Malaria case management across endemic regions of the globe relies on artemisinin (ART) combination therapies for the rapid treatment of acute Plasmodium infection and prevention of severe disease. However, the efficacy of combination therapy is threatened by reduced sensitivity of Plasmodium falciparum to ART and partner drugs, which, in turn, may compromise the progress of control and elimination campaigns. Clinically, this phenomenon is most prevalent in Southeast Asia, where it manifests in vivo as measurably slower clearance of parasitemia (1–3). There are also limited reports of patients with recrudescent infections of African origin (4–6); however, slow clearance, as measured by the microscopic criterion deployed in the Greater Mekong region, is not present. Much effort has gone into understanding the genetic basis of ART susceptibility. After the development of an in vitro correlate of clinical ART susceptibility by Witkowski et al. (7), it was demonstrated that variants in the pfk13 locus mediate the slow-clearance phenotype in Southeast Asia (8). The genetic diversity of this locus worldwide has since been studied extensively in vivo (9–12) and in vitro (7, 11). Further, reverse genetic approaches with genome editing technologies, including zinc-finger nucleases and CRISPR-Cas9, have validated the involvement of these pfkelch13 mutations in ring-stage ART resistance (12, 13). Now, in PNAS, Demas et al. (14) report and validate the role of the actin-binding protein Coronin in reducing P. falciparum ART susceptibility in vitro.
Conventional approaches to evolving and studying parasite resistance to ART have been challenging. Unlike other clinically approved antimalarial drugs, ART does not seem to inhibit a single target or pathway in the cell. Instead, the drug rapidly and promiscuously oxidizes intracellular material, overwhelming the parasite cell’s damage-response machinery (15). Despite its disseminated action, ART derivates are fully metabolized and cleared from in vivo circulation within hours, meaning that most of parasite development occurs in the absence of drug. Consistent with this, early studies of recrudescent parasites revealed that ART resistance results from a transient reduction in sensitivity during the first few hours of intraerythrocytic development, but that the same parasites are still fully susceptible to ART later in development (7, 15). Thus, in a treated febrile patient, a proportion of early-stage parasites may survive the brief exposure to ART that occurs after each dose, opening the door for recrudescent parasitemia.
So far, there is no evidence that the K13 protein itself is the target of ART. Instead, parasites expressing resistance-associated variants seem to be resilient to oxidative stress and have altered transcriptional activity in heat shock, redox, and endoplasmic reticulum (ER) stress response gene families (16). K13 has recently been implicated in phosphatidylinositol 3-phosphate–dependent vesicular traffic, and the function, regulation, and disruption of this pathway are of particular interest (17).
From a general perspective, these previous studies have provided valuable insight into the Southeast Asian ART resistance phenotype and the important role of pfkelch13 mutations. So far, however, there is no evidence of the same resistance-associated pfkelch13 mutations in parasite isolates from sub-Saharan Africa or the presence of other variants in this locus in documented cases of treatment failure (5, 6, 10). In addition, recent studies have reported pfkelch13-independent treatment failure in Southeast Asian patients, supporting the hypothesis that other loci are involved in modulation of ART susceptibility.
In their study, Demas et al. (14) provide concrete evidence for this by experimentally evolving ART resistance in recently culture-adapted, ART-sensitive clinical isolates from Pikine and Thiès, Senegal. Importantly, the authors pulsed parasite cultures with dihydroartemisinin (the active metabolite of ART) repeatedly over 4 y. Clones of the resulting parasite lineages, Pikine-R and Thiès-R, displayed significantly reduced ring-stage susceptibility phenotypes (7.8 ± 1.0% and 7.6 ± 1.5% ring-stage survival, respectively, compared with <1% in parental lineages), approaching the in vitro survival ability of the ART-R lineages in Southeast Asia. Parental lineages cultured continuously during the resistance evolution experiment remained fully sensitive to ART. Both evolved lineages harbored unchanged, parental pfkelch13 alleles. Neither lineage had mutations in ART-associated loci, including pfk13, mdr1, mdr2, arps10, and crt.
Compared with their progenitors, whole-genome sequencing of Pikine-R and Thiès-R revealed 10 unique SNPs outside of subtelomeric regions. Interestingly, Demas et al. (14) found mutations in PF3D7_1251200, which encodes PfCoronin, in both Pikine-R and Thiès-R. In Thiès-R, the authors found a mutation encoding PfCoronin (G50E), and in Pikine-R, they found two mutations encoding PfCoronin (R100K and E107V) (14).
Importantly, Demas et al. (14) then deployed Cas9 editing to validate the involvement of these mutations in modulating parasite susceptibility to ART. Introduction of the corresponding pfcoronin (G50E) mutation onto the Thiès background produced transgenic parasites with similar reduced ring-stage ART susceptibility. The same was found in clones of transgenic parasites expressing PfCoronin (R100K and E107V) on the Pikine background. Although the authors did not explore the evolved lineages further in this work, it would be interesting to understand the phenotypic contributions of each of the Pikine mutations. Individually, they perhaps have limited effects, but they synergize to cause a significant ART-R phenotype when introduced together. Alternatively, one of the two may be primarily responsible for the observed ART-R phenotype. Characterizing the contributions of the other mutations identified by whole-genome sequencing to ART susceptibility and parasite fitness will also be informative. Notably, other developmental stages of both ART-R lineages were fully sensitive, strongly paralleling pfk13-mediated reduced susceptibility. Susceptibility to other antimalarials, including commonly deployed ART partner drugs, was not reported.
The strengths of this work lie in the use by Demas et al. (14) of two recent isolates as the backbone of their selection experiment and validation of pfcoronin mutations with Cas9 editing. Although it is unclear whether in vitro ring-stage survival is predictive of clinical treatment outcome with these genotypes, the authors definitively show that pfcoronin is a contributor to ART susceptibility in African parasites in vitro. Ariey et al. (8) identified pfkelch13 mutants, which are now recognized as a major determinant of ART treatment failure, in a similar experimental approach. Characterizing the allelic diversity and evidence for directional selection of coronin variants in circulating patient isolates is important and will be a focus of both retro- and prospective studies in the near future.
This new Coronin story deepens the relevance of a profound biological question: What cellular processes render early-ring-stage P. falciparum parasites exquisitely susceptible to ART, and how are they exploited to evade or recover from drug action? From a cell biology perspective, it is tempting to speculate if and how Coronin, an actin-binding protein, might contribute to, interleave with, and diverge from the mechanisms underpinning the ART-resistance phenotype that has been defined in pfk13-mutant parasites. Recent evidence is supportive of a role for the inducible ER stress response in parasite susceptibility to ART, and other authors have noted that actin dynamics and intracellular traffic may be important extensions of this process (16, 18). K13 and Coronin both have β-propeller domains but have different functions in higher eukaryotes. However, KEAP1, the ortholog of K13 in mammals, is involved in regulation of a nuclear transcription factor, whereas K13 may be involved in intracellular traffic and protein turnover in Plasmodium (16, 17). Recent screens have suggested that Coronin is not required for asexual survival in P. falciparum, which makes the biological role of the mutations reported by Demas et al. (14) all the more curious.
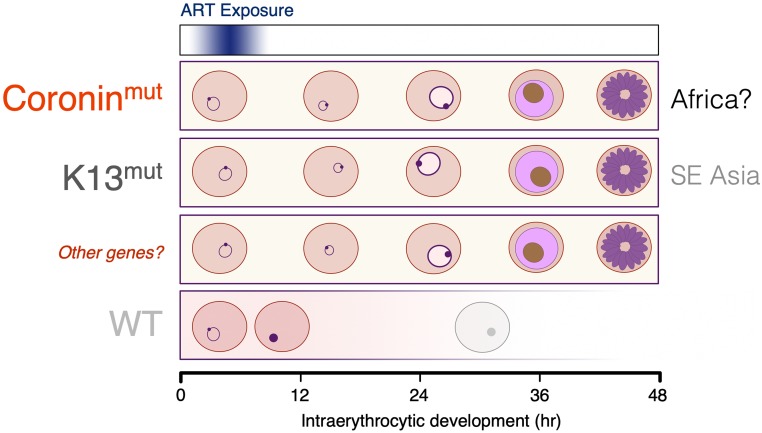
This work has important implications for surveillance of drug efficacy in endemic areas, particularly sub-Saharan Africa but also including Asia and South America, where pfk13-independent treatment failure has been reported. Demas et al. (14) demonstrate that variants of genes other than pfk13 can reduce ART susceptibility in vitro, and that simply monitoring for resistance through genotyping at the k13 locus will therefore miss novel genotypes (Fig. 1). The authors also indicate other loci that are implicated in reduced susceptibility but not as yet fully validated by reverse genetics, such as pfubp1 and pfap2mu (19, 20), which share with pfcoronin and pfk13 putative roles in protein dynamics and intracellular trafficking. These data strongly suggest that phenotypic surveillance of ART combination efficacy across malaria-endemic areas of the world is essential to ensure that emerging ART resistance in P. falciparum and in other species in the genus is identified in time to introduce corrective action.
Fig. 1.
Impact of transient ring-stage ART exposure on intraerythrocytic growth of P. falciparum. Parasites expressing certain genetic variants of Coronin (14) and Kelch 13 proteins (8) have increased chance of survival after a brief ring-stage pulse of ART in vitro. Other genes, such as pfap2mu and pfubp1, may also encode variant proteins conferring a similar phenotype but have not yet been fully validated. ART exposure at any other stage of intraerythrocytic development, later than 6 to 8 h postinvasion, is lethal for all genotypes. mut, mutant form; SE Asia, Southeast Asia.
Footnotes
The authors declare no conflict of interest.
See companion article on page 12799.
References
- 1.Noedl H, et al. Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 2.Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley EA, et al. Tracking Resistance to Artemisinin Collaboration (TRAC) Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beshir KB, et al. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis. 2013;208:2017–2024. doi: 10.1093/infdis/jit431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sondén K, et al. High rate of treatment failures in nonimmune travelers treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria in Sweden: Retrospective comparative analysis of effectiveness and case series. Clin Infect Dis. 2017;64:199–206. doi: 10.1093/cid/ciw710. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland CJ, et al. Pfk13-independent treatment failure in four imported cases of Plasmodium falciparum malaria treated with artemether-lumefantrine in the United Kingdom. Antimicrob Agents Chemother. 2017;61:e02382. doi: 10.1128/AAC.02382-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witkowski B, et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: In-vitro and ex-vivo drug-response studies. Lancet Infect Dis. 2013;13:1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ariey F, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ménard D, et al. KARMA Consortium A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374:2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muwanguzi J, et al. Lack of K13 mutations in Plasmodium falciparum persisting after artemisinin combination therapy treatment of Kenyan children. Malar J. 2016;15:36. doi: 10.1186/s12936-016-1095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper RA, et al. Lack of artemisinin resistance in Plasmodium falciparum in Uganda based on parasitological and molecular assays. Antimicrob Agents Chemother. 2015;59:5061–5064. doi: 10.1128/AAC.00921-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghorbal M, et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol. 2014;32:819–821. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- 13.Straimer J, et al. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015;347:428–431. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demas AR, et al. Mutations in Plasmodium falciparum actin-binding protein coronin confer reduced artemisinin susceptibility. Proc Natl Acad Sci USA. 2018;115:12799–12804. doi: 10.1073/pnas.1812317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klonis N, et al. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc Natl Acad Sci USA. 2013;110:5157–5162. doi: 10.1073/pnas.1217452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mok S, et al. Drug resistance. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science. 2015;347:431–435. doi: 10.1126/science.1260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharjee S, et al. Remodeling of the malaria parasite and host human red cell by vesicle amplification that induces artemisinin resistance. Blood. 2018;131:1234–1247. doi: 10.1182/blood-2017-11-814665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, et al. Inhibiting the Plasmodium eIF2α kinase PK4 prevents artemisinin-induced latency. Cell Host Microbe. 2017;22:766–776.e4. doi: 10.1016/j.chom.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt P, et al. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol Microbiol. 2007;65:27–40. doi: 10.1111/j.1365-2958.2007.05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriques G, et al. Artemisinin resistance in rodent malaria—Mutation in the AP2 adaptor μ-chain suggests involvement of endocytosis and membrane protein trafficking. Malar J. 2013;12:118. doi: 10.1186/1475-2875-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]