Abstract
Background:
Hepatitis C virus (HCV) infection causes hepatocellular carcinoma (HCC) and subtypes of non-Hodgkin lymphoma (NHL). Associations with other cancers are not established. We systematically assessed associations between HCV infection and cancers in the US elderly population.
Methods:
We conducted a registry-based case-control study using the SEER-Medicare data in US adults aged ≥66 years. Cases (n=1,623,538) were people with first cancers identified in SEER registries (1993–2011). Controls (n=200,000) were randomly selected cancer-free individuals frequency-matched to cases on age, sex, race, and calendar year. We determined associations with HCV using logistic regression.
Results:
HCV prevalence was higher in cases than controls (0.7% vs. 0.5%). HCV was positively associated with cancers of the liver (adjusted odds ratio [aOR]=31.5; 95%CI=29.0–34.3), intrahepatic bile duct (aOR=3.40; 95%CI=2.52–4.58), extrahepatic bile duct (aOR=1.90; 95%CI=1.41–2.57), pancreas (aOR=1.23; 95%CI=1.09–1.40), anus (aOR=1.97; 95%CI=1.42–2.73), and non-melanoma non-epithelial skin cancer (aOR=1.53; 95%CI=1.15–2.04), myelodysplastic syndrome (aOR=1.56; 95%CI=1.33–1.83), and diffuse large B-cell lymphoma (DLBCL) (aOR=1.57; 95%CI=1.34–1.84). Specific skin cancers associated with HCV were Merkel cell carcinoma (aOR=1.92; 95%CI=1.30–2.85) and appendageal skin cancers (aOR, 2.02; 95%CI=1.29–3.16). Inverse associations were observed with uterine cancer (aOR=0.64; 95%CI=0.51–0.80) and prostate cancer (aOR=0.73; 95%CI=0.66–0.82). Associations were maintained in sensitivity analyses conducted among people without documented alcohol abuse, cirrhosis, or hepatitis B or human immunodeficiency virus infections, and after adjustment for socioeconomic status. Associations of HCV with other cancers were not observed.
Conclusion(s):
HCV is associated with increased risk of cancers other than HCC in the US elderly population, notably bile duct cancers and DLBCL. These results support a possible etiologic role for HCV in an expanded group of cancers.
Keywords: Hepatitis C virus, cancers, hepatocellular carcinoma, cholangiocarcinoma, lymphoma, elderly, SEER-Medicare
Condensed abstract:
Chronic hepatitis C virus (HCV) infection is a cause of hepatocellular carcinoma, but associations with other cancers are uncertain. Using a nationally representative data of U.S. elderly population from the SEER-Medicare linked database, we found that HCV is associated with increased risk of intrahepatic and extrahepatic cholangiocarcinoma, and diffuse large B-cell lymphoma.
INTRODUCTION
Hepatitis C virus (HCV) infection is the most common chronic blood-borne infection in the US and approximately 3 million individuals are infected with this virus.1 About 55–85% of individuals newly infected with HCV develop chronic hepatitis, 20–30% of chronically infected people progress to cirrhosis and liver failure, and 2–5% develop hepatocellular carcinoma (HCC).2
Although HCV mainly affects the liver, extrahepatic manifestations are well-documented.3, 4 Based on strong epidemiological and clinical evidence, the International Agency for Research on Cancer classified HCV as a proven cause of not only HCC, but also B-cell non-Hodgkin lymphomas (NHLs).5 Furthermore, some epidemiological evidence suggests that chronic HCV infection is associated with cancers other than HCC and NHL, such as those of the oral cavity,6 oropharynx,7 intrahepatic bile duct,8 pancreas,9 and kidney.10 Adding biological plausibility, HCV antigens and/or RNA have been detected in some of these cancers.11–13 Whether the virus exerts direct oncogenic effects through its various proteins and modulation of cell signaling pathways, or indirect effects by inducing chronic antigenic stimulation or inflammation, is mostly unknown. Few large studies have been conducted to systematically assess associations with these cancers in the US.14, 15
Approximately 70% of HCV-infected individuals in the US were born between 1945 and 1965 (“baby boomers”), prompting the Centers for Disease Control and Prevention to recommend a one-time screening for HCV in this birth cohort.16 As baby boomers age, HCV-associated cancers in the elderly population may become an important public health issue in the US in the near future. To identify the cancers for which older HCV-infected people may be at increased risk, we utilized a large, nationally representative database of elderly individuals in the US and systematically evaluated the associations between HCV infection and all major cancer types.
MATERIALS AND METHODS
Data source: SEER-Medicare linked database
Surveillance, Epidemiology, and End Results (SEER) is a cancer surveillance program that collects information from 18 US cancer registries covering approximately 28% of the US population.17 Medicare is a federally funded program that provides health insurance to approximately 97% of the US elderly (age ≥65 years). All Medicare-eligible individuals are entitled to Part A coverage (for hospital inpatient care) and approximately 96% additionally subscribe to Part B coverage (for physician and outpatient care). Beneficiaries can elect to enroll in a health maintenance organization (HMO); Medicare does not receive claims for individual medical conditions for people enrolled in HMOs.
The SEER-Medicare database is an electronic linkage of SEER and Medicare that successfully links more than 94% of SEER cancer cases over 65 years of age with their Medicare claims data (1991 onward).18 Claims data for an additional 5% random sample of Medicare beneficiaries residing in SEER geographic areas are provided.
Study design and study population
We conducted a case-control study using the SEER-Medicare database to determine if HCV was associated with cancer risk.19 Eligible cases were people with a first cancer diagnosis identified in SEER, excluding basal cell and squamous cell skin carcinomas which are not captured by cancer registries. Cases diagnosed only at autopsy or by death certificate were excluded. Medicare did not cover claims for HCV infection before 1992. The 2014 SEER-Medicare linkage includes cancer cases diagnosed through 2011. To ensure adequate information on HCV status, we required that cases have at least 13 months of Medicare Part A, Part B, non-HMO coverage before cancer diagnosis. Therefore, only cases diagnosed between 1993 and 2011 and aged 66 years or older were included. Cancer sites were defined using the SEER site recode variable, and morphology codes were used to define histologic subtypes for some cancers.
We randomly selected 200,000 controls from the 5% random sample of Medicare beneficiaries who were alive and cancer-free as of July 1 of the calendar year of their selection.19 Like cases, controls were required to have at least 13 months of prior Medicare part A, part B, non-HMO insurance coverage. Controls were frequency matched to cases on age (categories of 66–69, 70–74, 75–79, 80–84, 85–99 years), calendar year of selection, sex, and race (whites, blacks, others). Controls could be sampled repeatedly across multiple calendar years (47,407 controls were sampled more than once) and also later become cases.
Ascertainment of HCV, other medical conditions, and socioeconomic status
Medicare claims files were examined for International Classification of Diseases, version 9 (ICD-9) diagnosis codes for HCV infection (see Supplementary Table 1 for ICD-9 codes). A diagnosis of HCV infection required at least one inpatient, physician or outpatient claim before cancer diagnosis/control selection, excluding the 12-month period before cancer diagnosis/control selection. This exclusion period was used to minimize the possibility of differential assessment of HCV in cases as part of medical work-up near the time of their cancer diagnosis. In a sensitivity analysis, we used a more stringent definition for HCV infection that required at least one inpatient claim or two physician or outpatient claims at least 30 days apart.
Since human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infections are associated with HCV and also increase risk of certain cancers, we identified cases and controls with at least one Medicare claim for HIV or HBV infection any time before death or last follow-up (Supplementary Table 1). Cirrhosis and diabetes mellitus were identified using ICD-9 codes (Supplementary Table 1) that required at least one inpatient claim or two physician/outpatient claims at least 30 days apart, more than 12 months before cancer diagnosis/control selection.
We used ICD-9 codes for diagnoses related to smoking and alcohol abuse to capture these behaviors (Supplementary Table 1), since direct information is not available in the SEER-Medicare database. Subjects were classified as smokers or alcohol abusers if at least one specified ICD-9 code was present, more than 12 months before cancer diagnosis/control selection.
Socioeconomic status (SES) is an important predictor of cancer incidence,20 and HCV infection is more common in low-income demographic groups.21 We used three variables available in SEER-Medicare that capture SES based on a person’s residential zip code: median household income, percentage of individuals 25+ years of age with less than12 years of education, and percentage of residents living below the poverty line.
Statistical analyses
Characteristics of cases and controls were compared using chi-squared tests. To select cancer sites for evaluation, we computed the expected number of individuals with HCV infection by multiplying the number of cases for each cancer site by the prevalence of HCV infection in controls in our study (0.5%). We included only those cancer sites where the expected count of individuals with HCV infection was more than 11 to be in compliance with the SEER-Medicare data use agreement which requires suppression of cell sizes less than 11. Major subtypes of NHL were selected for evaluation based on previous reports of their association with HCV infection. In all, forty-three cancer sites were evaluated.
We compared HCV prevalence in cases and controls by fitting separate unconditional logistic regression models for each cancer type. Odds ratios were adjusted (aOR) for age, sex, race, year of cancer diagnosis/control selection, average annual number of physician claims more than 12 months before cancer diagnosis/control selection (a measure of healthcare utilization), and smoking status. The variance of aORs obtained from these models was adjusted for repeated selection of some controls across calendar years and inclusion of some controls who later became cases.19 We utilized a two-sided alpha of 0.05 to describe confidence intervals, but to account for multiple testing, we selected cancers for further evaluation by using a false discovery rate of 10% according to the Benjamini and Hochberg method.22 We also used a more stringent Bonferroni criterion, which, based on assessment of 43 cancer outcomes, utilized a p-value threshold for statistical significance of 0.05/43 = 0.0012.
Cancers identified to be significantly associated with HCV by the false discovery rate method were analyzed further. Specifically, we assessed associations of HCV with histologic subtypes of non-melanoma non-epithelial skin cancers, and nodal vs. extranodal NHL. We conducted sensitivity analyses in which we assessed the associations of cancers in individuals without cirrhosis, in non-alcohol abusers, and in individuals without HBV or HIV infections. We conducted additional analyses to adjust for potential confounding by SES by introducing each SES variable individually in the models. We explored whether diabetes modified the association between HCV and selected cancers by calculating stratum-specific ORs and testing for heterogeneity. We also analyzed the data by using the more stringent definition of HCV infection. Finally, we calculated the population attributable fractions for certain cancers found to be associated with HCV infection for which there is a biologically plausible explanation for a causal association.23
RESULTS
We studied 1,623,538 cancer cases and 200,000 cancer-free controls (Table 1). Cases and controls were perfectly matched on age categories, sex, race, and calendar year of cancer diagnosis/control selection. Cases had slightly shorter duration of Medicare coverage and slightly fewer annual physician claims than controls. Although differences compared with controls were small, cases were also more likely to have HBV infection, cirrhosis, or diabetes mellitus; to be smokers or alcohol abusers; and to reside in zip codes with characteristics of high SES (Table 1). Cases and controls did not differ with respect to the proportion with HIV infection.
Table 1:
Characteristics of cancer cases and controls (1993-2011)
| Characteristics | Cases (N = 1,623,538) | Controls (N = 200,000) | p value | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Age, years* | - | ||||
| 66-69 | 265,364 | 16.3 | 32,690 | 16.4 | |
| 70-74 | 418,536 | 25.8 | 51,553 | 25.8 | |
| 75-79 | 393,987 | 24.3 | 48,536 | 24.3 | |
| 80-84 | 299,444 | 18.4 | 36,888 | 18.4 | |
| 85+ | 246,207 | 15.2 | 30,333 | 15.2 | |
| Sex* | - | ||||
| Female | 777,936 | 47.9 | 95,827 | 47.9 | |
| Male | 845,602 | 52.1 | 104,173 | 52.1 | |
| Race/ethnicity* | - | ||||
| White | 1,383,341 | 85.4 | 170,414 | 85.3 | |
| Black | 131,955 | 8.1 | 16,251 | 8.1 | |
| Other | 105,312 | 6.5 | 13,030 | 6.5 | |
| Year of cancer diagnosis/control selection* | - | ||||
| 1993 - 1999 | 367,339 | 22.6 | 45,242 | 22.6 | |
| 2000 - 2003 | 357,559 | 22.0 | 44,048 | 22.0 | |
| 2004 - 2007 | 454,391 | 28.0 | 55,978 | 28.0 | |
| 2008 - 2011 | 444,249 | 27.4 | 54,732 | 27.4 | |
| Total part A, part B, non-HMO Medicare coverage (months)† | |||||
| Median (IQR) | 53 (28-70) | 54 (30-66) | |||
| < 28 | 397,457 | 24.5 | 46,320 | 23.2 | < 0.0001 |
| 28 to 52 | 406,400 | 25.0 | 48,218 | 24.1 | |
| 53 to 69 | 392,033 | 24.2 | 58,341 | 29.2 | |
| 70+ | 427,648 | 26.3 | 47,121 | 23.6 | |
| Total number of physician claims/year† | |||||
| Median (IQR) | 22 (8-47) | 23 (8-48) | |||
| < 2.56 | 392,132 | 24.2 | 47,213 | 23.6 | < 0.0001 |
| 2.56 to < 5.57 | 399,687 | 24.6 | 49,077 | 24.5 | |
| 5.57 to < 10.07 | 418,491 | 25.8 | 51,382 | 25.7 | |
| 10.07 + | 413,228 | 25.5 | 52,328 | 26.2 | |
| Hepatitis C | 11,067 | 0.7 | 1,040 | 0.5 | < 0.0001 |
| Hepatitis B | 19,254 | 1.2 | 2,217 | 1.1 | 0.0025 |
| HIV | 6,976 | 0.4 | 880 | 0.4 | 0.5061 |
| Cirrhosis | 11,261 | 0.7 | 884 | 0.4 | < 0.0001 |
| Diabetes mellitus | 403,429 | 24.9 | 48,926 | 24.5 | 0.0002 |
| Smoking | 632,428 | 38.9 | 69,694 | 34.8 | < 0.0001 |
| Alcohol abuse | 159,001 | 9.8 | 14,253 | 7.1 | < 0.0001 |
| Median household income for the zip code (US $) | |||||
| Median (IQR) | 45,561 (35,114-59,202) | 45,013 (34,764-58,437) | |||
| < 35,097 | 396,608 | 24.8 | 50,371 | 25.6 | < 0.0001 |
| 35,097 - 45,537 | 398,753 | 25.0 | 49,811 | 25.4 | |
| 45,538 - 59,164 | 400,258 | 25.1 | 48,283 | 24.6 | |
| 59,164 + | 400,577 | 25.1 | 47,829 | 24.4 | |
| Percentage of non-high school graduates (age ≥ 25 years) in the zip code | |||||
| < 9.96 | 396,438 | 24.8 | 49,565 | 25.2 | 0.0010 |
| 9.96 - 15.78 | 399,793 | 25.1 | 48,676 | 24.8 | |
| 15.79 - 25.17 | 399,241 | 25.0 | 49,076 | 25.0 | |
| 25.18 + | 399,750 | 25.1 | 48,867 | 24.9 | |
| Percentage of residents living below the poverty line in the zip code | |||||
| < 5.23 | 392,548 | 24.6 | 47,861 | 24.4 | < 0.0001 |
| 5.23 - 8.91 | 402,349 | 25.2 | 48,786 | 24.8 | |
| 8.92 - 15.40 | 399,721 | 25.0 | 50,097 | 25.5 | |
| 15.41 + | 399,452 | 25.0 | 49,297 | 25.1 | |
Abbreviations: HMO, healthcare maintenance organization; HIV, human immunodeficiency virus; IQR, interquartile range
Note: Table entries are number of subjects (percentage) unless otherwise indicated.
Cases and controls were frequency-matched on age categories, sex, race/ethnicity, and year of cancer diagnosis/control selection.
Medicare coverage and physician claims were calculated excluding the 12-month period immediately before cancer diagnosis/control selection.
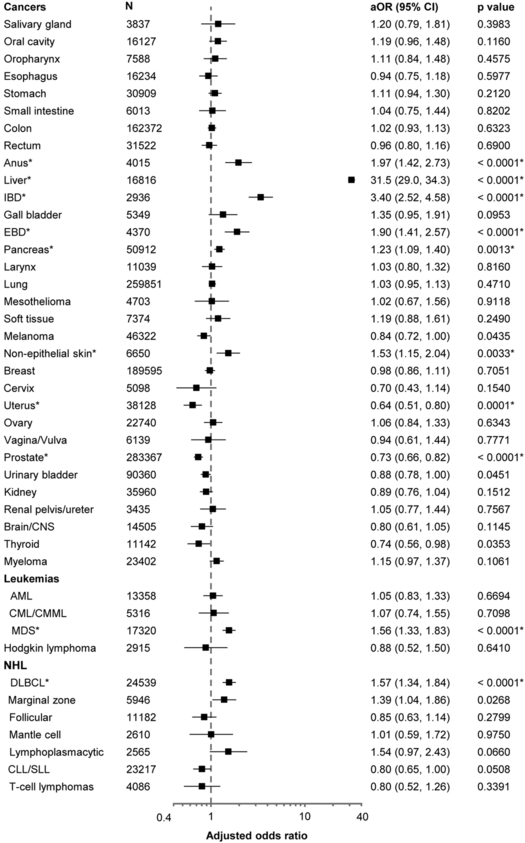
Overall, HCV prevalence was higher in cases than controls (0.7% vs. 0.5%; aOR=1.32; 95%CI=1.22–1.42; p<0.0001). We present results for 43 cancers separately in Figure 1. After correction for multiple comparisons by the Benjamini and Hochberg method, we observed significant positive associations between HCV infection and cancers of the liver (aOR=31.5; 95%CI=29.0–34.3), intrahepatic bile duct (aOR=3.40; 95%CI=2.52–4.58), extrahepatic bile duct (aOR=1.90; 95%CI=1.41–2.57), pancreas (aOR=1.23; 95%CI=1.09–1.40), anus (aOR=1.97; 95%CI=1.42–2.73), and non-melanoma non-epithelial skin cancer (aOR=1.53; 95%CI=1.15–2.04), myelodysplastic syndrome (MDS) (aOR=1.56; 95%CI=1.33–1.83), and diffuse large B-cell lymphoma (DLBCL) (aOR=1.57; 95%CI=1.34–1.84). We also observed inverse associations between HCV and cancers of the uterus (aOR=0.64; 95%CI=0.51–0.80) and prostate (aOR=0.73; 95%CI=0.66–0.82). Associations between HCV and marginal zone lymphoma (MZL) (aOR=1.39; 95%CI=1.04–1.86), and lymphoplasmacytic lymphoma (LPL) (aOR=1.54; 95%CI=0.97–2.43) were borderline significant. With the Bonferroni method, associations remained significant for most cancers, except for pancreatic and non-epithelial skin cancers.
Figure 1: Associations between HCV infection and risk of cancer.
The associations with HCV infection are presented for each cancer as an odds ratio and corresponding 95% confidence interval (horizontal axis, logarithmic scale). Odds ratios are adjusted for age categories (65–69, 70–74, 75–79, 80–84, 85+ years), sex, race/ethnicity, calendar year of cancer diagnosis/control selection (1993–1999, 2000–2003, 2004–2007, 2008–2011), number of physician claims per year (< 2.56, 2.56 to < 5.57, 5.57 to < 10.07, 10.07+), and smoking status (never/ever)
Abbreviations: AML, acute myeloid leukemia; aOR, adjusted odds ratio; CI, confidence intervals; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; EBD, extrahepatic bile ducts; IBD, intrahepatic bile ducts; MDS, myelodysplastic syndrome
* Asterisks indicate cancers that were identified to be significantly associated with hepatitis C virus infection after correction for multiple comparisons by Benjamini-Hochberg method.
Additional analyses were directed to assess specific subtypes of non-melanoma non-epithelial skin cancer. We observed associations between HCV and Merkel cell carcinoma (aOR=1.92; 95%CI=1.30–2.85; N=2,669 cases) and appendageal skin cancers (aOR, 2.02; 95%CI=1.29–3.16; N=1,969 cases); we did not detect an association with skin sarcomas, although the number of cases that could be assessed was small (data not shown). The associations between HCV and DLBCL were similar in magnitude for nodal cases (aOR=1.54; 95%CI=1.26–1.88; N=9,544 cases) and extranodal cases (aOR=1.62; 95%CI=1.28–2.06; N=14,995 cases).
In a sensitivity analyses, we excluded HBV or HIV-infected individuals. Since the prevalence of these two infections was low, there were no discernible differences in the aORs (Table 2). The associations were maintained when we conducted analyses among people without documented alcohol abuse or cirrhosis (Table 2). Additional adjustment for SES variables such as median household income did not affect the associations (Table 2). The association between HCV infection and liver cancer was attenuated among people with diabetes (aOR=18.9; 95%CI=16.7–21.4) as compared to those without diabetes (aOR=48.2; 95%CI=43.0–53.9). The associations did not differ according to diabetes status for bile duct or pancreatic cancers (Supplementary Table 3). On using the more stringent criterion for HCV diagnosis, the associations for most cancers were maintained; however, associations for pancreatic cancer and non-melanoma non-epithelial skin cancer were attenuated and no longer statistically significant (Supplementary Table 2).
Table 2:
Sensitivity analyses examining associations of HCV with select cancers
| Cancer | Excluding HBV and HIV infected individuals |
Never alcohol abusers | Non-cirrhotics | Additional adjustment for median household income |
||||
|---|---|---|---|---|---|---|---|---|
| aOR* (95% CI) | p value | aOR* (95% CI) | p value | aOR* (95% CI) | p value | aOR*† (95% CI) | p value | |
| Anus | 1.65 (1.09, 2.50) | 0.0173 | 1.59 (1.05, 2.40) | 0.0277 | 1.77 (1.22, 2.56) | 0.0027 | 1.97 (1.42, 2.73) | < 0.0001 |
| Liver | 32.2 (29.1, 35.5) | < 0.0001 | 32.5 (29.5, 35.7) | < 0.0001 | 23.8 (21.6, 26.2) | < 0.0001 | 31.6 (29.0, 34.3) | < 0.0001 |
| Intrahepatic bile duct | 2.99 (2.05, 4.34) | < 0.0001 | 3.11 (2.20, 4.40) | < 0.0001 | 2.99 (2.12, 4.20) | < 0.0001 | 3.38 (2.51, 4.56) | < 0.0001 |
| Extrahepatic bile duct | 2.06 (1.46, 2.90) | < 0.0001 | 1.99 (1.43, 2.76) | < 0.0001 | 1.92 (1.39, 2.64) | 0.0001 | 1.91 (1.41, 2.58) | < 0.0001 |
| Pancreas | 1.28 (1.11, 1.49) | 0.0009 | 1.24 (1.08, 1.44) | 0.0030 | 1.27 (1.11, 1.46) | 0.0005 | 1.23 (1.09, 1.40) | 0.0012 |
| Non-epithelial skin | 1.78 (1.31, 2.43) | 0.0002 | 1.67 (1.23, 2.26) | 0.0010 | 1.59 (1.18, 2.16) | 0.0027 | 1.54 (1.15, 2.04) | 0.0033 |
| Uterus | 0.66 (0.51, 0.86) | 0.0016 | 0.61 (0.48, 0.77) | 0.0001 | 0.62 (0.48, 0.79) | 0.0001 | 0.64 (0.51, 0.80) | 0.0001 |
| Prostate† | 0.73 (0.66, 0.82) | < 0.0001 | 0.76 (0.67, 0.87) | < 0.0001 | 0.77 (0.68, 0.86) | < 0.0001 | 0.73 (0.66, 0.82) | < 0.0001 |
| MDS | 1.56 (1.30, 1.88) | < 0.0001 | 1.64 (1.37, 1.96) | < 0.0001 | 1.43 (1.19, 1.70) | 0.0001 | 1.56 (1.33, 1.83) | < 0.0001 |
| DLBCL | 1.55 (1.29, 1.87) | < 0.0001 | 1.74 (1.46, 2.06) | < 0.0001 | 1.55 (1.30, 1.84) | < 0.0001 | 1.57 (1.33, 1.84) | < 0.0001 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence intervals; DLBCL, diffuse large B-cell lymphoma; HBV, hepatitis B virus; HIV, human immunodeficiency virus; MDS, myelodysplastic syndrome
Odds ratios assess the association of HCV with the specified cancer and were adjusted for age categories (65-69, 70-74, 75-79, 80-84, 85+ years), sex, race/ethnicity, calendar year of cancer diagnosis/control selection (1993-1999, 2000-2003, 2004-2007, 2008-2011), number of physician claims per year (< 2.56, 2.56 to < 5.57, 5.57 to < 10.07, 10.07+), and smoking status (never/ever)
Odds ratios assess the association of HCV with the specified cancer and were adjusted additionally for the zip code median household income categories (< 35,097, 35,097-45,537, 45,538-59,164, and 59,165+ US dollars). Models were separately adjusted for the education and poverty variables shown in Table 1, instead of median household income, with a resulting change in point estimates by <10% for all cancers (not shown).
The population attributable fractions for most cancers were very low, except for liver cancer (Table 3). Assuming that HCV infection is causally associated with the cancers, elimination of HCV infection would reduce the risk of the cancers of liver, intrahepatic bile ducts, extrahepatic bile ducts, pancreas, and MDS and DLBCL by 16.14%, 1.15%, 0.50%, 0.13%, 0.41%, and 0.28% respectively.
Table 3:
Population attributable fractions for HCV and selected cancers
| Cancer | aORs | Proportion of cases exposed to HCV infection |
Population attributable fraction, %* |
|---|---|---|---|
| Liver | 31.5 | 0.1667 | 16.1 |
| Intrahepatic bile duct | 3.40 | 0.0163 | 1.15 |
| Extrahepatic bile duct | 1.90 | 0.0105 | 0.50 |
| Pancreas | 1.23 | 0.0067 | 0.13 |
| Myelodysplastic syndrome | 1.56 | 0.0114 | 0.41 |
| Diffuse large B cell lymphoma | 1.57 | 0.0076 | 0.28 |
Abbreviations: aOR, adjusted odds ratio; HCV, hepatitis C virus
Population attributable fractions were calculated using the following formula, where Pd = proportion of cases exposed to HCV infection, and RR=adjusted relative risk (odds ratio) from the logistic regression model.24
DISCUSSION
Persistent HCV infection leads to liver fibrosis and eventually cirrhosis, which increases the risk for HCC.2 Chronic HCV infection also has important biological effects beyond the liver. In accord with two previous studies,14, 15 our analyses of a large population-based dataset of elderly individuals demonstrate that besides HCC, several additional cancers are associated with HCV infection.
HCV infection has previously been linked to hematological malignancies, including some subtypes of B-cell NHLs (such as DLBCL, MZL, and LPL) and MDS.24, 25 HCV is believed to cause NHL through chronic antigenic stimulation. We observed a significant association of HCV with DLBCL. The associations with MZL and LPL were borderline significant, perhaps due to lack of statistical power related to a low HCV prevalence in our study. Notably, HIV infection also causes NHLs, especially DLBCL.26 The association with DLBCL persisted in our study in individuals who lacked claims for HIV infection, although it is possible that some HIV-infected people were missed using this approach. A previous study conducted using SEER-Medicare database also found elevated risk of MDS in HCV-infected individuals.24 MDS is a heterogeneous group of malignant disorders characterized by ineffective blood cell production, and there is an increased risk of progression to acute myeloid leukemia.27 HCV can infect and replicate inside pluripotent hematopoietic stem cells, and HCV proteins and RNA have been isolated from these cells.12 Furthermore, a recent case report described the resolution of MDS in an HCV-infected individual after viral clearance with antiviral therapy.28
Prior epidemiological studies have found HCV to be associated with intrahepatic cholangiocarcinoma, with odds ratios in the range of 3.4–4.8.29–31 Detection of HCV RNA in bile duct epithelial cells,32 HCV core proteins and RNA in cholangiocarcinoma specimens,33 and the demonstration of increased cellular proliferation and decreased apoptosis in HCV-positive cholangiocarcinoma specimens,34 suggest that HCV may play a direct role in the development of cholangiocarcinoma. We also found an association between HCV and extrahepatic cholangiocarcinoma. Although that has not been observed in some prior studies, a recent meta-analysis found the pooled estimate to be borderline significant (OR=1.75, 95%CI=1.00–3.05).29 We found a moderate association between HCV infection and pancreatic cancer, which was not affected by adjustment for diabetes mellitus and was similar in strength to the result of a meta-analysis of 8 observational studies (OR=1.26, 95%CI=1.03–1.50).35 However, the association with pancreatic cancer became attenuated when we used a more stringent definition of HCV infection. The results of the sensitivity analysis suggest that the association with pancreatic cancer may reflect non-specific coding for HCV infection
Associations that we observed for HCV with anal cancer and non-epithelial skin cancers may be explained by confounding by shared risk factors. A high prevalence of HCV infection is seen in men who have sex with men (MSM) and injection drug users,1, 36 and MSM also have a high prevalence of anal human papillomavirus infection,37 the cause of anal cancer. MSM and injection drug users have an elevated prevalence of HIV infection which increases the risk of anal cancer.38 Similarly, the risk of non-epithelial skin cancers, including Merkel cell carcinoma and appendageal carcinomas, is increased in people with HIV infection.39 The associations with HCV in our study persisted in a sensitivity analysis in which we excluded people with documented HIV infection, but the Medicare claims may have missed some HIV infections.
The negative associations of HCV infection with uterine and prostate cancers are intriguing. A potential explanation for the negative association with uterine cancer is that some women included in our study could have undergone a hysterectomy, which is the most common non-obstetrical abdominal surgery in women. An estimated 33% women eligible for our study would have had a hysterectomy before the age of 60,40 which would not have been captured in Medicare claims. According to the age structure of our study population, most of these women would have undergone open abdominal hysterectomies in the 1970s and 80s, since laparoscopic techniques were developed only in the 1990s.41 Total abdominal hysterectomy is associated with a risk of hemorrhage requiring blood transfusion, and blood transfusions before 1992 conveyed a risk of HCV infection.1 Obviously, none of the cases with uterine cancer had previously had a hysterectomy, but prior hysterectomies among control women would have contributed to a relatively high HCV prevalence. In the US, a large fraction of prostate cancer cases are detected through screening.42 We believe that the deficit in prostate cancer cases may be due to lower rates of prostate cancer screening in HCV-infected people, since they often come from lower SES groups.43
We did not observe associations between HCV infection and cancers of the oral cavity, oropharynx, kidney, and thyroid, which were detected in other studies.6, 7, 10, 14, 44 Variation across studies may be due to differences in the populations, or differences in methods of exposure and outcome ascertainment. Alternatively, some prior studies were retrospective or used hospital-based controls, which may have biased their results. We found that the presence of diabetes attenuated the association between HCV infection and liver cancer. A similar negative interaction between HCV and diabetes has been previously reported.45 Although HCV infection and diabetes both contribute to the development of HCC, the biological mechanisms responsible for the negative interaction between the two conditions are unclear.
Despite a high prevalence of HCV infection in baby boomers, less than 50% of infected individuals are aware about their infection, and even fewer get treated for HCV.46 The first members of this birth cohort became eligible for Medicare (by virtue of being 65 years of age) in 2010, and in 15 years, approximately 90% of Medicare beneficiaries will belong to the baby boomer generation.47 This ageing population is already contributing to high resource utilization and healthcare costs in the US.48 Based on our calculations, HCV infection is responsible for approximately 16% of liver cancer cases in elderly adults, but it is likely that this burden will continue to rise. Although introduction of direct-acting antivirals has dramatically improved cure rates, HCC risk still remains relatively high in infected people who have cleared the virus, particularly elderly individuals.49 Whether effective antiviral therapy reduces the risk of cancers other than HCC is not known. Hence, physicians managing HCV-infected individuals need to be aware of a potential risk of non-HCC cancers.
A strength of our study is the systematic assessment of a large nationally representative population of elderly individuals. Previous large US studies have utilized data from either four urban health centers,14 or a large HMO organization,15 and compared cancer incidence in HCV-infected patients with cancer incidence in SEER registries. Since a large number of HCV-infected people are projected to be eligible for Medicare in the near future, assessment of cancer risk in the elderly US population is important. SEER registries have strict quality control measures for cancer ascertainment, which improved the reliability of our outcomes as compared to other large studies that relied on administrative claims data for identification of cancer outcomes. Given the size of our study, we were also able to assess the associations of HCV with a large number of cancer types and subtypes, many of which are uncommon.
We also acknowledge some limitations. First, since our study focused on elderly people, the results are not directly generalizable to a younger population. Second, the study is limited by the inability to accurately assess smoking, alcohol use, co-infections, obesity, and other cancer risk factors, which may have caused confounding. Third, since HCV infection was identified using administrative claims, under-ascertainment of HCV diagnosis is likely, e.g., if physicians did not test patients for infection. On the other hand, some misreporting ICD-9 codes for HCV infection by physicians also likely occurred. Additionally, some patients may have had HCV antibodies but no detectable HCV RNA in serum, indicating resolved infection. To improve the specificity of HCV diagnosis, we conducted an analysis using a stricter claims definition of HCV infection, which showed mostly similar results. We expect that both biases (i.e., under-ascertainment and false positive diagnoses) were non-differential between cases and controls, and would have driven associations that we measured towards the null. Fourth, we were unable to account for the effect of antiviral therapy. New interferon-free antiviral regimens have improved viral clearance rates, but the calendar period covered by our study implies that subjects were unlikely to have received these therapies. Finally, because we made multiple comparisons, some associations could be due to chance. We utilized a statistical procedure to minimize this possibility and emphasized the most significant associations.
To sum up, we observed significant associations between HCV infection and a number of cancers besides liver cancer, notably intrahepatic and extrahepatic cholangiocarcinomas, and DLBCL. Studies are needed to strengthen the evidence linking HCV infection to these cancers and further elucidate biological mechanisms.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Cancer Institute. We thank Ms. Winnie Ricker, Information Management Services Inc. (IMS) for assistance with management of the SEER-Medicare database. We also acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results (SEER) program tumor registries in the creation of the SEER-Medicare database.
Funding support: P. Mahale and E.A. Engels were supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
Footnotes
Conflict of interest disclosures: Dr. Torres reports grants from Gilead Sciences, grants from Merck & Co., Inc, other from Gilead Sciences, other from Janssen Pharmaceuticals, Inc., other from Merck & Co., Inc., outside the submitted work. All other authors have nothing to disclose.
REFERENCES
- 1.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20: 17–35. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36: 1439–1445. [DOI] [PubMed] [Google Scholar]
- 4.Negro F, Forton D, Craxi A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149: 1345–1360. [DOI] [PubMed] [Google Scholar]
- 5.Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100: 1–441. [PMC free article] [PubMed] [Google Scholar]
- 6.Su FH, Chang SN, Chen PC, et al. Positive association between hepatitis C infection and oral cavity cancer: a nationwide population-based cohort study in Taiwan. PLoS One. 2012;7: e48109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahale P, Sturgis EM, Tweardy DJ, Ariza-Heredia EJ, Torres HA. Association Between Hepatitis C Virus and Head and Neck Cancers. J Natl Cancer Inst 2016;108: djw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Magnusson M, Torner A, Ye W, Duberg AS. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: a nationwide study in Sweden. Br J Cancer. 2013;109: 2917–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon SC, Moonka D, Brown KA, et al. Risk for renal cell carcinoma in chronic hepatitis C infection. Cancer Epidemiol Biomarkers Prev. 2010;19: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 11.Carrozzo M, Quadri R, Latorre P, et al. Molecular evidence that the hepatitis C virus replicates in the oral mucosa. J Hepatol 2002;37: 364–369. [DOI] [PubMed] [Google Scholar]
- 12.Sansonno D, Lotesoriere C, Cornacchiulo V, et al. Hepatitis C virus infection involves CD34(+) hematopoietic progenitor cells in hepatitis C virus chronic carriers. Blood. 1998;92: 3328–3337. [PubMed] [Google Scholar]
- 13.Yan FM, Chen AS, Hao F, et al. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6: 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison RD, Tong X, Moorman AC, et al. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006–2010. J Hepatol 2015;63: 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyberg AH, Chung JW, Shi JM, et al. Increased cancer rates in patients with chronic hepatitis C: An analysis of the cancer registry in a large U.S. health maintenance organization. J Hepatol 2015;62: S220. [Google Scholar]
- 16.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61: 1–32. [PubMed] [Google Scholar]
- 17.Surveillance, Epidemiology, and End Results Program. Available from URL: http://seer.cancer.gov [accessed August 19, 2016]. [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40: Iv-3–18. [DOI] [PubMed] [Google Scholar]
- 19.Engels EA, Pfeiffer RM, Ricker W, Wheeler W, Parsons R, Warren JL. Use of surveillance, epidemiology, and end results-medicare data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol 2011;174: 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20: 417–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omland LH, Osler M, Jepsen P, et al. Socioeconomic status in HCV infected patients - risk and prognosis. Clin Epidemiol. 2013;5: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57: 289–300. [Google Scholar]
- 23.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson LA, Pfeiffer R, Warren JL, et al. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev. 2008;17: 3069–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Sanjose S, Benavente Y, Vajdic CM, et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008;6: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels EA. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. Aids. 2014;28: 2313–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109: 1536–1542. [DOI] [PubMed] [Google Scholar]
- 28.Maruyama S, Koda M, Oi S, Murawaki Y. Successful treatment of myelodysplastic syndrome and chronic hepatitis C using combined peginterferon-alpha-2b and ribavirin therapy. Hepatol Res. 2014;44: 1159–1164. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Hu B, Zhou ZQ, Guan J, Zhang ZY, Zhou GW. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: evidence from a systematic review and meta-analysis of 16 case-control studies. World J Surg Oncol. 2015;13: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Zhao Y, Li B, et al. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta-analysis. BMC Cancer. 2012;12: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fillipowicz EA, Xiao S, Sower LE, Weems J, Payne DA. Detection of HCV in bile duct epithelium by laser capture microdissection (LCM). In Vivo. 2005;19: 737–739. [PubMed] [Google Scholar]
- 33.Perumal V, Wang J, Thuluvath P, Choti M, Torbenson M. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006;37: 1211–1216. [DOI] [PubMed] [Google Scholar]
- 34.Chen RF, Li ZH, Zou SQ, Chen JS. Effect of hepatitis C virus core protein on modulation of cellular proliferation and apoptosis in hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2005;4: 71–74. [PubMed] [Google Scholar]
- 35.Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol. 2013;19: 4234–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson D, Fisher M, Sabin CA. Sexual transmission of hepatitis C in MSM may not be confined to those with HIV infection. J Infect Dis. 2008;197: 1213–1214, author reply 1214–1215. [DOI] [PubMed] [Google Scholar]
- 37.Goldstone S, Palefsky JM, Giuliano AR, et al. Prevalence of and risk factors for human papillomavirus (HPV) infection among HIV-seronegative men who have sex with men. J Infect Dis 2011;203: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 2009;101: 1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF Jr., Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. Aids. 2009;23: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 41.Reich H, DeCaprio J, McGlynn F. Laparoscopic Hysterectomy J Gynecol Surg. 1989;5: 213–216. [Google Scholar]
- 42.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 2009;101: 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Cozen W, Bernstein L, Ross RK, Deapen D. Changing relationship between socioeconomic status and prostate cancer incidence. J Natl Cancer Inst 2001;93: 705–709. [DOI] [PubMed] [Google Scholar]
- 44.Montella M, Pezzullo L, Crispo A, et al. Risk of thyroid cancer and high prevalence of hepatitis C virus. Oncol Rep. 2003;10: 133–136. [PubMed] [Google Scholar]
- 45.Yang JD, Ahmed Mohammed H, Cvinar JL, Gores GJ, Roberts LR, Kim WR. Diabetes Mellitus Heightens the Risk of Hepatocellular Carcinoma Except in Patients With Hepatitis C Cirrhosis. Am J Gastroenterol 2016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55: 1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayiner M, Wymer M, Golabi P, Ford J, Srishord I, Younossi ZM. Presence of hepatitis C (HCV) infection in Baby Boomers with Medicare is independently associated with mortality and resource utilisation. Aliment Pharmacol Ther. 2016;43: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 48.Galbraith JW, Donnelly JP, Franco RA, Overton ET, Rodgers JB, Wang HE. National estimates of healthcare utilization by individuals with hepatitis C virus infection in the United States. Clin Infect Dis. 2014;59: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.