;27:684–690. doi: 10.1097/MBC.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 15.Nenoff, P., Kruger, C., Ginter-Hanselmayer, G. & Tietz, H. J. Mycology - an update. Part 1: dermatomycoses: causative agents, epidemiology and pathogenesis. J Dtsch Dermatol Ges12, 188-209, quiz 210, 188–211, quiz 212, 10.1111/ddg.12245 (2014). [DOI] [PubMed]
- 16.Conte SM, Vale PR. Peripheral arterial disease. Heart Lung Circ. 2018;27:427–432. doi: 10.1016/j.hlc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Riss S, et al. The prevalence of hemorrhoids in adults. Int J Colorectal Dis. 2012;27:215–220. doi: 10.1007/s00384-011-1316-3. [DOI] [PubMed] [Google Scholar]
- 18.Gunduz T, et al. Onychomycosis in primary school children: association with socioeconomic conditions. Mycoses. 2006;49:431–433. doi: 10.1111/j.1439-0507.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- 19.Gupta AK, Daigle D, Foley KA. The prevalence of culture-confirmed toenail onychomycosis in at-risk patient populations. J Eur Acad Dermatol Venereol. 2015;29:1039–1044. doi: 10.1111/jdv.12873. [DOI] [PubMed] [Google Scholar]
- 20.Ameen M, Lear JT, Madan V, Mohd Mustapa MF, Richardson M. British Association of Dermatologists’ guidelines for the management of onychomycosis 2014. Br J Dermatol. 2014;171:937–958. doi: 10.1111/bjd.13358. [DOI] [PubMed] [Google Scholar]


