Abstract
Serotonin neurotransmitter deficits are reported in suicide, major depressive disorder (MDD) and alcohol use disorder (AUD). To compare pathophysiology in these disorders, we mapped brain serotonin transporter (SERT), 5-HT1A, and 5-HT2A receptor binding throughout prefrontal cortex and in anterior cingulate cortex postmortem. Cases and controls died suddenly minimizing agonal effects and had a postmortem interval ≤24 h to avoid compromised brain integrity. Neuropathology and toxicology confirmed absence of neuropathology and psychotropic medications. For most subjects (167 of 232), a DSM-IV Axis I diagnosis was made by psychological autopsy. Autoradiography was performed in right hemisphere coronal sections at a pre-genual level. Linear model analyses included sex and age with group and Brodmann area as interaction terms. SERT binding was lower in suicides (p = 0.004) independent of sex (females < males, p < 0.0001), however, the lower SERT binding was dependent on MDD diagnosis (p = 0.014). Higher SERT binding was associated with diagnosis of alcoholism (p = 0.012). 5-HT1A binding was greater in suicides (p < 0.001), independent of MDD (p = 0.168). Alcoholism was associated with higher 5-HT1A binding (p < 0.001) but only in suicides (p < 0.001). 5-HT2A binding was greater in suicides (p < 0.001) only when including MDD (p = 0.117) and alcoholism (p = 0.148) in the model. Reported childhood adversity was associated with higher SERT and 5-HT1A binding (p = 0.004) in nonsuicides and higher 5-HT2A binding (p < 0.001). Low SERT and more 5-HT1A and 5-HT2A binding in the neocortex in depressed suicides is dependent on Axis I diagnosis and reported childhood adversity. Findings in alcoholism differed from those in depression and suicide indicating a distinct serotonin system pathophysiology.
Introduction
Impaired serotonin (5-HT) neurotransmission is detectable in the brain of suicide decedents and in the cerebrospinal (CSF) fluid of nonfatal suicide attempters1,2, major depressive disorder (MDD) and alcohol use disorder (AUD)3,4. Biological findings in suicide include those related to comorbid diagnoses and those associated with the diathesis for suicide that explains why only a subgroup of people with MDD or AUD are at elevated risk for suicide5,6. Despite the fact that suicide-associated conditions such as MDD and AUD are frequently co-morbid, little work has compared 5-HT receptor abnormalities in suicide, MDD, and AUD (see ref. 1 for review).
Childhood adversity increases the risk of suicide, MDD, and AUD in adulthood7,8. What mediates the effect of childhood adversity on the risk for these diagnoses is not well understood, but epigenetic and gene-environment interactions are reported9–11. We hypothesize that childhood adversity affects the serotonin system and contributes to increased risk for suicide, MDD or AUD in adulthood.
In vivo and in vitro imaging of the SERT and 5-HT receptor subtype binding in suicide and nonfatal suicide suggest there are serotonin system abnormalities; but there is less agreement about the direction of the findings, the specificity of the findings for each of these conditions, including which brain areas are involved12–22. Explanations for discrepant results include small effect sizes, variability in outcome measures, and biodemographic variability related to sex and age; other factors are the heterogeneity in suicide behavior and the effects from comorbid psychiatric disorders. Abnormalities in the serotonin system are more pronounced with more lethal suicidal behavior16,22–25. In the present study, we sought to determine the effects of suicide on serotonin receptor binding and separate the effects of suicide from comorbid MDD, AUD, and early life adversity studying postmortem brain using quantitative autoradiography, in the hitherto largest published sample of postmortem suicides and controls.
Materials and methods
Subjects
The Division of Molecular Imaging and Neuropathology at the New York State Psychiatric Institute was the source of the brain samples. The Institutional Review Boards of the appropriate Institutions approved all procedures. Subjects had postmortem intervals (PMI) of 24 h or less and died suddenly. The Coroner or Medical Examiner diagnosed suicides on the basis of evidence of intent and a self-inflicted fatal act. There were 232 cases total (Table 1): suicide decedents (n = 83) and nonsuicides (n = 149). The majority of the cases and controls used have been published elsewhere in smaller, focused studies4,26–31.
Table 1.
All Subject demographics
| Group (n) | Age (y) Mean ± SEM | Sex (M:F) | Race W:B:A:H | PMI (h) Mean ± SEM | Brain pH Mean ± SEM | Axis I Dx (n) | Cause of death (n) |
|---|---|---|---|---|---|---|---|
| Nonsuicides (n = 149) | 44 ± 1 | 119:30 | 98:31:4:16 | 14.2 ± 0.4 | 6.54 ± 0.02 | None (48) AUD (35/18)* MDD (6) Unknown (43) Other (5) |
Respiratory failure (4), Liver failure (3), Cancer (1), Cardiac (71), hemorrhage (4), Gunshot wound (8), Stabbing (8), MVA (34), Fall from height (6), Industrial accident (2), Other accident (2), Drowning (1) Electrocution (1), Other (Natural/Accidental) (2), Asphyxiation (2) |
| Suicides (n = 83) | 45 ± 2 | 62:21 | 60:8:2:13 | 15.8 ± 0.7 | 6.37 ± 0.18 | None (3) AUD (14/6)* MDD (45) Unknown (22) Schizophrenia (4) |
Fall from height (13), Hanging (29), Gunshot wound (28), Subway (2), Drowning (4), Overdose (2), Asphyxiation (2), Poison ingestion (2), Other (1) |
AUD alcohol use disorder (dependent/abuser), MVA motor vehicle accident; *Some AUD subjects were comorbid for MDD
The next-of-kin of 167 subjects (Table 2) agreed to a psychological autopsy interview32. We fully implemented the psychological autopsy protocol only after the first 65 suicides and nonsuicides had been collected. Diagnoses were based on DSM-IV criteria and used the Structured Clinical Interview for DSM (SCID-I and SCID-II33), and the Brown-Goodwin Aggression History Scale34. AUD diagnosis was based on the psychological autopsy and autopsy findings such as liver cirrhosis combined with a blood or brain alcohol level of >0.15%. Medication use within three months of death were recorded and recent use was confirmed by toxicology. All diagnoses, or lack thereof for controls, were made at a consensus conference with experienced psychiatrists, psychologists and other researchers. In the 232 cases, 65 subjects did not have a psychological autopsy, but we were able to review charts and other medical examiner records and arrive at a consensus diagnosis.
Table 2.
Subjects with psychological autopsy
| Group (n) | Age (y) Mean ± SEM | Sex (M:F) | Race W:B:A:H | PMI (h) Mean ± SEM | Brain pH Mean ± SEM | Axis I Dx (n) | Cause of death (n) |
|---|---|---|---|---|---|---|---|
| Nonsuicides (n = 106) | 45 ± 2 | 91:15 | 68:22:3:13 | 14.6 ± 0.5 | 6.55 ± 0.03 | None (48) AUD (35/18)* MDD (6) Other (5) |
Respiratory failure (2), Liver failure (2), Cancer (1), Cardiac (59), Hemorrhage (4), Gunshot wound (2), Stabbing (6), MVA (19), Fall from height (3), Industrial accident (2), Drowning (1) Electrocution (1), Other (natural/accidental) (2) Asphyxiation (2) |
| Suicides (n = 61) | 45 ± 3 | 42:17 | 45:4:1:9 | 16.6 ± 0.8 | 6.31 ± 0.3 | None (3) AUD (14/5)* MDD (45) Other (4) |
Fall from height (8), Hanging (20), Gunshot wound (21), Drowning (4), Overdose (2), Asphyxiation (1), Poison (2), Other (1) |
AUD alcohol use disorder (dependent/abuser), MVA motor vehicle accident; *Some AUD subjects were comorbid for MDD
All cases were sudden-death cases
Brain collection
After brain removal, the brainstem was separated by a transverse cut at the rostral margin of the superior colliculus. The cerebellum was removed by severing the peduncles. The brain was bisected and the right hemicerebrum was cut into 2cm-thick coronal slabs. The slabs were placed on a glass plate, frozen in liquid Freon 12 (DuPont), placed in labeled plastic bags and transferred to a −80 °C freezer. Cerebellar tissue was collected for genetics and brain toxicology. The left hemisphere was placed in formalin for neuropathology.
Receptor autoradiography
Quantitative in vitro receptor autoradiography was done on frozen tissue sections as described elsewhere26,27. A single concentration of ligand was used which was based on the Kd reported in the literature and verified previously in our laboratories26,28. In brief, 20 µm sections were used for [3H]Cyanoimipramine, [3H]8-OH-DPAT and [3H]Ketanserin binding, to label SERT sites, 5-HT1A and 5-HT2A receptors, respectively (Fig. 1). Six tissue sections were used for each assay, three for total binding and three adjacent sections for non-specific binding. Sections were preincubated in buffer to remove endogenous ligands and incubated with radioligand under optimal conditions. Nonspecific binding was determined by incubation with appropriate displacers. Sections were then washed in buffer (4 °C), dipped in water, rapidly dried and transferred to a vacuum desiccator until exposure (24 h).
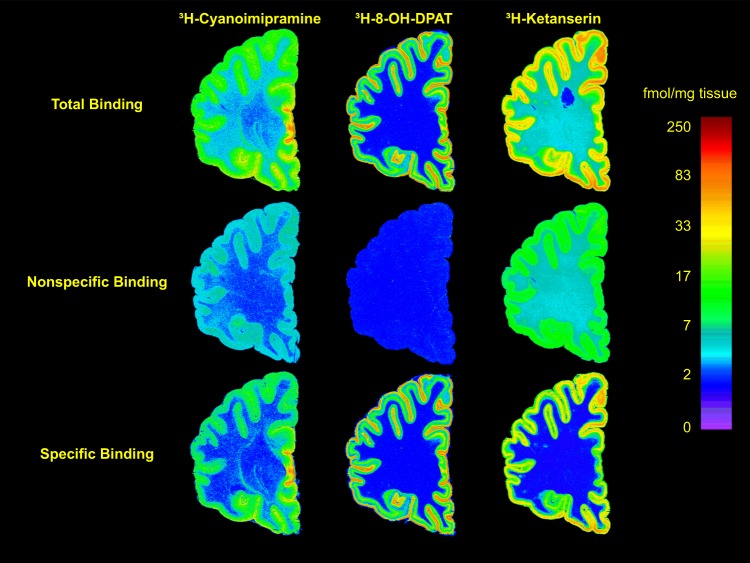
Fig. 1. Representative autoradiograms of receptor binding of [3H]-Cyanoimipramine to the serotonin transporter (left), [3H]-8-OH-DPAT to the 5-HT1A receptor (center) and [3H]-Ketanserin to the 5-HT2A receptor (right).
The autoradiograms are from sections cut from the right hemisphere of a representative nonpsychiatric control. The upper row has images of total binding, the middle row is of nonspecific binding and the lower row has subtracted images of specific binding. See methods for displacers and assay conditions. The images were calibrated to fmol/mg tissue using radioactivity standards and color-mapped to a single scale on the right
Dried slides were exposed to tritium-sensitive film (Hyperfilm from Amersham, or MS film from Kodak). Each film was exposed with tritium standards (American Radiolabeled Chemicals, Inc.). Films were developed (Kodak D-19) for 4 min at 17 °C, rinsed briefly, and fixed (Kodak Rapid Fixer) for 5 min The sections were fixed in buffered formalin and stained with thionin or cresyl violet.
Autoradiograms were quantified using an image analysis system (MCID, Imaging Research, Inc.). Images of standards were calibrated to femtomoles of radioligand per milligram of tissue. Samples of receptor binding were averaged from three sections to produce one binding measure for that individual.
[3H]Cyanoimipramine (CN-IMI) binding to serotonin transporter sites: Total SERT binding was determined with 0.4 nM 3H-CN-IMI and nonspecific binding using 10 μM sertraline26.
[3H]8-OH-DPAT binding to 5-HT1A receptors: 5-HT1A receptors were measured using our modifications of the protocol of Hoyer et al.35. Slides were incubated with 2 nM [3H]8-OH-DPAT and 100 nM sertraline (to block SERT sites).
[3H]-Ketanserin (Ket) binding to 5-HT2A receptors: Total binding was determined by incubation with 2 nM 3H-Ket, 1 μM prazosin and 1 μM tetrabenazine.
Statistical analyses
Statistical tests were done using SPSS (Version 24, IBM Analytics, NY) and R (Version 3.3.2, R Foundation for Statistical Computing; https://cran.r-project.org).
There were three primary hypotheses: (1) SERT are reduced in suicides; (2) there are more 5-HT1A receptors in the PFC in suicide; and, (3) there are more 5-HT2A receptors in the PFC in suicides. Linear models were used since the response variables were continuous (scalar) (SPSS Procedures UNIANOVA, REGRESSION, and T-TEST). Post hoc tests were performed only when main factors had a significant interaction with brain region. Suicide and MDD were fixed factors, brain regions were assigned as a random factor. All models tested included age and sex as covariates, and when found to be significant, correlation analysis was performed. Statistical tests were performed on raw values. The three receptors (SERT, 5-HT1A and 5-HT2A) were examined individually, and while uncorrected p-values are reported, a Bonferroni–adjusted significance level of 0.017 was used to preserve an experiment-wise Type I error rate of 0.05 for the primary analyses.
We sought to determine whether any differences in suicide, MDD, AUD or reported early life adversity (ELA) were widespread in PFC or anatomically discrete. The Brodmann areas present at this anatomical level included BA8, BA9, BA46, BA45, BA47, BA11, BA12, BA24, and BA32.
The primary hypotheses were re-tested in a sensitivity analysis limited to subjects with psychological autopsy: Two secondary analyses for each outcome tested whether the difference of binding in suicides is accounted for by the MDD or AUD; a third analysis tested whether binding is associated with aggression or reported early childhood adversity. These hypotheses were made a priori, but being secondary, no p-value adjustment was made for multiple testing other than using the 0.017 significance level to adjust for the three outcome measures.
Results
Serotonin transporter (SERT)
In nonsuicides (n = 143), SERT binding ranged from 5.9 ± 2.1 fmol/mg tissue in BA8 to 23.9 ± 12.0 fmol/mg tissue in BA24 (mean ± SD, Table 3).
Table 3.
Transporter, 5-HT1A and 5-HT2A binding in suicide and major depressive disorder
| All cases were sudden-death cases. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor | n | Group | BA 8 | BA 9 | BA 46 | BA 45 | BA 47 | BA 11 | BA 12 | BA 32 | BA 24 |
| SERT | 143 | NonSuicide | 5.86 ± 2.13 | 6.38 ± 2.6 | 6.83 ± 2.84 | 8.81 ± 4.42 | 9.62 ± 5.22 | 10.13 ± 5.55 | 11.21 ± 5.36 | 9.9 ± 4.29 | 23.91 ± 11.98 |
| 77 | Suicide | 5.35 ± 2.61 | 5.76 ± 2.96 | 5.81 ± 3.46* | 7.65 ± 4.31 | 8.8 ± 5.43 | 9.52 ± 6.15* | 9.34 ± 5.5* | 9.05 ± 6.53 | 20.49 ± 16.63 | |
| 5-HT1A | 143 | NonSuicide | 13.93 ± 4.77 | 14.66 ± 4.99 | 14.24 ± 4.79 | 14.75 ± 4.64 | 17.72 ± 5.57 | 19.18 ± 6.54 | 18.42 ± 6.2 | 16.15 ± 5.55 | 19.45 ± 7.18 |
| 79 | Suicide | 14.19 ± 4.03 | 14.59 ± 4.64 | 14.97 ± 4.61* | 15.19 ± 3.86 | 18.07 ± 4.87 | 19.68 ± 5.97 | 18.62 ± 5.43 | 16.26 ± 3.97 | 18.49 ± 6.08 | |
| 5-HT2A | 145 | NonSuicide | 29.38 ± 10.82 | 29.58 ± 10.82 | 29.36 ± 9.96 | 30.36 ± 10.55 | 32.76 ± 11.35 | 33.13 ± 11.55 | 33.22 ± 11.52 | 30.03 ± 10.05 | 25.48 ± 9.57 |
| 78 | Suicide | 30.05 ± 12.09 | 30.34 ± 12.54 | 31.41 ± 12.59 | 30.24 ± 11.48 | 33.65 ± 12.52 | 33.43 ± 12.55 | 32.93 ± 12.5 | 30 ± 11.69 | 23.21 ± 9.05 | |
| Only cases with psychological autopsy: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor | n | Group | BA 8 | BA 9 | BA 46 | BA 45 | BA 47 | BA 11 | BA 12 | BA 32 | BA 24 |
| SERT | 102 | NonSuicide | 5.7 ± 2.1 | 6.3 ± 2.58 | 6.73 ± 2.73 | 8.46 ± 4.53 | 9 ± 5.06 | 9.55 ± 4.79 | 10.92 ± 4.95 | 9.58 ± 4.17 | 22.54 ± 11.58 |
| 55 | Suicide | 4.58 ± 2.19 | 5.33 ± 2.64 | 5.4 ± 3.07 | 6.8 ± 3.64 | 8.11 ± 5.42 | 8.9 ± 6.5 | 8.15 ± 4.76 | 7.61 ± 4.55 | 15.63 ± 12.95 | |
| 5-HT1A | 100 | NonSuicide | 12.79 ± 3.76 | 14.01 ± 4.34 | 13.28 ± 3.84 | 14.06 ± 4.28 | 16.51 ± 5.32 | 17.98 ± 5.85 | 17.42 ± 5.95 | 14.81 ± 5.04 | 18.14 ± 6.43 |
| 56 | Suicide | 14.68 ± 4.06* | 14.88 ± 4.4 | 15.53 ± 4.34* | 15.26 ± 3.55 | 18.27 ± 4.85* | 20.06 ± 5.8* | 18.8 ± 4.86 | 16.09 ± 4.16 | 19.67 ± 5.96 | |
| 5-HT2A | 104 | NonSuicide | 27.37 ± 9.08 | 28.42 ± 10.37 | 29.14 ± 9.88 | 29.65 ± 10.12 | 32.1 ± 10.57 | 32.99 ± 11.3 | 32.96 ± 11.22 | 29.35 ± 9.34 | 24.6 ± 8.74 |
| 56 | Suicide | 31.09 ± 13.85* | 31.67 ± 13.68* | 32.82 ± 13.26* | 31.76 ± 12.19 | 35.41 ± 13.15* | 35.43 ± 13.03 | 34.31 ± 13.42 | 31.07 ± 12.92 | 23.43 ± 9.82 | |
| SERT | 112 | NoMDD | 5.65 ± 2.11 | 6.33 ± 2.62 | 6.65 ± 2.71 | 8.41 ± 4.49 | 9.11 ± 5.27 | 9.77 ± 5.24 | 10.85 ± 4.97 | 9.63 ± 4.21 | 21.83 ± 12.17 |
| 47 | MDD | 4.55 ± 2.17 | 5.1 ± 2.45* | 5.28 ± 3.14* | 6.6 ± 3.44* | 7.68 ± 4.81 | 8.2 ± 5.71 | 7.72 ± 4.52* | 7.04 ± 4.27* | 16.28 ± 11.94 | |
| 5-HT1A | 110 | NoMDD | 13 ± 3.63 | 14.14 ± 4.22 | 13.68 ± 3.93 | 14.31 ± 4.09 | 16.92 ± 5.03 | 18.54 ± 5.6 | 17.67 ± 5.81 | 15.04 ± 4.79 | 18.19 ± 6.07 |
| 48 | MDD | 14.72 ± 4.49* | 14.94 ± 4.7 | 15.15 ± 4.47* | 14.99 ± 3.93 | 17.71 ± 5.48 | 19.16 ± 6.52 | 18.61 ± 4.99 | 15.96 ± 4.67 | 19.77 ± 6.77* | |
| 5-HT2A | 112 | NoMDD | 28.03 ± 10.31 | 29.02 ± 11.12 | 29.7 ± 10.63 | 29.97 ± 10.4 | 32.79 ± 11.24 | 33.53 ± 11.67 | 33.28 ± 11.84 | 29.63 ± 9.83 | 24.91 ± 9.03 |
| 50 | MDD | 29.72 ± 12.27 | 30.8 ± 12.85 | 32.22 ± 12.57 | 31.35 ± 11.78 | 34.18 ± 12.23 | 34.19 ± 12.66 | 33.55 ± 12.34 | 30.61 ± 12.31 | 21.6 ± 8.39 | |
Values are expressed as fmol/mg tissue, mean ± SD
*p < 0.05
Suicide
Including all cases, and adjusting for sex and age, SERT binding was lower in suicides (F = 15.367, df = 1,9, p = 0.004). There was no interaction between suicide and Brodmann area (F = 0.828, df = 8,1665, p = 0.578) indicating the effect of suicide was comparable in all areas. Limiting the cases to only those with psychological autopsy, the effect of suicide remained significant (F = 11.464, df = 1,9, p = 0.008, Fig. 2a).
Fig. 2. Serotonin transporter (SERT) binding in the prefrontal cortex.
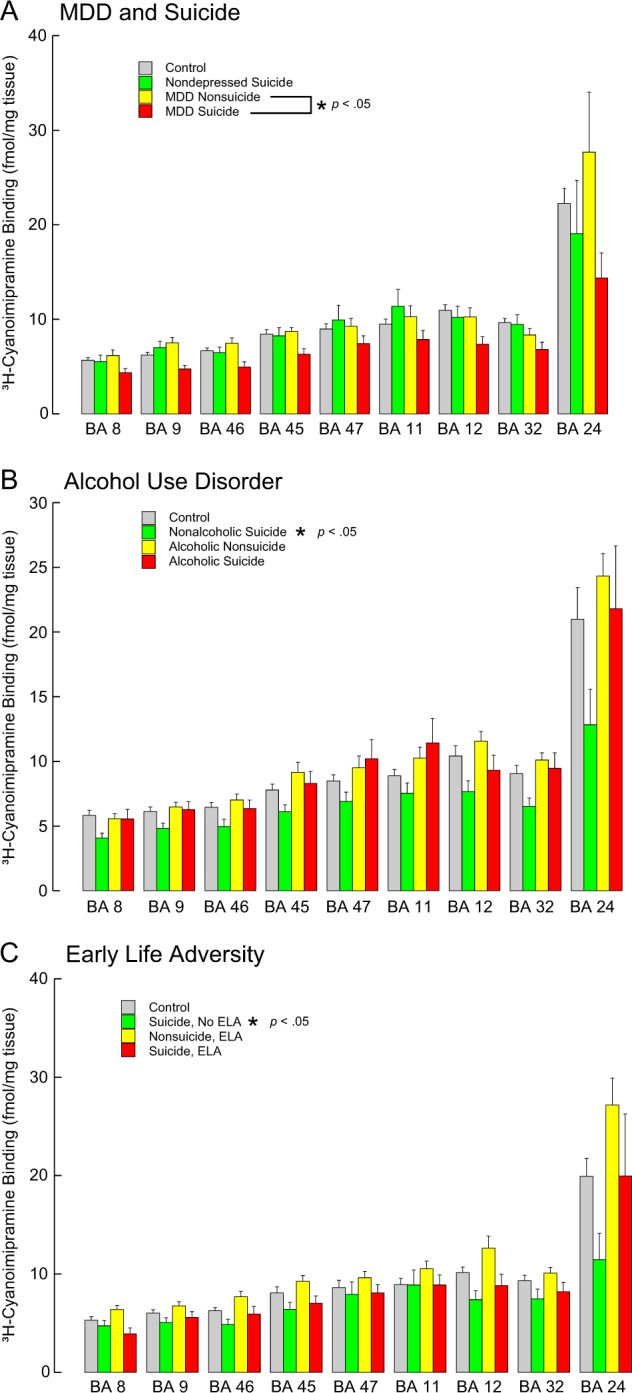
A suicide and major depressive disorder (MDD), B alcohol use disorder and C early life adversity (ELA). SERT sites were labeled with [3H]-Cyanoimipramine. Note that: SERT is less in depressed suicide decedents and with MDD. With AUD there is more SERT but only in suicides, and there is more in ELA but only in nonsuicides. Values are expressed as mean ± SEM
Major depressive disorder
There was lower SERT binding with MDD (F = 9.476, df = 1,13, p = 0.009), and the lower binding was found in all brain regions (region by depression interaction F = 0.378, df = 8,8, p = 0.878). In the model with suicide and MDD, the effect of suicide was not significant (F = 5.147, df = 1,9, p = 0.050) suggesting that lower SERT in suicide is attributable, at least in part, to the MDD diagnosis (Table 3, Fig. 2a).
Alcohol use disorder
There were cases with AUD in both nonsuicides (n = 53 with AUD) and suicides (n = 20 with AUD). The AUD diagnosis was associated with more SERT (Fig. 2b, Table 4; F = 8.135, df = 1,16, p = 0.012); the AUD interaction with brain region was not significant (F = 0.671, df = 8,10, p = 0.708) indicating the effect of AUD was comparable in all areas. In the same model, the suicide effect adjusted for AUD was not significant (F = 2.972, df = 1,10, p = 0.117), suggesting the AUD effect is in nonsuicides (Fig. 2b). Similarly, with MDD in the model, the diagnosis of MDD was also no longer significant (F = 3.418, df = 1,23, p = 0.078). This may reflect the lower rate of MDD in the alcoholics in our sample. In nonalcoholics, 34 of 40 suicides had MDD. In alcoholics, 14 of 58 had MDD.
Table 4.
Transporter, 5-HT1A and 5-HT2A binding in suicide and major depressive disorder in subjects with and without alcohol use disorder
| Only alcoholic cases: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor | n | Group | BA 8 | BA 9 | BA 46 | BA 45 | BA 47 | BA 11 | BA 12 | BA 32 | BA 24 |
| SERT | 52 | NonSuicide | 5.56 ± 2.01 | 6.48 ± 2.59 | 7.02 ± 2.95 | 9.14 ± 5.55 | 9.51 ± 6.36 | 10.24 ± 5.87 | 11.56 ± 4.48 | 10.1 ± 3.89 | 24.32 ± 8.93 |
| 20 | Suicide | 5.54 ± 2.57 | 6.27 ± 2.75 | 6.36 ± 2.57 | 8.29 ± 4.06 | 10.2 ± 6.65 | 11.42 ± 8.29 | 9.3 ± 4.99 | 9.46 ± 5.37 | 21.81 ± 16.08 | |
| 5-HT1A | 49 | NonSuicide | 13.04 ± 4.43 | 14.6 ± 4.65 | 13.65 ± 3.79 | 14.59 ± 4.39 | 17.07 ± 5.51 | 18.9 ± 5.97 | 17.19 ± 5.37 | 15.29 ± 5.41 | 18.81 ± 7.44 |
| 20 | Suicide | 15.96 ± 3.38 | 16.15 ± 3.89 | 16.31 ± 3.34 | 15.6 ± 2.8 | 19.72 ± 4.46 | 22.18 ± 6.24 | 18.77 ± 4.96 | 17.53 ± 3.85 | 22.24 ± 4.73 | |
| 5-HT2A | 52 | NonSuicide | 26.31 ± 10.13 | 28.46 ± 11.36 | 29.77 ± 9.91 | 28.65 ± 9.76 | 32.01 ± 10.6 | 33.14 ± 10.84 | 33.43 ± 10.21 | 28.74 ± 9.93 | 21.84 ± 8.28 |
| 19 | Suicide | 38.94 ± 12.19 | 36.59 ± 12.73 | 38.32 ± 12.07 | 35.53 ± 10.59 | 39.52 ± 11.59 | 38.9 ± 10.53 | 38.84 ± 12 | 34.99 ± 11.33 | 30.2 ± 10.99 | |
| SERT | 57 | NoMDD | 5.62 ± 2.08 | 6.49 ± 2.65 | 6.99 ± 2.88 | 9.18 ± 5.41 | 9.83 ± 6.59 | 10.75 ± 6.49 | 11.52 ± 4.73 | 10.39 ± 3.92 | 23.97 ± 10.57 |
| 14 | MDD | 5.35 ± 2.66 | 6.13 ± 2.68 | 6.12 ± 2.87 | 7.44 ± 3.88 | 9.12 ± 6.09 | 9.85 ± 7.55 | 8.13 ± 3.97 | 7.97 ± 5.54 | 19.91 ± 12.92 | |
| 5-HT1A | 55 | NoMDD | 13.33 ± 4.16 | 14.7 ± 4.43 | 14.15 ± 3.91 | 14.74 ± 4.17 | 17.42 ± 4.96 | 19.47 ± 5.63 | 17.41 ± 5.22 | 15.42 ± 4.98 | 18.97 ± 6.78 |
| 13 | MDD | 16.66 ± 4.52 | 16.53 ± 4.72 | 15.57 ± 3.61 | 15.65 ± 3.3 | 19.67 ± 6.71 | 21.51 ± 8.49 | 18.83 ± 5.74 | 18.24 ± 5.22 | 24.03 ± 6.03 | |
| 5-HT2A | 56 | NoMDD | 27.93 ± 12.35 | 29.78 ± 12.48 | 31.22 ± 11.23 | 29.54 ± 10.53 | 33.49 ± 11.81 | 34.32 ± 11.57 | 34.56 ± 11.52 | 29.74 ± 10.81 | 23.05 ± 9.42 |
| 14 | MDD | 36.21 ± 9.18 | 33.82 ± 11.3 | 35.97 ± 11.11 | 34.53 ± 9.52 | 36.68 ± 9.77 | 36.66 ± 8.93 | 36.97 ± 8.89 | 32.3 ± 10.01 | 26.92 ± 9.42 | |
| Only non-alcoholic cases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor | n | Group | BA 8 | BA 9 | BA 46 | BA 45 | BA 47 | BA 11 | BA 12 | BA 32 | BA 24 |
| SERT | 50 | NonSuicide | 5.82 ± 2.19 | 6.11 ± 2.59 | 6.45 ± 2.5 | 7.79 ± 3.17 | 8.49 ± 3.28 | 8.89 ± 3.38 | 10.41 ± 5.29 | 9.06 ± 4.42 | 21 ± 13.42 |
| 36 | Suicide | 4.07 ± 1.73 | 4.82 ± 2.42 | 4.97 ± 3.19 | 6.1 ± 3.2 | 6.9 ± 4.05 | 7.53 ± 4.67 | 7.66 ± 4.64 | 6.52 ± 3.51 | 12.83 ± 10.65 | |
| 5-HT1A | 51 | NonSuicide | 12.6 ± 3.16 | 13.44 ± 3.97 | 12.94 ± 3.9 | 13.57 ± 4.16 | 15.94 ± 5.11 | 17.11 ± 5.67 | 17.64 ± 6.48 | 14.37 ± 4.68 | 17.6 ± 5.59 |
| 37 | Suicide | 13.83 ± 4.22 | 14.19 ± 4.49 | 15.09 ± 4.72 | 15.03 ± 3.89 | 17.41 ± 4.85 | 18.84 ± 5.14 | 18.74 ± 4.82 | 15.27 ± 4.1 | 17.7 ± 5.98 | |
| 5-HT2A | 52 | NonSuicide | 28.25 ± 8.14 | 28.38 ± 9.39 | 28.61 ± 9.93 | 30.68 ± 10.47 | 32.2 ± 10.65 | 32.84 ± 11.83 | 32.55 ± 12.12 | 29.97 ± 8.76 | 26.72 ± 8.6 |
| 38 | Suicide | 26.92 ± 12.7 | 29.14 ± 13.43 | 30.39 ± 13 | 29.57 ± 12.48 | 32.96 ± 13.38 | 33.34 ± 13.83 | 31.88 ± 13.46 | 29.16 ± 13.21 | 18.85 ± 6.4 | |
| SERT | 55 | NoMDD | 5.68 ± 2.17 | 6.16 ± 2.6 | 6.33 ± 2.53 | 7.64 ± 3.15 | 8.36 ± 3.31 | 8.81 ± 3.41 | 10.29 ± 5.15 | 8.86 ± 4.39 | 20.02 ± 13.28 |
| 31 | MDD | 4.04 ± 1.82 | 4.57 ± 2.26 | 4.93 ± 3.3 | 6.15 ± 3.32 | 6.97 ± 4.14 | 7.48 ± 4.8 | 7.48 ± 4.82 | 6.52 ± 3.54 | 13.49 ± 11.53 | |
| 5-HT1A | 55 | NoMDD | 12.71 ± 3.14 | 13.58 ± 3.97 | 13.22 ± 3.94 | 13.87 ± 4.01 | 16.37 ± 5.1 | 17.58 ± 5.46 | 17.91 ± 6.38 | 14.66 ± 4.6 | 17.53 ± 5.42 |
| 33 | MDD | 13.81 ± 4.48 | 14.05 ± 4.58 | 14.87 ± 4.91 | 14.69 ± 4.27 | 16.86 ± 4.96 | 18.21 ± 5.62 | 18.39 ± 4.91 | 14.86 ± 4.24 | 17.91 ± 6.43 | |
| 5-HT2A | 56 | NoMDD | 28.1 ± 8.47 | 28.26 ± 9.63 | 28.42 ± 10.03 | 30.41 ± 10.36 | 32.06 ± 10.68 | 32.76 ± 11.83 | 32.14 ± 12.13 | 29.51 ± 8.82 | 26.36 ± 8.56 |
| 34 | MDD | 27.03 ± 12.85 | 29.44 ± 13.54 | 30.93 ± 13.16 | 29.9 ± 12.92 | 33.27 ± 13.57 | 33.54 ± 14.02 | 32.52 ± 13.6 | 29.89 ± 13.53 | 17.7 ± 5.63 | |
Values are expressed as fmol/mg tissue, mean ± SD
BA Brodmann area, MDD major depressive disorder, SERT serotonin transporter
Adversity
When childhood adversity was included in the model along with suicide, there were no SERT differences related to childhood adversity (F = 1.907, df = 1,9, p = 0.20, Table 5). The suicide effect of less SERT binding was significant (p = 0.006) and there was a suicide*adversity interaction (p = 0.007) with adversity associated with more SERT binding, but only in non-suicides (Fig. 2c). The interaction term with Brodmann area was significant with the difference localized to BA24.
Table 5.
Transporter, 5-HT1A and 5-HT2A binding in suicide and early life adversity
| Receptor | n | Group | BA 8 | BA 9 | BA 46 | BA 45 | BA 47 | BA 11 | BA 12 | BA 32 | BA 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SERT | 67 | Nonsuicide, No ELA | 5.3 ± 2.17 | 6.03 ± 2.67 | 6.26 ± 2.53 | 8.07 ± 4.99 | 8.63 ± 5.68 | 8.93 ± 4.85 | 10.15 ± 4.04 | 9.3 ± 4.63 | 19.94 ± 10.87 |
| 31 | Suicide, No ELA | 4.73 ± 2.33 | 5.06 ± 2.7 | 4.85 ± 2.76 | 6.4 ± 3.7 | 7.93 ± 6.55 | 8.88 ± 7.97 | 7.39 ± 4.67 | 7.48 ± 5.19 | 11.45 ± 10.04 | |
| 31 | Nonsuicide, ELA | 6.38 ± 1.87 | 6.76 ± 2.28 | 7.69 ± 2.85 | 9.26 ± 3.2 | 9.63 ± 3.45 | 10.52 ± 4.26 | 12.62 ± 6.15 | 10.1 ± 3.2 | 27.17 ± 11.65 | |
| 19 | Suicide, ELA | 3.91 ± 1.71 | 5.59 ± 2.54 | 5.93 ± 3.27 | 7.03 ± 3.21 | 8.07 ± 3.66 | 8.88 ± 4.51 | 8.81 ± 4.76a | 8.19 ± 3.86 | 19.96 ± 16.71 | |
| 5-HT1A | 67 | Nonsuicide, No ELA | 12.68 ± 3.81 | 13.47 ± 4.15 | 12.69 ± 3.77 | 13.47 ± 4.09 | 15.3 ± 4.87 | 16.48 ± 5.24 | 16.28 ± 5.96 | 14.08 ± 4.84 | 17.3 ± 6.62 |
| 31 | Suicide, No ELA | 14.5 ± 3.85 | 14.7 ± 4.98 | 14.81 ± 5.06 | 15.01 ± 3.97 | 18.52 ± 5.12 | 20.59 ± 6.44 | 19.04 ± 5.23 | 15.93 ± 4.76 | 19.69 ± 6.11 | |
| 30 | Nonsuicide, ELA | 12.64 ± 3.58 | 14.98 ± 4.64 | 14.45 ± 3.83 | 15.22 ± 4.51 | 18.33 ± 4.68 | 20.36 ± 5.3 | 19.51 ± 5.21 | 16.14 ± 5.19 | 19.1 ± 5.45 | |
| 20 | Suicide, ELA | 14.18 ± 2.95 | 14.86 ± 3.79 | 15.94 ± 3.02 | 15.19 ± 3.08 | 17.46 ± 4.38 | 19.04 ± 4.92 | 17.88 ± 4.25 | 15.64 ± 2.71 | 18.21 ± 5.83 | |
| 5-HT2A | 69 | Nonsuicide, No ELA | 27.74 ± 8.84 | 28.15 ± 10.11 | 28.49 ± 9.71 | 29.72 ± 10.34 | 31.79 ± 10.76 | 32.94 ± 11.31 | 32.43 ± 11.01 | 29.39 ± 8.77 | 25.74 ± 8.63 |
| 31 | Suicide, No ELA | 25.51 ± 11.68 | 27.07 ± 12.35 | 28.65 ± 12.75 | 27.07 ± 11.59 | 30.7 ± 12.58 | 31.16 ± 13.51 | 29.08 ± 11.61 | 26.08 ± 11.73 | 19.49 ± 8.54 | |
| 31 | Nonsuicide, ELA | 27.47 ± 9.92 | 29.5 ± 11.05 | 30.58 ± 10.54 | 30.19 ± 10.15 | 33.77 ± 10.01 | 34.03 ± 11.3 | 33.81 ± 11.94 | 30.13 ± 10.45 | 22.37 ± 8.89 | |
| 20 | Suicide, ELA | 37.18 ± 12.78 | 37.89 ± 12.89 | 37.01 ± 12.5 | 37.05 ± 10.66 | 41.2 ± 12.07 | 40.73 ± 10.35 | 39.34 ± 12.73 | 35.76 ± 11.89 | 29.21 ± 10.45 |
Values are expressed as fmol/mg tissue, mean ± SD
BA Brodmann area, ELA early live adversity, SERT serotonin transporter
Sex, age, aggression
Both sex and age were significant in every statistical model examined. Females (n = 49) had less SERT binding than males (n = 171, b =−0.33, t = −4.27, df = 216, p < 0.0001) in all BA areas, except BA24 (post hoc test, p = 0.069). SERT binding declined with age (t = −3.04, df = 215, p = 0.0027), and the effect was uniform across brain regions (age by BA interaction p > 0.05). SERT positively correlated with aggression in BA9 (r = 0.193, p = 0.0220), BA46 (r = 0.270, p = 0.0020), BA45 (r = 0.179, p = 0.0350), BA11 (r = 0.173, p = 0.0460) and BA32 (r = 0.198, p = 0.022) in cases overall.
5-HT1A receptors
In nonsuicides (n = 143), 5-HT1A binding ranged from 13.9 ± 4.8 fmol/mg tissue in BA8 to 19.5 ± 7.2 fmol/mg tissue in BA24 (Table 3).
Suicide
There was no significant effect of suicide (F = 0.163, df = 1,10, p = 0.695) and the suicide by brain region interaction term was not significant (F = 0.270, df = 8,1714, p = 0.976). However, in the sensitivity analysis limited to cases with psychological autopsy data, suicide was significant with more 5-HT1A binding (F = 60.049, df = 1,14, p < 0.001) and the suicide by brain region interaction was not significant (F = 0.320, df = 8,1179, p = 0.959, Fig. 3a).
Fig. 3. 5-HT1A receptor binding in the prefrontal cortex.
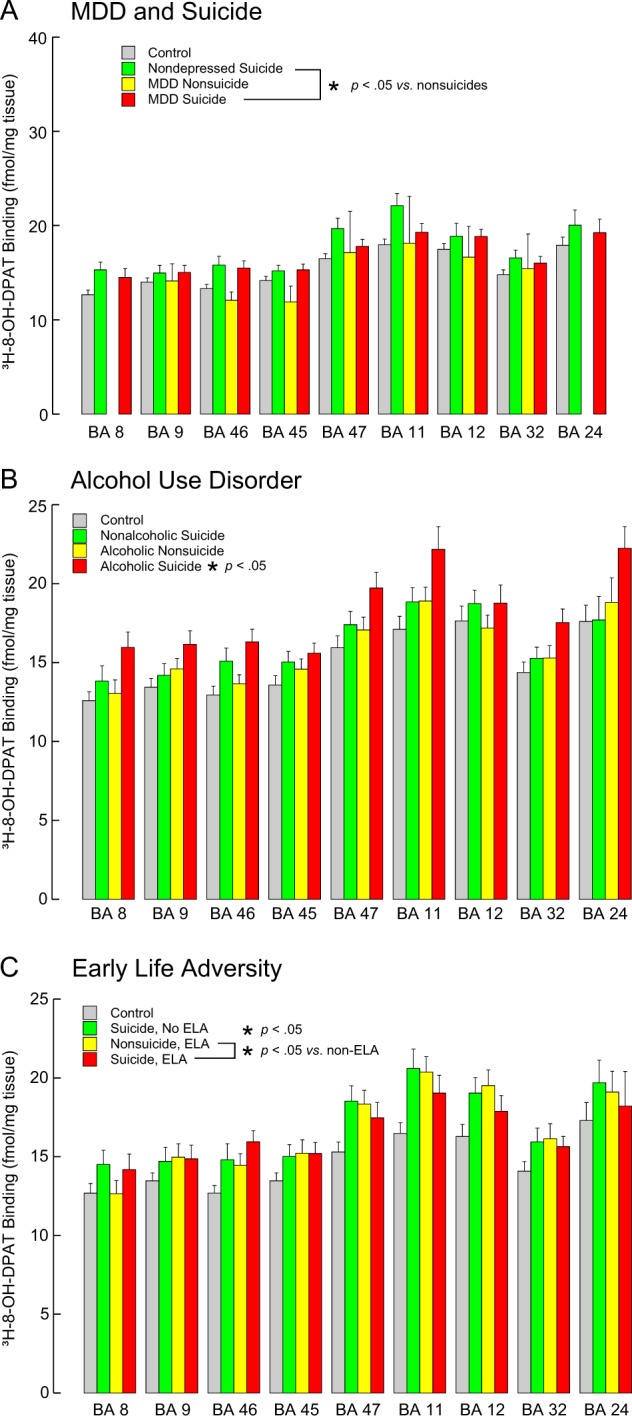
A suicide and major depressive disorder (MDD), B alcohol use disorder and C early life adversity (ELA). 5-HT1A receptors were labeled with [3H]-8-OH-DPAT. There is no data for BA8 or BA24 for MDD nonsuicides because there was only one case with these regions. Note: 5-HT1A binding was greater in suicides independent of MDD, AUD was associated with higher 5-HT1A binding but only in suicides, and ELA is associated with more 5-HT1A receptor binding. Values are expressed as mean ± SEM
Major depressive disorder
5-HT1A binding was not different in MDD (F = 2.148, df = 1,12, p = 0.168) and there was no significant interaction between suicide and MDD (F = 2.680, df = 1,12, p = 0.128) or interaction between MDD and Brodmann area (F = 0.909, df = 8,8, p = 0.552, Table 3, Fig. 3a).
Alcohol use disorder
With AUD in the model with suicide, MDD and sex, AUD was associated with more 5-HT1A binding (F = 35.703, df = 1,20, p < 0.001) and the interaction with brain region was not significant (F = 1.852, df = 8,2, p = 0.397, Fig. 3b, Table 4). The effect of suicide was still significant (F = 18.054, df = 1,12, p = 0.001) and there was a significant interaction between suicide and AUD (F = 16.562, df = 1,19, p < 0.001) with AUD associated with more 5-HT1A binding but only in suicides. There was no effect of MDD (p = 0.696) and there was no interaction between suicide and MDD (p = 0.087), but there was an interaction between MDD and AUD (F = 53.894, df = 1,18, p < 0.001) with more 5-HT1A binding only in MDD cases with AUD.
Adversity
With adversity in the model, suicide was associated with higher 5-HT1A binding (F = 27.697, df = 1,14, p < 0.001), adversity was not significant (F = 0.619, df = 1,28, p = 0.438, Table 5). There was no interaction between suicide and Brodmann area (F = 0.319, df = 8,8, p = 0.937). There was an interaction between suicide and adversity (F = 14.133, df = 1,9, p = 0.004) with adversity associated with more 5-HT1A binding, but only in non-suicides (Fig. 3c). There was no interaction between adversity and brain region (F = 0.273, df = 8,8, p = 0.958).
Sex, age, aggression
Sex was significant in select models particularly those examining the effect of suicide and AUD. 5-HT1A binding was negatively correlated with age in BA9, BA46 and BA32 (Pearson correlation values −0.141, −0.147, −0.151, p values 0.037, 0.037 and 0.029 respectively). 5-HT1A binding correlated positively with lifetime aggression in BA9 (r = .299, p < 0.001), BA46 r = .259, p = 0.003), BA45 (r = .194, p = 0.023), BA47 (r = .258, p = 0.002), BA11 (r = .212, p = 0.014), BA24 (r = .248, p = 0.036) and BA32 (r = .179, p = 0.036).
5-HT2A receptors
Suicide
There was no suicide effect detected overall (F = 2.139, df = 1,9, p = 0.176) and no suicide by region interaction (F = 0.477, df = 8,1687, p = 0.873). However, in the subgroup with psychological autopsies, there was more 5-HT2A binding in suicides (F = 41.226, df = 1,12, p < 0.001, Table 3). There was no suicide by region interaction (F = 0.386, df = 8,1193, p = 0.929) suggesting the greater amount of binding in suicide was widespread and the diagnosis of cases was important (Fig. 4a).
Fig. 4. 5-HT2A receptor binding in the prefrontal cortex.

A suicide and major depressive disorder (MDD), B alcohol use disorder and C early life adversity (ELA). 5-HT2A receptors were labeled with [3H]-Ketanserin. Note that: there is more 5-HT2A binding in suicides but no difference with MDD; there is more binding in AUD and ELA, but only in suicides. Values are expressed as mean ± SEM
Major depressive disorder
With MDD in the model, higher 5-HT2A binding was still significant with suicide (F = 18.695, df = 1,30, p < 0.001) and there was no suicide:Brodmann area interaction (F = 0.510, df = 8,8, p = 0.820). There was no significant effect of MDD (F = 2.570, df = 1,39, p = 0.117) and no MDD by brain region interaction (F = 0.361, df = 8,8, p = 0.914). This suggests that the diagnosis of MDD does not explain higher 5-HT2A binding in suicides (Fig. 4a).
Alcohol use disorder
There was no significant association between AUD and 5-HT2A binding (F = 2.302, df = 1,17, p = 0.148, Table 4); there was no interaction between AUD and Brodmann area on binding (F = 0.206, df = 8,1193, p = 0.990). With AUD in the model with suicide and MDD, the effect of suicide remained and MDD became significant (F = 17.59, df = 1,313, p < 0.001), and there was an interaction between AUD and suicide (F = 31.427, df = 1,130, p < 0.001) with more 5-HT2A binding in suicides with AUD (Fig. 4b), but not in suicides without AUD. There was no interaction between AUD and MDD (F = 0.687, df = 1,33, p = 0.413).
Adversity
Adversity was associated with more 5-HT2A binding (F = 25.484, df = 1,78, p < 0.001); the brain region by adversity interaction term was not significant (F = 0.743, df = 8,8, p = 0.658, Table 5). With adversity and suicide in the model, higher 5-HT2A binding remained associated with suicide (F = 37.658, df = 1,12, p < 0.001). There was an interaction between suicide and childhood adversity (F = 201.938, df = 1,21, p < 0.001) such that higher 5-HT2A binding was in suicides with adversity regardless of Brodmann area examined (Fig. 4c).
Sex, age, aggression
5-HT2A receptor binding negatively correlated with age in all regions (r = −0.227 to −0.441, p = 2.43 × 10−9 to 7.9 × 10−12). Females (n = 48) had lower 5-HT2A binding than males (n = 175) (b = −0.14, t = −2.32, df = 218, p = 0.0210). 5-HT2A binding correlated positively with aggression in all brain regions (r = 0.270–0.396, p < 0.05) except BA24 (r = 0.199, p = 0.085).
Discussion
The main findings in the present study were: (1) SERT binding was lower in suicides independent of sex, but dependent on MDD diagnosis; higher SERT binding is associated with AUD; (2) 5-HT1A binding was greater in suicides, independent of MDD, while AUD was associated with higher 5-HT1A binding but only in suicides; (3) 5-HT2A binding was greater in suicides only when accounting for the effects of MDD and AUD in the model. (4) Reported childhood adversity was associated with higher SERT, 5-HT1A binding, and 5-HT2A binding. These findings illustrate that the ability to detect differences in SERT, 5-HT1A, and 5-HT2A binding in the brain between cases with mood disorders and dying by suicide is dependent on Axis I diagnosis and reported childhood adversity, therein demonstrating the importance of clinical characterization of both cases and controls under investigation. Different findings in alcoholism from depression and suicide indicate distinct serotonin system pathophysiology.
Serotonin transporter
We previously found lower SERT binding in suicides in ventral PFC26,27,29. We confirm and extend this, finding the lower SERT is widespread and appears associated more with MDD than with suicide. Lower SERT in suicides is reported by some investigators, but not all (see12,36,37 for review). Our current study suggests one factor contributing to the discrepancy between studies is the proportion of MDD cases in the suicide group. Imaging studies in nonfatal attempters also report less SERT22,38–40. Interestingly, Miller et al.20 reported less SERT in depressed suicide attempters but not depressed nonattempters compared to controls, raising the possibility that SERT is related more to suicidal behavior than to depression. We observed a p-value of 0.053 which did not reach statistical significance but arguably is suggestive of an effect of suicide. Our findings here regarding suicide and MDD suggest that low SERT throughout the PFC is related to MDD more than to suicide, and the suicide effect may be the result of a more pronounced difference in the ventral PFC. Hypofunction of the ventral PFC may lead to increased suicide risk due to the inability to restrain the self-destructive act.
In AUD, we found more SERT binding, but others report lower SERT in cerebral cortex41. No difference in alcoholics or alcoholic-suicides in SERT mRNA was found in BA9 or BA2442, but there was a negative association between SERT mRNA in BA24 and anxiety symptoms. In in vivo imaging studies, alcoholics had less [11C]McN5652 binding to the SERT43. Others44,45 did not find any difference in [11C]DASB binding to the SERT in alcoholics. Comorbidity with mood disorders may contribute to the inconsistent findings and future studies of AUD should consider effects of comorbid MDD since the two diagnoses may have opposite effects on binding.
5-HT1A receptors
We observed more 5-HT1A binding in suicides, but only in cases that underwent psychological autopsy. We did not detect a difference in 5-HT1A binding in MDD, but we found more 5-HT1A binding associated with AUD. Several investigators, though not all, report higher 5-HT1A binding in depressed suicides (for review see ref. 36). We previously reported26,27 higher 3H-8-OH-DPAT binding in suicide that was anatomically restricted to ventrolateral prefrontal cortex, as were the increases reported by others46,47. Parsey et al.48 found that MDD patients whose depression did not remit had higher 5-HT1A binding and also an over-representation of GG genotype, suggesting that genotype may also affect the level of receptor binding, symptom progression and treatment response. Negative reports did not examine binding in anatomically discrete areas or did not examine ventral prefrontal cortex49–52. The lack of agreement about elevated 5-HT1A binding raises the possibility that the differences associated with suicide are anatomically discrete. The radioligand used may also affect receptor binding since antagonist but not agonist binding is decreased in MDD, suggesting binding differences may reflect the G-protein coupled or uncoupled state of the receptor53. Lower 5-HT1A receptor binding is reported in the cerebral cortex in alcoholics4,49,54. Lastly, we did not find differences in 5-HT1A binding when all cases and controls were examined together, greater 5-HT1A binding in suicides was only found when restricting the analysis to only those cases diagnosed by psychological autopsy. We believe this demonstrates how cases without psychological autopsy can bias, obscure or otherwise influence the outcome of an analysis to the point of affecting the conclusion reached.
In vivo PET imaging studies report higher 5-HT1A binding in depressed MDD subjects and remitted MDD;55 others find less binding56–58. The different results may be due to the method of estimating binding55. We observed more 5-HT1A binding associated with childhood adversity in nonsuicides, and a rodent study of stress in infants finds increased gene expression59.
We find more 5-HT1A binding with AUD, as did Thompson et al.42 who found higher 5-HT1A mRNA in BA9 in AUD but not AUD-suicides and not in BA24. Martinez and colleagues did not find any differences in 5-HT1A density in humans with alcohol dependence45. Taken together, the findings suggest brain region is important as is the potential for the presence of alcohol status to obscure effects of suicide. Genomic studies may be helpful for understanding 5HT1A regulation in suicide, MDD and AUD.
5-HT2A receptors
We found higher 5-HT2A binding in suicide, but only in cases with psychological autopsy. Other studies report higher28,60,61, lower62 and no difference52 in 5-HT2A binding in suicide. Both higher and lower binding are also reported in MDD in vivo (see ref. 15 for review). We detected more 5-HT2A binding in AUD in the present study but not previously31. No difference in 5-HT2A binding was reported in AUD by others63. Most studies did not separate effects of depression or alcoholism from suicide. Therefore, we extend previous observations by finding that 5-HT2A binding is greater in suicide alcoholics and in suicides with ELA but is not increased with MDD. Another possible explanation for the discrepancies is the use of an agonist versus antagonist ligand; higher binding in suicide was detected using the agonist LSD28,64, while the antagonist Ketanserin detected lower or no change in suicide. Another possible explanation for the discrepancy is the ligand specificity. It is known that ketanserin has affinity for tetrabenazine receptors, alpha1 adrenergic receptors and histamine receptors. We blocked for these receptors during the incubations. Some of the findings in the literature, including our own, were obtained by incubation of tissue using 125I-LSD28. A problem with 125I-labeled ligands is that differences in tissue thickness result in a darker image and higher measured receptor density. While this may pose less of a problem for small pieces of tissue, when sectioning entire hemispheres, inevitably there will be parts of the tissue that are thicker producing a darker image. We found a positive correlation between 5-HT2A binding and aggression raising the possibility that increased 5-HT2A binding in suicide is associated with the increased aggression commonly associated with suicide behavior30. Alternatively, the discrepant findings may be due to childhood adversity exposure. This not only implies a relationship between the 5-HT2A receptor and brain development and/or the development of AUD, but also demonstrates the importance of the diagnostic composition of the sample under study.
Early life adversity (ELA)
We found that reported ELA was associated with more SERT, 5-HT1A and 5-HT2A receptor binding. Reported ELA was associated with lower SERT in vivo in MDD65. Childhood adversity was not associated with 5-HT1A receptor density16, and early maternal separation, an animal model of childhood adversity in humans, was not associated with difference in 5-HT1A mRNA gene expression in rats66. In peer-reared rhesus monkeys, another animal model of childhood adversity, 5-HT1A receptor binding was less in father-reared compared with mother-reared animals, but greater in peer-reared females67.
Gene-environment interactions are increasingly found between early life stress and risk for psychiatric illness, including MDD (see ref. 68 for review) and suicide risk (see ref. 69). An interaction is widely reported between lower expressing alleles of the SERT gene promotor variant (5-HTTLPR), stressful early life events and increased risk for MDD70,71 and suicide72. Interestingly, it has been found that the S allele and the early adversity effects can be additive suggesting persistent changes in SERT expression may relate to altered serotonergic neurotransmission levels which could bring about increased disease risk73. No gene-environment interaction is reported between the 5-HT1A74 or 5-HT2A75 receptor gene polymorphisms and MDD and childhood stress. Alternatively, innate receptor binding level may mitigate the effects of gene x environment interactions.
Sex
We found females have less SERT and 5-HT2A binding than males. In contrast, sex was not a significant determinant in most of the models in which we examined the 5-HT1A receptor. Males and females differ in their prevalence in psychiatric illnesses (see refs. 68,76–78) and in serotonergic receptor densities79,80. We previously reported sex differences in SERT and 5-HT1A26. In vivo receptor binding studies as measured by PET, report that women have more 5-HT1A receptors81 and fewer SERT80 and 5-HT2A receptors82 compared with men. Others do not find sex differences49,83,84.
Conclusions
Reduced serotonergic neurotransmission has been a long-standing hypothesis in the etiology of suicide and mood disorders. The SERT is located on axons and axon terminals and are an indication of serotonergic innervation and intrasynaptic serotonin levels85. Less SERT in depressed suicides therefore suggests less innervation or greater SERT internalization secondary to less intra-synaptic 5-HT. 5-HT1A and 5-HT2A receptors in the PFC are located predominantly on cortical interneurons. We previously reported more 5-HT1A and 5-HT2A receptor binding in the PFC of depressed suicides, but only when we corrected for the density of cortical interneurons86. 5-HT1A receptor activation results in hyperpolarization and a decrease in neuronal activity in PFC87 on pyramidal neurons and cortical interneurons.
The causes of receptor differences in suicide is unclear. Evidence for receptor up- and down- regulation comes from several sources. Serotonin receptor down regulation has long been suggested as a mechanism of action for antidepressant drugs (see refs. 88–90). By the converse, in classic theories of receptor regulation, reduced 5-HT agonism or long term receptor blockade may lead to up-regulation of postsynaptic receptors, but the mechanisms of receptor regulation are complicated, differ by receptor and even by brain region for the same receptor and are not addressed in the current study (for reviews see refs. 90–94).
An increase in 5-HT1A receptors in PFC suggests an inhibition of excitatory output from cortical regions that mediate executive function and behavioral restraint. We hypothesize that reduced cortical activity may be a top-down cause of a reduction in restraint and increase in the risk for suicide behavior. Cognitive testing has been used to find that suicide attempters have worse inhibitory control in executive functioning skills;95 see ref. 96 for review). Neuroimaging studies of brain structure report prefrontal deficits in suicide behavior including impaired decision making and social assessment97,98. We now report altered 5-HT receptors in the same prefrontal cortex regions. Multimodal imaging studies of structure and connectivity in suicide attempters1, such as those used in connectomic studies99,100, support this model.
Strengths and limitations
In the present study we could compare effects across brain regions related to MDD, suicide, AUD, and childhood adversity and consider the impact of age and sex because we were able to draw upon the largest known collection of human postmortem cases with quantitative receptor autoradiography and psychological autopsy data. Our findings demonstrate the importance of clinical characterization of all cases, including the “controls”. We and others have shown how lack of reproducible findings in postmortem studies of suicide can be attributed to effects of antemortem factors, PMI, toxicology, neuropathology, clinical diagnosis, brain region identification and even freezer storage time13,101–103. The larger group size studied here, not only provided more statistical power for detecting differences, it accounted for potential receptor binding effects associated with comorbid diagnoses by using psychological autopsy to identify the presence of any comorbid illnesses and then including the diagnosis in the statistical model of the outcome measure. Effects detected in the sensitivity analysis, that were not detected in the analysis of the larger sample, which included cases that had not undergone psychological autopsy, further illustrates that undetected diagnoses can introduce bias in results that can obscure detection of differences between groups. The inclusion of alcoholics and subjects with other comorbid diagnoses provided the opportunity to begin to understand how additional illness burden can change the associated brain receptors, but a more clinically homogeneous sample increased the detectability of receptor differences. Regardless of the reliability and validity of the psychological autopsy procedure, it is not infallible, and the possibility remains that there are cases, for example suffering from an MDD episode at the time of death, that were not diagnosed or detected whether or not the psychological autopsy was performed, and these types of cases could affect the outcome. Postmortem studies have the inherent limitation of being cross-sectional and cannot address cause and effect relationships. Another limitation is not knowing the cellular sources giving rise to the receptors being measured as is coming through laser capture microdissection and cellular fractionation. Receptor binding is an endpoint measure and does nothing to address whether differences reflect changes in transcription, translation or posttranslational modification. Likewise, receptor expression is under genetic and epigenetic control and none of this is examined here, nor can it be.
Future work should examine SERT, 5HT1A, and 5HT2A binding in nonfatal suicide attempts in mood disorders and other diagnoses, and consider the effects of reported childhood adversity to determine the extent that any differences are part of these diagnostic entities or the diathesis for suicidal behavior. Indeed, there are several reports in the literature examining clinical similarities and differences between suicide attempters and completers104–106, and neurochemical similarities and differences107–109. The presence of differences in attempters to completers will suggest the differences are not only present antemortem, but will also be informative as to whether there is a continuum of differences in the brain in suicide and completers represent the most extreme form of the behavior. Future potential therapeutic approaches to the prevention of suicide, the treatment of MDD or AUD and reversing effects of childhood adversity, could then be developed to target these molecules.
Supplementary material
Acknowledgements
We are grateful to Virginia L. Johnson (autoradiography analysis) and Manuela Douglas (brain sectioning and staining) for their technical expertise. This work was supported by grants from the National Institute of Mental Health and the National Institute on Alcohol Abuse and Alcoholism (MH40210, AA09004, MH62185 and AA11293) and the Diane Goldberg Foundation. Some of the brain samples and their psychiatric characterization and storage were funded by NIH grants MH90964 and MH64168.
Conflict of interest
J.M. receives royalties for the commercial use of the C-SSRS from the Research Foundation for Mental Hygiene. V.A., M.D.U. A.J.D. S.A.K., M.J.B. declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Victoria Arango, Mark D. Underwood
Supplementary material
Supplementary Information accompanies this paper at (10.1038/s41398-018-0309-1).
References
- 1.van Heeringen K, Mann JJ. The neurobiology of suicide. Lancet Psychiatry. 2014;1:63–72. doi: 10.1016/S2215-0366(14)70220-2. [DOI] [PubMed] [Google Scholar]
- 2.Mann JJ, Arango V. Suicide: An Unnecessary Death. 2nd edn. Oxford OX2 6DP, United Kingdom: Oxford; 2015. [Google Scholar]
- 3.LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol. Psychiatry. 1994;36:326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 4.Underwood MD, Mann JJ, Arango V. Serotonergic and noradrenergic neurobiology of alcoholic suicide. Alcohol. Clin. Exp. Res. 2004;28:57s–69s. doi: 10.1097/01.ALC.0000127415.15000.CA. [DOI] [PubMed] [Google Scholar]
- 5.Pompili M, et al. Suicidal behavior and alcohol abuse. Int. J. Environ. Res. Public Health. 2010;7:1392–1431. doi: 10.3390/ijerph7041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker A, Ehret AM, Kirsch P. From the neurobiological basis of comorbid alcohol dependence and depression to psychological treatment strategies: study protocol of a randomized controlled trial. BMC Psychiatry. 2017;17:153. doi: 10.1186/s12888-017-1324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JG, et al. Childhood adversities, interpersonal difficulties, and risk for suicide attempts during late adolescence and early adulthood. Arch. Gen. Psychiatry. 2002;59:741–749. doi: 10.1001/archpsyc.59.8.741. [DOI] [PubMed] [Google Scholar]
- 8.Enns MW, et al. Childhood adversities and risk for suicidal ideation and attempts: a longitudinal population-based study. Psychol. Med. 2006;36:1769–1778. doi: 10.1017/S0033291706008646. [DOI] [PubMed] [Google Scholar]
- 9.Caspi A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 10.Labonte, B., Turecki, G. in The Neurobiological Basis of Suicide (ed Dwivedi, Y.) 275–296 (CRC Press, Boca Raton, 2012). [PubMed]
- 11.Yang BZ, et al. Child abuse and epigenetic mechanisms of disease risk. Am. J. Prev. Med. 2013;44:101–107. doi: 10.1016/j.amepre.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furczyk K, Schutová B, Michel TM, Thome J, Büttner A. The neurobiology of suicide - A review of post-mortem studies. J. Mol. Psychiatry. 2013;1:2. doi: 10.1186/2049-9256-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis DA. The human brain revisited. Opportunities and challenges in postmortem studies of psychiatric disorders. Neuropsychopharmacology. 2002;26:143–154. doi: 10.1016/S0893-133X(01)00393-1. [DOI] [PubMed] [Google Scholar]
- 14.Prabhakaran J, et al. Synthesis, in vitro and in vivo evaluation of [11C]MMTP: a potential PET ligand for mGluR1 receptors. Bioorg. Med. Chem. Lett. 2010;20:3499–3501. doi: 10.1016/j.bmcl.2010.04.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savitz JB, Drevets WC. Neuroreceptor imaging in depression. Neurobiol. Dis. 2013;52:49–65. doi: 10.1016/j.nbd.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan GM, et al. Positron emission tomography quantification of serotonin(1A) receptor binding in suicide attempters with major depressive disorder. JAMA Psychiatry. 2015;72:169–178. doi: 10.1001/jamapsychiatry.2014.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer JH, et al. Prefrontal cortex 5-HT2 receptors in depression: an [18F]setoperone PET imaging study. Am. J. Psychiatry. 1999;156:1029–1034. doi: 10.1176/ajp.156.7.1029. [DOI] [PubMed] [Google Scholar]
- 18.Audenaert K, Peremans K, Goethals I, van Heeringen C. Functional imaging, serotonin and the suicidal brain. Acta Neurol. Belg. 2006;106:125–131. [PubMed] [Google Scholar]
- 19.Parsey RV, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am. J. Psychiatry. 2006;163:52–58. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- 20.Miller JM, et al. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biol. Psychiatry. 2013;74:287–295. doi: 10.1016/j.biopsych.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh YW, et al. Incongruent reduction of serotonin transporter associated with suicide attempts in patients with major depressive disorder: a positron emission tomography study with 4-[18F]-ADAM. Int. J. Neuropsychopharmacol. 2014;18:pyu065. doi: 10.1093/ijnp/pyu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oquendo MA, et al. Positron emission tomographic imaging of the serotonergic system and prediction of risk and lethality of future suicidal behavior. JAMA Psychiatry. 2016;73:1048–1055. doi: 10.1001/jamapsychiatry.2016.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malone KM, Corbitt EM, Li S, Mann JJ. Prolactin response to fenfluramine and suicide attempt lethality in major depression. Br. J. Psychiatry. 1996;168:324–329. doi: 10.1192/bjp.168.3.324. [DOI] [PubMed] [Google Scholar]
- 24.Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol. Psychiatry. 1997;41:162–171. doi: 10.1016/S0006-3223(96)00217-X. [DOI] [PubMed] [Google Scholar]
- 25.Oquendo MA, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch. Gen. Psychiatry. 2003;60:14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- 26.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-S. [DOI] [PubMed] [Google Scholar]
- 27.Underwood MD, et al. Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int. J. Neuropsychopharmacol. 2012;15:435–447. doi: 10.1017/S1461145711000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arango V, et al. Autoradiographic Demonstration of Increased Serotonin 5-HT2 and Beta-Adrenergic-Receptor Binding-Sites in the Brain of Suicide Victims. Arch. Gen. Psychiatry. 1990;47:1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- 29.Mann JJ, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch. Gen. Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- 30.Oquendo MA, et al. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol. Psychiatry. 2006;59:235–243. doi: 10.1016/j.biopsych.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 31.Underwood MD, Mann JJ, Huang YY, Arango V. Family history of alcoholism is associated with lower 5-HT2A receptor binding in the prefrontal cortex. Alcohol. Clin. Exp. Res. 2008;32:593–599. doi: 10.1111/j.1530-0277.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 32.Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr. Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- 33.Spitzer, R. L., Williams, J. B. W., Gibbon, M. & First, M. B. Instruction Manual for the Structured Clinical Interview for DSM-III-R (SCID, 5/1/89 Revision). (Biometrics Research Department, New York State Psychiatric Institute: New York, 1989).
- 34.Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- 35.Hoyer D, Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain. I. Characterization and autoradiographic localization of 5-HT1A recognition sites. Apparent absence of 5-HT1B recognition sites. Brain Res. 1986;376:85–96. doi: 10.1016/0006-8993(86)90902-9. [DOI] [PubMed] [Google Scholar]
- 36.Arango, V. & Mann, J. J. in Neurobiology of Mental Illness, 3rd edn (eds Charney, D. & Nestler E. J) 515–529 (Oxford University Press: Oxford, UK, 2009).
- 37.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J. Psychiatr. Res. 2003;37:357–373. doi: 10.1016/S0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 38.Parsey RV, et al. Acute occupancy of brain serotonin transporter by sertraline as measured by [C-11]DASB and positron emission tomography. Biol. Psychiatry. 2006;59:821–828. doi: 10.1016/j.biopsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Parsey RV, et al. Metabolite considerations in the in vivo quantification of serotonin transporters using 11C-DASB and PET in humans. J. Nucl. Med. 2006;47:1796–1802. [PubMed] [Google Scholar]
- 40.Cannon DM, et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol. Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Mantere T, et al. Serotonin transporter distribution and density in the cerebral cortex of alcoholic and nonalcoholic comparison subjects: a whole-hemisphere autoradiography study. Am. J. Psychiatry. 2002;159:599–606. doi: 10.1176/appi.ajp.159.4.599. [DOI] [PubMed] [Google Scholar]
- 42.Thompson PM, Cruz DA, Olukotun DY, Delgado PL. Serotonin receptor, SERT mRNA and correlations with symptoms in males with alcohol dependence and suicide. Acta Psychiatr. Scand. 2012;126:165–174. doi: 10.1111/j.1600-0447.2011.01816.x. [DOI] [PubMed] [Google Scholar]
- 43.Szabo Z, et al. Positron emission tomography imaging of the serotonin transporter in subjects with a history of alcoholism. Biol. Psychiatry. 2004;55:766–771. doi: 10.1016/j.biopsych.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Brown AK, et al. PET [11C]DASB imaging of serotonin transporters in patients with alcoholism. Alcohol.: Clin. Exp. Res. 2007;31:28–32. doi: 10.1111/j.1530-0277.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 45.Martinez D, et al. Positron emission tomography imaging of the serotonin transporter and 5-HT(1A) receptor in alcohol dependence. Biol. Psychiatry. 2009;65:175–180. doi: 10.1016/j.biopsych.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. J. Neural Transm. Gen. Sect. 1991;85:181–194. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- 47.Joyce JN, et al. Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology. 1993;8:315–336. doi: 10.1038/npp.1993.32. [DOI] [PubMed] [Google Scholar]
- 48.Parsey RV, et al. Higher 5-HT(1A) receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31:1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- 49.Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Res. 1991;554:56–64. doi: 10.1016/0006-8993(91)90171-Q. [DOI] [PubMed] [Google Scholar]
- 50.Arranz B, Eriksson A, Mellerup E, Plenge P, Marcusson J. Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biol. Psychiatry. 1994;35:457–463. doi: 10.1016/0006-3223(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 51.Lowther S, et al. 5-HT1A receptor binding sites in post-mortem brain samples from depressed suicides and controls. J. Affect Disord. 1997;42:199–207. doi: 10.1016/S0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- 52.Stockmeier CA, et al. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 53.Stockmeier CA, et al. Antagonist but not agonist labeling of serotonin-1A receptors is decreased in major depressive disorder. J. Psychiatr. Res. 2009;43:887–894. doi: 10.1016/j.jpsychires.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Storvik M, Hakkinen M, Tupala E, Tiihonen J. 5-HT(1A) receptors in the frontal cortical brain areas in Cloninger type 1 and 2 alcoholics measured by whole-hemisphere autoradiography. Alcohol. Alcohol. 2009;44:2–7. doi: 10.1093/alcalc/agn090. [DOI] [PubMed] [Google Scholar]
- 55.Parsey RV, et al. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol. Psychiatry. 2010;68:170–178. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirvonen J, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int. J. Neuropsychopharmacol. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 57.Drevets WC, et al. PET imaging of serotonin 1A receptor binding in depression. Biol. Psychiatry. 1999;46:1375–1387. doi: 10.1016/S0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 58.Sargent PA, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch. Gen. Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 59.Le Francois B, et al. Chronic mild stress and antidepressant treatment alter 5-HT1A receptor expression by modifying DNA methylation of a conserved Sp4 site. Neurobiol. Dis. 2015;82:332–341. doi: 10.1016/j.nbd.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turecki G, et al. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am. J. Psychiatry. 1999;156:1456–1458. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- 61.Pandey GN, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am. J. Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- 62.Gross-Isseroff R, Salama D, Israeli M, Biegon A. Autoradiographic analysis of [3H]ketanserin binding in the human brain postmortem: effect of suicide. Brain Res. 1990;507:208–215. doi: 10.1016/0006-8993(90)90274-F. [DOI] [PubMed] [Google Scholar]
- 63.Erritzoe D, et al. Brain serotonin 2A receptor binding: relations to body mass index, tobacco and alcohol use. Neuroimage. 2009;46:23–30. doi: 10.1016/j.neuroimage.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 64.Pandey, G. N. et al. in Contemporary Neuropsychiatry (eds Miyoshi, K., Shapiro, C. M., Gaviria M. & Morita Y) 314–321 (Springer-Verlag: Tokyo, 2001).
- 65.Miller JM, et al. Reported childhood abuse is associated with low serotonin transporter binding in vivo in major depressive disorder. Synapse. 2009;63:565–573. doi: 10.1002/syn.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gartside SE, Johnson DA, Leitch MM, Troakes C, Ingram CD. Early life adversity programs changes in central 5-HT neuronal function in adulthood. Eur. J. Neurosci. 2003;17:2401–2408. doi: 10.1046/j.1460-9568.2003.02668.x. [DOI] [PubMed] [Google Scholar]
- 67.Spinelli S, et al. Effects of early-life stress on serotonin(1A) receptors in juvenile Rhesus monkeys measured by positron emission tomography. Biol. Psychiatry. 2010;67:1146–1153. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 69.Turecki G. Early-Life Adversity and Suicide Risk: The Role of Epigenetics. In: Pompili M (ed). Phenomenology of Suicide. Springer, Cham, Switzerland. 2018.
- 70.Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: a review and a hypothesis concerning gene-environment interaction. J. Affect Disord. 2008;111:1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Zalsman G, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am. J. Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 72.Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- 73.Wankerl M, et al. Effects of genetic and early environmental risk factors for depression on serotonin transporter expression and methylation profiles. Transl. Psychiatry. 2014;4:e402. doi: 10.1038/tp.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chipman P, Jorm AF, Tan XY, Easteal S. No association between the serotonin-1A receptor gene single nucleotide polymorphism rs6295C/G and symptoms of anxiety or depression, and no interaction between the polymorphism and environmental stressors of childhood anxiety or recent stressful life events on anxiety or depression. Psychiatr. Genet. 2010;20:8–13. doi: 10.1097/YPG.0b013e3283351140. [DOI] [PubMed] [Google Scholar]
- 75.Bukh JD, et al. Interaction between genetic polymorphisms and stressful life events in first episode depression. J. Affect Disord. 2009;119:107–115. doi: 10.1016/j.jad.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 76.Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch. Gen. Psychiatry. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- 77.Oquendo MA, et al. Sex differences in clinical predictors of suicidal acts after major depression: a prospective study. Am. J. Psychiatry. 2007;164:134–141. doi: 10.1176/ajp.2007.164.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cloninger CR, Christiansen KO, Reich T. Gottesman, II. Implications of sex differences in the prevalences of antisocial personality, alcoholism, and criminality for familial transmission. Arch. Gen. Psychiatry. 1978;35:941–951. doi: 10.1001/archpsyc.1978.01770320035002. [DOI] [PubMed] [Google Scholar]
- 79.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jovanovic H, et al. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 81.Parsey RV, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential using [11C]WAY-100635. Neuroimage. 2002;16:S106–S106. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 82.Soloff PH, Price JC, Mason NS, Becker C, Meltzer CC. Gender, personality, and serotonin-2A receptor binding in healthy subjects. Psychiatry Res. 2010;181:77–84. doi: 10.1016/j.pscychresns.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adams KH, et al. A database of [(18)F]-altanserin binding to 5-HT(2A) receptors in normal volunteers: normative data and relationship to physiological and demographic variables. Neuroimage. 2004;21:1105–1113. doi: 10.1016/j.neuroimage.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 84.Stein P, et al. The serotonin-1A receptor distribution in healthy men and women measured by PET and [carbonyl-11C]WAY-100635. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:2159–2168. doi: 10.1007/s00259-008-0850-x. [DOI] [PubMed] [Google Scholar]
- 85.Kovachich GB, Aronson CE, Brunswick DJ, Frazer A. Quantitative autoradiography of serotonin uptake sites in rat brain using 3H-cyanoimipramine. Brain Res. 1988;454:78–88. doi: 10.1016/0006-8993(88)90805-0. [DOI] [PubMed] [Google Scholar]
- 86.Underwood MD, Arango V. Evidence for Neurodegeneration and Neuroplasticity as Part of the Neurobiology of Suicide. Biol. Psychiatry. 2011;70:306–307. doi: 10.1016/j.biopsych.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Sprouse JS, Aghajanian GK. -)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur. J. Pharmacol. 1986;128:295–298. doi: 10.1016/0014-2999(86)90782-X. [DOI] [PubMed] [Google Scholar]
- 88.Stahl S. 5HT1A receptors and pharmacotherapy. Is serotonin receptor down-regulation linked to the mechanism of action of antidepressant drugs? Psychopharmacol. Bull. 1994;30:39–43. [PubMed] [Google Scholar]
- 89.Blakely RD, et al. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol. Psychiatry. 1998;44:169–178. doi: 10.1016/S0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 90.Hensler JG. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life. Sci. 2003;72:1665–1682. doi: 10.1016/S0024-3205(02)02482-7. [DOI] [PubMed] [Google Scholar]
- 91.Behm M, Ohman M. RNA editing: a contributor to neuronal dynamics in the mammalian brain. Trends Genet. 2016;32:165–175. doi: 10.1016/j.tig.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Leiser SC, et al. Serotonergic regulation of prefrontal cortical circuitries involved in cognitive processing: a review of individual 5-HT receptor mechanisms and concerted effects of 5-HT receptors exemplified by the multimodal antidepressant vortioxetine. ACS Chem. Neurosci. 2015;6:970–986. doi: 10.1021/cn500340j. [DOI] [PubMed] [Google Scholar]
- 93.Aznar S, Hervig Mel S. The 5-HT2A serotonin receptor in executive function: Implications for neuropsychiatric and neurodegenerative diseases. Neurosci. Biobehav. Rev. 2016;64:63–82. doi: 10.1016/j.neubiorev.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 94.Wirth A, Holst K, Ponimaskin E. How serotonin receptors regulate morphogenic signalling in neurons. Prog. Neurobiol. 2017;151:35–56. doi: 10.1016/j.pneurobio.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 95.Burton CZ, Vella L, Weller JA, Twamley EW. Differential effects of executive functioning on suicide attempts. J. Neuropsychiatry Clin. Neurosci. 2011;23:173–179. doi: 10.1176/jnp.23.2.jnp173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saffer BY, Klonsky ED. Do neurocognitive abilities distinguish suicide attempters from suicide ideators? A systematic review of an emerging research area. Clin. Psychol.: Sci. Pract. 2018;25:1. [Google Scholar]
- 97.Olie E, et al. Processing of decision-making and social threat in patients with history of suicidal attempt: a neuroimaging replication study. Psychiatry Res. 2015;234:369–377. doi: 10.1016/j.pscychresns.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 98.Ding Y, et al. Prefrontal cortex markers of suicidal vulnerability in mood disorders: a model-based structural neuroimaging study with a translational perspective. Transl. Psychiatry. 2015;5:e516. doi: 10.1038/tp.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol. Psychiatry. 2014;76:567–574. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 100.Singh MK, et al. Anomalous gray matter structural networks in major depressive disorder. Biol. Psychiatry. 2013;74:777–785. doi: 10.1016/j.biopsych.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferrer I, Martinez A, Boluda S, Parchi P, Barrachina M. Brain banks: benefits, limitations and cautions concerning the use of post-mortem brain tissue for molecular studies. Cell Tissue Bank. 2008;9:181–194. doi: 10.1007/s10561-008-9077-0. [DOI] [PubMed] [Google Scholar]
- 102.Fowler CJ, Cowburn RF, Hardy JA, Wester P, Winblad B. Neurotransmitter Function in Post-Mortem Human Brain: An Overview. Springer Berlin Heidelberg: Berlin, Heidelberg, 1990, pp 668-674.
- 103.McCullumsmith RE, Meador-Woodruff JH. Novel approaches to the study of postmortem brain in psychiatric illness: old limitations and new challenges. Biol. Psychiatry. 2011;69:127–133. doi: 10.1016/j.biopsych.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 104.Joo SH, et al. Factors associated with suicide completion: a comparison between suicide attempters and completers. Asia Pac. Psychiatry. 2016;8:80–86. doi: 10.1111/appy.12216. [DOI] [PubMed] [Google Scholar]
- 105.Davis FB. Relationship between suicide and attempted suicide - a review of literature. Psychiatr. Q. 1967;41:752-+. doi: 10.1007/BF01575635. [DOI] [Google Scholar]
- 106.Younes N, et al. Attempted and completed suicide in primary care: not what we expected? J. Affect Disord. 2015;170:150–154. doi: 10.1016/j.jad.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 107.Mathew DC, et al. Neurobiological aspects of suicide and suicide attempts in bipolar disorder. Transl Neurosci. 2013;4:203–216. doi: 10.2478/s13380-013-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pandey GN. Biological basis of suicide and suicidal behavior. Bipolar Disord. 2013;15:524–541. doi: 10.1111/bdi.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Balcioglu YH, Kose S. Neural substrates of suicide and suicidal behaviour: from a neuroimaging perspective. Psychiatry Clin. Psychopharmacol. 2018;0:1–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.