Abstract
Basal cell carcinomas (BCCs) represent one of the most common human neoplasias. The excellent prognosis of the diagnosed early lesions and the low metastasis rate are particularities that required the investigation of the mechanisms of carcinogenesis on these lesions. In this study we analyzed the proliferation rate for 53 cases of BCC in relation to the clinicopathological parameters of the lesions using Ki67, considered a true indicator of cellular proliferation. The results indicated statistically significant differences in Ki67 immunoexpression related to histological type and lesion stage. The highest Ki67 values were observed in the adenoid and morpheaform subtypes, and in advanced tumor stages. This aspects may be useful for stratification of lesions in terms of tumor aggressiveness.
Keywords: Ki67, proliferation, basal cell carcinoma
Introduction
Basal cell carcinoma (BCC) is the most common human neoplasia with a high healing rate and an excellent prognosis when is diagnosed in early stages [ 1]. Today are already known the risk factors associated with the lesions (sun exposure, chronic lesions), the age and gender groups which are affected (males over 40 years old) and preferential location of tumors (head and neck) [2, 3,4]. However, BCC aggressiveness is variable and dependent on histopathological parameters represented by subtype, size, tumor stage [5].
Although the BCS metastasis rate is relatively low, the lesions have local invasion and tissue destruction capacity, like any other malignant tumor. One of the biomolecular mechanisms that provide this behavior is the tumor cell proliferation and one of the most useful markers for quantifying this process is Ki67.
Ki67 is a protein encoded by MKI67 genes (antigen identified by monoclonal antibody Ki-67) associated with cell proliferation and ribosomal RNA (ribonucleic acid) transcription [ 6]. It is considered to be a true indicator of the proliferative tumor compartment, being a non-histone protein that is expressed in most cell cycle phases [ 7]. Ki67 overexpression is generally associated with aggressive malignant tumors with a high risk of progression and metastasis and a reduced survival rate [8]. At present, Ki67 is seen not only as a prognostic marker but also as a useful marker for diagnosis and as an effective therapeutic target for malignant tumors [8]. Also, Ki67 may be useful for assessing the risk of progression of pre-invasive lesions [ 7].
For BCC, Ki67 has been extensively studied and included in antibody panels useful for diagnosis or for identifying forms associated with aggressive lesion parameters. The data obtained so far are controversial, from results indicating broad variations of marker expression, unrelated to histopathological parameters, to associations with prognostic parameters of the lesions and recurrence of BCCs [ 9, 10].
In this study we analyzed the Ki67 immunoexpression in relation to the associated clinicopathological parameters.
Material and methods
In this study we analyzed 53 primary basal cell carcinomas (BCC) from patients admitted and operated in Plastic Surgery and Dermatology Clinics of Emergency County Hospital Craiova during 2013-2015. The lesions were diagnosed in the Pathology Laboratory of the same hospital, based on the histopathological criteria established by the working group for non-melanocytic tumors of the skin inside AJCC (American Joint Committee on Cancer) [5]. The surgical specimens were fixed in 10% neutral buffered formalin and processed by the classic histopathological technique of paraffin embedding and Hematoxylin-Eosin (HE) staining.
We analyzed clinicopathological parameters of BCC as gender, age, tumor location, tumor size, histologic type and the tumor stage in relation with imunoexpression of Ki67, which is considered an accurate indicator of tumor proliferation level.
For the immunohistochemical analysis were used serial sections which were processed by LSAB2-HRP (Labeled Streptavidin Biotin-Horseradish Peroxidase) amplification system (DAKO, Redox, Bucharest, code K0675). For the signal visualization was used as chromogen the 3,3’-diaminobenzidine tetrahydrochloride (DAB, Redox, Bucharest, code 3467). To validate the reactions, we use external negative (by omitting the primary antibody) and positive (tonsil) controls. In this study we use the monoclonal mouse antihuman Ki67 antibody (Dako), clone MIB-1, in dilution 1:100 and antigen retrieval represented by microwaving in Citrate buffer pH 6.
For the semiquantitative quantification we used the proliferation index (PI) of Ki67, which represents the average percent of positive (labeled) cells of the total cells from 20 microscopic fields (MF) of 200x, for each case, all cases being assessed by two pathologists (CS, AS).
The statistical analysis was performed within Statistical Package for the Social Sciences (SPSS) 10 software (ANOVA comparison tests) and p-values <0.05 were considered significant. In this study it was used for the image acquisition the Nikon Eclipse E600 microscope and Lucia 5 software. The study was approved by the local ethical committee and the written informed consent was obtained from all the patients.
Results
In our study, BCC was diagnosed mainly in male (73.6%) patients aged over 50 years (84.9%). Tumors were localized predominantly in the head and neck region (83%) and were more frequently under 2cm (52.8%) (Table 1).
Table 1.
Clinicopathologic aspects of the investigated basal cell carcinomas
| Clinicopathological parameters | Variable |
| Age | <50=8, >50=45 |
| Gender | males=39, females=14 |
| Tumor location | head and neck=44, trunk=6, members=3 |
| Tumor size (cm) | <2=28, >2=25 |
| Histologic type | nodular=28, adenoid=16, morpheaform=9 |
| Stage | I=30, II=19, III=3, IV=1 |
The histopathological analysis of the diagnosed BCC type indicated that most cases were classified as nodular BCC (52.8%), followed by adenoid type (30.2%) and morpheaform type (17%). At the same time, the most of lesions were classified in stage I and II tumors (56.6% and respectively 35.8%), compared to stages III and IV in which the lesions were lower in number (7.5%).
Histopathologically, the nodular BCCs were characterized by the presence of well-defined tumor cell islets consisting of monomorphic basal cells with basophilic or amphiphilic cytoplasm, and slightly elongated hyperchromatic nuclei. In the periphery of tumor islets, tumor cells showed palisading or slit-like appearance (Fig.1A).
Fig.1.
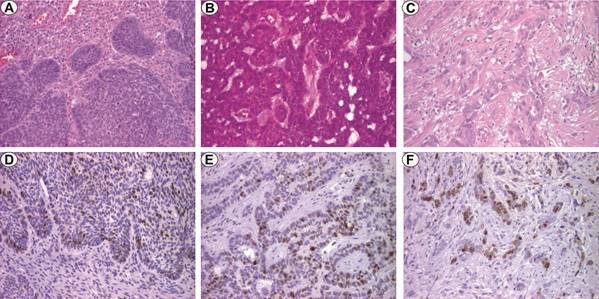
BCC, x100. A. Nodular type, HE staining; B. Adenoid type, HE staining; C. Morpheaform type, HE staining; D. Nodular type, Ki67 staining; E. Adenoid type, Ki67 staining; F. Morpheaform type, Ki67 staining
Tumor cell atypia and mitotic activity was relatively low. In some cases we have found cystic or micronodular focal growth patterns, especially at the level of the deep zones of the invasion front. The tumors present an inflammatory myxoid type stroma.
In the case of adenoid BCCs, in addition to classical histopathological aspects, we found anastomosed cellular strands and a more desmoplastic tumor stroma (Fig.1B). For morpheaform BCCs, the tumor parenchyma was represented by one-two cell clusters surrounded by a densely collagenous stroma with numerous fibroblastic elements (Fig.1C). In case of adenoid and morpheaform types, the cellular atypia was moderate.
Ki67 immunoexpression was identified in the tumor cell nucleus in all the analyzed BCCs. Also, the reaction was observed in stromal elements, such lymphocytes, and in rare basal cells of the skin adjacent to the tumors, as well as in the glandular structures and the hair follicles.
In this study we found differences in Ki67 immunoexpression in relation to histological type and tumor stage of BCC (Table 2).
Table 2.
IP Ki67 values depending on histologic types and stages of BCC
| Histologic type | Nodular | Adenoid | Morpheaform |
| PI Ki67 | 16.9±5.4 | 38.2±10.8 | 49.4±9.5 |
| Stage | I | II | III-IV |
| PI Ki67 | 21.4±10.3 | 34±14.5 | 55±9.1 |
*PI=proliferation index
The mean value of PI Ki67 for all analyzed cases was 28.4±15.2, the values varying large, between 10-65%. In relation to the histopathological type, the highest mean values of PI Ki67 were identified in the case of morpheaform and adenoid BCC, respectively 49.4±9.5 and 38.2±10.8.
By comparison PI Ki67 mean value for nodular BCCs was 16.9±5.4 ( Fig.1D-F). In these cases, the most Ki67 positive cells were present at the periphery of the tumor islets. In the case of morpheaform and adenoid types, the immunostaining had a heterogeneous appearance.
Regarding the tumor stage of BCC, the highest PI Ki67 values were identified for stages III-IV and II, respectively 55±9.1 and 34±14.5, compared to stage I where the value was 21.4±10.3.
The statistical analysis of the results showed significant differences in PI Ki67 values both in relation to the histopathological type of BCC (p=0.000, Anova test) and in relation to the tumor stage (p=0.000, Anova test) ( Fig.2A-B).
Fig.2.
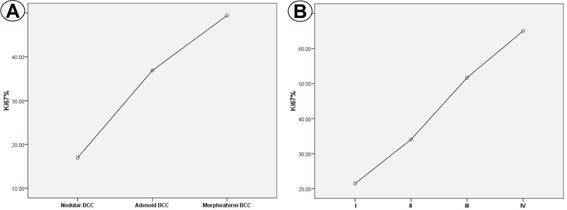
Ki67 proliferation index depending on histologic type (A) and stage (B)
In this study, we did not identify statistical relation of PI Ki67 values with age and gender of patients or tumor location.
Discussion
The incidence of BCC is highest after the age of 40, the lesions being diagnosed in patients presenting with risk factors as sun and chemical substances exposure, immunosuppression and chronic processes such as scarring and inflammation [1]. At the same time, BCC are more common in male patients, and head and neck are elective localizations [2,3,4]. In this study the lesions prevailed in male patients, over 50 years age and with head and neck location, aspects which are in concordance with the literature data.
The clinical appearance of BCC and tumor size can give information about the histological growth pattern and aggression of the lesions. Metastases are rare in the BCC, the aspect being observed especially for lesions over 5-10cm in size in which the risk is considered to be between 20-50%, compared to lesions under 5cm, where this risk is 1-2% [1,11]. In our study most lesions were less than 2 cm (28 cases), and lesions of over 2cm were observed in all three BCC histopathological subtypes.
The nodular, superficial and infiltrative types are the three major histological types of BCC, but are also are described growth patterns like micronodular, adenoid, morpheaform, pigmented, or fibroepithelial aspects [2,3].
The most common type of BCC is represented by the nodular type, which can have many components and differentiations such as the cystic, pigmented, sebaceous, eccrine ones [1,11]. In some classifications, the adenoid type is not considered as an individual histological form of BCC. The morpheaform (fibrosing, scarring, or desmoplastic) type is a histologically aggressive subtype of BCC, which, in addition to the particular aspect given by the desmoplastic tumor stroma, may have an increased number of mitosis, necrosis and infiltrative appearance [2,3].
In our study, the nodular type was the most common, followed by the adenoid and morpheaform types. The most important prognostic parameters of BCC are histological type, tumor size and stage. In this study, the advanced BCCs were observed in only four cases.
In this study we analyzed the Ki67 immunoexpression in relation to histopathological parameters of BCC. The results obtained indicated differences of PI Ki67 in relation to the histopathological type and tumor stage, the highest values of Ki67 being recorded for adenoid and morpheaform types and for advanced stage lesions.
There are numerous literature studies that have investigated Ki67 expression in BCC. Thus, in most studies, the Ki67 expression values were variable within wide limits, but most of BCC were positive for this marker [9,10,12]. Also in our study, the Ki67 immunostaining was present in all cases, with PI Ki67 values being variable. Ki 67 was also included in panels that were used to diagnose the lesions [12].
The results of the various studies related to Ki67 immunoexpression and BCC prognostic parameters are variable and controversial. Thus, Kramer E et al. has shown a highly variable proliferative activity ranging from the absence or minimal proliferation characteristic of dormant tumors to proliferation levels of up to 61% [9]. Also, the authors indicate the absence of PI Ki67 correlation with the histological subtype of BCC, concluding that other biomolecular mechanisms are involved in the tumor aggressiveness of BCC [9]. The same conclusion is reached by Corrêa Mde P et al., which recorded the value of 60% as maximum for Ki67 in the analyzed BCC [13]. On the contrary, other studies indicate a relation of Ki67 immunoexpression with prognostic histopathological factors of BCC, and in particular the tumor type. Thus, Koseoglu RD et al. indicates a Ki67 proliferation level associated with histopathological parameters of BCC, the values being superior for infiltrative lesions and having extensive fibrosis [14]. By comparison, in our study, the highest values of PI Ki67 were identified in the adenoid and morpheaform types, which were characterized by the presence of a desmoplastic stroma. Also, Healy E et al. indicated superior Ki67 immunoexpression in BCC that associated tumor recurrence [15]. The variation of Ki67 immunoexpression in various studies can be attributed to the use of the Ki67 clone, the working protocol and the tumor heterogeneity that is sometimes present in BCC.
In this context, in recent years Ki67 has been seen as a potential therapeutic target, for which experimental methods have been developed to inhibit the gene encoding the protein, direct inhibition with anti-Ki67 antibodies or antisense oligodeoxynucleotides (ASOs) [8]. Also, the involvement of tumor cell proliferation in the initiation and persistence of other biomolecular mechanisms are arguments for studying Ki67 expression in tumorigenesis, including BCC.
Conclusions
In this study, the Ki67 proliferation index indicated significant differences in relation to the histological type and tumor stage of BCC. The highest values of PI Ki67 were identified for the BCC adenoid and morpheaform types. The BCC proliferation rate can be used to stratify aggressive lesions for therapy and clinical follow-up.
References
- 1.Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol. 2006;19(2):S127–147. doi: 10.1038/modpathol.3800512. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg G., Golitz L.E., Fitzpatrick J. Histopathology of Skin Cancer. . In: Stockfleth E., Rosen T., Schumaak S., et al., editors. Managing Skin Cancer. 1. Berlin: Ed. Springer-Verlag Berlin; 2010. pp. 17–35. [Google Scholar]
- 3.Vantuchová Y, Čuřík R. Histological types of basal cell carcinoma. Scripta Medica (BRNO) 2006;79(5-6):261–270. [Google Scholar]
- 4.Chung S. Basal cell carcinoma. Arch Plast Surg. 2012;39(2):166–170. doi: 10.5999/aps.2012.39.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Joint Committee on Cancer. ; American Joint Committee on Cancer; American Cancer Society; American College of Surgeons. AJCC cancer staging manual. 2. New York: Springer; 2010. Cutaneous squamous cell carcinoma and other cutaneous carcinomas. ; pp. 301–314. [Google Scholar]
- 6.Bullwinkel J, Baron-Lühr B, Lüdemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206(3):624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Figueras MT, Puig L, Musulen E, Gilaberte M, Ferrándiz C, Lerma E, Ariza A. Prognostic significance of p27Kip1, p45Skp2 and Ki67 expression profiles in Merkel cell carcinoma, extracutaneous small cell carcinoma and cutaneous squamous cell carcinoma. Histopathology. 2005;46(6):614–621. doi: 10.1111/j.1365-2559.2005.02140.x. [DOI] [PubMed] [Google Scholar]
- 8.Li LT, Jiang G, Chen Q1, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review) Mol Med Rep. 2015;11(3):1566–1572. doi: 10.3892/mmr.2014.2914. [DOI] [PubMed] [Google Scholar]
- 9.Kramer E, Herman O, Frand J, Leibou L, Schreiber L, Vaknine H. Ki67 as a biologic marker of basal cell carcinoma: a retrospective study. Isr Med Assoc J. 2014;16(4):229–232. [PubMed] [Google Scholar]
- 10.Khodaeiani E, Fakhrjou A, Amirnia M, Babaei-Nezhad S, Taghvamanesh F, Razzagh-Karimi E, Alikhah H. Immunohistochemical evaluation of p53 and Ki67 expression in skin epithelial tumors. Indian J Dermatol. 2013;58(3):181–187. doi: 10.4103/0019-5154.110824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snow SN, Sahl W, Lo JS, Mohs FE, Warner T, Dekkinga JA, Feyzi J. Metastatic basal cell carcinoma, Report of five cases. Cancer. 1994;73(2):328–335. doi: 10.1002/1097-0142(19940115)73:2<328::aid-cncr2820730216>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Abdelsayed RA, Guijarro-Rojas M, Ibrahim NA, Sangueza OP. Immunohistochemical evaluation of basal cell carcinoma and trichepithelioma using Bcl-2, Ki67, PCNA and P53. J Cutan Pathol. 2000;27(4):169–175. doi: 10.1034/j.1600-0560.2000.027004169.x. [DOI] [PubMed] [Google Scholar]
- 13. Corrêa Mde P, Ferreira AP, Gollner AM, Rodrigues MF, Guerra MC. Markers expression of cell proliferation and apoptosis in basal cell carcinoma. An Bras Dermatol. 2009;84(6):606–614. doi: 10.1590/s0365-05962009000600006. [DOI] [PubMed] [Google Scholar]
- 14.Koseoglu RD, Sezer E, Eyibilen A, Aladag I, Etikan I. Expressions of p53, cyclinD1 and histopathological features in basal cell carcinomas. J Cutan Pathol. 2009;36(9):958–965. doi: 10.1111/j.1600-0560.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 15.Healy E, Angus B, Lawrence CM, Rees JL. Prognostic value of Ki67 antigen expression in basal cell carcinomas. Br J Dermatol. 1995;133(5):737–741. doi: 10.1111/j.1365-2133.1995.tb02748.x. [DOI] [PubMed] [Google Scholar]


