Studies reveal the mechanisms behind tumor metastasis and how to stymie it. But primary tumors still get the lion’s share of researchers’ attention.
When a cancer cell spreads from a primary tumor to the brain, it immediately meets a formidable opponent: the astrocyte. These stalwart defenders protect against any would-be infiltrators that don’t belong in the brain. But metastasizing cancer cells can and do persist there—brain metastases occur in an estimated 20 to 40% of advanced-stage cancers. And new research suggests that those cancer cells may even be getting help from the astrocytes.
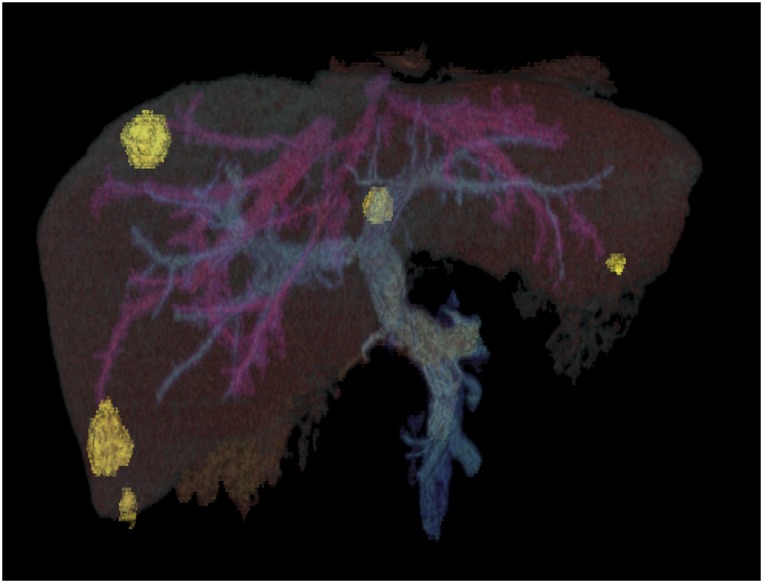
Researchers are starting to reveal the elusive mechanisms driving metastasis, such as the liver metastasis (yellow) that is seen originating from primary colorectal cancer tumors in this 3D computed tomographic reconstruction scan. Image courtesy of Science Source/Phanie.
When cancer cell biologist Joan Massagué and his team cultured astrocytes together with lung or breast cancer cells, they found the cancer cells form physical channels called gap junctions running to the astrocytes (1). The team watched as red dye loaded into the cancer cells moved across the junctions, turning the astrocytes red. But that wasn’t all that passed through. The cancer cells sent over the molecule cyclic guanosine monophosphate–adenosine monophosphate (cGAMP), triggering a series of reactions within the astrocyte that ultimately led it to release inflammatory signals that support tumor growth. The cancer cell seems to parasitize the astrocyte, explains Massagué, director of the Sloan Kettering Institute, the experimental research branch of the Memorial Sloan Kettering Cancer Center.
The team next examined mouse models of cancer that were genetically engineered to lack receptors for cGAMP molecules. When they inoculated these mice with breast cancer cells that have a propensity to metastasize in the brain, they found the metastases that did form were smaller than in wild-type mice, suggesting one possible target for keeping wayward tumor cells from gaining a foothold in the brain.
Massagué’s work is among the latest investigations delving into the details of how cancer cells spread to such deadly effect. Cancer research has long focused on understanding and defeating the primary tumor, and there’s much that researchers still don’t know about how metastasis works. How, for example, do a select few metastasizing cancer cells “decide” to travel to new tissues? How, once they arrive, do they differ from the original tumor? And what do these cells need to survive in their new setting?
The answers could have major implications for designing new therapies. Across cancer types, about 90% of cancer deaths are caused not by the primary tumor but by metastases (2). Although some drugs may shrink metastases along with primary tumors, no existing drugs treat or prevent metastasis directly. Without a targeted approach, metastatic tumors often reemerge. “We shrink them, we send them back to their residual state, and they reenact those survival functions and retention of regenerative powers that made them metastasis-initiating cells in the first place,” says Massagué. “That is what defeats us.”
The term metastasis comes from the Greek methistanai, meaning “to change” place or form (3). Today, Massagué and others are steadily revealing the elusive mechanisms driving that transformation and how they might be used to instead turn back cancer's spread.
A Cancer Cell Biography
Before a cancer cell can form metastases, it must break from the primary tumor, invade tissue, move into the bloodstream, colonize new tissue, and proliferate. “Only one in many millions of cells that went through all of this actually may turn out to regenerate the tumor at a distant organ,” says Massagué.
The molecular changes that cancer cells undergo during this process could offer clues to their success. But this “molecular biography” of a cancer cell, as cancer biologist Monte Winslow of Stanford University describes it, has been historically difficult to pin down. Researchers would like to search for differences in gene expression between cells in primary and secondary tumors. But samples from patients’ secondary tumors aren’t widely available. “If a patient has widespread metastatic disease, there is no clinical benefit, usually, to doing surgery,” Winslow explains. And in mouse models, the mice may die before metastases form.
For Winslow’s team, buying more time to follow the life course of a metastatic cell is key. “We’ve made some small alterations to the models and have been patient in letting the disease progress,” he says. In mouse models of lung cancer, the researchers deliver small amounts of virus to the lung to initiate only small numbers of primary tumors. The mice can survive up to a year—enough time for metastases to emerge.
In a 2016 study, Winslow teamed up with Julien Sage’s and William Greenleaf’s laboratories at Stanford University to examine differences in the configuration of chromatin—the complex of DNA and proteins that forms chromosomes—between small cell lung cancer cells in primary and secondary tumors in mice (4). Chromatin in the metastases was far more open, leaving stretches of DNA accessible to the cellular machinery that drives gene expression. The team identified a protein known as Nfib that is more highly expressed in the secondary tumor cells and maintains the chromatin in this accessible state. When the researchers inhibited the gene for Nfib in cancer cell lines and then injected the cells into mice, these Nfib knockdown cells were far less likely to form metastases than cells with a functional Nfib gene.
Nfib is a DNA-binding protein that isn’t easy to target with drugs. But this new understanding of Nfib’s role still has the potential to lead to new approaches to small cell lung cancers down the road, says Winslow. “Maybe something upstream is a target, or maybe it becomes a tool for early diagnosis.”
Cutting Off Support
Other researchers find clues to metastasis in a primary tumor's immediate environment—the surrounding healthy cells and extracellular matrix that glues them together. This microenvironment forms a support system that the tumor depends on, says cancer biologist Ashani Weeraratna of The Wistar Institute. “If we can cut off the support system to the tumor by targeting the microenvironment, we can make the tumor cell itself vulnerable.”
Weeraratna suspected that cellular changes may occur as a person ages that actually make the microenvironment of the primary tumor more conducive to metastasis. “Aging is the predominant prognostic factor for many cancers,” says Weeraratna. “It just made sense to me that that was not a coincidence.”
In a 2016 study, Weeraratna’s team tested whether the host’s age affects melanoma metastasis by injecting melanoma cells from a mouse model into the skin of mice that were either 8 or 52 weeks old (5). At any given time, a melanoma cell primarily puts energy into either spreading or dividing. In the younger mice, the cells formed primary tumors that grew more quickly. In older mice, they formed more metastases. “People often attribute the age-related changes in tumors to the chronic accumulation of genetic damage,” says Weeraratna. “We showed that, in fact, genetically identical tumors can be affected by the aged microenvironment.”
The researchers then tested whether the aging of dermal fibroblasts, cells that generate connective tissue in the skin, played a role in this changed behavior. They built a skin-like material by mixing melanoma cells with fibroblasts from healthy people who were either younger than 35 or older than 55 years. The melanoma cells in the primary tumor again grew more slowly but spread more readily in the older environment.
The team also discovered that the older fibroblasts produced more of a protein known as secreted frizzled-related protein 2 (sFRP2), which inhibits another protein called β-catenin that drives cancer cells to proliferate rather than spread. Melanoma cells with lower levels of β-catenin tend to be more resistant to a treatment called vemurafenib. When the team looked at a sample of 79 patients who received vemurafenib for melanoma, they found that, in patients older than 65 years, the total mass of existing tumors shrank by about 25%, whereas for patients younger than 65 years, it shrank by nearly half. “Our data showed that older patients respond differently to therapy than younger patients,” says Weeraratna.
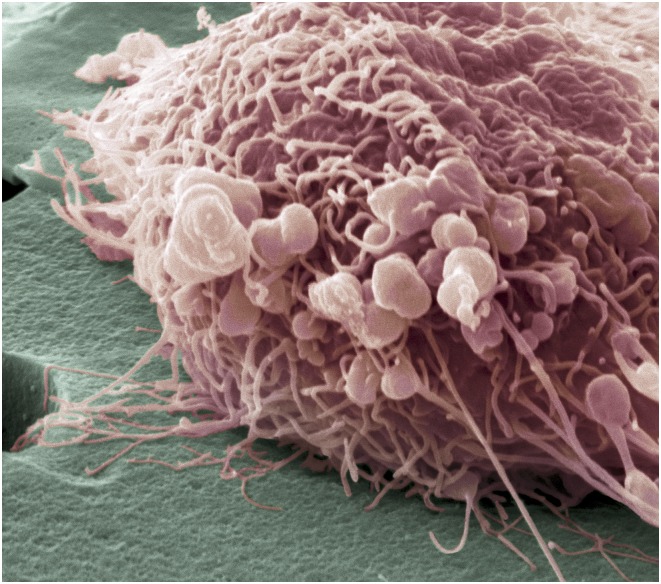
Oncologists are devising ways to better predict when metastasis initiates in breast cancer. Here, a migrating breast cancer cell, shown in a colored scanning electron micrograph, exhibits numerous thread-like formations and lumps, telltale characteristics of highly mobile cells. Image courtesy of Science Source/Steve Gschmeissner.
Blocking Cancer’s Doorway
“The microenvironment where cancer cells live is very, very important, and I think it is still underappreciated,” says pathologist and cancer cell biologist Maja Oktay of the Albert Einstein College of Medicine. In collaboration with Einstein colleagues John Condeelis, Joseph Sparano, and David Entenberg, Oktay studies another microenvironmental aspect of how cancer spreads—the sites where cancer cells enter blood vessels through a process known as intravasation.
Using a microscopy technique called intravital imaging, the group can see fluorescently tagged cells and blood vessels in the tumor microenvironment and watch cancer cells in real time as they enter the bloodstream from both primary and metastatic tumors in mouse models of breast cancer. They observed cancer cells enter the vasculature at specialized locations that they call tumor microenvironment of metastasis (TMEM) sites. Each entryway is made of three cells—a cancer cell, an endothelial cell that lines the blood vessel wall, and an immune cell called a proangiogenic macrophage that is specialized in promoting the growth of new blood vessels (6). The cancer cell inserts a tiny finger-like projection into the blood vessel while the macrophage releases a substance that causes the endothelial cell to contract, creating a temporary opening for other cancer cells to squeeze through. “We can actually see how the content of the blood vessel moves out and the cancer cells move in,” says Oktay.
The researchers found the risk that a common form of breast cancer will metastasize is greater for patients with a larger number of TMEM sites (7). And in mouse models, chemotherapy increased the number of these cancer doorways (8). It’s unclear whether humans respond similarly. But Oktay believes the effect of chemotherapy on the tumor microenvironment should be considered when testing treatment efficacy. “Everybody measures the response to chemotherapy as a shrinkage of tumors,” she says. “We also need to add a measurement of cancer cell dissemination.”
Oktay’s team is working with industry partners to develop therapies to disable TMEM sites. They found that an experimental drug called rebastinib limits cancer spread in mice by inhibiting a receptor present in high numbers on the TMEM macrophages (8). They’re now examining the safety of combining rebastinib with chemotherapy in a phase 1b clinical trial for patients with metastatic, human epidermal growth factor receptor 2–negative breast cancer. “If we could block the doorways and the proliferation, that may be a way to change it from a lethal to a chronic disease,” says Oktay.
Uncovering Cells in Hiding
For Massagué, one of the great metastasis mysteries is how cancer cells, even after establishing in a distant tissue, can sit inactive for years or decades. “How do they evade immune surveillance even as they sit as lonely single cells or in little tiny clusters not protected by a large tumor mass?” he wonders. In a 2016 study, his team uncovered an explanation that Massagué says, “very radically changed our perspective on what metastasis and its bottlenecks really are.”
To isolate dormant cells, Massagué’s team began by injecting cancer cells from human early-stage lung and breast carcinoma cell lines into the bloodstreams of mice (9). After 3 months, most mice hadn’t formed tumors. Because the cancer cells were tagged with a green fluorescent protein marker, the team could recover those that persisted in the tissue of tumor-free mice. When they cultured these cells in a dish, the cells multiplied, even though they hadn’t formed metastases in the mice.
The team then injected these cancer cells into the bloodstreams of mice again. Three months later, the vast majority of cells that had reached the lungs or brain were still not dividing to form tumors. Researchers analyzed the genes expressed by these cells and found their activity was much like that of another cell type that often remains dormant: stem cells.
“There are lots of different mechanisms, but it’s not chaos. It’s not millions of different ways. You can figure this out.”
—Monte Winslow
Healthy adult stem cells inhibit their own division except when the body needs to regenerate new tissue. By inhibiting division using similar molecular mechanisms, cancer cells can enter a stem-like dormant state. “As they enter quiescence, they gain something extremely valuable—they become immunoevasive,” explains Massagué. “Innate immunity will kill them if they try to grow but will not see them if, after a burst of growth, they return to the quiescent state.”
New Knowledge Needed
Less than 5% of cancer research funds worldwide go to studying metastatic disease, according to an estimate by members of the Tampa, Florida–based Metastasis Research Society. More resources, researchers, and novel approaches will be needed to untangle the many facets of this critical problem.
One major scientific challenge is that different forms of cancer seem to metastasize through different mechanisms. And, as Weeraratna showed, the same form of cancer may metastasize differently in different subsets of patients. It’s unlikely that one researcher is going to find one pathway that proves to be the key to metastasis, says Winslow.
Winslow would like to see researchers integrate findings to tease out which mechanisms are likely at play under different circumstances. “We all uncover different mechanisms that are important for metastasis, but what fraction of patients is that important in?” he asks. “What determines the probability that a tumor will use that mechanism?” The challenge is formidable but not insurmountable. “There are lots of different mechanisms, but it’s not chaos. It’s not millions of different ways,” Winslow says. “You can figure this out.”
Researchers might have a better shot at finding similarities between cancers if they could study multiple cancer types more freely, says bioengineer Hasini Jayatilaka. But to be considered a real expert in the cancer field, the current academic climate favors specialization. As an undergraduate and then a graduate student at Johns Hopkins University, Jayatilaka discovered that the density of a primary tumor helps determine whether its cells migrate (10). That work focused primarily on fibrosarcoma and breast cancer. Now, as a postdoctoral fellow at Stanford University School of Medicine, she studies pediatric cancers. Although she worries that this switch hurts her funding chances because she has less of a track record in this area, Jayatilaka is hopeful that by shifting focus commonalities may emerge. “I do believe there are general mechanisms to all types of cancers that we can potentially target,” she says. “I don’t think they’ve fully been explored.”
Oktay suggests one solution is to form large teams of diverse specialists. But regardless of how metastasis discoveries are achieved, translating many of the findings into therapies also presents unique hurdles. The fastest way to get a drug approved is to show in clinical trials that it’s effective on its own, notes Massagué. But in most cases, the doctor would also need to simultaneously treat the primary tumor. And because secondary tumors are often minuscule, measuring success by tumor shrinkage may not work. Alternatively, researchers could measure the incidence of metastasis after treatment—an approach that would be “more difficult,” says Massagué, “but not impossible.”
Challenges aside, current metastasis research is already inspiring possible therapies. When Massagué’s team made their gap junction discovery, the two physician-scientist lead authors, Adrienne Boire and Qing Chen, saw an opportunity: There was an FDA-approved antiinflammation drug on the market called meclofenamate that inhibits gap junctions. When the team administered it to mouse models, the drug diminished the proliferation of cancer cells within the brain (1). Boire immediately wrote a protocol for a clinical trial to determine the safety of such a drug in patients with brain metastases, and patients began enrolling even before the research was accepted for publication. Boire says that the trial showed “promising results,” and they are now planning a larger phase 2 clinical trial.
Without the gap junction discovery, the team couldn’t have pursued this possible treatment, says Massagué. “We need new knowledge,” he adds, “to come up with new therapies.”
References
- 1.Chen Q, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riggi N, Aguet M, Stamenkovic I. Cancer metastasis: A reappraisal of its underlying mechanisms and their relevance to treatment. Annu Rev Pathol. 2018;13:117–140. doi: 10.1146/annurev-pathol-020117-044127. [DOI] [PubMed] [Google Scholar]
- 3.Merriam-Webster, Inc. Merriam-Webster’s Collegiate Dictionary. 11th Ed Merriam-Webster; Springfield, MA: 2009. [Google Scholar]
- 4.Denny SK, et al. Nfib promotes metastasis through a widespread increase in chromatin accessibility. Cell. 2016;166:328–342. doi: 10.1016/j.cell.2016.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur A, et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532:250–254. doi: 10.1038/nature17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harney AS, et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by Tie2hi macrophage-derived VEGFA. Cancer Discov. 2015;5:932–943. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohan TE, et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst. 2014;106:dju136. doi: 10.1093/jnci/dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karagiannis GS, et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med. 2017;9:eaan0026. doi: 10.1126/scitranslmed.aan0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malladi S, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayatilaka H, et al. Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration. Nat Commun. 2017;8:15584. doi: 10.1038/ncomms15584. [DOI] [PMC free article] [PubMed] [Google Scholar]