Abstract
Chronic obstructive pulmonary disease (COPD) is a disease that affects the lungs and is defined by a variety of symptoms that combined with co-morbidities lead to a decline of the patients quality of life. The principal etiology of chronic obstructive pulmonary disease is smoking and air pollution that lead to oxidative and carbonyl stress. This review based on a search of PubMed, OxLIP+/SOLO (Bodleian Libraries) database (from 1991 to 2017) of relevant articles based on assessment of oxidative stress pathways involvement in COPD. Intracellular reactions that take place in organisms and aerobic cells have as by-products reactive oxygen species (ROS) and free radicals. Oxidative stress involved in pathogenesis of COPD is the result of lowered antioxidative potential combined with increased burden of oxidants. Molecular mechanisms underlying COPD pathways are not yet well understood, despite intensive research all over the world. A change in balance between Oxidants and antioxidants in the lungs as well as within the circulatory system, gene polymorphisms, and activation of transcription factors contribute to the molecular pathogenesis of COPD. Future research is needed in order to identify which patients will develop in time a susceptibility to damage caused by ROS and to determine if controlling ROS will have an effect on the progression of COPD.
Keywords: Chronic obstructive pulmonary disease, reactive oxygen species, oxidative stress
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease that affects the lungs and is defined by a variety of symptoms that combined with co-morbidities lead to a decline of the patients quality of life [ 1]. One out of eight emergency cases that require admission to the hospital are of patients with COPD, the fourth leading cause of death worldwide at the present time and it is expected to reach the third spot by 2030 [ 2].
Cigarette smoking is the main cause of COPD in the developed countries due to oxidative stress produced within the lungs [ 3]. Smoking and air pollution are the most significant initiating and risk factors for COPD and cell apoptosis described in COPD airways, and these have been attributed to oxidative and carbonyl stress [ 4]. A significant contributing role in the pathogenesis of COPD is the imbalance of oxidant burden and antioxidant capacity [ 3]. COPD is defined by mucous inflammation, apoptosis of the cells forming the epithelium of the airway lining, glandular hyperplasia that are produced by free radicals released and oxidative stress [ 3].
We have conducted this review based on a search of the PubMed database (from 1991 to 2017) of relevant articles based on assessment of oxidative stress pathways involvement in COPD and in their references as well.
Balance oxidants-antioxidants in COPD
Superoxides and hydroxyl radicals, alpha leukotrienes, interleukins, pro-inflammatory cytokines (tumor necrosis factor and activated transcriptional factors) are reactive oxygen species that serve the biggest pro-inflammatory role in the organism [3].
High concentration of inflammatory mediators like interleukin (IL)-8 and leukotriene B4 (LTB4) can produce a constant recruitment of neutrophils to the lungs and activate them at this level [ 5]. Free radicals, cytokines and proteases (proinflammatory enzymes) released as a result of neutrophils activating is believed to be a factor in COPD changes such as mucus hyper secretion [ 6], loss of lung elasticity [ 7] and also the impairment of surrounding tissues [ 7].
Changes in oxidant-antioxidant balance in the lungs and the circulatory system, gene polymorphisms and transcription factors like nuclear factor kappa B NF-κB becoming active lead to the molecular pathogenesis of COPD [ 1, 8, 9]. Acute exacerbations of COPD has also been associated with the down regulation of antioxidant pathways. A possible therapeutic method for COPD could be administering redox-protective antioxidants [3]. In the case of patients with COPD that smoke, it is possible that maintaining a balance between oxidants and antioxidant and associated pathogenic pathways could serve a great role in the progression of COPD and also on the search for novel therapeutic approaches [8,10].
Intracellular reactions that take place in organisms and aerobic cells have as by-products reactive oxygen species (ROS) and free radicals. Being molecules with high reactive properties, ROS could play a part in various diseases pathogenesis (idiopathic pulmonary fibrosis, Adult Respiratory Distress Syndrome (ARDS), arterial hypertension, diabetes and cancer), not exclusive to COPD, as well as for damage to cell structures (carbohydrates, nucleic acids, lipids, proteins) [ 10, 11]. In lung fibrosis, high levels of ROS can be produced by inflammatory cells and increased amounts of oxidants [ 10, 11].
Oxidative stress involved in pathogenesis of COPD is not the result of just increased burden of oxidants, but also due to a decrease in antioxidative potential. Several studies described an important decline of protection due to antioxidant’s level in alveolar macrophages [ 12], a sign that antioxidative mechanisms are not sufficiently adapted in the case of inflammatory respiratory diseases, so oxidants could take over as the leading role. Oxidative stress is involved in COPD pathogenesis by several mechanisms. The most important mechanism that correlates COPD with oxidative stress is represented by the chronic inflammation induced by proinflammatory genes increased expression [12, 13,14].
Association between smoke and oxidative stress
The lungs are very susceptible to injuries produced due to ROS. The situation is more severe in the case of smokers, since the smoke from burned cigarettes (composed from a variety of chemical substances), is the most important and preventable risk factor responsible for COPD [13, 15]. This environmental oxidant burden effect is increased by the additional release of endogenous oxidants, resulted from inflammatory airway cells, especially in COPD patients that smoke [16]. Besides tobacco smoke, urban, occupational and industrial air pollution are a source of various oxidants that could be the cause of an increased prevalence rate of COPD in nonsmokers in developing countries [17].
One of the most important pathways to COPD is high radical burden associated with low antioxidant capacity [ 18]. Thus, cigarette smoke is responsible for high oxidant burden and an important decrease of antioxidant capacity even in the plasma. ROS from the environment produced from cigarette smoke, combustion of organic matter, gases like ozone and nitrogen dioxide are common at the lung epithelium, [19], and can decrease oxidant defenses [20,21], increasing the oxidative burden on the lungs.
Inflammation play’s a great part in excessive ROS generation that leads to oxidant stress. Thus, there is an increased risk of developing COPD for heavy smokers for whom treatment has little effect [ 22]. The redox status balance is altered even more for smoking COPD patients due to excessive ROS produced both exogenous and endogenous [22].
Smoking exposure has the potential to decrease antioxidants, fact already proven in a study performed on smokers, passive smokers, and nonsmokers [ 23]. The study evidentiated that in smokers and passive smokers there were higher α-tocopherol concentrations and lower ascorbic acid and ß-carotene plasmatic level concentrations than in the case of nonsmokers [23]. Smoking COPD patients have higher risk of developing osteoporosis due to cigarette smoke radicals within the lungs that can influence the metabolism of Vitamin D at this level [24].
The evidence that cigarette smoking depletes antioxidants is evidentiated in other studies [ 25], that revealed decreased concentrations of ascorbate and vitamin E among smokers [ 26, 27]. Since the levels of ascorbate and vitamin E in the serum returns to normal soon after the patient stops smoking proof that dietary intake isn’t the cause but rather due to oxidative stress [28].
Cigarette smoke contains high amounts of nitric oxide, also produced by inflammatory cells, is known for it’s anti-inflammatory actions and antioxidant potency, but also plays a part in oxidative reactions [ 29] (Fig.1). NO found in cigarette smoke can form peroxynitrite by reacting with O2−, which has a negative effect on antioxidant capacity and increases oxidative stress [1,30,31].
Fig.1.
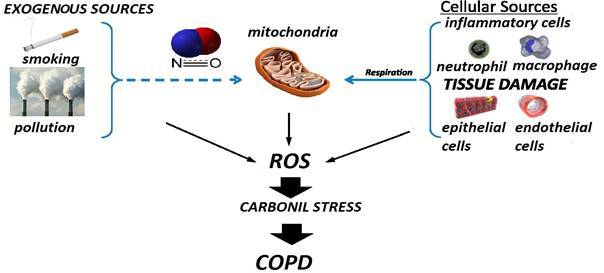
Exogenous and endogenous sources of ROS
Association between COPD and oxidative stress
It has been shown in multiple methods and studies that compared to non-smokers, smokers with COPD present obvious signs of oxidative burden and oxidative stress in the lungs [2,32,33].
Smoking is considered the root cause for COPD in patients as a byproduct of imbalance of oxidants and antioxidants that leads to oxidative stress [26]. Cigarette smoke leads to lowered antioxidant defense and lung cell injuries as a cause of oxidative stress from inflammatory cells or resulted from cigarette smoke [34].
Oxidants including H2O2, have been found in cigarette smoke by particulate fractions directly measured [35]. Studies in vitro performed on phagocytes collected from patients that smoke have shown unlike non-smoking patients phagocytes they possess the ability to release increased amounts of O2− and H2O2 spontaneously [36]. High levels of H2O2 have been found in smokers in tests performed on fluid collected from bronchoalveolar lavage and on condensation produced from exhaled breath [37], due to the release of O2− and lower respiratory tract increased population of macrophages [38]. There are evidences that supports the role of oxidants in smoking COPD patients caused by white blood cells peripheral release of O2− correlated with bronchial hyperreactivity [39].
Several mechanisms link COPD and oxidative stress, certain proinflammatory genes expressed more than usual has been shown to be a contributing factor in COPD chronic inflammation and the most important mechanism to support the relation between the two [14].
Further studies on biomarkers of oxidative stress and there relation in COPD especially that would be reproducible and that could reflect changes caused by therapeutic intervention or due to the pathology are ongoing [40].
Endogenous defense against ROS
Enzymatic and non-enzymatic means can lead to the inactivation of ROS and these are abundantly present in healthy subjects. Antioxidant molecules (ascorbic acid), unsaturated lipids, sacrificial proteins and metal binding proteins are examples of non-enzymatic antioxidants that constitute a defense mechanism. Vitamin C (ascorbate) and vitamin E (tocopherol) are found in the fluid epithelial lining of the lung and serve the role of antioxidant molecules. H2O and vitamin E radical are produced from tocopherol (antioxidant) that donates and electron its aromatic ring to OH. Vitamin C acts by donating electrons to lipid peroxyls [2,41].
Superoxide dismutases (SOD), glutathion peroxidases (GPx) and catalases (CAT) are a few examples of enzymatic antioxidant that can react with the hydrogen peroxide from the airways found in ample physiological reserve [42].
Superoxide dismutase has a very high affinity for O2− that leads to the production of H2O2. Its role is the removal of superoxide anions, serving the function of an antioxidant. SOD are enzymes that counteract superoxide radicals and are named so due to the metals found in there composition (CuZnSOD, MnSOD, FeSOD, extracellular SOD [EcSOD]) [42]. In smokers with COPD there are found increased sputum levels of EcSOD [43]. Several studies performed on patients with COPD found that polymorphisms in the EcSOD gene were shown to be less susceptible with COPD than with emphysema [44,45]. Several studies have confirmed the antioxidant efficiency of MnSOD against damage to the lung cells and could be induced by TNF-α and oxidants found in tobacco smoke. EcSOD and MnSOD are believed to have a significant protective role in COPD [1,46]. It is proved that experimental EcSOD deficiencies lead to emphysema [45,46].
On a cellular level the most important redox sensor is the GSH system. Even in the human lung the GSH system is considered to be one of the most important non-enzymatic antioxidants and it is homeostasis is dependent on other enzymatic interactions [47].
Reduced plasmatic levels of glutathione peroxidase in COPD patients have been found according to certain studies [47], and these are correlated significantly with FEV1 [47]. The reported decreased activity of glutathione peroxidase in blood [48,49] supports either a reduction in total glutathione peroxidase activity or a decrease in efficacy, with a reduced antioxidant function [50].
CAT is found in inflamed respiratory cells and alveolar macrophage type II and serves the role of a highly reactive intracytoplasmic enzyme against H2O2. COPD patients compared to smokers and non-COPD subjects have been shown to have a significantly reduced catalase activity [51,52]. Several studies performed by Betsuyaku [53] showed that smoking COPD subjects unlike non-COPD smoking subjects and non-smoking controls presented significantly decreased immunohistochemical staining for catalase in bronchiolar epithelial cells [53]. COPD patients have reduced catalase activity in bronchiolar epithelium [53] and peripheral lung cells [54], presenting increased opportunity for oxidative stress conditions to occur.
COPD is a complex disease process that combines the effect of recurrent inflammation, oxidative stress (oxidant/antioxidant imbalance), environmental impairment and host genetics [55]. Smoking and is the major source of oxidants/reactive oxygen species (ROS) to the lungs and to the entire body and it is the major risk factor for developing COPD [56,57] (Fig.2). There are evidences that extensive tissue damage and disease exacerbation susceptibility can be caused by environmental sources of oxidative stress from (cigarette smoking) and persistent inflammation [55,57,58].
Fig.2.
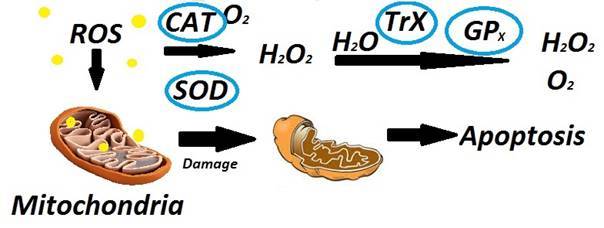
Involvement of mitochondria, ROS and other factors in the process of apoptosis
One-year experience from a medical clinic in a Romanian emergency county hospital
A total of 248 patients with COPD were admitted into the 2nd Department of Internal Medicine and Pneumology compartment of County Clinical Emergency Hospital of Craiova during January 1st 2017- January 1st 2018.
From these, 114 patients were hospitalized for chronic respiratory insufficiency; 70 patients with interstitial pneumonia; 56 patients with bacterial pneumonia and 8 patients with acute respiratory failure (Fig.3). All patients that were admitted had complications. Out of the total number of patients with COPD and chronic respiratory insufficiency 32 patients developed various clinical forms of pulmonary embolism and 64 patients were diagnosed with chronic pulmonary cord (Fig.4). The complications were diagnosed using imaging methods: pulmonary X-Ray, Doppler echocardiography, blood gas analysis and various biological laboratory explorations.
Fig.3.
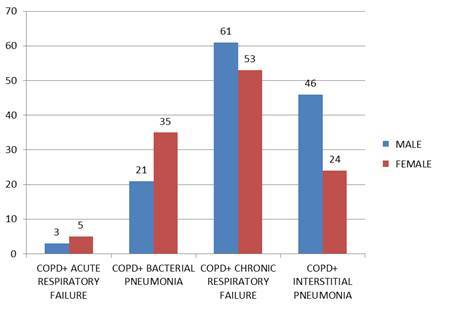
Association of COPD with other diagnoses, in an emergency county hospital
Fig.4.
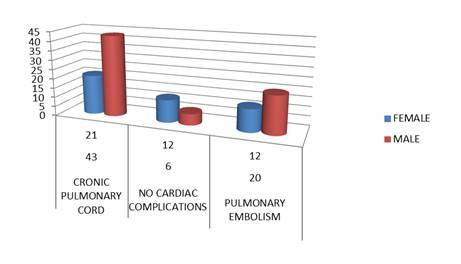
The most common complications of COPD patients that presented with chronic respiratory failure in our clinic
Conclusions
Molecular mechanisms underlying COPD pathways are not yet well understood, despite intensive research all over the world. The molecular pathogenesis of COPD is caused by oxidant-antioxidant imbalances in the lungs and the circulation that leads to activation of transcription factors and gene polymorphisms. It is not yet clear if oxidant/anti-oxidants expression is uniform amongst COPD. In order to stratify the groups of patients which may benefit most from anti-oxidant therapies, understanding these mechanisms is a priority. Future research is required in order to identify the patients that become susceptible to ROS related damage and to establish if targeted ROS control can be a contributing factor in COPD management. Therefore, this mechanism needs to be further with modern tools.
References
- 1.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl. J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 2.Nacul L, Soljak M, Samarasundera E, Hopkinson NS, Lacerda E, Indulkar T, Flowers J, Walford H, Majeed A. COPD in England: A comparison of expected, model-based prevalence and observed prevalence from general practice data. J Public Health. 2011;33:108–116. doi: 10.1093/pubmed/fdq031. [DOI] [PubMed] [Google Scholar]
- 3.Lin JL, Thomas PS. Current perspectives of oxidative stress and its measurement in chronic obstructive pulmonary disease. COPD. 2010;4(7):291–306. doi: 10.3109/15412555.2010.496818. [DOI] [PubMed] [Google Scholar]
- 4.Cohen BH, Ball WC, Brashears S, Diamond EL, Kreiss P, Levy DA, Menkes HA, Permutt S, Tockman MS. Risk factors in Chronic Obstructive Pulmonary Disease (COPD) Am J Epidemiol. 1997;105:223–232. doi: 10.1093/oxfordjournals.aje.a112378. [DOI] [PubMed] [Google Scholar]
- 5.Stone H, McNab G, Wood AM, Stockley RA, Sapey E. Variability of sputum inflammatory mediators in COPD and a1-antitrypsin deficiency. Eur Respir J. 2012;40:561–569. doi: 10.1183/09031936.00162811. [DOI] [PubMed] [Google Scholar]
- 6.Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases muc5ac mRNA and protein expression in respiratory epithelial cells. Am J Physiol. 1999;5 Pt 1(276):L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 7.Russell RE, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, Fitzgerald M, Barnes PJ. Alveolar macrophage-mediated elastolysis: Roles of matrix metalloproteinases, cysteine, and serine proteases. Am J Physiol Lung Cell Mol Physiol. 2002;4(283):L867–L873. doi: 10.1152/ajplung.00020.2002. [DOI] [PubMed] [Google Scholar]
- 8.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Respir Crit Care Med. 1997;2 Pt 1(156):341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 9.MacNee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;5 Pt 2(160):S58–S65. doi: 10.1164/ajrccm.160.supplement_1.15. [DOI] [PubMed] [Google Scholar]
- 10.Birben E, Sahiner UM, Sackesen C, Erzurum S, Katayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;1(5):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domej W, Földes-Papp Z, Flögel E, Haditsch B. Chronic obstructive pulmonary disease and oxidative stress. Curr Pharm Biotechnol. 2006;2(7):117–123. doi: 10.2174/138920106776597676. [DOI] [PubMed] [Google Scholar]
- 12.Täger M, Piecyk A, Köhnlein T, Thiel U, Ansorge S, Welte T. Evidence of a defective thiol status of alveolar macrophages from COPD patients and smokers. Chronic obstructive pulmonary disease. Free Radic Biol Med. 2000;11(29):1160–1165. doi: 10.1016/s0891-5849(00)00424-x. [DOI] [PubMed] [Google Scholar]
- 13.Churg DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loukides S, Bakakos P, Kostikas K. Oxidative stress in patients with COPD. Curr Drug Targets. 2011;4(12):469–477. doi: 10.2174/138945011794751573. [DOI] [PubMed] [Google Scholar]
- 15.Vlahos R, Bozinovski S. Glutathione peroxidase-1 as a novel therapeutic target for COPD. Redox Rep. 2013;4(18):142–149. doi: 10.1179/1351000213Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacNee W. Oxidants and COPD. Curr Drug Targets Inflamm Allergy. 2005;6(4):627–641. doi: 10.2174/156801005774912815. [DOI] [PubMed] [Google Scholar]
- 17.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;3(128):1239–1244. doi: 10.1378/chest.128.3.1239. [DOI] [PubMed] [Google Scholar]
- 18.Mak JC. Pathogenesis of COPD. Part II. Oxidative-antioxidative imbalance. Int J Tuberc Lung Dis. 2008;4(12):368–374. [PubMed] [Google Scholar]
- 19.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985;(64):111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols BG, Woods JS, Luchtel DL, Corral J, Koenig JQ. Effects of ozone exposure on nuclear factor-kappaB activation and tumor necrosis factor-alpha expression in human nasal epithelial cells. Toxicol. Sci. 2001;2(60):356–362. doi: 10.1093/toxsci/60.2.356. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B, Hu ML, Louie S, Duvall TR, Tarkington BK, Motchnik P, Cross CE. Interaction of nitrogen dioxide with human plasma. Antioxidant depletion and oxidative damage. FEBS Lett. 1992;1(13):62–66. doi: 10.1016/0014-5793(92)81185-o. [DOI] [PubMed] [Google Scholar]
- 22.O’Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD implications and relevance for treatment. Int J Chron Obstruct Pulm Dis. 2014;(9):1207–1224. doi: 10.2147/COPD.S51226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich M, Block G, Norkus EP. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intake. Am J Clin Nutr. 2003;1(77):160–166. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 24.Hansdottir S, Monick MM, Lovan N, Powers LS, Hunninghake GW. Smoking disrupts vitamin D metabolism in the lungs. Am J Respir Crit Care Med. 2010;Meeting Abstracts(181):A1425–A1425. [Google Scholar]
- 25.Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;(16):33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 26.Fischer BM, Voynow JA, Ghio AJ. OPD: balancing oxidants and antioxidants Int J Chron Obstruct Pulmon Dis, 015,;(0:):61–276. doi: 10.2147/COPD.S42414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duthie GG, Arthur JR, James WP. Effects of smoking and vitamin E on blood antioxidant status. Am J Clin Nutr. 1991;Suppl 4(53):1061S–1063S. doi: 10.1093/ajcn/53.4.1061S. [DOI] [PubMed] [Google Scholar]
- 28.Lykkesfeldt J, Priemé H, Loft S, Poulsen HE. Effect of smoking cessation on plasma ascorbic acid concentration. BMJ. 1996;7049(313):91–91. doi: 10.1136/bmj.313.7049.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med. 1996;5(21):669–681. doi: 10.1016/0891-5849(96)00155-4. [DOI] [PubMed] [Google Scholar]
- 30.Van der Vliet A, Smith D, O’Neill CA. Interactions of peroxynitrite with human plasma and its constituents: oxidative damage and antioxidant depletion. Biochem J. 1994;Pt 1(303):295–301. doi: 10.1042/bj3030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padmaja S, Huie RE. The reaction of nitric oxide with organic peroxyl radicals. Biochem Biophys Res Commun. 1993;2(195):539–544. doi: 10.1006/bbrc.1993.2079. [DOI] [PubMed] [Google Scholar]
- 32.Birben E, Sahiner UM, Sackesen C, Erzurum S, Katayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;1(5):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGuinness AJA, Sapey E. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. J Clin Med. 2017;2(6):21–21. doi: 10.3390/jcm6020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cano M, Thimmalappula R, Fujihara M. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and age-related macular degeneration. Vision Res. 2010;7(50):652–664. doi: 10.1016/j.visres.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zang LY, Stone K, Pryor WA. Detection of free radicals in aqueous extracts of cigarette tar by electron spin resonance. Free Radic Biol Med. 1995;2(19):161–167. doi: 10.1016/0891-5849(94)00236-d. [DOI] [PubMed] [Google Scholar]
- 36.van Antwerpen VL, Theron AJ, Richards GA. Vitamin E, pulmonary functions, and phagocyte-mediated oxidative stress in smokers and nonsmokers. Free Radic Biol Med. 1995;5(18):935–941. doi: 10.1016/0891-5849(94)00225-9. [DOI] [PubMed] [Google Scholar]
- 37.Dekhuijzen PN, Aben KK, Dekker I. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;3 Pt 1(154):813–816. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- 38.Schaberg T, Haller H, Rau M, Kaiser D, Fassbender M, Lode H. Superoxide anion release induced by platelet-activating factor is increased in human alveolar macrophages from smokers. Eur Respir J. 1992;4(5):387–393. [PubMed] [Google Scholar]
- 39.Rogers DF. Mucus hypersecretion in chronic obstructive pulmonary disease . In: Chadwick D, Goode JA, editors. Chronic Obstructive Pulmonary Disease: Pathogenesis to Treatment. Chichester: John Wiley & Sons; 2001. pp. 65–83. [Google Scholar]; Rogers DF. Mucus hypersecretion in chronic obstructive pulmonary disease. In: Chadwick D, Goode JA. (edit):s. Ch–s. Ch. doi: 10.1002/0470868678.ch5. [DOI] [PubMed] [Google Scholar]
- 40.Lakhdar R, Denden S, Kassab A. Update in chronic obstructive pulmonary disease: role of antioxidant and metabolizing gene polymorphisms. Exp Lung Res. 2011;6(37):364–375. doi: 10.3109/01902148.2011.580416. [DOI] [PubMed] [Google Scholar]
- 41.Lu JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010;4(14):840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;12(167):1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 43.Regan EA, Mazur W, Meoni E. Smoking and COPD increase sputum levels of extracellular superoxide dismutase. Free Radic Biol Med. 2011;3(51):726–732. doi: 10.1016/j.freeradbiomed.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Yao H, Arunachalam G, Hwang JW. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc Natl Acad Sci USA. 2010;35(107):15571–15576. doi: 10.1073/pnas.1007625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorheim IC, DeMeo DL, Washo G. Polymorphisms in the superoxide dismutase-3 gene are associated with emphysema in COPD. COPD. 2010;4(7):262–268. doi: 10.3109/15412555.2010.496821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gongora MC, Lob HE, Landmesser U. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am J Pathol. 2008;4(173):915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vibhuti A, Arif E, Deepak D, Singh B, Qadar Pasha MA. Correlation of oxidative status with bmi and lung function in COPD. Clin. Biochem. 2007;(40):958–963. doi: 10.1016/j.clinbiochem.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 48.Kluchova Z, Petrasova D, Joppa P, Dorkova Z, Tkacova R. The association between oxidative stress and obstructive lung impairment in patients with COPD. Physiol. Res. 2007;(56):51–56. doi: 10.33549/physiolres.930884. [DOI] [PubMed] [Google Scholar]
- 49.Santos MC, Oliveira AL, Viegas-Crespo AM, Vicente L, Barreiros A, Monteiro P, Pinheiro T, Bugalho De Almeida A. Systemic markers of the redox balance in chronic obstructive pulmonary disease. Biomarkers. 2004;9:461–469. doi: 10.1080/13547500400024768. [DOI] [PubMed] [Google Scholar]
- 50.Tavilani H, Nadi E, Karimi J, Goodarzi MT. Oxidative stress in COPD patients, smokers, and non-smokers. Respir. Care. 2012;57:2090–2094. doi: 10.4187/respcare.01809. [DOI] [PubMed] [Google Scholar]
- 51.Tavilani H, Nadi E, Karimi J, Goodarzi MT. Oxidative stress in COPD patients, smokers, and non-smokers. Respir. Care. 2012;57:2090–2094. doi: 10.4187/respcare.01809. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad A, Shameem M, Husain Q. Altered oxidant-antioxidant levels in the disease prognosis of chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2013;17:1104–1109. doi: 10.5588/ijtld.12.0512. [DOI] [PubMed] [Google Scholar]
- 53.Betsuyaku T, Fuke S, Inomata T, Kaga K, Morikawa T, Odajima N, Adair-Kirk T, Nishimura M. Bronchiolar epithelial catalase is diminished in smokers with mild COPD. Eur Respir J. 2013;42:42–53. doi: 10.1183/09031936.00058912. [DOI] [PubMed] [Google Scholar]
- 54.Tomaki M, Sugiura H, Koarai A, Komaki Y, Akita T, Matsumoto T, Nakanishi A, Ogawa H, Hattori T, Ichinose M. Decreased expression of antioxidant enzymes and increased expression of chemokines in COPD lung. Pulm Pharmacol Ther. 2007;(20):596–605. doi: 10.1016/j.pupt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;(6):413–421. doi: 10.2147/COPD.S10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;(64):111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pryor WA. Cigarette smoke and the involvement of free radical reactions in chemical carcinogenesis. Br J Cancer Suppl. 1987;(8):19–23. [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;(343):269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]


