Abstract
Background
Angiogenesis plays a crucial role in myocardial infarction (MI) treatment by ameliorating myocardial remodeling, thus improving cardiac function and preventing heart failure. Muscone has been reported to have beneficial effects on cardiac remodeling in MI mice. However, the effects of muscone on angiogenesis in MI mice and its underlying mechanisms remain unknown.
Material/Methods
Mice were randomly divided into sham, MI, and MI+muscone groups. The MI mouse model was established by ligating the left anterior descending coronary artery. Mice in the sham group received the same procedure except for ligation. Mice were administered muscone or an equivalent volume of saline for 4 consecutive weeks. Cardiac function was evaluated by echocardiograph after MI for 2 and 4 weeks. Four weeks later, all mice were sacrificed and Masson’s trichrome staining was used to assess myocardial fibrosis. Isolectin B4 staining was applied to evaluate the angiogenesis in mouse hearts. Immunohistochemistry, Western blot analysis, and quantitative real-time polymerase chain reaction (qPCR) were performed to analyze expression levels of HIF-1α and its downstream genes.
Results
Compared with the MI group, muscone treatment significantly improved cardiac function and reduced myocardial fibrosis. Moreover, muscone enhanced angiogenesis in the peri-infarct region and p-VEGFR2 expression in the vascular endothelial cells. Western blot analysis and qPCR showed that muscone upregulated expression levels of HIF-1α and VEGFA.
Conclusions
Muscone improved cardiac function in MI mice through augmented angiogenesis. The potential mechanism of muscone treatment in regulating angiogenesis of MI mice was upregulating expression levels of HIF-1α and VEGFA.
MeSH Keywords: Angiogenesis Inducing Agents; Hypoxia-Inducible Factor 1, alpha Subunit; Myocardial Infarction; Vascular Endothelial Growth Factor A
Background
Myocardial infarction (MI) caused by coronary blockage may lead to cardiac arrhythmia and heart failure, and is the leading cause of cardiovascular-related deaths [1–3]. Previous studies showed that MI is usually accompanied by hypoxia, myocardial cell apoptosis, and death [4,5]. Oxidative stress, inflammation, and fibrosis are significantly involved in the progression of MI [6–10].
Traditional Chinese medicine (TCM) has received great attention in treating MI in recent years. Musk, a high-value traditional Chinese medicine, is an odoriferous material obtained from the ventral glandular secretion of male musk deer. Long-term clinical practices have extensively used musk for treating stroke, angina pectoris, and myocardial infarction [11,12]. Muscone, the synthetic equivalent of musk, shows anti-fibrotic, anti-inflammatory, anti-oxidant, and anti-apoptotic effects on the ischemic myocardium [13].
Angiogenesis exerts a vital role in cell growth and development, as well as in wound healing and the formation of granulation tissues [14]. The essential role of angiogenesis in MI has been accepted for the past several years [15]. Novel treatments in MI models based on angiogenesis, such as administration of exogenous angiogenic growth factors, transplantation of mesenchymal stromal cells (MSCs), and induced pluripotent stem cells (iPSCs), have been reported to promote angiogenesis and ameliorate myocardial remodeling [16–18]. Vascular endothelial growth factor (VEGF) is the most important angiogenic factor [19]. Muscone has also been reported to increase VEGF expression and angiogenesis in skin flaps [20]. Therefore, we speculated that muscone would promote cardiac angiogenesis and improve cardiac function.
In the present study, we explored the involvement of muscone in the repair of ischemic myocardium and its underlying mechanisms, using an MI mouse model. We demonstrated that muscone plays a significant role in cardiac remodeling and heart function restoration through regulating angiogenesis via the hypoxia-inducible factor 1 alpha (HIF-1α)/VEGFA pathway.
Material and Methods
Animals
Male C57BL/6J mice weighing 20–25 g were obtained from the Model Animal Research Center of Nanjing University, Nanjing, China. They were maintained in a 12 h/12 h light–dark cycle (lights on: 07: 00–19: 00), with a room temperature thermostatically maintained at 23±2°C under hygienic conditions. Mice were given free access to water and food. All mice were adaptively kept under this condition for 1 week before myocardial infarction procedure. All experiments were performed according to the guidelines for animal care set by the Institute for Laboratory Animal Research of Nanjing Medical University. Animal experiments were approved by the Animal Ethics and Welfare Committee of Nanjing Medical University (Approval No. IACUC-1709001).
Drugs
Muscone was purchased from Nanjing Zelang Biological Technology Company (Nanjing, China). Muscone (dissolved in 0.05% normal saline) was administrated once a day at a dose of 2 mg/kg through intragastric gavage according to methods described in our previous study [13] for 4 consecutive weeks.
Experimental procedure
Mice were randomly divided into a sham group (n=12), an MI group (n=12), and an MI+muscone group (n=12). We ligated the left anterior descending coronary artery to establish an MI mouse model, following methods previously described [21]. Briefly, mice were anesthetized by the intraperitoneal injection of pentobarbital sodium (50 mg/kg) and then placed in a supine position. After endotracheal intubation, artificial mechanical ventilation was performed (tidal volume of 1.8 ml, an inspiratory and expiratory ration of 2: 1, and a respiratory rate of 130 breaths per min). After the heart exposure, an 8-0 nylon suture was passed approximately 2–3 mm below the tip of the left auricle and used for permanent ligation of the left anterior descending coronary artery. The color of the infarcted zone was changed from red to white. Mice in the sham group underwent the same procedure except for ligation. Mice were administered muscone for 4 weeks in the MI+muscone group, whereas equivalent volumes of saline were administered to mice in the sham and MI groups.
Cardiac structure or function detection by echocardiography
Heart function of each group was evaluated using the Vevo 2100 ultrasound system (Visual Sonic, Canada) after the procedure for 2 and 4 weeks, respectively, as previously described [13]. The ultrasonic probe was 30 MHz. The left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were calculated. Each index was measured for 3 cardiac cycles.
Masson’s trichrome staining
Hearts were collected in different groups, fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4-μm-thick sections. Slides were stained with Masson’s trichrome to measure the extent of cardiac fibrosis. The collagen tissue area was calculated using Image Pro Plus software (version 6.0) and is expressed as the percentage of the full ventricle area.
Immunohistochemistry
Paraffin sections were deparaffinized and rehydrated, then antigen retrieval was achieved in 10 mM sodium citrate buffer (pH 6.0) for 20 min using a microwave oven. Sections were incubated with primary antibodies at 4ºC overnight following blocking of endogenous peroxidase with 3% hydrogen peroxide and preincubation with serum. Two-step technique (Maixin Biotech, Fuzhou, China) was used for visualization, and DAB was used as a chromogen. Sections were counterstained with hematoxylin and sealed with neutral balsam. Primary antibodies for immunohistochemistry were: HIF-1α (sc-10790, 1: 200, Santa Cruz Biotechnology, CA, USA), p-VEGFR2 (#2478, 1: 300, Cell Signaling Technology, Boston, USA), and p-AKT (#4060, 1: 100, Cell Signaling Technology, Boston, USA).
Isolectin B4 staining
Cardiac capillary density was measured by biotinylated Isolectin B4 staining as previously described [22]. Slides were incubated with the fluorescein-labeled Griffonia Simplicifolia Lectin I (GSLI) Isolectin B4 (FL-1201, 1: 50, Vector Laboratories, USA) overnight at 4°C and sealed with DAPI Fluoromount-G® mounting medium (Southern Biotech, USA). To measure the capillary density quantitatively, slides were examined in a blinded way using a fluorescence microscope under 400× magnification. Isolectin B4-positive cells were captured by Image Pro Plus software (version 6.0) in 3 tissue sections per group. Three randomly selected fields in the infarct, peri-infarct, and remote zone were examined in each section. The capillary density was expressed in capillaries per square millimeter.
Western blotting
Left ventricular myocardial tissues were lysed with radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Hangzhou, China). After being centrifuged at 13 000 rpm at 4°C for 10 min, supernatants were submitted to Western blotting. Protein concentrations were measured using the BCA Protein Assay Kit (KeyGEN BioTECH, Nanjing, China). Equal amounts of protein (25 μg/lane) were electrophoresed and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, USA). After being blocked with 5% skim milk in TBST at room temperature for 2 h, the membranes were incubated with primary antibodies at 4°C overnight. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1: 5000, Santa Cruz Biotechnology, CA, USA) for 1 h. Finally, the membranes were exposed using the ECL system (Millipore, Bedford, USA). Primary antibodies were used as follows: GAPDH (#2118, 1: 1000, Cell Signaling Technology, Boston USA), HIF-1α (sc-10790, 1: 200, Santa Cruz Biotechnology, CA, USA), VEGFA (ab46154, 1: 1000, Abcam, Cambridge, UK), AKT (#9272, 1: 1000, Cell Signaling Technology, Boston, USA), p-AKT (#4060, 1: 1000, Cell Signaling Technology, Boston, USA).
RNA preparation and quantitative real-time polymerase chain reaction (qPCR)
We extracted total RNA from left ventricular myocardial tissues using TRIzol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. Reverse transcription was performed using the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, Ottawa, Canada) according to the manufacturer’s instructions. qPCR was performed using a Hieff™ qPCR SYBR® Green Master Mix (High Rox Plus, Yeasen, China) following the manufacturer’s protocol. The primers were designed as follows: HIF-1α forward: 5′-CGCCTCTGGACTTGTCTCTT-3′; reverse: 5′-TCGACGTTCAGAACTCATCCT-3′; VEGFA forward: 5′-TATTC AGCGGACTCACCAGC-3′; reverse: 5′-AACCAACCTCCTCAAACCGT-3′; GAPDH forward: 5′-CATTTCACTCAAGGTTGTCAGC-3; reverse: 5′-ATCATACTTGGCAGGTTTCTCC-3′. mRNA quantification was processed using the 2−ΔΔCt method and normalized to GAPDH as an endogenous control.
Statistical analysis
Data from at least 3 independent experiments were used to calculate mean ± standard deviation (SD). Statistical analyses between groups were carried out using one-way ANOVA or unpaired t test. A two-sided value of P<0.05 was considered to be statistically significant. All statistical analyses were conducted using SPSS software (version 22.0) or GraphPad Prism software (version 6.02).
Results
Effect of muscone on the cardiac function in MI mice
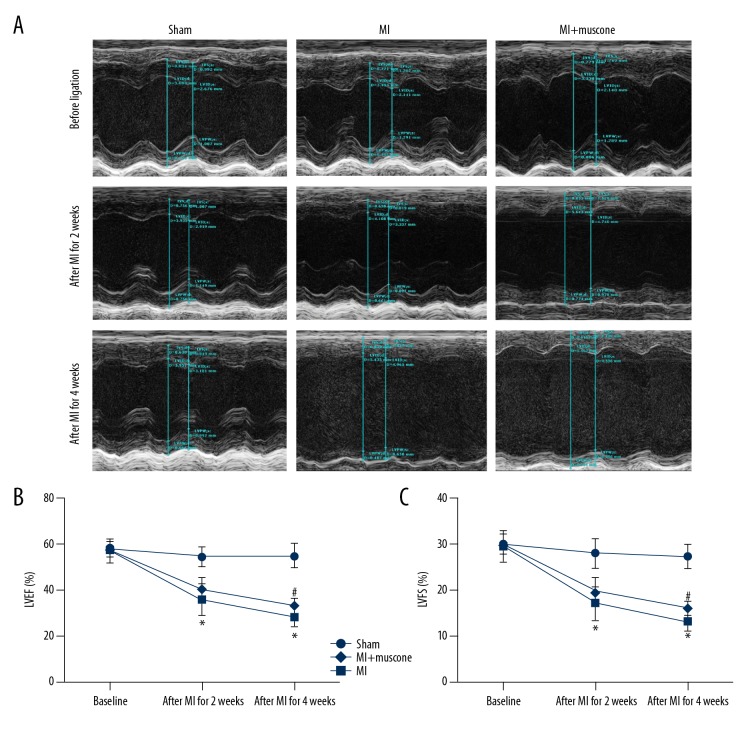
Four weeks later, there were no deaths in the sham group. However, only 8 mice survived in the MI group and 10 mice survived in the MI+muscone group. At the 2nd and 4th week after MI, LVEF and LVFS were measured by echocardiography to evaluate the heart ejection function and myocardial contractility, respectively. As shown in Figure 1, LVEF and LVFS were significantly decreased in the MI group and MI+muscone group when compared with the sham group after the procedure for 2 and 4 weeks (P<0.05). After MI for 2 weeks, there were no significant differences in LVEF and LVFS between MI group and MI+muscone group (LVEF: 35.661±6.998% vs. 40.204±5.054%, P>0.05; LVFS: 17.073±3.726% vs. 19.734±2.788%, P>0.05). Four weeks later, markedly elevations in both LVEF and LVFS were found in the MI+muscone group when compared with the MI group (LVEF: 33.128±3.077% vs. 28.198±5.123%, P<0.05; LVFS: 15.940±1.538% vs. 13.175±2.564%, P<0.05). These observations suggested that muscone treatment improved the heart ejection function and myocardial contractility in MI mice after 4 weeks.
Figure 1.
Muscone improved cardiac function after MI for 4 weeks. (A) Representative M-mode images by echocardiography after MI for 2 and 4 weeks, respectively. The improvement of left ventricular wall motion in MI+muscone group after MI for 4 weeks was observed. Left ventricular ejection fraction (LVEF) (B) and left ventricular fractional shortening (LVFS) (C) were measured by echocardiography. Data are represented as mean ±SD, n=8 per group (* p<0.05 versus sham group, # p<0.05 versus MI group).
Effect of muscone on the fibrosis in MI mice
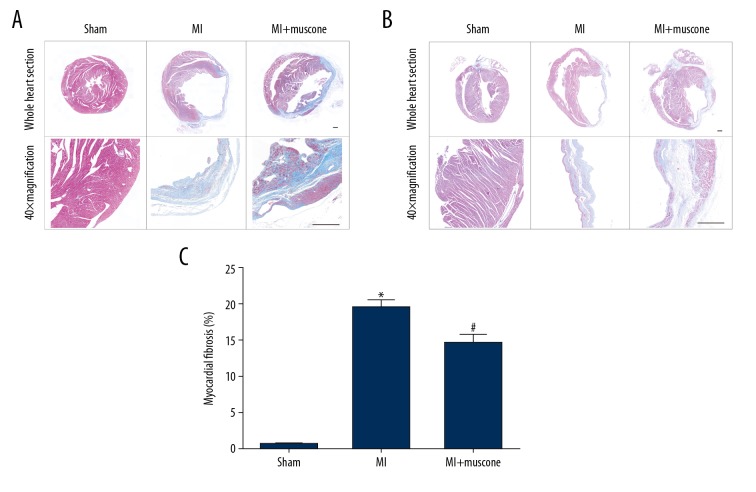
After mice were exposed to the coronary ligation for 4 weeks, Masson’s trichrome staining was applied to measure the collagen deposition to evaluate the myocardial fibrosis in MI mice. As shown in Figure 2, the Masson’s trichrome staining density was significantly increased in the MI group and MI+muscone group when compared with the sham group after the procedure for 4 weeks (P<0.05). After 4 weeks of treatment, a marked decrease in Masson’s trichrome staining density was found in the MI+muscone group when compared with the MI group (14.675±1.135% vs. 19.550±0.933%, P<0.05). These observations suggest that muscone treatment reduced the fibrosis in MI mice after 4 weeks.
Figure 2.
Muscone reduced fibrosis in left ventricular (LV) myocardium after MI for 4 weeks. Representative histological photomicrographs showed the collagen deposition (blue) on the infarct region in transverse sections (A) and longitudinal sections (B) in each group as shown by Masson’s trichrome staining. Scale bars=500 μm. (C) Quantitative analysis of fibrotic area by Masson’s trichrome staining. Data are represented as mean ±SD, n=4 per group (* p<0.05 versus sham group, # p<0.05 versus MI group).
Effect of muscone on the angiogenesis in MI mice
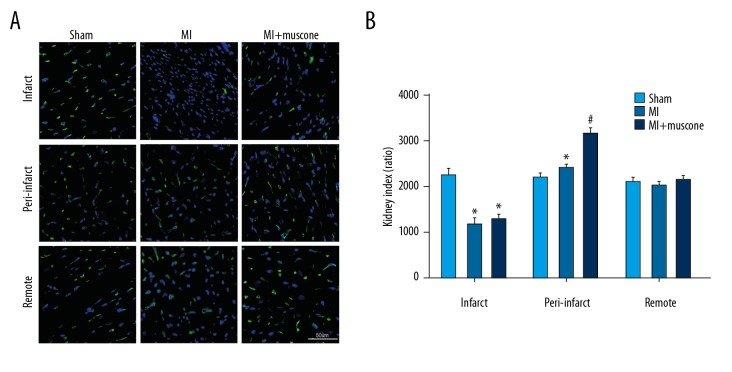
After mice were exposed to the coronary ligation for 4 weeks, Isolectin B4 staining was applied to measure the capillary density to evaluate the angiogenesis in MI mice. As shown in Figure 3, Isolectin B4-positive cells were remarkably increased in the peri-infarct area in the MI+muscone group when compared with the MI group (3156.46±131.97 vs. 2416.33±65.31, P<0.05). These observations suggest that muscone treatment promoted angiogenesis in MI mice after 4 weeks.
Figure 3.
Muscone promoted angiogenesis in left ventricular (LV) after MI for 4 weeks. (A) Representative photomicrographs showed the capillary density in the infarct, peri-infarct, and remote region in the 3 groups as shown by Isolectin B4 staining. Bar=50 μm. (B) Quantitative summary of capillary density by Isolectin B4 staining. Data are represented as mean ±SD, n=12 per group (* p<0.05 versus sham group, # p<0.05 versus MI group).
Effect of muscone on the expression levels of HIF-1α, VEGFA, and p-AKT in MI mice
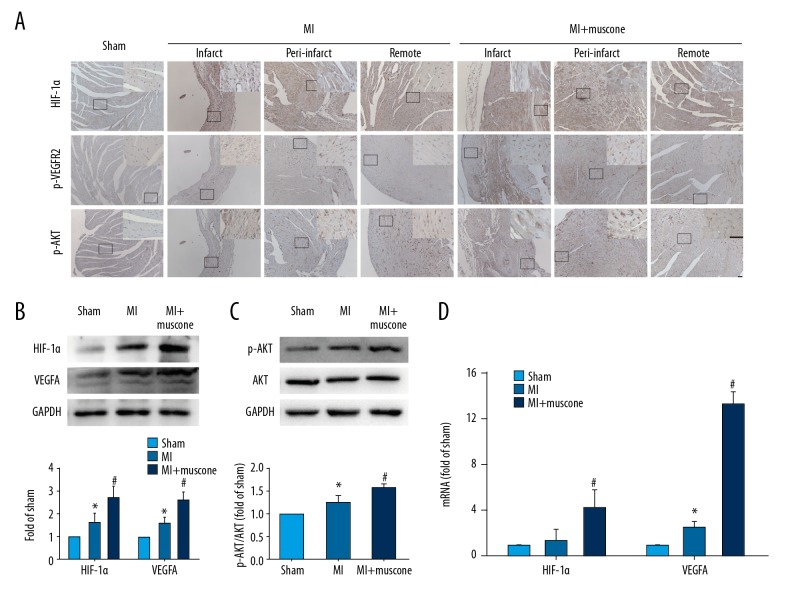
To further elucidate the detailed mechanisms, we investigated the expression levels of HIF-1α, VEGFA and p-AKT in MI mice after 4 weeks, all of which were well documented to be involved in angiogenesis. Immunohistochemical staining was used to identify the distribution of HIF-1α, p-VEGFR2 (activated mainly by VEGFA), and p-AKT. Western blot analysis and qPCR were applied to measure expression levels of HIF-1α, VEGFA, and p-AKT in mice of different groups. As shown in Figure 4A, based on the morphological characteristics, HIF-1α-positive cells and p-AKT-positive cells were distributed in the cardiomyocytes, while p-VEGFR2-positive cells were distributed in the vascular endothelial cells in the MI group and MI+muscone group. Four weeks later, protein and mRNA levels of HIF-1α (protein level: 3.04±0.37 vs. 1.84±0.31, P<0.05; mRNA level: 4.34±1.45 vs. 1.42±0.90, P<0.05) and VEGFA (protein level: 3.00±0.28 vs. 1.82±0.09, P<0.05; mRNA level: 13.31±1.02 vs. 2.50±0.52, P<0.05) were upregulated in the MI+muscone group when compared with the MI group (Figure 4B, 4D). p-AKT/AKT (0.90±0.05 vs. 0.73±0.05, P<0.05) was also upregulated by muscone treatment in MI mice (Figure 4C). These results demonstrate that muscone treatment upregulates the expression levels of HIF-1α, VEGFA, and p-AKT in MI mice after 4 weeks.
Figure 4.
Muscone increased expression levels of HIF-1α, VEGFA and p-AKT in left ventricular (LV) after MI for 4 weeks. (A) Representative photomicrographs of HIF-1α, p-VEGFR2, and p-AKT distribution in the infarct, peri-infarct, and remote region in the 3 groups by immunohistochemical staining. Identified according to the morphological characteristics, the cardiomyocytes showed HIF-1α and p-AKT-positive staining, and the vascular endothelial cells showed p-VEGFR2-positive staining. Bar=50 μm. (B) Western blot analysis of HIF-1α and VEGFA in the 3 groups. GAPDH served as the loading control. Quantification was shown. (C) Western blot analysis of AKT and p-AKT in the 3 groups. Quantification of p-AKT/AKT ratio is shown. (D) The mRNA expression levels of HIF-1α and VEGFA. Data are represented as mean ±SD, n=12 per group (* p<0.05 versus sham group, # p<0.05 versus MI group).
Discussion
This study is the first to demonstrate the important role of angiogenesis in the protective effect of muscone on MI mice. Our previous study found that muscone treatment improves cardiac function and reduces myocardial fibrosis in MI mice [13]. In the present study, the results of LVEF, LVFS, and Masson’s trichrome staining were similar to those of our previous study, suggesting the successful construction of an MI mouse model treated with muscone. The data presented herein also show the upregulated expression levels of HIF-1α, VEGFA, and p-AKT in muscone-treated MI mice.
AKT is a well-characterized target of PI3K, and its phosphorylation protects MI-induced heart function by promoting angiogenesis, as well as inhibiting apoptosis in the heart [23–25]. In the present study, muscone treatment upregulated p-AKT in MI mice. Meanwhile, the immunohistochemical staining identified that p-AKT was mainly distributed in cardiomyocytes but not in vascular endothelial cells. These results suggest that the possible effect of muscone on upregulating p-AKT was inhibition of MI-induced myocardial apoptosis, as described in our previous study [13]. Myocardial apoptosis is mainly involved in the acute phase after MI. Apoptosis inhibition can protect cardiac function at a very early stage and prevent heart failure. However, no significant difference in cardiac function was found between the MI group and MI+muscone group in the early stage (after MI for 2 weeks). All these results indicate that muscone exerted its protective role in preserving cardiac function after MI, primarily by other important mechanisms rather than AKT activation.
HIF-1α is an important transcription factor that regulates the cellular response to hypoxia, and the increased expression of HIF-1α is one of the first adaptations of human myocardium to ischemia [26,27]. Previous studies using a transgenic model have found that over-expression of HIF-1α can promote angiogenesis, reduce infarct size, and improve cardiac function [28–30]. VEGFA, usually referred to as VEGF, acts as a key contributor in angiogenesis [31]. Multiple lines of evidence suggest that VEGFA directly induced by HIF-1α is cardioprotective and promotes repair of the infarcted heart [29,32–34]. In the present study, HIF-1α and its downstream VEGFA were upregulated in muscone-treated MI mice. p-VEGFR2, the main activated receptor of VEGFA and representing the extent of angiogenesis [35], was mainly distributed in the vascular endothelial cells as shown by immunohistochemical staining. Moreover, muscone treatment improved the cardiac function 4 weeks later, not within 2 weeks after MI. Taken together, our results show that muscone improved cardiac function primarily by promoting angiogenesis through the HIF-1α/VEGFA pathway.
In addition, muscone treatment may be better-tolerated than those of direct cell therapy and delivery of angiogenic growth factors in MI patients with poor cardiac function. Further in-depth studies are warranted to determine the potential mechanisms of HIF-1α expression responsible for muscone treatment.
Conclusions
Our study is the first to confirm that muscone improves cardiac function after MI by stimulating angiogenesis via upregulating HIF-1α and VEGFA, which may provide a potential strategy for post-MI treatment.
Footnotes
Source of support: This study was funded by the Wuxi Municipal Committee for Health and Family Planning (MS201514), the National High Technology Research and Development Program of China (863 Program), the National Natural Science Foundation of China (No. 81170102 & No. 81441011 & No. 81670328), the Doctoral Scientific Fund Project of the Ministry of Education of China (20123234110015), the Fourth Period Project “333” of Jiangsu Province (BRA2012207), the Chinese Medical Association of the Sunlight Foundation (SCRFCMDA201217), the Collaborative Innovation Center of Nanjing Medical University, and the Jiangsu Provincial Science and Technology Department Basic Research Program (Natural Science Foundation of China BK20141020)
Conflict of interest
None.
References
- 1.Cannon RR. Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nat Clin Pract Cardiovasc Med. 2005;2:88–94. doi: 10.1038/ncpcardio0096. [DOI] [PubMed] [Google Scholar]
- 2.Muzumdar RH, Huffman DM, Calvert JW, et al. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30:1940–48. doi: 10.1161/ATVBAHA.110.205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian H, Cimini M, Fedak PW, et al. TIMP-3 deficiency accelerates cardiac remodeling after myocardial infarction. J Mol Cell Cardiol. 2007;43:733–43. doi: 10.1016/j.yjmcc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Wang Z, Feng SJ, et al. PEDF improves cardiac function in rats with acute myocardial infarction via inhibiting vascular permeability and cardiomyocyte apoptosis. Int J Mol Sci. 2015;16:5618–34. doi: 10.3390/ijms16035618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang D, Zhu L, Li C, et al. Sialyltransferase7A, a Klf4-responsive gene, promotes cardiomyocyte apoptosis during myocardial infarction. Basic Res Cardiol. 2015;110:28. doi: 10.1007/s00395-015-0484-7. [DOI] [PubMed] [Google Scholar]
- 6.Karabag Y, Cagdas M, Rencuzogullari I, et al. Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur J Clin Invest. 2018;48:e12928. doi: 10.1111/eci.12928. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Jin L, Zhuang Y, et al. Cardioprotective effect of rosuvastatin against isoproterenol-induced myocardial infarction injury in rats. Int J Mol Med. 2018;41:3509–16. doi: 10.3892/ijmm.2018.3572. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, Cheng P, Huang GY, et al. Effects of Xin-Ji-Er-Kang on heart failure induced by myocardial infarction: Role of inflammation, oxidative stress and endothelial dysfunction. Phytomedicine. 2018;42:245–57. doi: 10.1016/j.phymed.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Askari H, Rajani SF, Poorebrahim M, et al. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol Res. 2018;129:44–55. doi: 10.1016/j.phrs.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Jin X, Hu CF, et al. Amphiregulin enhances cardiac fibrosis and aggravates cardiac dysfunction in mice with experimental myocardial infarction partly through activating EGFR-dependent pathway. Basic Res Cardiol. 2018;113:12. doi: 10.1007/s00395-018-0669-y. [DOI] [PubMed] [Google Scholar]
- 11.Wei G, Chen DF, Lai XP, et al. Muscone exerts neuroprotection in an experimental model of stroke via inhibition of the fas pathway. Nat Prod Commun. 2012;7:1069–74. [PubMed] [Google Scholar]
- 12.Wu Q, Li H, Wu Y, et al. Protective effects of muscone on ischemia-reperfusion injury in cardiac myocytes. J Ethnopharmacol. 2011;138:34–39. doi: 10.1016/j.jep.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Meng H, Chen P, et al. Beneficial effects of muscone on cardiac remodeling in a mouse model of myocardial infarction. Int J Mol Med. 2014;34:103–11. doi: 10.3892/ijmm.2014.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birbrair A, Zhang T, Wang ZM, et al. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 2015;128:81–93. doi: 10.1042/CS20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: Maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–71. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 16.Tao Z, Chen B, Tan X, et al. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci USA. 2011;108:2064–69. doi: 10.1073/pnas.1018925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim M, Wang W, Liang L, et al. Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res Ther. 2018;9:129. doi: 10.1186/s13287-018-0888-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song G, Li X, Shen Y, et al. Transplantation of iPSc restores cardiac function by promoting angiogenesis and ameliorating cardiac remodeling in a post-infarcted swine model. Cell Biochem Biophys. 2015;71:1463–73. doi: 10.1007/s12013-014-0369-7. [DOI] [PubMed] [Google Scholar]
- 19.Blazquez C, Gonzalez-Feria L, Alvarez L, et al. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004;64:5617–23. doi: 10.1158/0008-5472.CAN-03-3927. [DOI] [PubMed] [Google Scholar]
- 20.Kailiang Z, Yihui Z, Dingsheng L, Xianyao T. Effects of muscone on random skin flap survival in rats. J Reconstr Microsurg. 2016;32:200–7. doi: 10.1055/s-0035-1565264. [DOI] [PubMed] [Google Scholar]
- 21.Cheng W, Wu P, Du Y, et al. Puerarin improves cardiac function through regulation of energy metabolism in Streptozotocin-Nicotinamide induced diabetic mice after myocardial infarction. Biochem Biophys Res Commun. 2015;463:1108–14. doi: 10.1016/j.bbrc.2015.06.067. [DOI] [PubMed] [Google Scholar]
- 22.Zhou N, Fu Y, Wang Y, et al. P27 kip1 haplo-insufficiency improves cardiac function in early-stages of myocardial infarction by protecting myocardium and increasing angiogenesis by promoting IKK activation. Sci Rep. 2014;4:5978. doi: 10.1038/srep05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siragusa M, Katare R, Meloni M, et al. Involvement of phosphoinositide 3-kinase gamma in angiogenesis and healing of experimental myocardial infarction in mice. Circ Res. 2010;106:757–68. doi: 10.1161/CIRCRESAHA.109.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patten RD, Pourati I, Aronovitz MJ, et al. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–99. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 25.Lu C, Wang X, Ha T, et al. Attenuation of cardiac dysfunction and remodeling of myocardial infarction by microRNA-130a are mediated by suppression of PTEN and activation of PI3K dependent signaling. J Mol Cell Cardiol. 2015;89:87–97. doi: 10.1016/j.yjmcc.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SH, Wolf PL, Escudero R, et al. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–33. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 27.Semenza GL. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 2000;88:1474–80. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 28.Tang JM, Wang JN, Zhang L, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91:402–11. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kido M, Du L, Sullivan CC, et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2005;46:2116–24. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 30.Jianqiang P, Ping Z, Xinmin F, et al. Expression of hypoxia-inducible factor 1 alpha ameliorate myocardial ischemia in rat. Biochem Biophys Res Commun. 2015;465:691–95. doi: 10.1016/j.bbrc.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 31.Zhao T, Zhao W, Chen Y, et al. Vascular endothelial growth factor (VEGF)-A: Role on cardiac angiogenesis following myocardial infarction. Microvasc Res. 2010;80:188–94. doi: 10.1016/j.mvr.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Fu L, Han Y, et al. Testosterone replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing expression of cytokines HIF-1a, SDF-1a and VEGF. Eur J Pharmacol. 2012;684:116–24. doi: 10.1016/j.ejphar.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 34.Fei L, Zhang J, Niu H, et al. Effects of rosuvastatin and MiR-126 on myocardial injury induced by acute myocardial infarction in rats: Role of vascular endothelial growth factor A (VEGF-A) Med Sci Monit. 2016;22:2324–34. doi: 10.12659/MSM.896983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klinnikova MG, Bakarev MA, Nikityuk DB, Lushnikova EL. Immunohistochemical study of the expression of vascular endothelial growth factor receptor-2 (KDR/Flk-1) during myocardial infarction. Bull Exp Biol Med. 2017;163:500–5. doi: 10.1007/s10517-017-3838-3. [DOI] [PubMed] [Google Scholar]