Abstract
Background
Intranasal calcitonin gene-related peptide (CGRP) delivery offers a noninvasive method of bypassing the blood-brain barrier for the delivery of CGRP to the brain. Here, we first reported the therapeutic benefits of intranasal CGRP delivery in rats following middle cerebral artery occlusion (MCAO).
Material/Methods
Real-time quantitative polymerase chain reaction (RT-qPCR) assay, enzyme-linked immunosorbent assay (ELISA), rat MCAO model, TTC (2, 3, 5-triphenyltetrazolium chloride) staining, hematoxylin and eosin (H & E) staining, Morris water maze test, TUNEL assay, immunofluorescence, and western blot assay were used to investigate the role of CGRP in rats. Cell Counting Kit-8 assay, colony formation assay, cell cycle assay, apoptosis assay, western blot assay, and TOP/FOP assay were used to investigate the role of CGRP in normal human astrocytes (NHA) cells.
Results
The CGRP-MCAO-NDDS (nasal drug delivery system) group showed a significant reduction in the infarct volume and improvement in neurologic deficit tests of motor, sensory, reflex and vestibulo-motor functions compared to those rats in the CGRP-MCAO-IV group. CGRP markedly inhibited apoptosis and increased the expression of vascular endothelial growth factor (VEGF) and bFGF and decreased the expression of GAP43 in the cortex of MCAO rats. CGRP promoted cell proliferation and cell cycle process and inhibited cell apoptosis through the Wnt/β-catenin pathway in NHA cells.
Conclusions
This noninvasive, simple, and cost-effective method is a potential treatment strategy for focal cerebral ischemic injury.
MeSH Keywords: Apoptosis; Calcitonin Gene-Related Peptide; Ischemic Attack, Transient; Wnt Signaling Pathway
Background
Cerebral ischemia is a main cause of disability in high-income countries and ranks second as a cause of death worldwide [1]. It can induce secondary cerebral injury, which can then trigger a series of biological processes, such as neuronal necrosis and inflammatory changes [2]. Apoptosis is considered the major source for neuronal necrosis after cerebral ischemia. It involves a series of pathophysiological changes secondary to disorder of energy metabolism, which finally results in irreversible brain injuries, especially damages to the cerebral cortex [3].
Calcitonin gene-related peptide (CGRP) is an excellent endogenous vasoactive substance reported to involved in the attenuation of cerebral ischemia [4]. Moreover, CGRP could improve the neurobehavioral function of ischemic rats and reduce the infarction range through the regulation of the c-Jun NH2-terminal kinase (JNK), extracellular regulated MAP kinase (ERK), and p38 phosphorylation [5]. Nevertheless, the penetration of CGRP to the blood brain barrier is limited due to its large molecular weight, which thus hampers the efficiency of conventional medications. Nasal drug delivery system (NDDS) has been utilized in the drug delivery for cerebral diseases as it offers an interesting alternative for achieving systemic drug effects compared with intravenous route [6]. Therefore, we hypothesized that intranasal drug delivery approach to noninvasively target CGRP to the central nervous system may contribute to the brain exposure and protect against focal cerebral ischemic damage.
In this study, we found that NDDS-CGRP induced significant reduction in the infarct volume and improved all the neurologic deficit tests of motor, sensory, reflex, and vestibulomotor functions compared to those of the CGRP, middle cerebral artery occlusion (MCAO), intravenous injection (CGRP-MCAO-IV) group. In addition, CGRP markedly inhibited apoptosis and increased the expression of VEGF (vascular endothelial growth factor), GAP43 (growth associated protein 43), and bFGF (fibroblast growth factor 2) in the cortex from MCAO rats. Furthermore, CGRP promoted cell proliferation and cell cycle process and inhibited cell apoptosis through the Wnt/β-catenin pathway in normal human astrocytes (NHA) cells. Thus, we studied this noninvasive, simple, and cost-effective method as a potential treatment strategy for focal cerebral ischemic injury.
Material and Methods
Animals
Male Wistar rats (SPF grade, 12 weeks old, weighing: 260±20 g) were purchased from Charles River Co., Ltd. (Peking, China, Animal No. 11400700063442). All experimental procedures were compliant with the Guidelines for Laboratory Animal Use and Care from the Chinese Center for Disease Control and Prevention and the Rules for Medical Laboratory Animals from the Chinese Ministry of Health. Rats were randomly divided into 1) sham group (n=20) subjected to sham operation and received vehicle intraperitoneally; 2) MCAO group (n=20) subjected to MCAO induction; 3) CGRP-MCAO group subjected to MCAO induction, followed by CGRP via NDDS (CGRP-MCAO-NDDS group; n=20); and 4) intravenous injection (CGRP-MCAO-IV group; n=20). The study protocols were approved by the Ethical Committee of Taishan Medical University (TMU20150326).
MCAO induction
MCAO rats were induced according to the previous description by Koizumi et al. [7]. Briefly, the rats were anesthetized with 10% chloral hydrate (360 mg/100 g). Following chloral hydrate anesthesia and disinfection, the carotid, internal carotid, and external carotid arteries were separated. One thread of nylon monofilament line coated with poly-L-lysine was inserted along the internal carotid artery; when resistance was sensed, the monofilament was assumed to have reached the initial site of the middle cerebral artery. Muscle and skin were temporarily full-layer sutured. After 2 hours of cerebral ischemia, the nylon line was removed, and the incision was sutured. Subsequently, reperfusion was performed for 3 hours. Following surgery, the rats were placed under an illuminating lamp to maintain the body temperature of the rats between 37–37.5°C.
Evaluation of neurobehavioral score
The neurobehavioral score for each rat was evaluated according to the standards described by Berderson et al. [8] as follows: grade 0, no observable deficit; grade 1, forelimb flexion; grade 2, decreased resistance to lateral push without circling; and grade 3, same behavior grade 2 with circling; grade 4, soft paralysis, no spontaneous activity or death. Only the animals with a score of 0–3 were selected for the subsequent experiments.
Morris water maze test
Cognitive function was determined using the Morris water test according to the conventional descriptions [9]. The maze consisted of a 1.5 m diameter, 40 cm deep water pool (25±2°C) and a submerged and hidden platform in one quadrant. Briefly, on day 7 post MCAO induction, the rats were habituated to water by placing into the pool and removing the platform. The test was initiated on day 14 post MCAO, starting at 4 locations randomly assigned, and the test lasting for 5 days. The cognitive outcome was evaluated as follows: 1) the time spent in the marginal area, referred to as thigmotaxis; and 2) the distance to find the platform as an indicator for visual-spatial learning.
Determination of cerebral infarction volume
Six rats in each group were sacrificed to obtain the olfactory bulb and brainstem. Then 2, 3, 5-triphenyltetrazolium chloride (TTC) staining was performed for the evaluation of infarction volume as conventionally described. The infarction area was analyzed using the Image-pro Plus 6.0 software (Media Cybernetics, USA).
Hematoxylin and eosin (H & E) staining
The resting rats in each group were then sacrificed for the hematoxylin and eosin (H & E) staining. The tissues with infarction were fixed using 4% paraformaldehyde, and then embedded in paraffin. Subsequently, the sections were subject to conventional H & E staining. The images were observed under a microbioscope (BX51) to determine the pathological changes of the brain tissues.
TUNEL assay
TUNEL assay was performed to determine the apoptosis of neurons. Briefly, the sections (10 μm) were obtained based from the brain tissues at about 3–6 mm from the frontal pole. TUNEL assay was carried out using a commercial kit, according to the manufacturer’s instructions. For the image observation, 10 fields were randomly selected from each section under a BX51 microscope. The cells with nucleus stained in a brown color were defined as neurons underwent apoptosis.
Immunofluorescence
Immunofluorescence of NeuN, bFGF, GAP43, and VEGF was performed using commercial kits, according to the manufacturer’s instructions. In brief, the sections (40 μm) were incubated with the primary antibody including NeuN (Abcam Biotech Co., Ltd., Boston, MA, USA), bFGF (Abcam Biotech Co., Ltd., Boston, MA, USA), GAP43 (Abcam Biotech Co., Ltd., Boston, MA, USA), and VEGF (Abcam Biotech Co., Ltd., Boston, MA, USA). Subsequently, the mixture was incubated with the secondary antibodies (Beyotime Biotech Co., Ltd., Nanjing, China). The images were observed under confocal microscope.
Western blot analysis
Brain tissue specimens (100 mg) near the ischemic cortex were homogenized in RIPA lysis buffer (Sigma) containing protease inhibitors. The protein concentration was determined using the bicinchoninic acid (BCA) method (Beyotime Biotech Co., Ltd., Nanjing, China). Proteins were separated by electrophoresis on 10% SDS-PAGE gel and transferred to a PVDF membrane. The membrane was blocked in 5% nonfat milk and incubated with an primary antibodies of NeuN (Abcam, USA) and bFGF (Abcam, USA), GAP43 (Abcam, USA), VEGF (Abcam, USA), caspase-3 (Wanlei Biotech Co., Ltd., Shenyang, China), BCL2 (Wanlei Biotech Co., Ltd., Shenyang, China), BAX (Wanlei Biotech Co., Ltd., Shenyang, China), and PARP (Wanlei Biotech Co., Ltd., Shenyang, China) overnight at 4°C. Then incubated with the peroxidase-conjugated rabbit anti-goat secondary antibodies (Cell Signaling Technology, Boston, MA, USA) for 2 hours at room temperature. The same membrane was probed for GAPDH for loading control.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from brain tissue specimens using TRIzol agent (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to manufacturer’s instructions. Total RNAs were reversely transcribed into cDNA using the Reverse Transcription System Kit (Takara, Dalian, China). Real-time quantitative polymerase chain reaction (RT-qPCR) was conducted using SYBR-green on the BioRad system with the primers listed in Table 1. The PCR conditions consisted of denaturation at 94°C for 4 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds. The amplification results for real-time PCR was calculated as 2(−ΔΔCt) method.
Table 1.
Real-time quantitative polymerase chain reaction (RT-qPCR) primers used in this study.
| Gene | Sequences (5′-------------3′) |
|---|---|
| NeuN-qPCR-Fwd | 5′ GAAGCCGTCCAACAGTAGCC 3′ |
| NeuN-qPCR-Rev | 5′ TTCCCTACTTCAGTGCTCCCT 3′ |
| bFGF-qPCR-Fwd | 5′ GGAATTCACCATGGCAGCCGGGA 3′ |
| bFGF-qPCR-Rev | 5′ GCTCTAGAATCAGCTCTTAGCAGACA 3′ |
| GAP43-qPCR-Fwd | 5′ CGACAGGATGAGGGTAAAGAAGA 3′ |
| GAP43-qPCR-Rev | 5′ GTGGAGCAGGACAGGAGAGGAA 3′ |
| VEGF-qPCR-Fwd | 5′ GGCTTTACTGCTGTACCTCCAC 3′ |
| VEGF-qPCR-Rev | 5′ TTTTTGCAGGAACATTTACACG 3′ |
| β-actin-qPCR-Fwd | 5′ CTGCCGCATCCTCTTCCTC 3′ |
| β-actin-qPCR-Rev | 5′ CTCCTGCTTGCTGATCCACTA 3′ |
Statistical analysis
SPSS 19.0 software was used for the data analysis. All data were presented as mean ± standard deviation. Student’s t-test was used for the inter-group comparison. For comparisons of 3 or more groups, one-way analysis of variance (ANOVA) was followed by the Bonferroni post-hoc test. P<0.05 was considered to be statistically significant.
Results
The neuroprotective role of CGRP in MCAO model
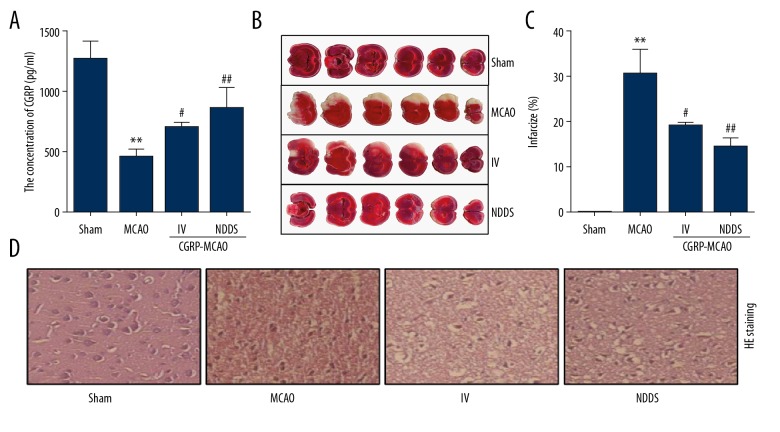
After intranasal administration of CGPR, a high level of CGRP was observed in the brain tissue. First intranasally administered CGRP resulted in the cortical CGRP concentration at 997±274 pg/mL in the MCAO model. In comparison, intravenous administration of a similar dose resulted in CGRP concentration at 773±193 pg/mL in the MCAO model (Figure 1A). Compared with the sham group, the cerebral infarction volume in the MCAO group was significant elevated. Whereas, the infarction area showed significant reduction after CGRP treatment when comparing with that of MCAO group, especially in the CGRP-MCAO-NDDS group (Figure 1B, 1C). In the MCAO group, obvious edema was noticed in the cerebral tissues adjacent to the ischemic sites, combined with sharp decrease in the number of neurons and gliocytes, as well as widening of the intercellular space, irregular morphology of cytoplasm, and cell membrane of the neurons. CGRP treatment contributed to the improvements in cell number especially in CGRP-MCAO-NDDS group (Figure 1D).
Figure 1.
Neuroprotective effect of intranasal CGRP treatment. (A) ELISA detected the protein level of CGRP. (B) TTC staining showed the infarct volume of the indicated groups. (C) H & E staining showed the pathological injury of the indicated groups. * P<0.05, compared to the sham group; # P<0.05, ## P<0.01, compared to the MCAO group. (D) H & E staining showed the pathological injury of the indicated groups. * P<0.05, compared to the sham group; # P<0.05, ## P<0.01, compared to the MCAO group. CGRP – calcitonin gene-related peptide; ELISA – enzyme-linked immunosorbent assay; TTC – 2, 3, 5-triphenyltetrazolium chloride; H & E – hematoxylin and eosin; MCAO – middle cerebral artery occlusion.
The analysis of neurobehavioral score and Morris water maze findings
The neurobehavioral score in the sham group was 0, due to the absence of neurologic impairment. The score of the MCAO group was 2.6±0.35, indicating the presence of neurologic impairment in these animals. Compared with the MCAO group, the score was significantly attenuated in the CGRP-MCAO-IV group and the CGRP-MCAO-NDDS group, respectively (Table 2). In addition, compared with the sham group, the escape latency in the MCAO group showed significant increase (42.23±6.88 versus 25.70±5.04), together with that of the distance traveled (1576.27±270.14 versus 3688.91±526.70). Compared with the sham group, significant decrease was noticed at the time, percentage of platform quadrant journey and the speed in the MCAO group. Compared with the MCAO group, the escape latency after CGRP treatment showed significant decrease, especially in the CGRP-MCAO-NDDS group. Furthermore, significant increase was noticed at the time going through the platform after CGRP treatment compared with the MCAO group. The speed showed significant increase in the CGRP-MCAO-NDDS group and the CGRP-MCAO-IV group compared with that of the MCAO group. Significant decrease was noticed in the distance traveled in the CGRP-MCAO-NDDS group and the CGRP-MCAO-IV group compared with that of the MCAO group.
Table 2.
Effects of calcitonin gene-related peptide (CGRP) on neurological behavior.
| Group | Sham | MCAO | CGRP-MCAO | |
|---|---|---|---|---|
| IV | NDDS | |||
| Score | 0.00±0.00 | 2.60±0.35## | 2.20±0.37* | 1.40±0.41** |
| Time 1 | 25.70±5.04 | 42.34±6.88## | 37.86±4.64 | 31.97±5.13** |
| Time 2 | 1.97±0.51 | 0.22±0.11## | 1.29±0.77* | 1.67±0.82** |
| Percentage | 31.71±7.87 | 20.89±8.12# | 27.66±5.09* | 28.09±3.84* |
| Speed (cm/s) | 36.54±7.99 | 16.22±4.07## | 21.44±5.92 | 27.33±7.59* |
| Distance traveled | 1576.27±270.14 | 3688.91±526.7## | 2882.01±416.97* | 2371.01±406.66* |
P<0.05 compared to the sham group;
P<0.05 compared to the MCAO group;
P<0.01 compared to the MCAO group.
Three animals per group. Score, neurobehavioral score; Time 1 – time for average escape latency; Time 2 – the times go through the platform (n); percentage, The percentage of platform quadrant journey (%).
CGRP regulated the expression of neuron protective proteins
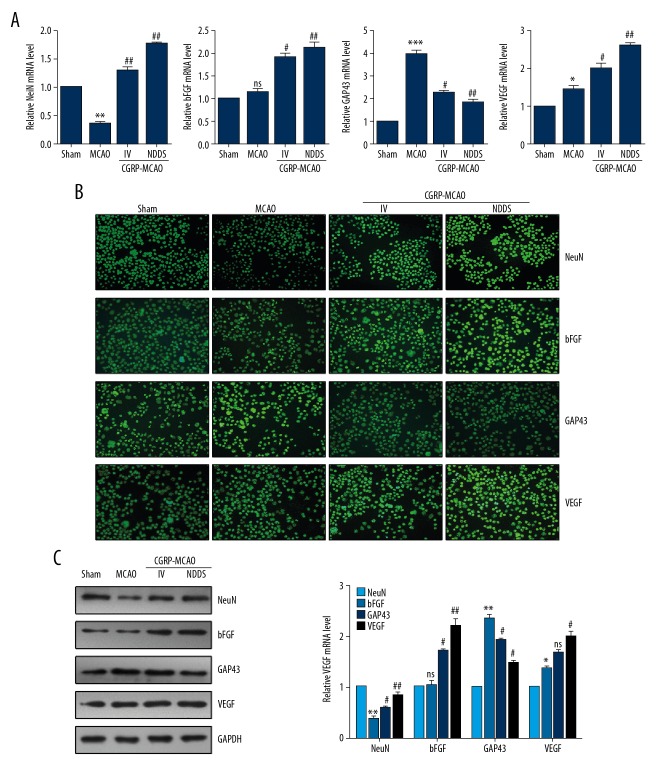
Compared with the sham group, the mRNA and protein levels of NeuN in the MCAO group showed significant decrease. In contrast, the expression of NeuN in the CGRP group showed significant increase compared with the MCAO group, especially the CGRP-MCAO-NDDS group. Although no statistical difference was observed in the expression of bFGF in the MCAO group compared with the sham group, the statistical elevation was noticed in the expression after CGRP treatment. Significant upregulation was identified in the expression of VEGF after MCAO induction compared with that of the sham group. After CGRP treatment, the expression of GAP43 showed significant downregulation compared with that of the MCAO group, however, significant upregulation was noticed in VEGF compared with that of the MCAO group (Figure 2A–2C).
Figure 2.
Intranasal CGRP treatment regulated expression of neuron protective proteins. (A) RT-qPCR assay showed the mRNA level of NeuN, bFGF, GAP43, and VEGF. (B) Immunostaining for the NeuN, bFGF, GAP43, and VEGF in MCAO rats. (C) Western blot assay showed the protein level of NeuN, bFGF, GAP43, and VEGF. * P<0.05, ** P<0.01, *** P<0.001 compared to the sham group; # P<0.05, ## P<0.01, compared to the MCAO group. CGRP – calcitonin gene-related peptide; VEGF – vascular endothelial growth factor; MCAO – middle cerebral artery occlusion; GAP43 – growth associated protein 43; bFGF – fibroblast growth factor 2.
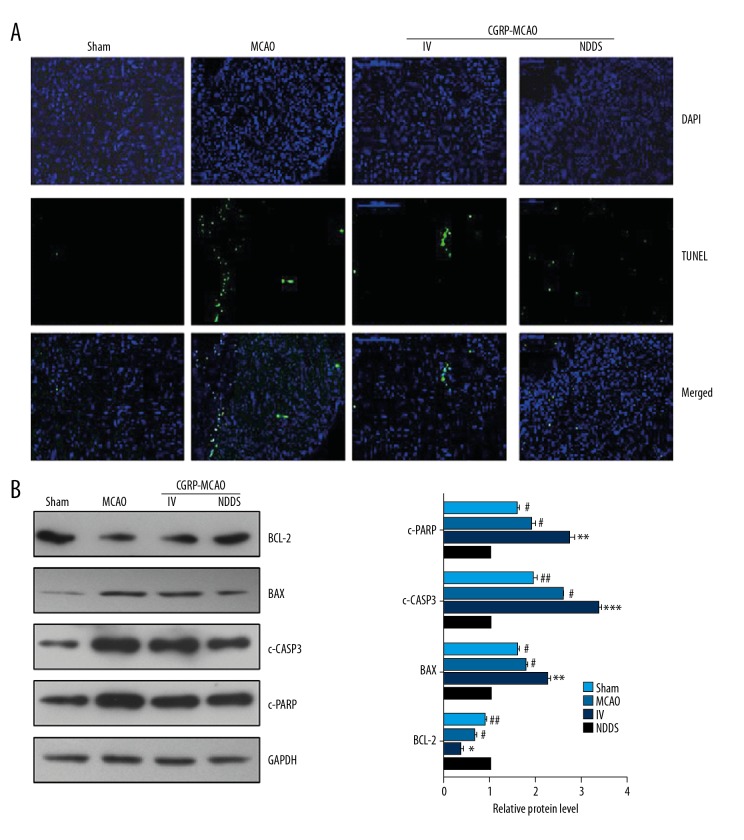
CGRP attenuated apoptosis in ischemic sites
TUNEL assay showed that the apoptotic cells were observed in the peripheral tissues in the MCAO group. CGRP treatment induced significant decrease in the apoptosis, especially the CGRP-MCAO-NDDS group (Figure 3A). The protein levels of BCL2 in the MCAO group showed significant decrease compared with that of the sham group. Significant elevation was noticed in the BCL2 expression after CGRP treatment, especially the CGRP-MCAO-NDDS group. The expression of BAX (BCL2 associated X), c-CASP3 (cleaved CASP3), and c-PARP [cleaved Poly-(ADP-ribose) polymerase] showed significant increase after MCAO induction compared with that of the sham group. Significant decrease was noticed in the BAX, c-CASP3, and c-PARP expression after CGRP treatment, especially the CGRP-MCAO-NDDS group (Figure 3B).
Figure 3.
Intranasal CGRP inhibited apoptosis in MCAO rats. (A) Representative pictures of tunnel staining in the indicated groups. (B) Western blot assay showed the protein level of BCL2, BAX, c-CASP3 and c-PARP. c – cleaved. * P<0.05, ** P<0.01, *** P<0.001 compared to the sham group; # P<0.05, ## P<0.01, compared to the MCAO group. CGRP – calcitonin gene-related peptide; MCAO – middle cerebral artery occlusion.
CGRP plays the neuroprotective role via WNT/β-catenin pathway
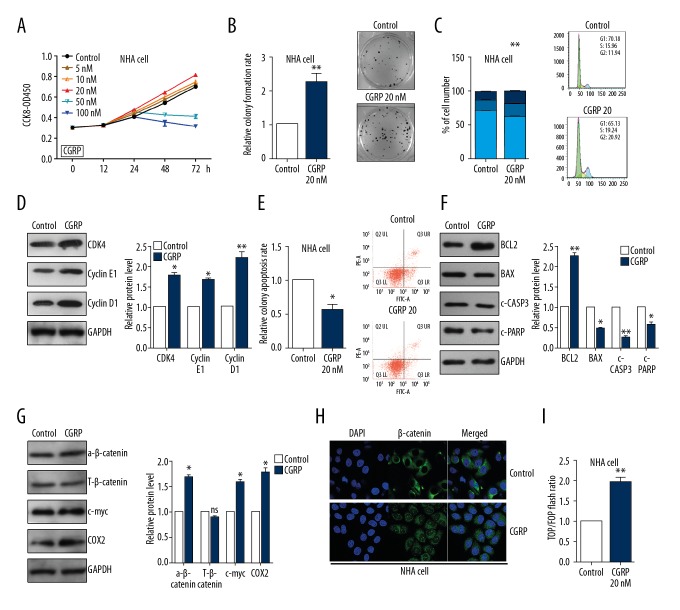
CCK8 assay showed that CGRP promoted cell viability in NHA cells in a dose- and time-dependent manner (Figure 4A). Colony formation assays demonstrated that CGRP (20 nM) increased cell proliferation in NHA cells (Figure 4B). Cell cycle assay showed that decreased the proportion of NHA cells in the G0/G1 phase and increased the proportion of G2/S phase cells and then increased the PI index (Figure 4C). In addition, CGRP markedly promoted cyclin D1, CDK4, and cyclin E1 expression level in NHA cells using western blot assay (Figure 4D). The apoptosis assay by flow cytometry showed that the percentage of apoptotic cells was significantly lower in cells treated with CGRP (Figure 4E). Western blot assay showed that CGRP treatment reduced the expression of cleaved CASP3, and PARP and BAX and increased BCL2 expression in NHA cells (Figure 4F). As shown in Figure 4G, CGRP treatment significantly enhance protein levels of the WNT/β-catenin target genes, such as activated β-catenin, c-myc, and COX2 in NHA cells (Figure 4G). The nuclear distribution of β-catenin protein was increased in the CGRP treatment group in NHA cells (Figure 4H). TOP/FOP luciferase reporter assays identified the activation of WNT/β-catenin by CGRP treatment in NHA cells (Figure 4I).
Figure 4.
CGRP plays the neuroprotective role via WNT/β-catenin pathway. (A) CCK8 assay showed the cell viability of NHA cells treated with CGRP at different concentrations. (B) Colony formation assay showed the proliferation ability of NHA cells treated with 20 nM CGRP. (C) Cell cycle assays showed that CGRP increased the proliferation index in NHA cells. (D) Western blot showed the protein level of cyclin D1, cyclin E1 and CDK4 in NHA cells. (E) Cell apoptosis assays showed that CGRP reduced the apoptosis of NHA cells. (F) Western blot showed the protein level of BCL2, BAX, c-CASP3 and c-PARP in NHA cells. (G) Western blot showed the protein level of β-catenin, c-myc and COX2 in NHA cells. (H) Immunofluorescence assay showed the distribution of β-catenin. (I) Top/Fop luciferase reporter assays were performed to detect the β-catenin activity treated with CGRP. * P<0.05, ** P<0.01 compared to the control group. CGRP – calcitonin gene-related peptide; CCK8 – cell counting kit-8.
Discussion
CGRP, a polypeptide composed of 37 amino acids, is extensively expressed in the central and peripheral nervous systems [10]. Previous studies have shown that during cerebral ischemia/reperfusion, the CGRP level in the neuronal cells in the brain tissue exhibits a relative increase [5]. However, when the cerebral ischemia is relatively severe, the CGRP produced by the brain tissues is not adequate to defend the injury. Thus, exogenous CGRP may relieve ischemia/reperfusion brain injury. This study demonstrated that intranasally administered CGRP could target the central nervous system and reduce the infarct volume after MCAO in rats. In addition, CGRP could remarkably attenuate the neuronal apoptosis and the NeuN decrease in brain.
Blood-brain barrier is a major concern for developing a treatment for stroke as it prevents a number of potential therapeutic agents from reaching the brain [11]. Delivery issues remain substantial obstacles to the practical use of potential therapeutics such as CGRP-I. CGRP-ICV administration or a higher dose of CGRP is not adequate for the large number of individuals requiring treatment for stroke or head injury. Our data suggested intranasally administered CGRP reduces the infarct volume and neurologic deficits following reversible MCAO in rats.
Recently, CGRP has been reported to show protective effects on the anoxic neuronal death as it contributes to the cerebral vessel dilatation and attenuation of ischemic lesions in brain [12,13]. Nevertheless, the potential mechanisms are still not well defined. An in vivo study revealed intravenous injection of CGRP could alleviate the cerebral infarction as it triggered the dilatation of cerebral vessels, especially the cerebral capillary [14]. To the best of our knowledge, few studies have focused on the potential efficiency of CGRP after NDDS delivery. In our study, a rat MCAO model was established, and then CGRP was administrated via NDDS in order to investigate the protective effects of CGRP on the cortex and peripheral neurons. Our data showed CGRP administration via NDDS induced significant decrease in the neurobehavioral scores, and cognitive function and memory showed significant improvements. In addition, the size of cerebral infarction volume was significant reduced, while H & E staining revealed CGRP contributed to the morphologic recovery of cortical nerve cells. These data demonstrated that CGRP contributed to the integrity of nerve cells, morphology of neurons, as well as the physiopathologic improvements of the neurons.
NeuN, a soluble nucleoprotein, is a marker for mature neurons. It is a specific protein that is expressed in the nucleus and cytoplasm of the majority of neurons. Therefore, it is considered a specific marker for neurons [15]. Recently, extensive studies have been carried out to investigate the role of NeuN in the cerebral ischemia. For example, Lu et al. reported that NeuN protein served as a specific target for the protective effects of cerebral ischemia [16]. Morancho et al. showed tissue MMP9 had a crucial role in EPC-induced vascular remodeling after stroke [17]. In our study, our results demonstrated NeuN protein was downregulated in MCAO rats, while significant upregulation was noticed after CGRP treatment. BCL2 is reported to be involved in the regulation of differentiation and development of nerve cells, which can obviously inhibit apoptosis and play a role in neuroprotective effects as well [18]. However, BAX and CASP3 were reported to induce apoptosis both in vitro and in vivo [19]. In this study, administration of CGRP via NDDS showed protective effects on neuron as it could obviously attenuate apoptosis in the cortex and peripheral nerve cells. All these data demonstrated that CGRP could inhibit apoptosis of nerve cells in the cerebral cortex, which then exerted neuroprotective effects.
VEGF plays important roles in the angiogenesis and neurotrophic/neuroprotective processes, and apoptosis could induce the secretion of VEGF in tissue as a feedback mechanism [20]. Furthermore, the expression of VEGF and receptors was upregulated in cerebral ischemia tissue, which then resulted in the proliferation of VECs (vascular epithelial cell) and the attenuation of infarction volume [21]. In our study, compared with VEGF protein expression in the cerebral cortex in MCAO rats, its expression was significantly elevated after CGRP administration via NDDS. As a specific neural protein localized in the axolemma, GAP43 participated in the growth of nerve cells, synapse development, and regeneration of nerve cells. A previous study found that GAP43 and CASP3 may act in a coordinated way in the regulation of early brain response and repair after stroke [22]. In our study, the expression of bFGF showed significant elevation in the MCAO group, which showed persistent elevation in the presence of CGRP. Compared with the MCAO group, the expression of GAP43 in the cerebral cortex showed significant decrease after CGRP treatment. On this basis, we speculated that GAP43 activity showed elevation in the neurons of the cortex under cerebral ischemia conditions, which then activated the expression of bFGF and the subsequent nervous repairment after obstructive brain injury. In the presence of CGRP, the activated bFGF showed persistent upregulation, while the GAP43 activity decreased. Meanwhile, such a process might synergize with the persistent elevation of VEGF, which then might inhibit the activity of signaling pathways of apoptosis and contributed to the recovery of nervous function.
Tapia-Rojas et al. highlighted the importance of Wnt/β-catenin signaling dysfunction in the onset of Alzheimer’s disease and proposed that the loss of canonical Wnt signaling was a triggering factor of Alzheimer’s disease [23]. Recently, Wang et al. reported that CGRP protected against hyperoxia-induced lung injury in premature rats through the Wnt7b/β-catenin signaling pathway [24]. We hypothesized if the neuroprotective role of CGRP might be correlated with the Wnt/β-catenin pathway. In our study, we demonstrated that CGRP could promote cell proliferation and cell cycle process and inhibited cell apoptosis in NHA cells through the Wnt/β-catenin pathway.
Conclusions
We first reported that intranasal CGRP was effective for treating focal cerebral ischemic injury. Intranasal CGRP can effectively reduce infarct size and neurologic deficits in experimental stroke studies. Furthermore, CGRP was found to markedly inhibited apoptosis and increased the expressions of VEGF and bFGF in the cortex of MCAO rats. CGRP could activated the Wnt/β-catenin pathway in NHA cells. As this noninvasive method of bypassing the blood-brain barrier has multiple advantages compared with existing conventional methods, such as preferentially delivered to the brain and reducing unwanted systemic effects, it may serve as a promising candidate for treating stroke and other central nervous system disorders.
Footnotes
Source of support: This project was supported by the Science and Technology Project of Higher Education of Shandong Province (No.J12LK07)
References
- 1.Vermeij JD, Westendorp WF, Dippel DW, et al. Antibiotic therapy for preventing infections in people with acute stroke. Cochrane Database Syst Rev. 2018;1:CD008530. doi: 10.1002/14651858.CD008530.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu SH, Yin MS, Liu B, et al. Tetramethylpyrazine-2′-O-sodium ferulate attenuates blood-brain barrier disruption and brain oedema after cerebral ischemia/reperfusion. Hum Exp Toxicol. 2017;36:670–80. doi: 10.1177/0960327116657401. [DOI] [PubMed] [Google Scholar]
- 3.Li F, Shi W, Zhao EY, et al. Enhanced apoptosis from early physical exercise rehabilitation following ischemic stroke. J Neurosci Res. 2017;95:1017–24. doi: 10.1002/jnr.23890. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki Y, Ogihara S, Harada S, Tokuyama S. Activation of cerebral sodium-glucose transporter type 1 function mediated by post-ischemic hyperglycemia exacerbates the development of cerebral ischemia. Neuroscience. 2015;310:674–85. doi: 10.1016/j.neuroscience.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Yang SI, Yuan Y, Jiao S, et al. Calcitonin gene-related peptide protects rats from cerebral ischemia/reperfusion injury via a mechanism of action in the MAPK pathway. Biomed Rep. 2016;4(6):699–703. doi: 10.3892/br.2016.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y, Duan M, Liang S, et al. Senkyunolide I protects rat brain against focal cerebral ischemia-reperfusion injury by up-regulating p-Erk1/2, Nrf2/HO-1 and inhibiting caspase 3. Brain Res. 2015;1605:39–48. doi: 10.1016/j.brainres.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema, I: A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 8.Bederson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–76. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 9.Gordan ML, Jungwirth B, Ohl F, et al. Evaluation of neurobehavioral deficits following different severities of cerebral ischemia in rats: A comparison between the modified hole board test and the Morris water maze test. Behav Brain Res. 2012;235:7–20. doi: 10.1016/j.bbr.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Evans RM, Amara S, Rosenfeld MG. Molecular events in developmental regulation of neuroendocrine genes: Characterization of the novel neuropeptide CGRP. Cold Spring Harb Symp Quant Biol. 1983;1:413–17. doi: 10.1101/sqb.1983.048.01.045. [DOI] [PubMed] [Google Scholar]
- 11.Sorby-Adams AJ, Marcoionni AM, Dempsey ER, et al. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral edema following acute central nervous system (CNS) injury. Int J Mol Sci. 2017;18(8) doi: 10.3390/ijms18081788. pii: E1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker CS, Hay DL. CGRP in the trigeminovascular system: A role for CGRP, adrenomedullin and amylin receptors. Br J Pharmacol. 2013;170:1293–307. doi: 10.1111/bph.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Close LN, Eftekhari S, Wang M, et al. Cortical spreading depression as a site of origin for migraine: Role of CGRP. Cephalalgia. :2018. doi: 10.1177/0333102418774299. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toshima M, Kassell NF, Tanaka Y, Dougherty DA. Effect of intracisternal and intravenous calcitonin gene-related peptide on experimental cerebral vasospasm in rabbits. Acta Neurochir (Wien) 1992;119:134–38. doi: 10.1007/BF01541797. [DOI] [PubMed] [Google Scholar]
- 15.Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, et al. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: A cautionary note. Brain Res. 2004;1015:169–74. doi: 10.1016/j.brainres.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Lu Q, Tucker D, Dong Y, et al. Neuroprotective and functional improvement effects of methylene blue in global cerebral ischemia. Mol Neurobiol. 2016;53:5344–55. doi: 10.1007/s12035-015-9455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morancho A, Ma F, Barcelo V, et al. Impaired vascular remodeling after endothelial progenitor cell transplantation in MMP9-deficient mice suffering cortical cerebral ischemia. J Cereb Blood Flow Metab. 2015;35:1547–51. doi: 10.1038/jcbfm.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H, Liu P, Tang H, et al. Oleuropein, a natural extract from plants, offers neuroprotection in focal cerebral ischemia/reperfusion injury in mice. Eur J Pharmacol. 2016;775:113–19. doi: 10.1016/j.ejphar.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Wang GH, Lan R, Zhen XD, et al. An-Gong-Niu-Huang Wan protects against cerebral ischemia induced apoptosis in rats: up-regulation of Bcl-2 and down-regulation of Bax and caspase-3. J Ethnopharmacol. 2014;154:156–62. doi: 10.1016/j.jep.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 20.Yousuf S, Atif F, Sayeed I, et al. Neuroprotection by progesterone after transient cerebral ischemia in stroke-prone spontaneously hypertensive rats. Horm Behav. 2016;84:29–40. doi: 10.1016/j.yhbeh.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhao XC, Zhang LM, Tong DY, et al. Propofol increases expression of basic fibroblast growth factor after transient cerebral ischemia in rats. Neurochem Res. 2013;38:530–37. doi: 10.1007/s11064-012-0945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorup D, Bohacek I, Milicevic T, et al. Increased expression and colocalization of GAP43 and CASP3 after brain ischemic lesion in mouse. Neurosci Lett. 2015;597:176–82. doi: 10.1016/j.neulet.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Tapia-Rojas C, Inestrosa NC. Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen Res. 2018;13(10):1705–10. doi: 10.4103/1673-5374.238606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Dang H, Xu F, et al. The Wnt7b/β-catenin signaling pathway is involved in the protective action of calcitonin gene-related peptide on hyperoxia-induced lung injury in premature rats. Cell Mol Biol Lett. 2018;23:4. doi: 10.1186/s11658-018-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]