Graphical abstract
Highlights
-
•
Enteroviral infection has been long-associated with type 1 diabetes in epidemiological studies.
-
•
β-Cells express a specific enteroviral receptor isoform, CAR-SIV, mainly on secretory granules.
-
•
β-Cells respond to enteroviruses by allowing the establishment of a persistent infection.
-
•
Enteroviral vaccines are under development that might be effective in type 1 diabetes.
Abstract
The development of islet autoimmunity and type 1 diabetes has long been linked with enteroviral infection but a causal relationship has proven hard to establish. This is partly because much of the epidemiological evidence derives from studies of neutralising antibody generation in blood samples while less attention has been paid to the pancreatic beta cell as a site of infection. Nevertheless, recent studies have revealed that beta cells express specific enteroviral receptors and that they can sustain a productive enteroviral infection. Importantly, they can also mount antiviral responses which attenuate viral replication and may favour the establishment of a persistent enteroviral infection. Together, these responses combine to create the Trojan horse by which enteroviruses might precipitate islet autoimmunity.
Current Opinion in Pharmacology 2018, 43:11–19
This review comes from a themed issue on Endocrine & metabolic diseases
Edited by Shanta J Persaud and James E Bowe
For a complete overview see the Issue and the Editorial
Available online 29th July 2018
https://doi.org/10.1016/j.coph.2018.07.006
1471-4892/© 2018 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Type 1 diabetes (T1D) is characterised by the selective loss of insulin producing beta cells from the islets of Langerhans in the pancreas, meaning that affected individuals must administer exogenous insulin throughout their lives. The incidence of the disease is increasing [1] and although previously considered to be predominantly a disease of the young, it is now known to develop in all decades of life [2••]. As a consequence, there are likely to be significant numbers of older individuals with T1D who have been mis-diagnosed with Type 2 diabetes (T2D). These two observations suggest that the number of people affected by T1D may be larger than previously thought.
Type 1 diabetes arises from a complex interaction between genetic, immune and environmental factors which, as emphasized in comprehensive recent reviews [3,4] are poorly understood. In particular, the environmental influences have proven hard to identify, although studies as far back as the 1960s have implicated viral infection, particularly by human enteroviruses (HEV; single-stranded RNA (+) viruses from the picornavirus family), as a potentially important factor both in the triggering of islet autoimmunity and the onset of clinical disease. In support of this, a 2011 meta-analysis of 26 earlier studies provided evidence that enteroviral infection occurred 3.7 times more commonly in individuals with islet autoimmunity and was 9.8 times more likely at disease onset when compared to matched controls [5]. Since that time, additional studies have emerged to support this hypothesis [6]. In particular, evidence that enterovirus infections are more frequent prior to the appearance of islet autoantibodies has been found in several large prospective cohort studies [7, 8, 9, 10]. Importantly, recent studies of unique pancreas biopsy samples from Norwegian patients with T1D (the DiViD samples [11]) have provided strong evidence for both the presence of HEV and enhanced islet anti-viral responses in newly-diagnosed patients [12,13•,14•,15]. In addition, ever more sensitive technologies are being developed to detect or interrogate viral infection and anti-viral responses in blood [8,16••,17, 18, 19, 20], islets [12,21•,22•], stool [9] and other tissues [23, 24, 25, 26, 27, 28, 29, 30, 31]. These are currently being applied in new collaborative studies involving multiple laboratories who are employing differing expertise and complementary technologies to examine blinded tissue samples available from the network of Pancreatic Organ Donors with Diabetes (nPOD). The first results are due for publication soon and are expected to provide additional support for the enteroviral hypothesis in T1D.
Enteroviruses and beta cells: an unfortunate conjunction
Human beta cells are known to be susceptible to infection with HEVs, particularly members of the Coxsackievirus B family. Thus, isolated human islets can be productively infected with a range of different EV-B family members (CVBs and Echoviruses, many of which have been associated with T1D; Table 1) in vitro. Furthermore, among the various islet cells, it is the beta cells that are preferentially susceptible to infection [32, 33, 34], and this leads to a dramatic decrement in glucose-induced insulin secretion [21•,35••,36]. Tropism of HEVs for the islets has also been demonstrated in vivo in the pancreata of neonates who died following a lethal CVB infection [6,34,37] and in the pancreas of individuals with T1D [38,39]. This then raises the question: `so why the beta cells?’
Table 1.
Examples of enterovirus serotypes associated with Type 1 diabetes or which have the ability to infect human islets in vitro
The tropism of enteroviruses for the beta cell is likely to be driven by at least two factors; first, these cells express receptors necessary for the binding and subsequent internalisation of the virus and secondly, they contain specific host factors which the virus can hijack to facilitate successful infection, replication and, perhaps, persistence. This latter point is interesting since the traditional view states that enteroviruses are not likely to establish persistent infections and this concept will be explored further below. The various potential receptors utilised by enteroviruses and their expression in human islets are summarised in Table 2 but one that is receiving particular attention is the Coxsackie and Adenovirus Receptor (CAR). This molecule is utilised as an entry vehicle by many of the viruses that are associated with T1D in epidemiological studies and, very recently, we have shown that a specific isoform of CAR, having a unique C-terminal PDZ binding domain (CAR-SIV) is selectively and highly expressed within the beta cell [40••]. Studies by Ylipaasto et al. have also demonstrated that infection of human islets with CVB4 and CVB5 was effectively prevented in the presence of an antibody that blocks CAR [41]. Intriguingly, in our work, the subcellular localisation of CAR-SIV was unusual in that it was not present primarily at the plasma membrane of beta cells, as might be expected, but rather it was located mainly in insulin secretory granules. This unexpected localisation implies that the virus could selectively enter the beta cell by a Trojan horse mechanism in which secretory granule proteins are hijacked as they emerge onto the cell surface during exocytosis, such that virus particles are then internalized by the endocytic machinery during membrane recovery (Figure 1). In support of this, electron microscopy studies by Frisk et al. of human islets infected with CVBs clearly show the presence of viral replication complexes and newly synthesised virions at, or near, insulin granule membranes [35••].
Table 2.
Relevant enteroviral receptors and their expression in human beta cells/islets
| Potential Enterovirus receptors and role [78] | Enteroviruses that utilise these receptors | Transcriptomic data suggesting expression in beta cellsa | Protein expression in isletsb |
|---|---|---|---|
| CAR Uncoating |
Coxsackievirus B1-6 | +++ | +++ [40••] |
| DAF (CD55) Attachment |
Coxsackievirus A21, B1, B3 & B5 Echovirus 3, 6, 7, 11–13, 20, 21, 25, 29, 30 |
++ | HPA — not detected; [41] |
| ICAM1 Uncoating |
Coxsackievirus A13, A18, A21 Rhinovirus Major group (91 serotypes) |
Low | HPA — not detected in healthy controls; some evidence of upregulation in inflamed T1D islets [108] |
| ICAM5 Uncoating |
Enterovirus D68 | Negative | HPA — not detected |
| SCARB2 Uncoating |
Enterovirus 71 Coxsackievirus A16 |
+++ | HPA — ++ |
| PSGL1 Attachment |
Enterovirus 71 Coxsackievirus A16 |
Negative | HPA — not detected |
| α2β1 (VLA2) Attachment |
Echovirus 1, 8 |
ITGA2 — negative ITGB1 — +++ |
HPA — not detected |
| α5β3 Attachment |
Coxsackievirus A9, Echovirus 1, 9 |
ITGA5 — negative ITGB3 — negative |
HPA — not detected + in isolated islets [41] |
CAR, Coxsackie and adenovirus receptor; DAF, complement decay accelerating factor; ICAM1, intercellular adhesion molelcule-1; SCARB2, scavenger receptor class B member 2; PSGL1, P-selectin glycoprotein ligand 1; VLA2, very late antigen 2.
Source: Transcriptomics of human islets. http://sandberg.cmb.ki.se/pancreas/.
Source: Human Protein Atlas (HPA) or references. https://www.proteinatlas.org/.
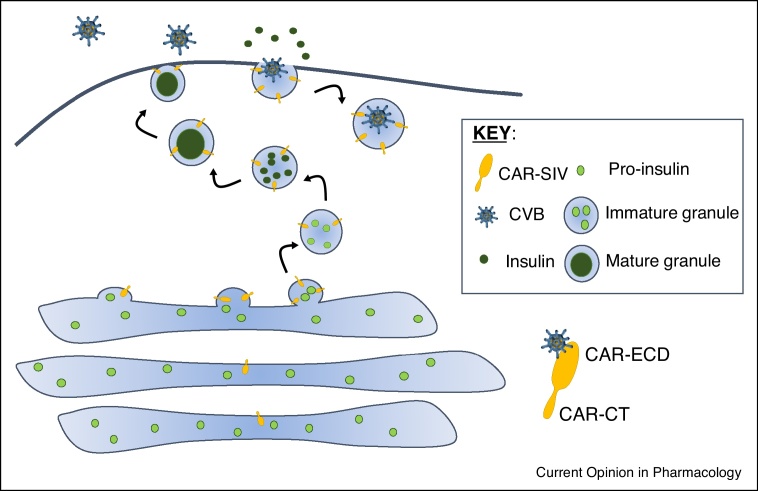
Figure 1.
A model for CVB entry into beta cells via a specific CAR isoform, CAR-SIV. Recent data demonstrate that CAR-SIV is present at high levels on insulin secretory granules. Based on its structural organization, we predict that the C-terminus (CT) of CAR-SIV faces the cytoplasmic environment and importantly, the putative `extracellular domain’ (ECD) which is required for the binding of enteroviruses, faces the granule lumen during biogenesis and maturation. This suggests that during exocytosis of insulin, the extracellular domain of CAR-SIV will be displayed on the external face of the plasma membrane and would then be available to bind to enteroviruses. During subsequent endocytosis of the granule membrane for recycling, the virus would be transported inside the cell, where it could initiate infection.
In recent years, a series of critical host factors required for successful HEV infections have been identified. These include PLA2G16 [42••] which is essential for virion-mediated genome delivery into the cytoplasm; phosphatidylinositol-4-kinase IIIβ (PI4KIIIβ) and its product phosphatidylinositol-4-phosphate (PI4P), which are critical for the generation of specialised organelles required for efficient viral replication [43,44]); polypyrimidine tract-binding protein 1 (PTBP1), which is utilized by the virus to promote cap-independent translation of viral RNA [36] and heat shock protein 90 (HSP90), which is required for the correct processing of the capsid precursor P1 [45]. Importantly, many of these proteins are expressed in human beta cells and play a key role in pathways unique to beta cells (reviewed in [46]). For example, PTBP1, has an important role in glucose-stimulated cap-independent translation of insulin granule proteins [36]; PI4KIIIβ acts as a metabolic sensor in beta cells and regulates the priming of secretory granules [47] and HSP90 is a chaperone that regulates surface expression of ATP-sensitive potassium (KATP) channels [48]. Together these results suggest that human beta cells express specific virus entry receptors as well as key host factors that aid the virus at various points in its lifecycle. This may therefore help to explain their exquisite sensitivity to infection.
In parallel with these considerations, it is also important to note that beta cells are terminally differentiated and studies of their neogenesis and proliferation suggest that these processes are vanishingly rare in humans after the age of 10y [49,50]. This means that the human host must develop strategies to effectively manage, or preferably clear, any beta cell viral infection, whilst doing everything possible to minimise the destruction of these largely irreplaceable cells. In this context, it is well known that beta cells are extremely sensitive to interferons (IFNs), the principal anti-viral cytokines produced in response to an infection [51,52]. As viremia (viruses in the bloodstream) must occur before infection of the beta cells it is likely that any cells targeted during the initial acute phase of infection will elaborate IFNs. Thus, the pancreas will be exposed to IFNs prior to any encounter with the virus and this may serve to prime the beta cells to resist infection. Indeed, pre-treatment of islets with Type I and III IFNs promotes an anti-viral state and significantly reduces viral replication following infection in vitro [51,52]. However, IFNs do not necessarily block viral entry, which could yield a scenario in which virus has entered the cell, yet the host cell has succeeded in upregulating a range of anti-viral proteins that will counter any attempt by that virus to establish a productive, lytic, infection [53]. Conceivably, a battle then ensues between the host (beta) cell and the virus which culminates in a mutual compromise where viral persistence is established and the host cells remain viable (Figure 2). In support of this hypothesis, risk-associated single nucleotide polymorphisms (SNPs) for T1D are found in key anti-viral response genes such as IFIH1 and TYK2. Individuals carrying these SNPs exhibit altered IFN responses [54, 55, 56, 57, 58, 59, 60,61••] and the risk variants have been associated with an increased frequency of HEV infection [62]. Furthermore, of the 51 identified candidates genes associated with T1D, 42 are expressed in human beta cells and when Ingenuity Pathway Analysis was performed on these genes, the three highest scoring canonical pathways were — Interferon signalling, Role of JAK1, JAK2 and TYK in interferon signalling and Role of pattern recognition receptors in recognition of virus and bacteria [54]. These pathways are all activated in response to viral infections and this provides a possible link between genetic predisposition to T1D and host anti-viral responses.
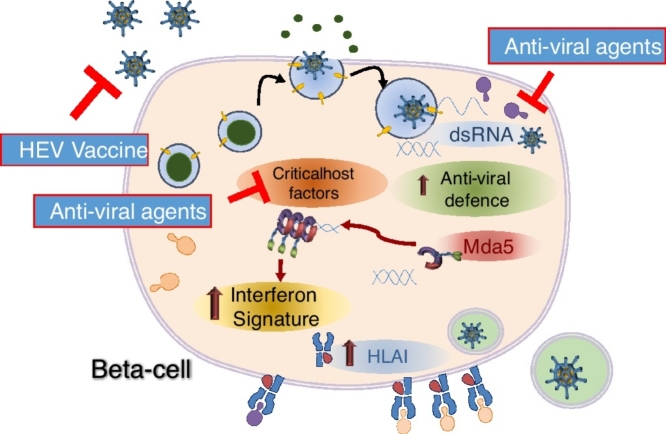
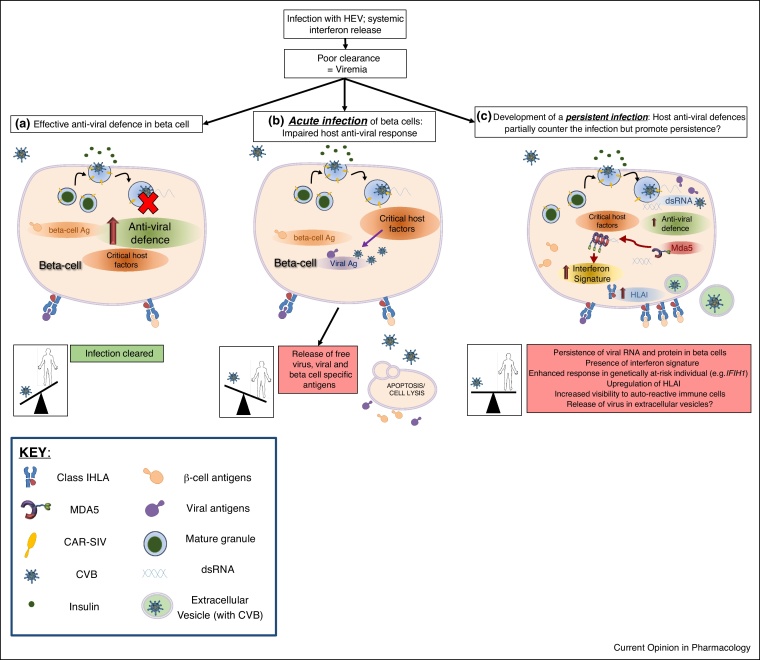
Figure 2.
A model of different beta cell responses to HEV infection. Following an infection with a HEV, systemic release of interferons primes the pancreas to respond to the likelihood of a local viral infection. (a) In most individuals this will lead to the induction of an anti-viral defence program which prevents the development of a sustained and productive infection of the beta cells. The virus is cleared and the host wins the battle. (b) In some individuals (possibly neonates?) who have an impaired anti-viral defence, enterovirus enters the cells and utilises critical host factors to establish a productive, lytic, infection. This can result in the release of free virus and/or viral and beta cell specific antigens. In individuals who are genetically predisposed to T1D, this damage may trigger the activation of islet autoreactive immune cells. (c) If the host anti-viral defence program only partially inhibits viral replication, then a persistent infection might develop. Persistent infections are associated with 5′UTR deletions of the viral genome and the formation of dsRNA. dsRNA can activate host pathogen recognition receptors (PRRs) such as Mda5 (encoded by IFIH1) and stimulate an enhanced interferon signature in cells. This will, in turn, lead to the upregulation of HLAI and enhanced presentation of beta cell and viral antigens at the cell surface. In `at-risk’ individuals this might then result in destruction by auto-reactive immune cells. Virus could be disseminated to other cells via extracellular vesicles, although this remains to be determined for human beta cells.
Evidence for persistence of HEV infection
Traditionally, HEVs are thought to induce an acute infection in which large numbers of new viral particles are rapidly synthesized and release from infected cells to spread to other nearby host cells. One of the most effective host mechanisms to control HEVs infection is the generation of neutralising antibodies which help to clear the virus from the circulation and affected tissues. However, there is mounting evidence that HEVs can evade these primary defense mechanisms to establish a lower level, persistent, infection under certain circumstances. Indeed, this type of infection has now been associated with several diseases such as Chronic Fatigue Syndrome (CFS) [63]; chronic myocarditis and dilated cardiomyopathy [64] and Amyotrophic Lateral Sclerosis (ALS; reviewed in [65]) as well as Type 1 diabetes. In order to understand this previously unrecognized aspect of EV biology, a number of mechanisms have been proposed to explain how the virus might persist. These include the activation of processes to restrict viral RNA replication, including by deletion of nucleotides from within the 5′UTR of the viral RNA genome [64,66, 67, 68] and equalization of the proportions of positive and negative strands to form double stranded RNA (dsRNA) molecules [69,70]. A key additional requirement is the need for the virus to minimise host cell lysis, which would not only promote inflammation and the activation of antiviral immune cells, but also lead to the release of free virus particles that are susceptible to neutralization by anti-EV antibodies. One mechanism by which this may be avoided is suggested by recent evidence that HEVs, including CVB3, can be shed from cells within extracellular vesicles [71,72]. In principle, this could shield the virions from neutralizing antibodies and provide a means by which they can evade immune surveillance.
Tackling HEV infection in individuals with, or who are at-risk of developing, T1D
Two main strategies are being explored to tackle HEV infection in T1D; vaccination and treatment with anti-viral agents. Both have the potential to slow disease progression, yet each also has significant obstacles that must be overcome before it could be utilized in clinical practice. Islet autoimmunity in at-risk children peaks at two different ages and the specificity of the first autoantibody also differs at each age [73]. The first peak of autoimmunity occurs during the second year of life and is associated with the development of insulin autoantibodies (IAA), while the second is seen between 3 and 5y and is associated preferentially with the emergence of GADA autoantibodies [73]. Given that these initial signs of islet autoimmunity occur early in life, children would probably need to be vaccinated within the first few months of life in order to offer effective protection against HEV infection. This will require the development of safe and effective vaccines that can target multiple HEVs associated with the disease and there is precedent for this approach given the proven success of neonatal vaccination against poliomyelitis (another enterovirus). Moreover, encouraging progress has already been made on this front, with a new formalin-inactivated CVB1 vaccine successfully developed and tested in animal models [74,75••,76]. Multivalent CVB1-6 vaccines are also now being generated (Hytönen and Flodström-Tullberg, personal communication). Epidemiological data support the idea that a vaccination approach might be effective as a means to reduce the incidence of T1D since Finnish children infected early in life with CVB3 or CVB6 appear to be immuno-protected against a subsequent infection with different HEVs which might, otherwise, precipitate T1D [7]. On the basis of such evidence, one company, `Provention’ has recently announced exciting plans to launch a first phase clinical trial to assess the safety and efficacy of a CVB vaccine in humans, with the intention of developing an effective approach to reduce the incidence of T1D (www.proventionbio.com).
Alternative approaches also being explored include the development of virus like particles (VLPs) as antigens. These resemble the viral capsid of HEVs but do not contain infectious genome [77]. Vaccines and/or VLP rely predominantly on the host developing neutralizing antibodies against virus. These will therefore be most effective when given to individuals prior to any exposure to diabetogenic viruses and will hopefully ensure that the immune response is sufficiently robust to prevent the spread of infectious virus to the pancreas. What, though, might be done to tackle infection in people who already have evidence of islet autoimmunity and/or clinical diabetes and who may be harbouring a persistent infection?
This could be a fertile realm for anti-viral agents; although at the present time very little is known about whether these are effective against persistent enteroviral infections. Anti-viral agents are available (many of which have been re-purposed from use in other conditions), or are in development, that are effective against HEVs associated with T1D (comprehensively reviewed recently in [78]), but the majority of these have been tested only under acute infection settings. These agents can be subdivided into two broad categories; those that target viral proteins and others which affect host proteins required for efficient viral replication, translation and release. Examples of the former include pleconaril which targets the viral capsid (reviewed in [79]); fluoxetine, commonly known as Prozac, which inhibits the viral protease 2C; and Gemcitabine, which binds to the viral RNA-dependent RNA polymerase, 3Dpol [80]. The second group includes Enviroxime, which targets the PI4K pathway [79]. Encouragingly, recent evidence has suggested that fluoxetine is effective against persistent enteroviral infection in cell models [81], but more research is required to test the activity of other drugs in this setting. Extensive efforts are underway to identify new anti-viral agents and these are aided by an increasing knowledge of the structure and function of viral proteins, as well as the identification of essential host factors. A further avenue of exploration is the use of combinations of different anti-viral agents that have additive or synergistic responses, which together can increase anti-viral potency, minimise the emergence of resistance and reduce drug toxicity/side effects. This could be achieved by, for example, combining one drug that targets a viral protein, with another that targets an essential host factor. Alternatively, since it is well established that some anti-viral drugs have a low barrier to resistance (meaning that a single mutation within the virus can rapidly lead to drug resistance) whereas, for others, this is much higher, a combination approach employing each type of reagent might also yield clinical benefit.
In summary, evidence for a viral aetiology in T1D is a long-established concept that has remained unproven. Nevertheless, supportive evidence continues to emerge at increasing pace and effective strategies which would minimize the risks deriving from HEV infection in susceptible individuals are being developed with increasing momentum. Arguably, it is only when the outcomes of these studies are known that it will be possible to confirm once-and-for-all whether T1D has an enteroviral component.
Funding
We are pleased to acknowledge financial support via a JDRF Career Development Award (5-CDA-2014-221-A-N) to SJR, an MRC Project Grant (MR/P010695/1) to SJR & NGM and project grants from Diabetes UK (15/0005156 & 16/0005480) to NGM & SJR.
Conflict of interest statement
Nothing declared.
Author contributions
S.J.R and N.G.M. wrote the manuscript and are the guarantors of this work.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This research was performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD: 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (Grant #2018PG-T1D053). Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org//for-partners/npod-partners/. We are grateful for the contributions of colleagues who have stimulated great discussions about the roles viruses may play in the development of Type 1 diabetes and would like to acknowledge the valuable work of many within the field that have not been cited in this review due to space restrictions.
Contributor Information
Sarah J Richardson, Email: s.richardson@exeter.ac.uk.
Noel G Morgan, Email: n.g.morgan@exeter.ac.uk.
References
- 1.Patterson C.C., Dahlquist G.G., Gyurus E., Green A., Soltesz G., Group E.S. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 2••.Thomas N.J., Jones S.E., Weedon M.N., Shields B.M., Oram R.A., Hattersley A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6:122–129. doi: 10.1016/S2213-8587(17)30362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used a Type 1 diabetes genetic risk score to demonstrate that almost half of cases of Type 1 diabetes represented in the UK Biobank were diagnosed between 31 and 60y of age. These individuals are frequently misdiagnosed with Type 2 diabetes and receive inappropriate therapy.
- 3.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiMeglio L.A., Evans-Molina C., Oram R.A. Type 1 diabetes. Lancet. 2018;391:2449–2462. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung W.C., Rawlinson W.D., Craig M.E. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan N.G., Richardson S.J. Enteroviruses as causative agents in type 1 diabetes: loose ends or lost cause? Trends Endocrinol Metab. 2014;25:611–619. doi: 10.1016/j.tem.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Laitinen O.H., Honkanen H., Pakkanen O., Oikarinen S., Hankaniemi M.M., Huhtala H., Ruokoranta T., Lecouturier V., Andre P., Harju R. Coxsackievirus B1 is associated with induction of beta-cell autoimmunity that portends type 1 diabetes. Diabetes. 2014;63:446–455. doi: 10.2337/db13-0619. [DOI] [PubMed] [Google Scholar]
- 8.Oikarinen S., Tauriainen S., Hober D., Lucas B., Vazeou A., Sioofy-Khojine A., Bozas E., Muir P., Honkanen H., Ilonen J. Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes. 2014;63:655–662. doi: 10.2337/db13-0620. [DOI] [PubMed] [Google Scholar]
- 9.Honkanen H., Oikarinen S., Nurminen N., Laitinen O.H., Huhtala H., Lehtonen J., Ruokoranta T., Hankaniemi M.M., Lecouturier V., Almond J.W. Detection of enteroviruses in stools precedes islet autoimmunity by several months: possible evidence for slowly operating mechanisms in virus-induced autoimmunity. Diabetologia. 2017;60:424–431. doi: 10.1007/s00125-016-4177-z. [DOI] [PubMed] [Google Scholar]
- 10.Hyoty H. Viruses in type 1 diabetes. Pediatr Diabetes. 2016;17(Suppl. 22):56–64. doi: 10.1111/pedi.12370. [DOI] [PubMed] [Google Scholar]
- 11.Krogvold L., Edwin B., Buanes T., Ludvigsson J., Korsgren O., Hyoty H., Frisk G., Hanssen K.F., Dahl-Jorgensen K. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. 2014;57:841–843. doi: 10.1007/s00125-013-3155-y. [DOI] [PubMed] [Google Scholar]
- 12.Krogvold L., Edwin B., Buanes T., Frisk G., Skog O., Anagandula M., Korsgren O., Undlien D., Eike M.C., Richardson S.J. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes. 2015;64:1682–1687. doi: 10.2337/db14-1370. [DOI] [PubMed] [Google Scholar]
- 13•.Lundberg M., Krogvold L., Kuric E., Dahl-Jorgensen K., Skog O. Expression of interferon-stimulated genes in insulitic pancreatic islets of patients recently diagnosed with type 1 diabetes. Diabetes. 2016;65:3104–3110. doi: 10.2337/db16-0616. [DOI] [PubMed] [Google Scholar]; Demonstrates the presence of an interferon signature at the transcriptomic level using laser capture microdissected islets from recent-onset Type 1 diabetes patients.
- 14•.Richardson S.J., Rodriguez-Calvo T., Gerling I.C., Mathews C.E., Kaddis J.S., Russell M.A., Zeissler M., Leete P., Krogvold L., Dahl-Jorgensen K. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia. 2016;59:2448–2458. doi: 10.1007/s00125-016-4067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes how a hallmark feature of Type 1 diabetes, hyperexpression of HLAI within insulin-containing islets, is associated with expression of STAT1.
- 15.Kuric E., Seiron P., Krogvold L., Edwin B., Buanes T., Hanssen K.F., Skog O., Dahl-Jorgensen K., Korsgren O. Demonstration of tissue resident memory CD8 T cells in insulitic lesions in adult patients with recent-onset type 1 diabetes. Am J Pathol. 2017;187:581–588. doi: 10.1016/j.ajpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 16••.Genoni A., Canducci F., Rossi A., Broccolo F., Chumakov K., Bono G., Salerno-Uriarte J., Salvatoni A., Pugliese A., Toniolo A. Revealing enterovirus infection in chronic human disorders: an integrated diagnostic approach. Sci Rep. 2017;7:5013. doi: 10.1038/s41598-017-04993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes the development of a technique to identify the presence of chronic (persistent) enterovirus infection in human samples.
- 17.Salvatoni A., Baj A., Bianchi G., Federico G., Colombo M., Toniolo A. Intrafamilial spread of enterovirus infections at the clinical onset of type 1 diabetes. Pediatr Diabetes. 2013;14:407–416. doi: 10.1111/pedi.12056. [DOI] [PubMed] [Google Scholar]
- 18.Cinek O., Stene L.C., Kramna L., Tapia G., Oikarinen S., Witso E., Rasmussen T., Torjesen P.A., Hyoty H., Ronningen K.S. Enterovirus RNA in longitudinal blood samples and risk of islet autoimmunity in children with a high genetic risk of type 1 diabetes: the MIDIA study. Diabetologia. 2014;57:2193–2200. doi: 10.1007/s00125-014-3327-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee H.S., Briese T., Winkler C., Rewers M., Bonifacio E., Hyoty H., Pflueger M., Simell O., She J.X., Hagopian W. Next-generation sequencing for viruses in children with rapid-onset type 1 diabetes. Diabetologia. 2013;56:1705–1711. doi: 10.1007/s00125-013-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oikarinen S., Martiskainen M., Tauriainen S., Huhtala H., Ilonen J., Veijola R., Simell O., Knip M., Hyoty H. Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes. 2011;60:276–279. doi: 10.2337/db10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Nyalwidhe J.O., Gallagher G.R., Glenn L.M., Morris M.A., Vangala P., Jurczyk A., Bortell R., Harlan D.M., Wang J.P., Nadler J.L. Coxsackievirus-induced proteomic alterations in primary human islets provide insights for the etiology of diabetes. J Endocr Soc. 2017;1:1272–1286. doi: 10.1210/js.2017-00278. [DOI] [PMC free article] [PubMed] [Google Scholar]; Utilised unbiased proteomic approaches to characterize the impact of CVB4 infection on human islets.
- 22•.Nyalwidhe J.O., Grzesik W.J., Burch T.C., Semeraro M.L., Waseem T., Gerling I.C., Mirmira R.G., Morris M.A., Nadler J.L. Comparative quantitative proteomic analysis of disease stratified laser captured microdissected human islets identifies proteins and pathways potentially related to type 1 diabetes. PLOS ONE. 2017;12:e0183908. doi: 10.1371/journal.pone.0183908. [DOI] [PMC free article] [PubMed] [Google Scholar]; Proteomic study of LCM islets from controls, autoantibody positive and T1D donors identifying key proteins and pathways involved in disease pathogenesis.
- 23.Busse N., Paroni F., Richardson S.J., Laiho J.E., Oikarinen M., Frisk G., Hyoty H., de Koning E., Morgan N.G., Maedler K. Detection and localization of viral infection in the pancreas of patients with type 1 diabetes using short fluorescently-labelled oligonucleotide probes. Oncotarget. 2017;8:12620–12636. doi: 10.18632/oncotarget.14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oikarinen M., Tauriainen S., Penttila P., Keim J., Rantala I., Honkanen T., Hyoty H. Evaluation of immunohistochemistry and in situ hybridization methods for the detection of enteroviruses using infected cell culture samples. J Clin Virol. 2010;47:224–228. doi: 10.1016/j.jcv.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Richardson S.J., Leete P., Dhayal S., Russell M.A., Oikarinen M., Laiho J.E., Svedin E., Lind K., Rosenling T., Chapman N. Evaluation of the fidelity of immunolabelling obtained with clone 5D8/1, a monoclonal antibody directed against the enteroviral capsid protein, VP1, in human pancreas. Diabetologia. 2014;56:185–193. doi: 10.1007/s00125-013-3094-7. [DOI] [PubMed] [Google Scholar]
- 26.Richardson S.J., Willcox A., Hilton D.A., Tauriainen S., Hyoty H., Bone A.J., Foulis A.K., Morgan N.G. Use of antisera directed against dsRNA to detect viral infections in formalin-fixed paraffin-embedded tissue. J Clin Virol. 2010;49:180–185. doi: 10.1016/j.jcv.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Laiho J.E., Oikarinen M., Richardson S.J., Frisk G., Nyalwidhe J., Burch T.C., Morris M.A., Oikarinen S., Pugliese A., Dotta F. Relative sensitivity of immunohistochemistry, multiple reaction monitoring mass spectrometry, in situ hybridization and PCR to detect Coxsackievirus B1 in A549 cells. J Clin Virol. 2016;77:21–28. doi: 10.1016/j.jcv.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laiho J.E., Oikarinen S., Oikarinen M., Larsson P.G., Stone V.M., Hober D., Oberste S., Flodstrom-Tullberg M., Isola J., Hyoty H. Application of bioinformatics in probe design enables detection of enteroviruses on different taxonomic levels by advanced in situ hybridization technology. J Clin Virol. 2015;69:165–171. doi: 10.1016/j.jcv.2015.06.085. [DOI] [PubMed] [Google Scholar]
- 29.Saarinen N.V.V., Laiho J.E., Richardson S.J., Zeissler M., Stone V.M., Marjomaki V., Kantoluoto T., Horwitz M.S., Sioofy-Khojine A., Honkimaa A. A novel rat CVB1-VP1 monoclonal antibody 3A6 detects a broad range of enteroviruses. Sci Rep. 2018;8:33. doi: 10.1038/s41598-017-18495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ettischer-Schmid N., Normann A., Sauter M., Kraft L., Kalbacher H., Kandolf R., Flehmig B., Klingel K. A new monoclonal antibody (Cox mAB 31A2) detects VP1 protein of coxsackievirus B3 with high sensitivity and specificity. Virchows Arch. 2016;469:553–562. doi: 10.1007/s00428-016-2008-8. [DOI] [PubMed] [Google Scholar]
- 31.Laitinen O.H., Svedin E., Kapell S., Hankaniemi M.M., Larsson P.G., Domsgen E., Stone V.M., Maatta J.A.E., Hyoty H., Hytonen V.P. New Coxsackievirus 2A(pro) and 3C(pro) protease antibodies for virus detection and discovery of pathogenic mechanisms. J Virol Methods. 2018;255:29–37. doi: 10.1016/j.jviromet.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Anagandula M., Richardson S.J., Oberste M.S., Sioofy-Khojine A.B., Hyoty H., Morgan N.G., Korsgren O., Frisk G. Infection of human islets of langerhans with two strains of coxsackie B virus serotype 1: assessment of virus replication, degree of cell death and induction of genes involved in the innate immunity pathway. J Med Virol. 2014;86:1402–1411. doi: 10.1002/jmv.23835. [DOI] [PubMed] [Google Scholar]
- 33.Frisk G., Tuvemo T. Enterovirus infections with beta-cell tropic strains are frequent in siblings of children diagnosed with type 1 diabetes children and in association with elevated levels of GAD65 antibodies. J Med Virol. 2004;73:450–459. doi: 10.1002/jmv.20111. [DOI] [PubMed] [Google Scholar]
- 34.Marroqui L., Lopes M., dos Santos R.S., Grieco F.A., Roivainen M., Richardson S.J., Morgan N.G., Op de Beeck A., Eizirik D.L. Differential cell autonomous responses determine the outcome of coxsackievirus infections in murine pancreatic alpha and beta cells. Elife. 2015;4:e06990. doi: 10.7554/eLife.06990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Hodik M., Skog O., Lukinius A., Isaza-Correa J.M., Kuipers J., Giepmans B.N., Frisk G. Enterovirus infection of human islets of Langerhans affects beta-cell function resulting in disintegrated islets, decreased glucose stimulated insulin secretion and loss of Golgi structure. BMJ Open Diabetes Res Care. 2016;4:e000179. doi: 10.1136/bmjdrc-2015-000179. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates the presence of viral replication complexes in close association with secretory granules in enterovirus infected beta cells.
- 36.Knoch K.P., Nath-Sain S., Petzold A., Schneider H., Beck M., Wegbrod C., Sonmez A., Munster C., Friedrich A., Roivainen M. PTBP1 is required for glucose-stimulated cap-independent translation of insulin granule proteins and Coxsackieviruses in beta cells. Mol Metab. 2014;3:518–530. doi: 10.1016/j.molmet.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilton D.A., Variend S., Pringle J.H. Demonstration of Coxsackie virus RNA in formalin-fixed tissue sections from childhood myocarditis cases by in situ hybridization and the polymerase chain reaction. J Pathol. 1993;170:45–51. doi: 10.1002/path.1711700108. [DOI] [PubMed] [Google Scholar]
- 38.Yoon J.W., Austin M., Onodera T., Notkins A.L. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 39.Dotta F., Censini S., van Halteren A.G., Marselli L., Masini M., Dionisi S., Mosca F., Boggi U., Muda A.O., Del Prato S. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A. 2007;104:5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Ifie E., Russell M.A., Dhayal S., Leete P., Sebastiani G., Nigi L., Dotta F., Marjomaki V., Eizirik D.L., Morgan N.G. Unexpected subcellular distribution of a specific isoform of the Coxsackie and Adenovirus receptor, CAR-SIV, in human pancreatic beta cells. Diabetologia. 2018 doi: 10.1007/s00125-018-4704-1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]; Reports the expression of a specific isoform of CAR in human beta cells and its unique localisation within the insulin secretory granule, which could have important implications for the mechanism of enterovirus infection.
- 41.Ylipaasto P., Klingel K., Lindberg A.M., Otonkoski T., Kandolf R., Hovi T., Roivainen M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia. 2004;47:225–239. doi: 10.1007/s00125-003-1297-z. [DOI] [PubMed] [Google Scholar]
- 42••.Staring J., von Castelmur E., Blomen V.A., van den Hengel L.G., Brockmann M., Baggen J., Thibaut H.J., Nieuwenhuis J., Janssen H., van Kuppeveld F.J. PLA2G16 represents a switch between entry and clearance of Picornaviridae. Nature. 2017;541:412–416. doi: 10.1038/nature21032. [DOI] [PubMed] [Google Scholar]; This study identifies PLA2G16 as an essential host factor required for enterovirus genome release.
- 43.Hsu N.Y., Ilnytska O., Belov G., Santiana M., Chen Y.H., Takvorian P.M., Pau C., van der Schaar H., Kaushik-Basu N., Balla T. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Schaar H.M., van der Linden L., Lanke K.H., Strating J.R., Purstinger G., de Vries E., de Haan C.A., Neyts J., van Kuppeveld F.J. Coxsackievirus mutants that can bypass host factor PI4KIIIbeta and the need for high levels of PI4P lipids for replication. Cell Res. 2012;22:1576–1592. doi: 10.1038/cr.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Linden L., Wolthers K.C., van Kuppeveld F.J. Replication and Inhibitors of Enteroviruses and Parechoviruses. Viruses. 2015;7:4529–4562. doi: 10.3390/v7082832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petzold A., Solimena M., Knoch K.P. Mechanisms of beta cell dysfunction associated with viral infection. Curr Diab Rep. 2015;15:73. doi: 10.1007/s11892-015-0654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsen H.L., Hoy M., Zhang W., Bertorello A.M., Bokvist K., Capito K., Efanov A.M., Meister B., Thams P., Yang S.N. Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic beta cells. Proc Natl Acad Sci U S A. 2003;100:5187–5192. doi: 10.1073/pnas.0931282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan F.F., Pratt E.B., Chen P.C., Wang F., Skach W.R., David L.L., Shyng S.L. Role of Hsp90 in biogenesis of the beta-cell ATP-sensitive potassium channel complex. Mol Biol Cell. 2010;21:1945–1954. doi: 10.1091/mbc.E10-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam C.J., Jacobson D.R., Rankin M.M., Cox A.R., Kushner J.A. beta cells persist in T1D pancreata without evidence of ongoing beta-cell turnover or neogenesis. J Clin Endocrinol Metab. 2017;102:2647–2659. doi: 10.1210/jc.2016-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregg B.E., Moore P.C., Demozay D., Hall B.A., Li M., Husain A., Wright A.J., Atkinson M.A., Rhodes C.J. Formation of a human beta-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97:3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hultcrantz M., Huhn M.H., Wolf M., Olsson A., Jacobson S., Williams B.R., Korsgren O., Flodstrom-Tullberg M. Interferons induce an antiviral state in human pancreatic islet cells. Virology. 2007;367:92–101. doi: 10.1016/j.virol.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Lind K., Richardson S.J., Leete P., Morgan N.G., Korsgren O., Flodstrom-Tullberg M. Induction of an antiviral state and attenuated Coxsackievirus replication in type III interferon-treated primary human pancreatic islets. J Virol. 2013;87:7646–7654. doi: 10.1128/JVI.03431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flodstrom-Tullberg M., Hultcrantz M., Stotland A., Maday A., Tsai D., Fine C., Williams B., Silverman R., Sarvetnick N. RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J Immunol. 2005;174:1171–1177. doi: 10.4049/jimmunol.174.3.1171. [DOI] [PubMed] [Google Scholar]
- 54.Marroqui L., Dos Santos R.S., Floyel T., Grieco F.A., Santin I., Op de Beeck A., Marselli L., Marchetti P., Pociot F., Eizirik D.L. TYK2, a candidate gene for type 1 diabetes, modulates apoptosis and the innate immune response in human pancreatic beta-cells. Diabetes. 2015;64:3808–3817. doi: 10.2337/db15-0362. [DOI] [PubMed] [Google Scholar]
- 55.Gorman J.A., Hundhausen C., Errett J.S., Stone A.E., Allenspach E.J., Ge Y., Arkatkar T., Clough C., Dai X., Khim S. The A946T variant of the RNA sensor IFIH1 mediates an interferon program that limits viral infection but increases the risk for autoimmunity. Nat Immunol. 2017;18:744–752. doi: 10.1038/ni.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S., Wang H., Jin Y., Podolsky R., Reddy M.V., Pedersen J., Bode B., Reed J., Steed D., Anderson S. IFIH1 polymorphisms are significantly associated with type 1 diabetes and IFIH1 gene expression in peripheral blood mononuclear cells. Hum Mol Genet. 2009;18:358–365. doi: 10.1093/hmg/ddn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nejentsev S., Walker N., Riches D., Egholm M., Todd J.A. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324:387–389. doi: 10.1126/science.1167728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rice G.I., del Toro Duany Y., Jenkinson E.M., Forte G.M., Anderson B.H., Ariaudo G., Bader-Meunier B., Baildam E.M., Battini R., Beresford M.W. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet. 2014;46:503–509. doi: 10.1038/ng.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smyth D.J., Cooper J.D., Bailey R., Field S., Burren O., Smink L.J., Guja C., Ionescu-Tirgoviste C., Widmer B., Dunger D.B. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 60.Winkler C., Lauber C., Adler K., Grallert H., Illig T., Ziegler A.G., Bonifacio E. An interferon-induced helicase (IFIH1) gene polymorphism associates with different rates of progression from autoimmunity to type 1 diabetes. Diabetes. 2011;60:685–690. doi: 10.2337/db10-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Domsgen E., Lind K., Kong L., Huhn M.H., Rasool O., van Kuppeveld F., Korsgren O., Lahesmaa R., Flodstrom-Tullberg M. An IFIH1 gene polymorphism associated with risk for autoimmunity regulates canonical antiviral defence pathways in Coxsackievirus infected human pancreatic islets. Sci Rep. 2016;6:39378. doi: 10.1038/srep39378. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that a common IFIH1 polymorphism associated with Type 1 diabetes alters the way human islets respond to CVB-infection. The risk variant was associated with increased expression of type III interferons.
- 62.Cinek O., Tapia G., Witso E., Kramna L., Holkova K., Rasmussen T., Stene L.C., Ronningen K.S. Enterovirus RNA in peripheral blood may be associated with the variants of rs1990760, a common type 1 diabetes associated polymorphism in IFIH1. PLOS ONE. 2012;7:e48409. doi: 10.1371/journal.pone.0048409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chia J.K. The role of enterovirus in chronic fatigue syndrome. J Clin Pathol. 2005;58:1126–1132. doi: 10.1136/jcp.2004.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapman N.M., Kim K.S. Persistent coxsackievirus infection: enterovirus persistence in chronic myocarditis and dilated cardiomyopathy. Curr Top Microbiol Immunol. 2008;323:275–292. doi: 10.1007/978-3-540-75546-3_13. [DOI] [PubMed] [Google Scholar]
- 65.Xue Y.C., Feuer R., Cashman N., Luo H. Enteroviral infection: the forgotten link to amyotrophic lateral sclerosis? Front Mol Neurosci. 2018;11:63. doi: 10.3389/fnmol.2018.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman N.M., Kim K.S., Drescher K.M., Oka K., Tracy S. 5′ terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart. Virology. 2008;375:480–491. doi: 10.1016/j.virol.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim K.S., Chapman N.M., Tracy S. Replication of coxsackievirus B3 in primary cell cultures generates novel viral genome deletions. J Virol. 2008;82:2033–2037. doi: 10.1128/JVI.01774-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim K.S., Tracy S., Tapprich W., Bailey J., Lee C.K., Kim K., Barry W.H., Chapman N.M. 5′-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J Virol. 2005;79:7024–7041. doi: 10.1128/JVI.79.11.7024-7041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cunningham L., Bowles N.E., Lane R.J., Dubowitz V., Archard L.C. Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA. J Gen Virol. 1990;71(Pt 6):1399–1402. doi: 10.1099/0022-1317-71-6-1399. [DOI] [PubMed] [Google Scholar]
- 70.Tam P.E., Messner R.P. Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J Virol. 1999;73:10113–10121. doi: 10.1128/jvi.73.12.10113-10121.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inal J.M., Jorfi S. Coxsackievirus B transmission and possible new roles for extracellular vesicles. Biochem Soc Trans. 2013;41:299–302. doi: 10.1042/BST20120272. [DOI] [PubMed] [Google Scholar]
- 72.Robinson S.M., Tsueng G., Sin J., Mangale V., Rahawi S., McIntyre L.L., Williams W., Kha N., Cruz C., Hancock B.M. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014;10:e1004045. doi: 10.1371/journal.ppat.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ilonen J., Hammais A., Laine A.P., Lempainen J., Vaarala O., Veijola R., Simell O., Knip M. Patterns of beta-cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013;62:3636–3640. doi: 10.2337/db13-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larsson P.G., Lakshmikanth T., Laitinen O.H., Utorova R., Jacobson S., Oikarinen M., Domsgen E., Koivunen M.R., Chaux P., Devard N. A preclinical study on the efficacy and safety of a new vaccine against Coxsackievirus B1 reveals no risk for accelerated diabetes development in mouse models. Diabetologia. 2015;58:346–354. doi: 10.1007/s00125-014-3436-0. [DOI] [PubMed] [Google Scholar]
- 75••.Stone V.M., Hankaniemi M.M., Svedin E., Sioofy-Khojine A., Oikarinen S., Hyoty H., Laitinen O.H., Hytonen V.P., Flodstrom-Tullberg M. A Coxsackievirus B vaccine protects against virus-induced diabetes in an experimental mouse model of type 1 diabetes. Diabetologia. 2018;61:476–481. doi: 10.1007/s00125-017-4492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work describes the testing of a new CVB1 vaccine in diabetes relevant rodent models and provides proof-of-concept for further studies in humans.
- 76.Hankaniemi M.M., Laitinen O.H., Stone V.M., Sioofy-Khojine A., Maatta J.A.E., Larsson P.G., Marjomaki V., Hyoty H., Flodstrom-Tullberg M., Hytonen V.P. Optimized production and purification of Coxsackievirus B1 vaccine and its preclinical evaluation in a mouse model. Vaccine. 2017;35:3718–3725. doi: 10.1016/j.vaccine.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 77.Koho T., Koivunen M.R., Oikarinen S., Kummola L., Makinen S., Mahonen A.J., Sioofy-Khojine A., Marjomaki V., Kazmertsuk A., Junttila I. Coxsackievirus B3 VLPs purified by ion exchange chromatography elicit strong immune responses in mice. Antiviral Res. 2014;104:93–101. doi: 10.1016/j.antiviral.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 78.Baggen J., Thibaut H.J., Strating J.R., van Kuppeveld F. The life cycle of non-polio enteroviruses and how to target it. Nat Rev Microbiol. 2018;16:368–381. doi: 10.1038/s41579-018-0005-4. [DOI] [PubMed] [Google Scholar]
- 79.Benschop K.S., van der Avoort H.G., Duizer E., Koopmans M.P. Antivirals against enteroviruses: a critical review from a public-health perspective. Antivir Ther. 2015;20:121–130. doi: 10.3851/IMP2939. [DOI] [PubMed] [Google Scholar]
- 80.Kang H., Kim C., Kim D.E., Song J.H., Choi M., Choi K., Kang M., Lee K., Kim H.S., Shin J.S. Synergistic antiviral activity of gemcitabine and ribavirin against enteroviruses. Antiviral Res. 2015;124:1–10. doi: 10.1016/j.antiviral.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 81.Alidjinou E.K., Sane F., Bertin A., Caloone D., Hober D. Persistent infection of human pancreatic cells with Coxsackievirus B4 is cured by fluoxetine. Antiviral Res. 2015;116C:51–54. doi: 10.1016/j.antiviral.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 82.Dahlquist G.G., Forsberg J., Hagenfeldt L., Boman J., Juto P. Increased prevalence of enteroviral RNA in blood spots from newborn children who later developed type 1 diabetes: a population-based case–control study. Diabetes Care. 2004;27:285–286. doi: 10.2337/diacare.27.1.285. [DOI] [PubMed] [Google Scholar]
- 83.Coutant R., Carel J.C., Lebon P., Bougneres P.F., Palmer P., Cantero-Aguilar L. Detection of enterovirus RNA sequences in serum samples from autoantibody-positive subjects at risk for diabetes. Diabet Med. 2002;19:968–969. doi: 10.1046/j.1464-5491.2002.00807_2.x. [DOI] [PubMed] [Google Scholar]
- 84.Elfaitouri A., Berg A.K., Frisk G., Yin H., Tuvemo T., Blomberg J. Recent enterovirus infection in type 1 diabetes: evidence with a novel IgM method. J Med Virol. 2007;79:1861–1867. doi: 10.1002/jmv.21008. [DOI] [PubMed] [Google Scholar]
- 85.Richardson S.J., Leete P., Bone A.J., Foulis A.K., Morgan N.G. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia. 2013;56:185–193. doi: 10.1007/s00125-012-2745-4. [DOI] [PubMed] [Google Scholar]
- 86.Richardson S.J., Willcox A., Bone A.J., Foulis A.K., Morgan N.G. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 87.Willcox A., Richardson S.J., Bone A.J., Foulis A., Morgan N.G. Immunohistochemical analysis of the relationship between islet cell proliferation and the production of the enteroviral capsid protein, VP1, in the islets of patients with recent-onset type 1 diabetes. Diabetologia. 2011;54:2417–2420. doi: 10.1007/s00125-011-2192-7. [DOI] [PubMed] [Google Scholar]
- 88.Stene L.C., Oikarinen S., Hyoty H., Barriga K.J., Norris J.M., Klingensmith G., Hutton J.C., Erlich H.A., Eisenbarth G.S., Rewers M. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010;59:3174–3180. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andreoletti L., Hober D., Hober-Vandenberghe C., Belaich S., Vantyghem M.C., Lefebvre J., Wattre P. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J Med Virol. 1997;52:121–127. doi: 10.1002/(sici)1096-9071(199706)52:2<121::aid-jmv1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 90.Clements G.B., Galbraith D.N., Taylor K.W. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346:221–223. doi: 10.1016/s0140-6736(95)91270-3. [DOI] [PubMed] [Google Scholar]
- 91.Diaz-Horta O., Bello M., Cabrera-Rode E., Suarez J., Mas P., Garcia I., Abalos I., Jofra R., Molina G., Diaz-Diaz O. Echovirus 4 and type 1 diabetes mellitus. Autoimmunity. 2001;34:275–281. doi: 10.3109/08916930109014696. [DOI] [PubMed] [Google Scholar]
- 92.Dotta F., Censini S., van Halteren A.G., Marselli L., Masini M., Dionisi S., Mosca F., Boggi U., Muda A.O., Prato S.D. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A. 2007;104:5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Banatvala J.E., Bryant J., Schernthaner G., Borkenstein M., Schober E., Brown D., De Silva L.M., Menser M.A., Silink M. Coxsackie B, mumps, rubella, and cytomegalovirus specific IgM responses in patients with juvenile-onset insulin-dependent diabetes mellitus in Britain, Austria, and Australia. Lancet. 1985;1:1409–1412. doi: 10.1016/s0140-6736(85)91843-4. [DOI] [PubMed] [Google Scholar]
- 94.Ashton M.P., Eugster A., Walther D., Daehling N., Riethausen S., Kuehn D., Klingel K., Beyerlein A., Zillmer S., Ziegler A.G. Incomplete immune response to coxsackie B viruses associates with early autoimmunity against insulin. Sci Rep. 2016;6:32899. doi: 10.1038/srep32899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hodik M., Lukinius A., Korsgren O., Frisk G. Tropism analysis of two Coxsackie B5 strains reveals virus growth in human primary pancreatic islets but not in exocrine cell clusters in vitro. Open Virol J. 2013;7:49–56. doi: 10.2174/1874357901307010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Friman G., Fohlman J., Frisk G., Diderholm H., Ewald U., Kobbah M., Tuvemo T. An incidence peak of juvenile diabetes. Relation to Coxsackie B virus immune response. Acta Paediatr Scand Suppl. 1985;320:14–19. doi: 10.1111/j.1651-2227.1985.tb10132.x. [DOI] [PubMed] [Google Scholar]
- 97.Williams C.H., Oikarinen S., Tauriainen S., Salminen K., Hyoty H., Stanway G. Molecular analysis of an echovirus 3 strain isolated from an individual concurrently with appearance of islet cell and IA-2 autoantibodies. J Clin Microbiol. 2006;44:441–448. doi: 10.1128/JCM.44.2.441-448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Otonkoski T., Roivainen M., Vaarala O., Dinesen B., Leipala J.A., Hovi T., Knip M. Neonatal Type I diabetes associated with maternal echovirus 6 infection: a case report. Diabetologia. 2000;43:1235–1238. doi: 10.1007/s001250051518. [DOI] [PubMed] [Google Scholar]
- 99.Smith C.P., Clements G.B., Riding M.H., Collins P., Bottazzo G.F., Taylor K.W. Simultaneous onset of type 1 diabetes mellitus in identical infant twins with enterovirus infection. Diabet Med. 1998;15:515–517. doi: 10.1002/(SICI)1096-9136(199806)15:6<515::AID-DIA608>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 100.Paananen A., Ylipaasto P., Rieder E., Hovi T., Galama J., Roivainen M. Molecular and biological analysis of echovirus 9 strain isolated from a diabetic child. J Med Virol. 2003;69:529–537. doi: 10.1002/jmv.10341. [DOI] [PubMed] [Google Scholar]
- 101.Cabrera-Rode E., Sarmiento L., Tiberti C., Molina G., Barrios J., Hernandez D., Diaz-Horta O., Di Mario U. Type 1 diabetes islet associated antibodies in subjects infected by echovirus 16. Diabetologia. 2003;46:1348–1353. doi: 10.1007/s00125-003-1179-4. [DOI] [PubMed] [Google Scholar]
- 102.Cabrera-Rode E., Sarmiento L., Molina G., Perez C., Arranz C., Galvan J.A., Prieto M., Barrios J., Palomera R., Fonseca M. Islet cell related antibodies and type 1 diabetes associated with echovirus 30 epidemic: a case report. J Med Virol. 2005;76:373–377. doi: 10.1002/jmv.20368. [DOI] [PubMed] [Google Scholar]
- 103.Paananen A., Ylipaasto P., Smura T., Lempinen M., Galama J., Roivainen M. A single amino acid substitution in viral VP1 protein alters the lytic potential of clone-derived variants of echovirus 9 DM strain in human pancreatic islets. J Med Virol. 2013;85:1267–1273. doi: 10.1002/jmv.23574. [DOI] [PubMed] [Google Scholar]
- 104.Sarmiento L., Frisk G., Anagandula M., Cabrera-Rode E., Roivainen M., Cilio C.M. Expression of innate immunity genes and damage of primary human pancreatic islets by epidemic strains of Echovirus: implication for post-virus islet autoimmunity. PLoS One. 2013;8:e77850. doi: 10.1371/journal.pone.0077850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarmiento L., Frisk G., Anagandula M., Hodik M., Barchetta I., Netanyah E., Cabrera-Rode E., Cilio C.M. Echovirus 6 infects human exocrine and endocrine pancreatic cells and induces pro-inflammatory innate immune response. Viruses. 2017;9 doi: 10.3390/v9020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarmiento L., Medina A., Aziz K., Anagandula M., Cabrera-Rode E., Fex M., Frisk G., Cilio C.M. Differential effects of three echovirus strains on cell lysis and insulin secretion in beta cell derived lines. J Med Virol. 2016;88:971–978. doi: 10.1002/jmv.24438. [DOI] [PubMed] [Google Scholar]
- 107.Frisk G., Nilsson E., Tuvemo T., Friman G., Diderholm H. The possible role of Coxsackie A and echo viruses in the pathogenesis of type I diabetes mellitus studied by IgM analysis. J Infect. 1992;24:13–22. doi: 10.1016/0163-4453(92)90814-m. [DOI] [PubMed] [Google Scholar]
- 108.Somoza N., Vargas F., Roura-Mir C., Vives-Pi M., Fernandez-Figueras M.T., Ariza A., Gomis R., Bragado R., Marti M., Jaraquemada D. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J Immunol. 1994;153:1360–1377. [PubMed] [Google Scholar]