Significance
Electrocatalytic CO2 reduction to fuels using renewable electricity provides an entry into sustainable carbon-neutral fuels. Most reactions require proton equivalents, but direct proton reduction to H2 leads to low product selectivity, a significant challenge in the field. The issue of product selectivity arises from shared catalytic intermediates for both CO2 reduction and H2 evolution. We describe how thermodynamic relationships regarding the reactivity of H+ and CO2 with one of these shared intermediates, homogeneous metal hydrides, can be applied to identify catalyst candidates and conditions where H2 evolution is suppressed in favor of highly selective CO2 reduction. The conceptual framework is used to outline operational conditions for electrocatalytic reduction of CO2 to formate (HCO2−) by [Pt(dmpe)2](PF6)2 with negligible H2 production.
Keywords: electrocatalysis, CO2 reduction, solar fuel, formate production, hydride
Abstract
A critical challenge in electrocatalytic CO2 reduction to renewable fuels is product selectivity. Desirable products of CO2 reduction require proton equivalents, but key catalytic intermediates can also be competent for direct proton reduction to H2. Understanding how to manage divergent reaction pathways at these shared intermediates is essential to achieving high selectivity. Both proton reduction to hydrogen and CO2 reduction to formate generally proceed through a metal hydride intermediate. We apply thermodynamic relationships that describe the reactivity of metal hydrides with H+ and CO2 to generate a thermodynamic product diagram, which outlines the free energy of product formation as a function of proton activity and hydricity (∆GH−), or hydride donor strength. The diagram outlines a region of metal hydricity and proton activity in which CO2 reduction is favorable and H+ reduction is suppressed. We apply our diagram to inform our selection of [Pt(dmpe)2](PF6)2 as a potential catalyst, because the corresponding hydride [HPt(dmpe)2]+ has the correct hydricity to access the region where selective CO2 reduction is possible. We validate our choice experimentally; [Pt(dmpe)2](PF6)2 is a highly selective electrocatalyst for CO2 reduction to formate (>90% Faradaic efficiency) at an overpotential of less than 100 mV in acetonitrile with no evidence of catalyst degradation after electrolysis. Our report of a selective catalyst for CO2 reduction illustrates how our thermodynamic diagrams can guide selective and efficient catalyst discovery.
The emerging availability of inexpensive renewable electricity has motivated interest in using electrolytic methods to generate sustainable fuels. Electrocatalytic CO2 reduction provides an entry to carbon-neutral fuels, but product selectivity remains a significant challenge (1, 2). Nearly all reductive reactions of interest involve protons as well as electrons, introducing the complication of direct proton reduction to H2 under electrolytic conditions. Diversion of electron equivalents into proton reduction results in lower Faradaic efficiency for the desired CO2 reduction reaction. Various strategies to inhibit or suppress H2 evolution for heterogeneous (3–7) and homogeneous (8, 9) catalysts have been explored.
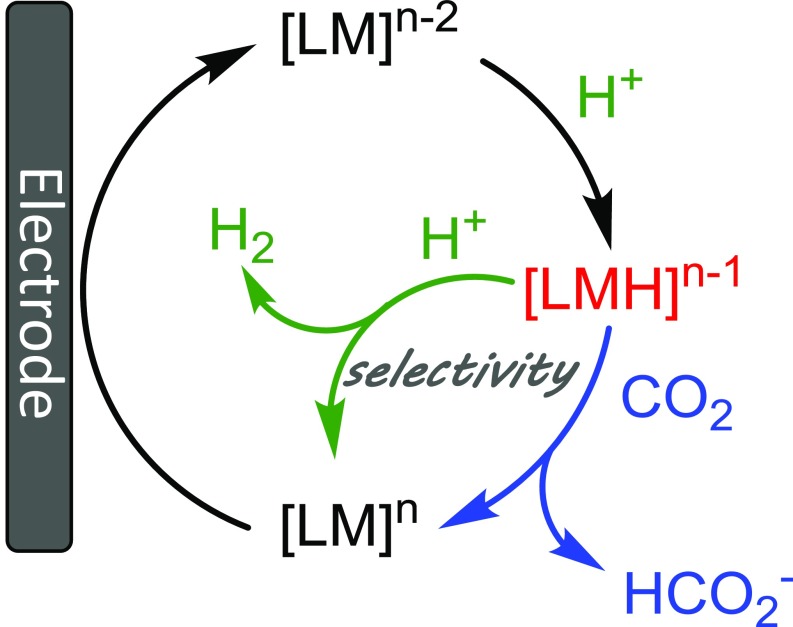
To understand the factors that determine selectivity between CO2 and H+ reduction, we have been investigating the reactivity of metal hydrides. Selectivity for formate production is particularly challenging because metal hydride intermediates are common to both reaction pathways (Fig. 1). As a result, very few heterogeneous (10–13) or homogeneous (14–16) catalysts have been reported with high (>90%) Faradaic efficiency for formate production. Understanding the reactivity of metal hydrides is key to controlling the bifurcating reaction pathways that ultimately determine selectivity. Most selective catalysts for CO2 reduction utilize kinetic inhibition (or a high-transition state barrier) to minimize H2 evolution.
Fig. 1.
Proposed catalytic cycles for H+ and CO2 reduction.
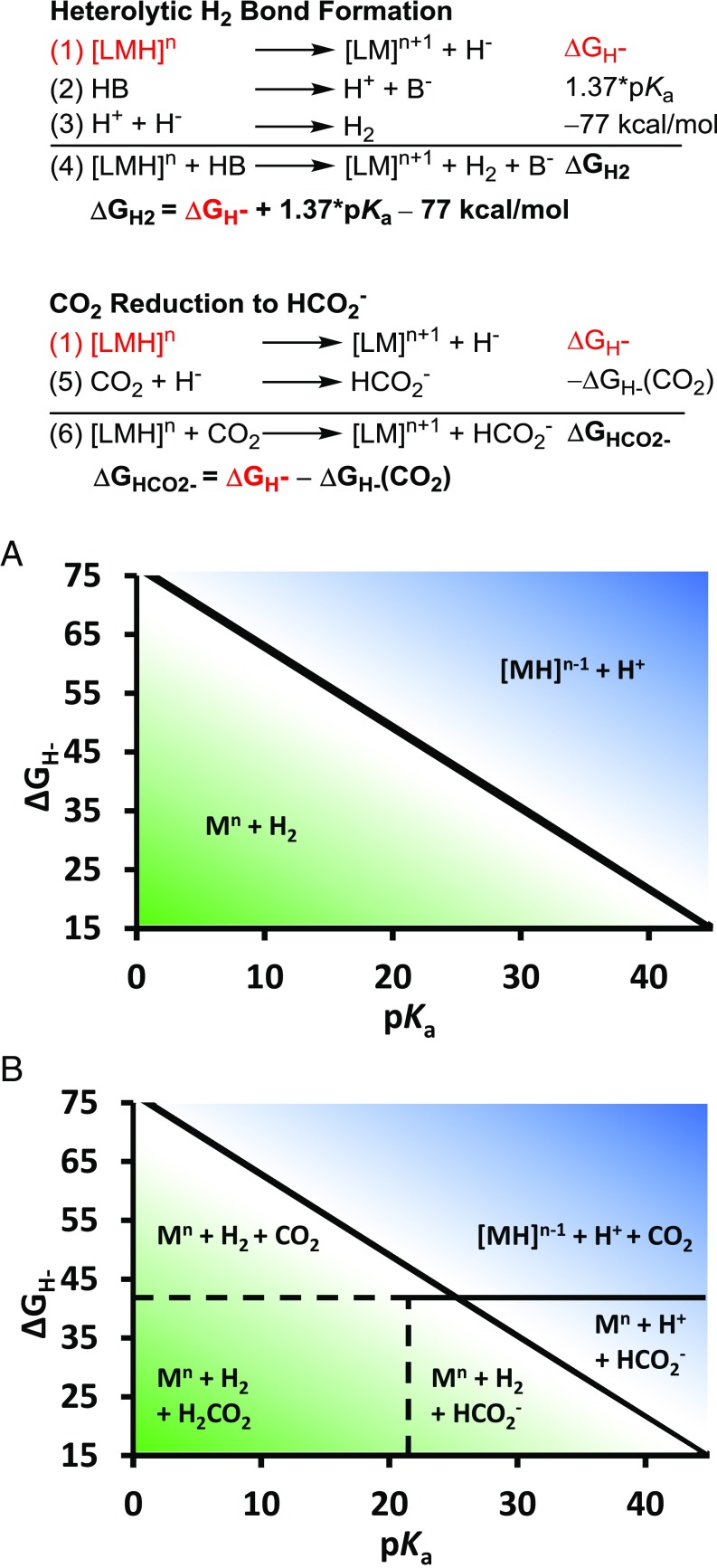
In this report, we utilize a thermodynamic approach to describe the reactivity of metal hydrides with H+ and CO2 by applying known free energy relationships (14, 17–28). We depict our findings in a diagram (Fig. 2) that describes the thermodynamic products as a function of hydricity (∆GH−) (Eq. 1) of the metal hydride intermediate vs. proton activity (pKa). Notably, a region is delineated in which select combinations of hydricity and pKa lead to thermodynamic favorability for selective CO2 reduction to formate over hydrogen evolution. Since hydricity values have been measured for many classes of compounds and can be predictably tuned through metal and ligand design (29), we believe that the diagrams provide instructive guidelines for targeting the discovery of selective reduction catalysts.
Fig. 2.
Free energy calculations for H2 and HCO2− generation from metal hydrides and aqueous thermodynamic product diagrams (A and B).
We establish the utility of our diagram by using it to select a known metal hydride [HPt(dmpe)2]+ with an appropriate hydricity in acetonitrile to access conditions in which selective CO2 reduction to formate is favorable. We demonstrate that the resting state catalyst [Pt(dmpe)2](PF6)2 reduces CO2 to formate with high Faradaic efficiency and low overpotential.
Results and Discussion
Thermodynamic Product Diagrams.
We first describe the construction of the thermodynamic product diagram in acetonitrile (CH3CN), but the relationships hold in other solvents provided that the correct constants are used (vide infra). The thermodynamic cycle describing the free energy for the reaction of a metal hydride toward H+ to evolve H2 is shown in Fig. 2. The free energy of H2 evolution (Eq. 4) is dependent on the hydricity, pKa of the acid, and the heterolytic bond forming energy of H2, which is 77 kcal/mol in acetonitrile (30). Eq. 4 has successfully been applied to identify and optimize electrocatalysts for H2 evolution (26, 31–38) and H2 oxidation (31–34, 39–42), and to achieve reversible reactivity (43, 44).
The relationship between pKa, hydricity, and H2 evolution in acetonitrile is quantitatively depicted in Fig. 2A. The black line differentiates the boundary in which the free energy of Eq. 4 in Fig. 2 (ΔGH2) equals zero and MH/H+/H2 exist in equilibrium, analogous to lines in a Pourbaix diagram. Protonation to evolve hydrogen is exergonic for hydride complexes of a given hydricity under conditions below the black line (green zone) and endergonic above the line (blue zone).
The thermodynamic requirement for reduction of CO2 to formate, a net hydride transfer, is also dictated by the hydricity (∆GH−) of the donor as shown in Eq. 6 (Fig. 2). Eq. 6 has been applied to rationalize or predict the activity of the iron-based CO2 reduction electrocatalyst by Berben and coworkers (20) and CO2 hydrogenation catalysts (45–50) as well as formate oxidation electrocatalysts (51). Transition metal hydricity values lower than that of formate will result in exergonic hydride transfer to CO2. This information can be mapped onto the thermodynamic product diagram illustrating metal hydride reactivity with protons (Fig. 2B). The thermodynamic product distribution that results from stoichiometric mixtures of metal hydride and acid of specific pKa values under 1 atm of CO2 is described in this diagram. The pKa of formic acid is estimated to be ∼20.9 in acetonitrile (52); therefore, it is expected to be protonated at lower pKa values.
In Fig. 2B, a region is defined where specific metal hydricity and pKa combinations will result in selective reduction of CO2 to formate without concomitant H2 evolution (near the bottom right of Fig 2B). The challenge of selective CO2 reduction is often introduced by comparing the thermodynamic potentials for H+ reduction with CO2 reduction to formate [−0.028 V (53) vs. −0.150 V (22) vs. Fe(C5H5)2+/0 in CH3CN]. Since the former is more positive than the latter, hydrogen is assumed to be the more favorable thermodynamic product upon reduction. However, these potentials are listed at the standard state (1 M H+). A full Pourbaix diagram that spans a larger range of proton activity (pKa) provides a more complete picture (SI Appendix, Fig. S1).The thermodynamic potential for proton reduction shifts with the pKa of the solution by 59 mV per unit according to the Nernst equation for a 2 e−, 2H+ process. Similarly, CO2 reduction to formic acid follows the same relationship until the solution pKa matches that of formic acid to generate formate. For CO2 reduction to formate, the thermodynamic potential shifts will deviate in accordance with a 2 e−, 1H+ process or 29.5 (59/2) mV per pKa unit. The smaller decline in thermodynamic potential vs. pKa compared with H+ reduction results in a crossing of potentials, where CO2 reduction to formate occurs at a more positive potential at higher pKa values and can thus be the more favorable thermodynamic product. The crossing point in the Pourbaix diagram (SI Appendix, Fig. S1) matches the pKa (25.1), which defines the initial point in which selective CO2 reduction can be achieved in our diagram. A similar crossing in the 2H+/H2 and CO2/HCO2− is observed in Pourbaix diagrams in water (54), although it is complicated by CO2 equilibria with hydroxide at high pH (55). We believe that our redrawn thermodynamic product diagram, where potential is replaced by hydricity, provides a more instructive guide for targeting catalysts to access the region where selective CO2 reduction is possible, which we demonstrate experimentally herein.
A recently published perspective by Kubiak and coworkers (22) described a primarily linear relationship between hydricity and the one-electron reduction potentials of the parent complex for previously reported groups 8–10 metal hydrides. The perspective provides valuable insight into the relationship between hydricity and reversible redox properties as they pertain to H2 evolution and CO2 reduction. However, the authors note that their correlation is qualitative and dependent on similar hydrogen atom transfer bond dissociation free energies among their complexes. Additionally, the authors detail why some metal hydrides deviate significantly from their observed trend and were excluded from the linear fit. We note that the diagrams shown in Fig. 2 are based directly on thermodynamic hydricity values and are thus applicable to all metal hydrides, regardless of metal identity, oxidation state, or ligand environment. The thermodynamic hydricity can also be accurately measured for complexes without reversible redox potentials using methods that have previously been described (19).
Application in other solvents.
The diagrams shown in Fig. 2 can be drawn for any solvent provided that the appropriate constants (ΔGH2 and ΔGHCO2−) are known in the respective solvent. For example, the diagram illustrating the relationships between metal hydricity and proton activity (similar to Fig. 2A) in dimethylsulfoxide (DMSO) is shown in SI Appendix, Fig. S2 (ΔGH2 = 60.7 kcal/mol in DMSO) (30). The hydricity of formate (ΔGHCO2−) in DMSO is estimated to be 42 kcal/mol (56). The equivalent diagram shown in Fig. 2B for DMSO is shown in SI Appendix, Fig. S3. We note that we were unable to find a published value for the pKa of formic acid in DMSO, and therefore, the line between CO2/HCO2− is drawn across the whole range of pKa values shown. Applying thermodynamic relationships with metal hydricity is most useful in organic solvents with self-consistent data on acid pKa values. Most measurements of this type have been made in CH3CN (57–61) and to a lesser extent DMSO (62), but additional values in various solvents are continually being reported.
ΔGH2 and ΔGHCO2− have also been reported in aqueous solution (34.2 and 24.1 kcal/mol, respectively) (17). The analogous diagrams in Fig. 2 A and B are shown in SI Appendix, Figs. S4 and S5, respectively. However, the diagrams do not account for the equilibria between CO2 and OH− to form HCO3− and CO32−, which become more significant at higher pH values.
The hydricity value (∆GH−) for metal hydrides also has a solvent dependence. Multiple studies have measured how solvent affects the hydricity values (14, 18, 19, 23–25, 27, 35). Hydricity values for transition metal hydrides tend to be lower and span a narrower range in solvents with higher dielectric constants, but their absolute values do not change in a predictable fashion by solvent. However, a notable trend is that hydricity values for transition metal hydrides in organic solvents tend to decline to a greater magnitude (become better donors) in water compared to HCO2− (∆GHCO2−). As a result, there are a few examples where CO2 reduction to HCO2− is exergonic in water but endergonic in organic solvents (14, 27, 48). Additionally, detailed studies by Miller and coworkers (63) determined that aqueous hydricity can also be dependent on anions commonly found in aqueous buffers as well as hydroxide at higher pH values.
Kinetic vs. thermodynamic selectivity.
The diagrams depicted in this study delineate how to achieve thermodynamic selectivity for CO2 reduction. In CH3CN, DMSO, and H2O, thermodynamic selectivity can only be achieved at modest or low proton activity (pKa > 25.9 in CH3CN, pKa > 13.6 in DMSO, and pH > 8.1 in H2O). However, kinetic barriers for protonation can be applied to achieve selectivity for CO2 reduction under more acidic conditions. The difference in kinetic selectivity will still have to compensate for larger free energies of protonation of the metal hydride to form hydrogen at lower pKa/pH values. Consistent with the free energy associated with H2 and HCO2− formation (Eqs. 4 and 7, respectively) the only two molecular catalysts with >90% selectivity for formate generation function optimally at modest to high pKa/pH conditions, with decreasing selectivity at lower proton activities (16, 20). Yet, both maintain fairly high selectivity under more acidic conditions, indicating that the product distribution is also under kinetic control (64).
Application of Thermodynamic Product Diagrams for Catalyst Discovery.
To target the region in Fig. 2B where selective CO2 reduction to HCO2− in CH3CN is dictated by the thermodynamic Eqs. 4 and 6, we selected [Pt(dmpe)2](PF6)2 (3) as a possible catalyst. The corresponding hydride [HPt(dmpe)2](PF6) (2) has an experimentally measured hydricity (∆GH−) (Eq. 1) of 41.4 kcal/mol (Fig. 2) (19, 65). Consistent with Eq. 6, [HPt(dmpe)2](PF6) reacts stoichiometrically with CO2 to give HCO2− and [Pt(dmpe)2](PF6)2 (3) (21). The preparation and characterization of the hydride precursor Pt(dmpe)2 (1) along with 2 and 3 have been previously reported (65, 66). Complexes 1, 2, and 3 are diamagnetic with easily distinguishable 31P{1H}NMR spectral signatures.
According to Eq. 4 and Fig. 2, the threshold for H2 evolution with [HPt(dmpe)2](PF6) (2) is at a pKa of 25. Acids with pKa values lower than 25 will be exergonic for H2 evolution. As expected, stoichiometric reaction of [HPt(dmpe)2](PF6) (2) with acids, such as anilinium tetrafluoroborate (pKa = 10.62) and protonated 1,8-diazabicyclo[5.4.0]undec-7-ene (pKa = 24.34) in CH3CN (60), results in protonation to give H2 and [Pt(dmpe)2](PF6)2 (3) (SI Appendix, Figs. S6 and S7). In contrast, [HPt(dmpe)2](PF6) (2) is stable to protonation using acids with higher pKa values (ΔGH2 > 0), which we demonstrate experimentally. No reaction is observed with [HPt(dmpe)2](PF6) (2) upon addition of phenol (pKa = 29.1) (SI Appendix, Fig. S8) (62). According to Eq. 4, H2 evolution with [HPt(dmpe)2](PF6) (2) and phenol has an endergonic free energy of 5.5 kcal/mol.
The pKa (31.1) (65) of the metal hydride provides an upper bound on acids that can be used for metal hydride generation. As a result, [HPt(dmpe)2](PF6) (2) can be selectively generated at an electrode using proton sources with pKa values between 31.1 and 25. Therefore, phenol (pKa = 29.1) is sufficiently acidic to generate [HPt(dmpe)2](PF6) (2) without additional protonation to evolve H2.
Selective electrocatalytic reduction of CO2 to formate.
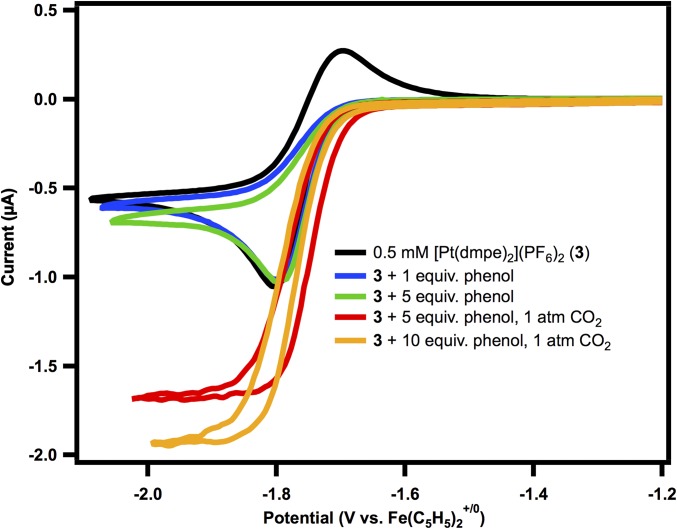
[Pt(dmpe)2](PF6)2 (3) exhibits a 2 e− reduction to [Pt(dmpe)2] (1) at −1.73 V vs. Fe(C5H5)2+/0 in acetonitrile (Fig. 3, black trace). Electrochemical reduction of 3 to 1 in the presence of phenol results in a loss of reversibility (blue trace in Fig. 3), which we attribute to formation of the hydride 2 [electron transfer (E) followed by a chemical step (C), protonation, or an EC step]. Higher concentrations of phenol do not result in an increase in current (green trace in Fig. 3).
Fig. 3.
Cyclic voltammetry under 1 atm of N2 of 0.5 mM solution of [Pt(dmpe)2](PF6)2 (3) (black); after addition of 1 equivalent of phenol (blue); and 5 equivalents of phenol (green); and under 1 atm of CO2 with 5 equivalents of phenol (red); and 10 equivalents of phenol (orange). Conditions: 0.1 M Et4NPF6, 1 mM Fe(C5H5)2 present as an internal reference, glassy carbon working and auxiliary electrode, Ag/AgCl pseudoreference electrode, 10 mV/s scan rate.
According to Eq. 5, [HPt(dmpe)2](PF6) (2) is sufficiently hydritic to react with CO2 to generate formate (ΔG° in Eq. 6 = −2.6 kcal/mol). The cyclic voltammogram on addition of 1 atm of CO2 to 3 with phenol is shown by the red trace in Fig. 3. Titration with increasing concentrations of phenol results in an increase in current (Fig. 3), which is indicative of electrocatalysis. The current reaches a maximum at 10 equivalents.
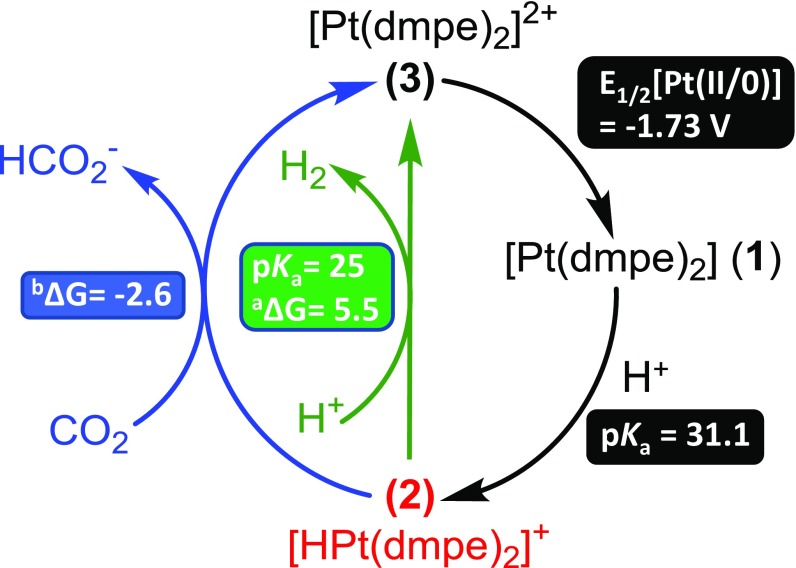
A proposed catalytic cycle for CO2 reduction to formate with thermodynamic parameters (E1/2 for e− transfer and pKa for protonation reactions) for each step is depicted in Fig. 4.
Fig. 4.
Proposed catalytic cycle and thermodynamic properties. aFree energy for H2 evolution (kilocalories per mole) calculated from Eq. 4 with an equivalent of phenol. bFree energy of hydride transfer (kilocalories per mole) to form formate under 1 atm of CO2 calculated using Eq. 6.
Electrolysis was performed at −2.4 V vs. Fe(C5H5)2+/0 under 1 atm of CO2 (1 mM 3 in 10 mM phenol) for 1 h (SI Appendix, Figs. S9 and S10). The concentration of formate in solution after electrolysis was measured using two different methods. The first method used an internal dimethylforamide (DMF) standard to quantify formate by 1H NMR spectroscopy (SI Appendix, Fig. S11). The second method required acidifying the postelectrolysis solution with HCl and quantifying the formic acid with a DMF internal standard using 1H NMR spectroscopy (SI Appendix, Fig. S12). The Faradaic efficiency for formate production from either method was greater than 90%. The headspace of the cell was analyzed by GC to detect and quantify H2 and CO production. A small amount of H2 (<0.1%) was sometimes observed, but CO was never detected. 31P{1H} NMR spectra of the pre- and postelectrolysis solution display a single resonance that corresponds to 3. Quantification by integration relative to the PF6− anion (SI Appendix, Figs. S13 and S14) confirms retention of the catalyst after electrolysis.
The maximum catalytic current observed in the cyclic voltammetry (Fig. 3, orange trace) and the peak current under noncatalytic conditions (Fig. 3, black trace) was used to estimate an observed rate constant (kobs) of 0.5 s−1 (SI Appendix, Eq. S4) (67, 68) for CO2 reduction to formate. The standard potential for CO2 reduction to formate in acetonitrile was recently estimated (22). The thermodynamic potential under our conditions (pKa of 29.1) is E° = −1.64 V. Using the half-wave potential E°1/2 = −1.73 V (69), the overpotential for catalysis is 90 mV.
To quantify any potential H2 or formate generated directly at the electrode, an equivalent electrolysis in the absence of any Pt compound was performed (SI Appendix, Fig. S15). Analysis of the headspace by GC detected the presence of <0.1% mL H2, no CO, and no formate in solution by 1H NMR spectroscopy. Calibration curves for H2 and formate quantification are shown in SI Appendix, Figs. S16 and S17, respectively.
General considerations.
The diagrams in Fig. 2 informed our choice of [Pt(dmpe)2](PF6)2 (3) as a good catalyst candidate for selective CO2 reduction to formate. The hydricity of the corresponding hydride [HPt(dmpe)2](PF6) (2) is sufficient to reduce CO2, and there are appropriate acids to access the region of selective reduction. Although the thermodynamic framework described herein proved effective for [Pt(dmpe)2]2+ (3), some considerations on its application to other potential catalysts are discussed.
Although nearly all molecular hydrogen evolution reaction (HER) catalysts involve a metal hydride intermediate, H–H bond formation can occur through two distinct mechanisms: bimolecular (homolytic) or heterolytic (protonation; as described here). There are cases where both homolytic and heterolytic H2-forming pathways are viable, such as in the well-studied cobaloxime HER catalysts (70–72), with the predominant operating mechanism dependent on the specific reaction conditions (73–80). The free energy of homolytic H–H bond formation is dependent on the homolytic bond energy of the metal hydride and H2 and is not expected to have a dependence on the proton activity.
Consequently, the thermodynamic framework described in Fig. 2 is most useful for metal hydrides that are stable to homolytic H2 bond formation. This is generally true for many classes of transition metal hydrides, including those in the [Ni(P2N2)2]2+ class of catalysts (70, 71, 78–91), the water soluble [HNi(DHMPE)2]2+, and the subject of these studies [HPt(dmpe)2](PF6)2 (2). [The 31P{1H}NMR spectra of [HPt(dmpe)2](PF6)2 (2) in acetonitrile show no change over 23 h, confirming that there is no appreciable homolytic pathway for H2 generation (SI Appendix, Fig. S18).] Application of these guidelines to metal hydrides prone to homocoupling to form H2 would be expected to impact the pH-dependent H2 evolution activity, present challenges to isolating the metal hydride under electrolytic conditions without generating H2, and lead to lower overall selectivity.
The reduction potential required to generate [HPt(dmpe)2](PF6) (2) from the resting-state catalyst [Pt(dmpe)2](PF6)2 (3) (shown as Ered1 in Eq. 7) is positive of the reduction potential for 2 (Ered2 in Eq. 7):
| [7] |
Facile electrolytic generation of [HPt(dmpe)2](PF6) (2) is possible, because Ered1 is greater than Ered2 (−1.7 vs. −2.8 V vs. Fe(C5H5)2+/0) (shown in SI Appendix, Fig. S19). As a result, additional reduction of [HPt(dmpe)2](PF6) (2) to another intermediate is not possible at the electrochemical potential required to reduce 3. Several other group 10 metal hydride complexes (26) along with multiple cobalt hydride complexes (84, 88, 92–94) have the same property; reduction of the hydride complex ([LMH]n in Eq. 7) is more challenging than reduction of the parent complex ([LM]n+1 in Eq. 7) to generate the hydride (or Ered1 > Ered2). In cases where this property does not hold [i.e., the reduction potential of the hydride intermediate (Ered2) is positive of the parent complex], it will likely be reduced under electrolytic conditions to generate a stronger hydride donor. For example, cobaloxime hydride complexes will generally be reduced further at potentials necessary to generate the hydride [LMH]n in Eq. 7 (or Ered2 > Ered1). In these cases, the hydricity of the reduced complex ([LMH]n−1 in Eq. 7) can be applied to understand the pKa-dependent hydrogen evolution catalysis, as [LMH]n−1 will likely be generated under electrolytic conditions.
Conclusion
Despite immense interest in electrolytic fuel generation, there are few guidelines for the rational design of catalysts for selective CO2 reduction. We use thermodynamic relationships to understand the reactivity of metal hydrides, a branch point in the reactivity for formation of either H2 or HCO2−. We applied our analysis to construct a diagram that defines catalyst parameters for achieving selective CO2 reduction by targeting an appropriate hydricity. Hydricity is akin to an activity descriptor for H2 and HCO2− generation—a thermodynamic quantity that describes the bond energy of a key intermediate. Like an activity descriptor, hydricity is general across many classes of compounds, expanding its utility. Identifying descriptors in molecular systems is particularly advantageous, as bond energies are easily tuned through metal–ligand design.
We applied our thermodynamic product diagram to identify a metal hydride with appropriate hydricity to reduce CO2 to formate under conditions that minimized concomitant H2 evolution. We expect that the thermodynamic framework described herein will continue to stimulate the discovery of more selective and efficient catalysts necessary for a carbon-neutral energy economy.
Methods
Experimental details, cyclic voltammograms, NMR spectra, data from electrolysis, H2 (GC) and HCO2− (NMR) calibration curves, and additional thermodynamic product diagrams in DMSO and H2O are provided in SI Appendix. SI Appendix includes synthetic methods and materials, physical methods, electrochemical methods (including controlled potential electrolysis and product analysis), additional thermodynamic product diagrams, and NMR spectra.
Supplementary Material
Acknowledgments
This material is based on work supported by US Department of Energy, Office of Science, Office of Basic Energy Sciences Award DE-SC0012150. B.M.C. acknowledges support from National Science Foundation Graduate Research Fellowship Grant DGE 1321846. J.Y.Y. acknowledges support as a Sloan Foundation Fellow and a Canadian Institute for Advanced Research (CIFAR) Azrieli Global Scholar in the Bio-Inspired Solar Energy Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811396115/-/DCSupplemental.
References
- 1.Seh ZW, et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science. 2017;355:eaad4998. doi: 10.1126/science.aad4998. [DOI] [PubMed] [Google Scholar]
- 2.Inglis JL, MacLean BJ, Pryce MT, Vos JG. Electrocatalytic pathways towards sustainable fuel production from water and CO2. Coord Chem Rev. 2012;256:2571–2600. [Google Scholar]
- 3.Li CW, Ciston J, Kanan MW. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature. 2014;508:504–507. doi: 10.1038/nature13249. [DOI] [PubMed] [Google Scholar]
- 4.Mariano RG, McKelvey K, White HS, Kanan MW. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science. 2017;358:1187–1192. doi: 10.1126/science.aao3691. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Liu J, Wang Y, Al-Enizi AM, Zheng G. Tuning of CO2 reduction selectivity on metal electrocatalysts. Small. 2017;13:1701809. doi: 10.1002/smll.201701809. [DOI] [PubMed] [Google Scholar]
- 6.Feaster JT, et al. Understanding selectivity for the electrochemical reduction of carbon dioxide to formic acid and carbon monoxide on metal electrodes. ACS Catal. 2017;7:4822–4827. [Google Scholar]
- 7.Cave ER, et al. Trends in the catalytic activity of hydrogen evolution during CO2 electroreduction on transition metals. ACS Catal. 2018;8:3035–3040. [Google Scholar]
- 8.Elgrishi N, Chambers MB, Fontecave M. Turning it off! Disfavouring hydrogen evolution to enhance selectivity for CO production during homogeneous CO2 reduction by cobalt-terpyridine complexes. Chem Sci (Camb) 2015;6:2522–2531. doi: 10.1039/c4sc03766a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fogeron T, et al. A bioinspired nickel(bis-dithiolene) complex as a homogeneous catalyst for carbon dioxide electroreduction. ACS Catal. 2018;8:2030–2038. [Google Scholar]
- 10.Podlovchenko BI, Kolyadko EA, Lu S. Electroreduction of carbon dioxide on palladium electrodes at potentials higher than the reversible hydrogen potential. J Electroanal Chem. 1994;373:185–187. [Google Scholar]
- 11.Min X, Kanan MW. Pd-catalyzed electrohydrogenation of carbon dioxide to formate: High mass activity at low overpotential and identification of the deactivation pathway. J Am Chem Soc. 2015;137:4701–4708. doi: 10.1021/ja511890h. [DOI] [PubMed] [Google Scholar]
- 12.Lee CH, Kanan MW. Controlling H+ vs CO2 reduction selectivity on Pb electrodes. ACS Catal. 2015;5:465–469. [Google Scholar]
- 13.Zhang S, Kang P, Meyer TJ. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J Am Chem Soc. 2014;136:1734–1737. doi: 10.1021/ja4113885. [DOI] [PubMed] [Google Scholar]
- 14.Taheri A, Thompson EJ, Fettinger JC, Berben LA. An iron electrocatalyst for selective reduction of CO2 to formate in water: Including thermochemical insights. ACS Catal. 2015;5:7140–7151. [Google Scholar]
- 15.Roy S, et al. Molecular cobalt complexes with pendant amines for selective electrocatalytic reduction of carbon dioxide to formic acid. J Am Chem Soc. 2017;139:3685–3696. doi: 10.1021/jacs.6b11474. [DOI] [PubMed] [Google Scholar]
- 16.Kang P, et al. Selective electrocatalytic reduction of CO2 to formate by water-stable iridium dihydride pincer complexes. J Am Chem Soc. 2012;134:5500–5503. doi: 10.1021/ja300543s. [DOI] [PubMed] [Google Scholar]
- 17.Connelly SJ, Wiedner ES, Appel AM. Predicting the reactivity of hydride donors in water: Thermodynamic constants for hydrogen. Dalton Trans. 2015;44:5933–5938. doi: 10.1039/c4dt03841j. [DOI] [PubMed] [Google Scholar]
- 18.Muckerman JT, Achord P, Creutz C, Polyansky DE, Fujita E. Calculation of thermodynamic hydricities and the design of hydride donors for CO2 reduction. Proc Natl Acad Sci USA. 2012;109:15657–15662. doi: 10.1073/pnas.1201026109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiedner ES, et al. Thermodynamic hydricity of transition metal hydrides. Chem Rev. 2016;116:8655–8692. doi: 10.1021/acs.chemrev.6b00168. [DOI] [PubMed] [Google Scholar]
- 20.Loewen ND, Neelakantan TV, Berben LA. Renewable formate from C-H bond formation with CO2: Using iron carbonyl clusters as electrocatalysts. Acc Chem Res. 2017;50:2362–2370. doi: 10.1021/acs.accounts.7b00302. [DOI] [PubMed] [Google Scholar]
- 21.DuBois DL, Berning DE. Hydricity of transition-metal hydrides and its role in CO2 reduction. Appl Organomet Chem. 2000;14:860–862. [Google Scholar]
- 22.Waldie KM, Ostericher AL, Reineke MH, Sasayama AF, Kubiak CP. Hydricity of transition-metal hydrides: Thermodynamic considerations for CO2 reduction. ACS Catal. 2018;8:1313–1324. [Google Scholar]
- 23.Matsubara Y, Fujita E, Doherty MD, Muckerman JT, Creutz C. Thermodynamic and kinetic hydricity of ruthenium(II) hydride complexes. J Am Chem Soc. 2012;134:15743–15757. doi: 10.1021/ja302937q. [DOI] [PubMed] [Google Scholar]
- 24.Creutz C, Chou MH. Rapid transfer of hydride ion from a ruthenium complex to C1 species in water. J Am Chem Soc. 2007;129:10108–10109. doi: 10.1021/ja074158w. [DOI] [PubMed] [Google Scholar]
- 25.Tsay C, Livesay BN, Ruelas S, Yang JY. Solvation effects on transition metal hydricity. J Am Chem Soc. 2015;137:14114–14121. doi: 10.1021/jacs.5b07777. [DOI] [PubMed] [Google Scholar]
- 26.Tsay C, Yang JY. Electrocatalytic hydrogen evolution under acidic aqueous conditions and mechanistic studies of a highly stable molecular catalyst. J Am Chem Soc. 2016;138:14174–14177. doi: 10.1021/jacs.6b05851. [DOI] [PubMed] [Google Scholar]
- 27.Ceballos BM, Tsay C, Yang JY. CO2 reduction or HCO2- oxidation? Solvent-dependent thermochemistry of a nickel hydride complex. Chem Commun (Camb) 2017;53:7405–7408. doi: 10.1039/c7cc02511d. [DOI] [PubMed] [Google Scholar]
- 28.Taheri A, Berben LA. Tailoring electrocatalysts for selective CO2 or H(+) reduction: Iron carbonyl clusters as a case study. Inorg Chem. 2016;55:378–385. doi: 10.1021/acs.inorgchem.5b02293. [DOI] [PubMed] [Google Scholar]
- 29.Brereton KR, et al. Aqueous hydricity from calculations of reduction potential and acidity in water. J Phys Chem B. 2016;120:12911–12919. doi: 10.1021/acs.jpcb.6b09864. [DOI] [PubMed] [Google Scholar]
- 30.Wayner DDM, Parker VD. Bond energies in solution from electrode potentials and thermochemical cycles. A simplified and general approach. Acc Chem Res. 1993;26:287–294. [Google Scholar]
- 31.Wilson AD, et al. Hydrogen oxidation and production using nickel-based molecular catalysts with positioned proton relays. J Am Chem Soc. 2006;128:358–366. doi: 10.1021/ja056442y. [DOI] [PubMed] [Google Scholar]
- 32.Fraze K, Wilson AD, Appel AM, Rakowski DuBois M, DuBois DL. Thermodynamic properties of the Ni−H bond in complexes of the type [HNi(P2RN2R‘)2](BF4) and evaluation of factors that control catalytic activity for hydrogen oxidation/production. Organometallics. 2007;26:3918–3924. [Google Scholar]
- 33.Rakowski DuBois M, DuBois DL. Development of molecular electrocatalysts for CO2 reduction and H2 production/oxidation. Acc Chem Res. 2009;42:1974–1982. doi: 10.1021/ar900110c. [DOI] [PubMed] [Google Scholar]
- 34.Yang JY, Bullock RM, DuBois MR, DuBois DL. Fast and efficient molecular electrocatalysts for H2 production: Using hydrogenase enzymes as guides. MRS Bull. 2011;36:39–47. [Google Scholar]
- 35.Brereton KR, Pitman CL, Cundari TR, Miller AJM. Solvent-dependent thermochemistry of an iridium/ruthenium H2 evolution catalyst. Inorg Chem. 2016;55:12042–12051. doi: 10.1021/acs.inorgchem.6b02223. [DOI] [PubMed] [Google Scholar]
- 36.Wiese S, Kilgore UJ, DuBois DL, Bullock RM. [Ni(PMe2NPh2)2](BF4)2 as an electrocatalyst for H2 production. ACS Catal. 2012;2:720–727. [Google Scholar]
- 37.Kilgore UJ, et al. Studies of a series of [Ni(P(R)2N(Ph)2)2(CH3CN)]2+ complexes as electrocatalysts for H2 production: Substituent variation at the phosphorus atom of the P2N2 ligand. Inorg Chem. 2011;50:10908–10918. doi: 10.1021/ic201461a. [DOI] [PubMed] [Google Scholar]
- 38.Tsay C, Ceballos BM, Yang JY. pH-dependent reactivity of a water-soluble nickel complex: Hydrogen evolution vs selective electrochemical hydride generation. Organometallics. October 8, 2018 doi: 10.1021/acs.organomet.8b00558. [DOI] [Google Scholar]
- 39.Curtis CJ, et al. [Ni(Et2PCH2NMeCH2PEt2)2]2+ as a functional model for hydrogenases. Inorg Chem. 2003;42:216–227. doi: 10.1021/ic020610v. [DOI] [PubMed] [Google Scholar]
- 40.Yang JY, et al. Hydrogen oxidation catalysis by a nickel diphosphine complex with pendant tert-butyl amines. Chem Commun (Camb) 2010;46:8618–8620. doi: 10.1039/c0cc03246h. [DOI] [PubMed] [Google Scholar]
- 41.Liu T, Dubois DL, Bullock RM. An iron complex with pendent amines as a molecular electrocatalyst for oxidation of hydrogen. Nat Chem. 2013;5:228–233. doi: 10.1038/nchem.1571. [DOI] [PubMed] [Google Scholar]
- 42.Liu T, et al. Iron complexes bearing diphosphine ligands with positioned pendant amines as electrocatalysts for the oxidation of H2. Organometallics. 2015;34:2747–2764. [Google Scholar]
- 43.Smith SE, Yang JY, DuBois DL, Bullock RM. Reversible electrocatalytic production and oxidation of hydrogen at low overpotentials by a functional hydrogenase mimic. Angew Chem Int Ed Engl. 2012;51:3152–3155. doi: 10.1002/anie.201108461. [DOI] [PubMed] [Google Scholar]
- 44.Priyadarshani N, et al. Achieving reversible H2/H+ interconversion at room temperature with enzyme-inspired molecular complexes: A mechanistic study. ACS Catal. 2016;6:6037–6049. [Google Scholar]
- 45.Jeletic MS, Mock MT, Appel AM, Linehan JC. A cobalt-based catalyst for the hydrogenation of CO2 under ambient conditions. J Am Chem Soc. 2013;135:11533–11536. doi: 10.1021/ja406601v. [DOI] [PubMed] [Google Scholar]
- 46.Zall CM, Linehan JC, Appel AM. A molecular copper catalyst for hydrogenation of CO2 to formate. ACS Catal. 2015;5:5301–5305. [Google Scholar]
- 47.Cammarota RC, et al. A bimetallic nickel-gallium complex catalyzes CO2 hydrogenation via the intermediacy of an anionic d10 nickel hydride. J Am Chem Soc. 2017;139:14244–14250. doi: 10.1021/jacs.7b07911. [DOI] [PubMed] [Google Scholar]
- 48.Burgess SA, Appel AM, Linehan JC, Wiedner ES. Changing the mechanism for CO2 hydrogenation using solvent-dependent thermodynamics. Angew Chem Int Ed Engl. 2017;56:15002–15005. doi: 10.1002/anie.201709319. [DOI] [PubMed] [Google Scholar]
- 49.Burgess SA, Kendall AJ, Tyler DR, Linehan JC, Appel AM. Hydrogenation of CO2 in water using a bis(diphosphine) Ni–H complex. ACS Catal. 2017;7:3089–3096. [Google Scholar]
- 50.Lilio AM, et al. Incorporation of pendant bases into Rh(diphosphine)2 complexes: Synthesis, thermodynamic studies, and catalytic CO2 hydrogenation activity of [Rh(P2N2)2](+) complexes. J Am Chem Soc. 2015;137:8251–8260. doi: 10.1021/jacs.5b04291. [DOI] [PubMed] [Google Scholar]
- 51.Galan BR, et al. Electrocatalytic oxidation of formate by [Ni(P(R)2N(R′)2)2(CH3CN)]2+ complexes. J Am Chem Soc. 2011;133:12767–12779. doi: 10.1021/ja204489e. [DOI] [PubMed] [Google Scholar]
- 52.Stirling MJ, Sweeney G, MacRory K, Blacker AJ, Page MI. The kinetics and mechanism of the organo-iridium-catalysed enantioselective reduction of imines. Org Biomol Chem. 2016;14:3614–3622. doi: 10.1039/c6ob00245e. [DOI] [PubMed] [Google Scholar]
- 53.Roberts JAS, Bullock RM. Direct determination of equilibrium potentials for hydrogen oxidation/production by open circuit potential measurements in acetonitrile. Inorg Chem. 2013;52:3823–3835. doi: 10.1021/ic302461q. [DOI] [PubMed] [Google Scholar]
- 54.Costentin C, Robert M, Savéant J-M. Current issues in molecular catalysis illustrated by iron porphyrins as catalysts of the CO2-to-CO electrochemical conversion. Acc Chem Res. 2015;48:2996–3006. doi: 10.1021/acs.accounts.5b00262. [DOI] [PubMed] [Google Scholar]
- 55.Keene FR. Thermodynamic, kinetic, and product considerations in carbon dioxide reactivity. In: Sullivan BP, editor. Electrochemical and Electrocatalytic Reactions of Carbon Dioxide. Elsevier; Amsterdam: 1993. pp. 1–18. [Google Scholar]
- 56.Moret S, Dyson PJ, Laurenczy G. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat Commun. 2014;5:4017. doi: 10.1038/ncomms5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kütt A, et al. A comprehensive self-consistent spectrophotometric acidity scale of neutral Brønsted acids in acetonitrile. J Org Chem. 2006;71:2829–2838. doi: 10.1021/jo060031y. [DOI] [PubMed] [Google Scholar]
- 58.Eckert F, et al. Prediction of acidity in acetonitrile solution with COSMO-RS. J Comput Chem. 2009;30:799–810. doi: 10.1002/jcc.21103. [DOI] [PubMed] [Google Scholar]
- 59.Izutsu K. International Union of Pure and Applied Chemistry. Commission on Electroanalytical Chemistry . Acid-Base Dissociation Constants in Dipolar Aprotic Solvents. Blackwell Scientific Publications; Hoboken, NJ: 1990. [Google Scholar]
- 60.Kaljurand I, et al. Extension of the self-consistent spectrophotometric basicity scale in acetonitrile to a full span of 28 pKa units: Unification of different basicity scales. J Org Chem. 2005;70:1019–1028. doi: 10.1021/jo048252w. [DOI] [PubMed] [Google Scholar]
- 61.Lõkov M, et al. On the basicity of conjugated nitrogen heterocycles in different media. Eur J Org Chem. 2017;2017:4475–4489. [Google Scholar]
- 62.Kütt A, et al. Pentakis(trifluoromethyl)phenyl, a sterically crowded and electron-withdrawing group: Synthesis and acidity of pentakis(trifluoromethyl)benzene, -toluene, -phenol, and -aniline. J Org Chem. 2008;73:2607–2620. doi: 10.1021/jo702513w. [DOI] [PubMed] [Google Scholar]
- 63.Pitman CL, Brereton KR, Miller AJM. Aqueous hydricity of late metal catalysts as a continuum tuned by ligands and the medium. J Am Chem Soc. 2016;138:2252–2260. doi: 10.1021/jacs.5b12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson SI, Nielsen RJ, Goddard WA. Selectivity for HCO2– over H2 in the electrochemical catalytic reduction of CO2 by (POCOP)IrH2. ACS Catal. 2016;6:6362–6371. [Google Scholar]
- 65.Curtis CJ, Miedaner A, Ellis WW, DuBois DL. Measurement of the hydride donor abilities of [HM(diphosphine)2]+ complexes (M = Ni, Pt) by heterolytic activation of hydrogen. J Am Chem Soc. 2002;124:1918–1925. doi: 10.1021/ja0116829. [DOI] [PubMed] [Google Scholar]
- 66.Berning DE, Noll BC, DuBois DL. Relative hydride, proton, and hydrogen atom transfer abilities of [HM(diphosphine)2]PF6 complexes (M = Pt, Ni) J Am Chem Soc. 1999;121:11432–11447. [Google Scholar]
- 67.Nicholson RS, Shain I. Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal Chem. 1964;36:706–723. [Google Scholar]
- 68.Saveant JM, Vianello E. Potential-sweep chronoamperometry: Kinetic currents for first-order chemical reaction parallel to electron-transfer process (catalytic currents) Electrochim Acta. 1965;10:905–920. [Google Scholar]
- 69.Appel AM, Helm ML. Determining the overpotential for a molecular electrocatalyst. ACS Catal. 2014;4:630–633. [Google Scholar]
- 70.Muckerman JT, Fujita E. Theoretical studies of the mechanism of catalytic hydrogen production by a cobaloxime. Chem Commun (Camb) 2011;47:12456–12458. doi: 10.1039/c1cc15330g. [DOI] [PubMed] [Google Scholar]
- 71.Solis BH, Hammes-Schiffer S. Theoretical analysis of mechanistic pathways for hydrogen evolution catalyzed by cobaloximes. Inorg Chem. 2011;50:11252–11262. doi: 10.1021/ic201842v. [DOI] [PubMed] [Google Scholar]
- 72.Dempsey JL, Winkler JR, Gray HB. Kinetics of electron transfer reactions of H2-evolving cobalt diglyoxime catalysts. J Am Chem Soc. 2010;132:1060–1065. doi: 10.1021/ja9080259. [DOI] [PubMed] [Google Scholar]
- 73.Thoi VS, Sun Y, Long JR, Chang CJ. Complexes of earth-abundant metals for catalytic electrochemical hydrogen generation under aqueous conditions. Chem Soc Rev. 2013;42:2388–2400. doi: 10.1039/c2cs35272a. [DOI] [PubMed] [Google Scholar]
- 74.Zee DZ, Chantarojsiri T, Long JR, Chang CJ. Metal-polypyridyl catalysts for electro- and photochemical reduction of water to hydrogen. Acc Chem Res. 2015;48:2027–2036. doi: 10.1021/acs.accounts.5b00082. [DOI] [PubMed] [Google Scholar]
- 75.Kandemir B, Kubie L, Guo Y, Sheldon B, Bren KL. Hydrogen evolution from water under aerobic conditions catalyzed by a cobalt ATCUN metallopeptide. Inorg Chem. 2016;55:1355–1357. doi: 10.1021/acs.inorgchem.5b02157. [DOI] [PubMed] [Google Scholar]
- 76.Eckenhoff WT, Brennessel WW, Eisenberg R. Light-driven hydrogen production from aqueous protons using molybdenum catalysts. Inorg Chem. 2014;53:9860–9869. doi: 10.1021/ic501440a. [DOI] [PubMed] [Google Scholar]
- 77.Beyene BB, Mane SB, Hung C-H. Highly efficient electrocatalytic hydrogen evolution from neutral aqueous solution by a water-soluble anionic cobalt(II) porphyrin. Chem Commun (Camb) 2015;51:15067–15070. doi: 10.1039/c5cc05582b. [DOI] [PubMed] [Google Scholar]
- 78.Mondal B, et al. Cobalt corrole catalyst for efficient hydrogen evolution reaction from H2O under ambient conditions: Reactivity, spectroscopy, and density functional theory calculations. Inorg Chem. 2013;52:3381–3387. doi: 10.1021/ic4000473. [DOI] [PubMed] [Google Scholar]
- 79.McKone JR, Marinescu SC, Brunschwig BS, Winkler JR, Gray HB. Earth-abundant hydrogen evolution electrocatalysts. Chem Sci (Camb) 2014;5:865–878. [Google Scholar]
- 80.Costentin C, Dridi H, Savéant J-M. Molecular catalysis of H2 evolution: Diagnosing heterolytic versus homolytic pathways. J Am Chem Soc. 2014;136:13727–13734. doi: 10.1021/ja505845t. [DOI] [PubMed] [Google Scholar]
- 81.Wiedner ES, Brown HJS, Helm ML. Kinetic analysis of competitive electrocatalytic pathways: New insights into hydrogen production with nickel electrocatalysts. J Am Chem Soc. 2016;138:604–616. doi: 10.1021/jacs.5b10853. [DOI] [PubMed] [Google Scholar]
- 82.Elgrishi N, McCarthy BD, Rountree ES, Dempsey JL. Reaction pathways of hydrogen-evolving electrocatalysts: Electrochemical and spectroscopic studies of proton-coupled electron transfer processes. ACS Catal. 2016;6:3644–3659. [Google Scholar]
- 83.Roubelakis MM, Bediako DK, Dogutan DK, Nocera DG. Proton-coupled electron transfer kinetics for the hydrogen evolution reaction of hangman porphyrins. Energy Environ Sci. 2012;5:7737–7740. [Google Scholar]
- 84.Solis BH, et al. Theoretical analysis of cobalt hangman porphyrins: Ligand dearomatization and mechanistic implications for hydrogen evolution. ACS Catal. 2014;4:4516–4526. [Google Scholar]
- 85.Bediako DK, et al. Role of pendant proton relays and proton-coupled electron transfer on the hydrogen evolution reaction by nickel hangman porphyrins. Proc Natl Acad Sci USA. 2014;111:15001–15006. doi: 10.1073/pnas.1414908111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhattacharjee A, et al. A computational study of the mechanism of hydrogen evolution by cobalt(diimine-dioxime) catalysts. Chemistry. 2013;19:15166–15174. doi: 10.1002/chem.201301860. [DOI] [PubMed] [Google Scholar]
- 87.Lee CH, Dogutan DK, Nocera DG. Hydrogen generation by hangman metalloporphyrins. J Am Chem Soc. 2011;133:8775–8777. doi: 10.1021/ja202136y. [DOI] [PubMed] [Google Scholar]
- 88.Marinescu SC, Winkler JR, Gray HB. Molecular mechanisms of cobalt-catalyzed hydrogen evolution. Proc Natl Acad Sci USA. 2012;109:15127–15131. doi: 10.1073/pnas.1213442109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solis BH, Hammes-Schiffer S. Substituent effects on cobalt diglyoxime catalysts for hydrogen evolution. J Am Chem Soc. 2011;133:19036–19039. doi: 10.1021/ja208091e. [DOI] [PubMed] [Google Scholar]
- 90.Razavet M, Artero V, Fontecave M. Proton electroreduction catalyzed by cobaloximes: Functional models for hydrogenases. Inorg Chem. 2005;44:4786–4795. doi: 10.1021/ic050167z. [DOI] [PubMed] [Google Scholar]
- 91.Baffert C, Artero V, Fontecave M. Cobaloximes as functional models for hydrogenases. 2. Proton electroreduction catalyzed by difluoroborylbis(dimethylglyoximato)cobalt(II) complexes in organic media. Inorg Chem. 2007;46:1817–1824. doi: 10.1021/ic061625m. [DOI] [PubMed] [Google Scholar]
- 92.Fang M, et al. Cobalt complexes containing pendant amines in the second coordination sphere as electrocatalysts for H2 production. Organometallics. 2014;33:5820–5833. [Google Scholar]
- 93.Koelle U, Paul S. Electrochemical reduction of protonated cyclopentadienylcobalt phosphine complexes. Inorg Chem. 1986;25:2689–2694. [Google Scholar]
- 94.Wiedner ES, Bullock RM. Electrochemical detection of transient cobalt hydride intermediates of electrocatalytic hydrogen production. J Am Chem Soc. 2016;138:8309–8318. doi: 10.1021/jacs.6b04779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.