Abstract
Background:
Low adherence can undermine the efficacy of daily oral pre-exposure prophylaxis (PrEP). Mental health conditions, particularly depression, could be associated with low PrEP adherence, especially for women.
Setting:
We analyzed data from 1013 Kenyan and Ugandan HIV-uninfected participants in the Partners Demonstration Project, an open-label study of PrEP delivered to HIV-uninfected members of serodiscordant couples.
Methods:
Participants completed quarterly visits over 2 years and were encouraged to use PrEP until their partners living with HIV had ≥6 months of antiretroviral therapy use (when viral suppression was expected). PrEP adherence was measured daily with electronic medication event monitoring system caps and dichotomized into low (<80% of expected bottle openings) and high adherence. Depression was assessed annually using the 16-item Hopkins Symptom Checklist screening tool; scores >1.75 indicate “probable depression.” The association between probable depression and PrEP adherence was assessed separately for men and women using generalized estimating equations and marginal structural models.
Results:
At enrollment, 39 (11.7% of 334) women and 64 (9.4% of 679) men reported symptoms indicating probable depression, and these proportions decreased during follow-up (P < 0.001 for women and men). Probable depression was significantly associated with low PrEP adherence among women (adjusted risk ratio = 1.77; 95% confidence interval: 1.14 to 2.77; P = 0.01); there was no association between depression and adherence among men (P = 0.50). Marginal structural models and sensitivity analyses confirmed these findings.
Conclusions:
Depression was relatively uncommon in this population and was an independent risk factor for low PrEP adherence among women. For PrEP programs targeting African women, integration of depression screening may improve PrEP effectiveness.
Keywords: depression, HIV, pre-exposure prophylaxis, Africa, women
INTRODUCTION
Oral emtricitabine/tenofovir disoproxil fumarate pre-exposure prophylaxis (PrEP) has demonstrated efficacy to prevent HIV transmission, with reductions in HIV incidence estimated to be >90% when daily adherence is high.1–6 A recent open-label study conducted in South Africa (HPTN 067/ADAPT) found that high-risk women are generally able to adhere to daily oral PrEP regimens, providing reassurance about the usability of daily PrEP among women when efficacy is known.7–9 However, there remains a need to more fully understand factors influencing adherence as PrEP availability continues to expand throughout sub-Saharan Africa.10,11 Although PrEP use could fluctuate appropriately to align with sexual behavior and HIV exposure, qualitative work suggests that salience of HIV (whether information about HIV comes to mind at times of risk), perceived risk of infection, stigma, social support, and fears about disclosure are important barriers to PrEP use among African women during periods of heightened HIV risk.10–14 Further research is needed to identify psychosocial factors related to PrEP use in the context of open-label PrEP delivery, explore gender-based differences in associations, and understand whether interventions targeting these constructs can improve PrEP adherence.
Mental health conditions, including depression, are known barriers to health care engagement and daily medication adherence, but their relationship to PrEP adherence has not been well studied. Depression is highly prevalent worldwide, particularly among women who are 1.5–3 times more likely to experience lifetime depression than men, and depressive symptoms are related to increased HIV risk behaviors (eg, multiple sexual partners, condomless sex), poor social support, and substance abuse.15–18 Among adults living with HIV, those with depressive symptoms are approximately 55% less likely to achieve optimal daily antiretroviral therapy (ART) adherence.19,20 Depression also has an impact on adherence to preventative behaviors such as daily contraceptive use and engagement in prenatal health care services, although the mechanisms underlying these associations may differ from the relationship between depression and ART adherence.21–23 Among general populations of reproductive-age women in sub-Saharan Africa and the United States, depression has been significantly associated with interruptions and cessation in effective contraception use over a 6-month period and failure to use dual protection methods, after accounting for fertility desires and sexual behavior.21–23
Given the considerable burden of depression among individuals at high risk of HIV and associations between depression, health-seeking behavior, and daily medication use, depression may also be linked with PrEP adherence. One recent study examined the influence of depressive symptoms on PrEP adherence, and concluded that depression modestly reduced PrEP adherence in men who have sex with men and transgender women.24 Similar research is yet to be conducted among cisgender women in African settings. To contribute information to this gap, we evaluated the association between depression and PrEP adherence among a cohort of 1013 HIV-uninfected individuals participating in a PrEP demonstration project in Kenya and Uganda. We hypothesized that depression would be associated with low PrEP adherence and that there may be important gender-based differences in this relationship.
METHODS
Study Populatio
The Partners Demonstration Project was a prospective, open-label implementation study to evaluate delivery of daily oral PrEP integrated into existing ART services among high-risk, heterosexual HIV serodiscordant couples in Kenya and Uganda.25 The study was implemented from 2012 to 2016 at 4 clinical care research sites in Thika and Kisumu, Kenya and Kampala and Kabwohe, Uganda. Eligible couples were aged 18 years and older, sexually active, and planned to remain a couple for ≥1 year. PrEP, prescribed as daily coformulated emtricitabine/tenofovir disoproxil fumarate, was offered to HIV-uninfected participants and PrEP discontinuation was encouraged if their partners initiated and used ART for ≥6 months (when viral suppression would be expected) and if there were no concerns about their partner’s ART adherence, or other considerations such as plans to become pregnant.25
Data Collection
HIV-uninfected participants attended quarterly study visits for up to 24 months and received HIV testing, PrEP refills (as needed), and HIV prevention counseling at each visit. Information on sexual behavior (eg, condomless sex acts in the previous month), fertility intentions (eg, intending to become pregnant in the next year), and contraceptive use were collected with standardized questionnaires, administered by interviewers in the participant’s preferred language. Their partners living with HIV received ART on-site or were given referrals to a public health clinic of their choice, based on national ART eligibility guidelines.
At baseline and annual follow-up visits, sociodemographic data were collected and participants were assessed for depression, internalized stigma, heavy alcohol use, perceived social support, and relationship satisfaction with validated questionnaires. Depression was assessed using the 16-item Hopkins Symptoms Checklist for Depression (HSCL-D) screening tool.26 Overall scores were calculated as a mean value (range 0–4), and a score .1.75 was indicative of “probable depression.”26 This cutoff value has been previously validated in sub-Saharan Africa and corresponds well to depression diagnosis based on other screening tools and local informant interviews.27,28 Depression was analyzed as a time-dependent exposure; we used linear interpolation methods to estimate participants’ HSCL-D scores throughout the study period by fitting linear regression equations for the change in scores between annual visits and then calculating HSCL-D scores at intervening quarterly visits using the model coefficients.29,30 As part of routine counseling procedures, participants who screened positive for depression were referred to local specialists for mental health care.
Internalized stigma was measured by summing scores from a 4-item scale (adapted from the Internalized AIDS-Related Stigma Scale), with a value closer to 16 indicating greater stigma.31 Heavy alcohol use was defined as a response of “yes” to any of the 4 items from the Rapid Alcohol Problems Screen.32 Social support was measured as a continuous mean score from the 10-item Functional Social Support Questionnaire (range 1–4), with a higher score indicating greater perceived social support.33 Relationship satisfaction was based on the summed score from an 8-item dyadic adjustment scale (range 8–48) and a higher score indicated greater satisfaction.34 Stigma, alcohol use, social support, and relationship satisfaction were also analyzed as time-dependent covariates with scores carried forward between annual visits.
PrEP Adherence
The primary outcome was PrEP adherence among participants choosing to take PrEP, measured using medication event monitoring system (MEMS) caps, which electronically captured a date-and-time stamp with each bottle opening. These data were downloaded at each visit after PrEP initiation. MEMS data were collected as a research procedure and were not used in adherence counseling. Adherence was calculated as the number of pill bottle openings divided by the number of expected bottle openings during the period between visits and is reported as a percentage. Openings by the study staff during visits were excluded from the numerator. The number of expected openings excluded days for which PrEP was not dispensed due to missed visits, protocol-defined stops (eg, related to adverse events or sustained ART use by a partner living with HIV), or refill refusals. We also excluded missing data due to broken or lost MEMS caps and instances where participants had >120% of expected bottle openings during the period between study visits, which could indicate cap malfunction or repeated openings without removing a dose. Participants were considered to have high PrEP adherence during periods where MEMS data indicated that ≥80% of expected openings occurred between quarterly visits. This cutoff corresponds to 5–6 doses per week and plasma tenofovir concentrations >40 ng/mL, which is consistent with steady-state daily dosing and has been associated with high levels of protection against HIV.35,36 In this cohort, MEMS data corresponded well with blood plasma tenofovir concentrations in a random sample of 140 participants.25,37 Approximately 81% of samples had detectable tenofovir concentrations consistent with dosing in the previous week, whereas MEMS data indicated that PrEP was taken during 82% of days and 71% of periods between study visits had adherence ≥80%.25,37
Statistical Analyses
We used descriptive statistics to summarize the sample by gender and compared participant characteristics during periods of probable depression and those without probable depression using generalized estimating equations (GEE) extension to logistic regression. The multivariable model of factors associated with probable depression included any variables for which the univariable P-value was ≤0.10.
To assess the effect of depression on low PrEP adherence, we used GEE with a log link, Poisson distribution, and robust standard errors to estimate risk ratios. Our primary model included “probable depression” as a categorical (yes/no) exposure, as has been done in studies of depressive symptoms and ART adherence to allow for a meaningful interpretation of results.24,38,39 Study site, age, and time-dependent measures of stigma, social support, fertility intentions, and any condomless sex acts since the previous study visit were included in the models based on a priori knowledge that these factors may be related to both depression and PrEP adherence.10,12,40 We assessed several additional covariates for confounding, including education, income, marital status, partnership duration, time known to be in an HIV serodiscordant relationship, and parity (measured at enrollment), as well as time-dependent measures of sexually transmitted infection symptoms, heavy alcohol use, current relationship status, relationship satisfaction with study partner, reported abuse, and plasma HIV RNA viral load (log10 copies/mL) and CD4 count (cells/mL) of the partner living with HIV. Of these covariates, any that resulted in a substantial change in the effect estimate (>10%) were included in the multivariable model. Separate models were run for men and women.
We also conducted several sensitivity analyses of our primary GEE models to explore whether our findings were robust to changes in depression and adherence definitions. First, we examined the dose–response relationship between depression and PrEP adherence by varying the HSCL-D score cutoff to >1.60 and >1.50 to indicate depression, and we conducted additional analyses using continuous HSCL-D mean score as the exposure of interest. Second, we altered the definition of high PrEP adherence using cutoffs of ≥70%, ≥85%, and ≥90% of expected openings using MEMS data. Third, we ran our models among the subset of participants with available plasma tenofovir levels (N = 140) and defined our adherence outcome using a cutoff of >35 ng/mL.
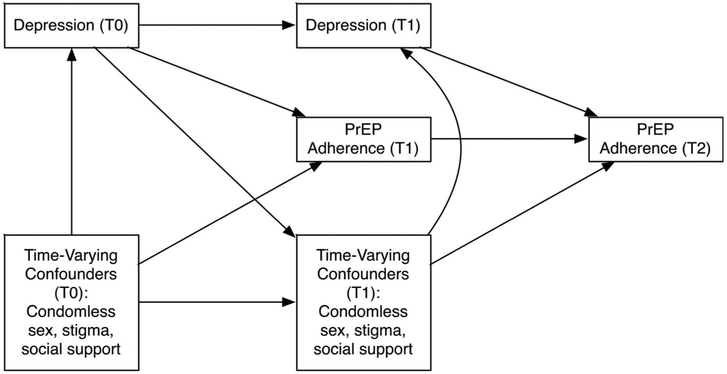
We repeated our primary analyses using marginal structural models to refine the adjustment for time-dependent confounding by stigma, social support, and sexual risk behavior, guided by hypothesized relationships between these factors (Fig. 1).41 We computed stabilized inverse probability weights using logistic regression to predict the probability of categorical depression exposure at each visit.42 The weights were calculated with baseline measures of marital status, age, heavy alcohol use, relationship satisfaction with study partner, and plasma HIV RNA viral load of the partner living with HIV, as well as HSCL-D score at the previous visit (exposure history) and time-dependent measures of stigma, social support, and condomless sex. Exposure weights were computed separately for women (mean 0.99, range 0.23–3.13) and men (mean 1.00, range 0.49–1.91) and were not truncated. These weights were included in regression models with a log link, Poisson distribution, and robust standard errors.
FIGURE 1.
Hypothesized relationships between depression, PrEP adherence, and time-varying confounding variables during study follow-up. T0, enrollment; T1, quarterly follow-up visits; T2, final study visit. PrEP, pre-exposure prophylaxis.
All analyses were conducted using SAS 9.4 (Cary, NC).
Ethical Statement
Protocols were approved by ethical review boards at the University of Washington and collaborating institutions in Kenya and Uganda. All participants provided written informed consent in their preferred language.
RESULTS
Participant Characteristics
The Partners Demonstration Project enrolled 1013 HIV-uninfected individuals, including 334 (33.0%) women and 679 (67.0%) men. The median age at enrollment was 30 years [interquartile range (IQR) 26–36 years].25 The median reported duration of partnership was 5.9 years (IQR 1.9–11.6) for women and 2.4 years (IQR 0.8–5.8) for men (Table 1). Most participants reported learning that they were in an HIV serodiscordant relationship shortly before study enrollment (median time = 1.2 months). Approximately 198 women(59.3%) and 458 men (67.5%) reported at least one condomless sex act with their study partner in the month before enrollment and the median plasma HIV RNA viral load for participants’ partners living with HIV was 4.6 log10 copies per milliliter (IQR 3.8–5.0).
TABLE 1.
Characteristics of the Study Sample at Enrollment (N = 1013 Unless Otherwise Indicated)
| HIV-Uninfected Women (N = 334) |
HIV-Uninfected Men (N = 679) |
|
|---|---|---|
| Age, yrs | 29 (24–35) | 30 (26–37) |
| Unmarried | 9 (2.7) | 32 (4.7) |
| Education, yrs | 8 (5–11) | 8 (7–12) |
| Any income reported | 217 (65.0) | 653 (96.2) |
| Relationship duration with study partner, yrs | 5.9 (1.9–11.6) | 2.4 (0.8–5.8) |
| Time known to be in an HIV serodiscordant relationship, yrs | 0.1 (0.1–0.6) | 0.1 (0.0–0.2) |
| No. of children with study partner | 1 (0–2) | 0 (0–1) |
| No. of more children desired | 1 (0–2) | 2 (1–3) |
| Timing of next pregnancy (N = 887 nonpregnant participants) | ||
| Currently trying to get pregnant | 23 (6.9) | 41 (6.0) |
| Not currently trying to get pregnant | 185 (55.4) | 514(75.7) |
| No. of sex acts with study partner | 4.5 (3.0–8.0) | 6.0 (3.0–12.0) |
| Any condomless sex with study partner | 198 (59.3) | 458 (67.5) |
| Any sex with outside partner(s) | 5 (1.5) | 79 (11.6) |
| STI symptoms | 20 (6.0) | 11 (1.6) |
| CD4 count (cells/μL) of partner living with HIV | 403 (221–595) | 451 (285–660) |
| Plasma HIV RNA (log10 copies/mL) of partner living with HIV | 4.8 (4.3–5.3) | 4.4 (3.5–4.9) |
| Probable depression* | 39 (11.7) | 64 (9.4) |
| HIV-related stigma | 6.0 (4.0–8.0) | 8.0 (5.0–9.0) |
| Heavy alcohol use | 50 (15.0) | 152 (22.4) |
| Social support | 3.6 (3.2–3.9) | 3.7 (3.2–4.0) |
| Relationship satisfaction with study partner | 31.0 (27.0–34.0) | 32.0 (28.0–35.0) |
| Happiness in relationship (N = 1012)† | ||
| Happy | 292 (87.4) | 621 (91.6) |
| Unhappy | 42 (12.6) | 57 (8.4) |
| Reported abuse | 1 (0.3) | 2 (0.3) |
Data are presented as number (%) for categorical variables and median (IQR) for continuous variables.
Cognitive, behavioral disengagement, and somatic depressive symptoms were assessed with a Likert response scale ranging from 1 (“Not at all”) to 4 (“Extremely”). A mean score >1.75 was indicative of “probable depression.”
Happiness in the relationship was assessed with a single item asking participants to rate the degree of happiness in their partnership. Responses of “happy,” “very happy,” “extremely happy,” and “perfect” were coded as “Happy”; responses of “a little unhappy,” “fairly unhappy,” and “extremely unhappy” were coded as “Unhappy.”
STI, sexually transmitted infection.
During the 2-year study period, 982 participants initiated PrEP, a majority at enrollment (98.2% of women and 97.1% of men). In addition, 233 women (74.9%) and 425 men (70.7%) had a partner initiate ART within the first 6 months of follow-up. Retention rates were high throughout the study (>83% at all visits), and 962 participants had at least one follow-up visit after PrEP initiation. More than half of the participants stopped PrEP by their 12-month visit (N = 196; 58.7% for women and N = 372; 54.8% for men). The most frequent reason for stopping PrEP during follow-up was ≥6 months of ART use by the partner living with HIV (N = 519; 51.3%).
Prevalence and Factors Associated With Depression
A total of 103 HIV-uninfected participants (10.2%) had probable depression at enrollment and this proportion decreased during study follow-up. Among women, 11.7%,8.2%, and 3.1% were classified as having probable depression at enrollment, 12-month visit, and 24-month visit, respectively (P-value for trend <0.001). Among men, 9.4%, 2.6%, and 2.0% were classified as having probable depression at each of the annual visits (P-value for trend <0.001). In a multivariable model, being female [adjusted odds ratio (aOR) 2.16, 95% confidence interval (CI): 1.35 to 3.43], being unmarried (aOR 2.77, 95% CI: 1.33 to 5.77), reporting condomless sex in the previous month (aOR 1.33, 95% CI:1.01 to 1.77), and having a partner with higher plasma HIV RNA viral load (aOR 1.16, 95% CI: 1.04 to 1.31) were all independently associated with higher odds of probable depression during the study (Table 2). Several psychosocial and behavioral characteristics were also associated with probable depression during follow-up. Higher levels of HIV-related stigma (aOR 1.11, 95% CI: 1.04 to 1.19), heavy alcohol use (aOR 1.83, 95% CI: 1.18 to 2.82), lower levels of social support (aOR 0.72, 95% CI: 0.52 to 1.00), lower reported relationship satisfaction with a study partner (aOR0.91, 95% CI: 0.89 to 0.94), and reporting abuse from a study partner (aOR 2.73, 95% CI: 1.48 to 5.02) were more likely during periods with probable depression than during periods without probable depression.
TABLE 2.
Associations Between Participant Characteristics and Probable Depression During Study Follow-up (n = 1013)
| Frequency of Visits* | Factors Associated With Probable Depression | |||||
|---|---|---|---|---|---|---|
| Probable depression† (N = 431 Visits) |
No Probable Depression (N = 8386 Visits) |
Univariable OR (95% CI) |
P | Multivariable OR‡ (95% CI) |
P | |
| Demographic characteristics | ||||||
| Female | 219 (50.8) | 2770 (33.0) | 2.41 (1.61 to 3.61) | <0.001 | 2.16 (1.35 to 3.43) | 0.001 |
| Age, yrs | 30 (25–35) | 30 (25–35) | 1.00 (0.97 to 1.02) | 0.84 | ||
| Unmarried | 31 (7.2) | 291 (3.5) | 2.12 (0.92 to 4.90) | 0.08 | 2.77 (1.33 to 5.77) | 0.01 |
| Education, yrs | 9 (7–12) | 8 (6–12) | 1.02 (0.97 to 1.08) | 0.42 | ||
| Any income reported | 335 (77.7) | 7256 (86.5) | 0.56 (0.33 to 0.94) | 0.03 | 0.84 (0.48 to 1.47) | 0.54 |
| Couple characteristics | ||||||
| Relationship duration with study partner, yrs | 3.4 (0.7–8.2) | 3.5(1.1–8.1) | 1.00 (0.97 to 1.03) | 0.87 | ||
| Time known to be in an HIV serodiscordant relationship, yrs | 0.1 (0.0–0.2) | 0.1 (0.1–0.3) | 1.04 (0.80 to 1.36) | 0.77 | ||
| No. of children with study partner | 0 (0–1) | 0 (0–2) | 1.03 (0.88 to 1.22) | 0.69 | ||
| No. of more children desired | 1 (0–2) | 1 (0–2) | 0.94 (0.83 to 1.05) | 0.28 | ||
| Currently pregnant or trying to become pregnant | 71 (18.2) | 1320 (19.4) | 0.86 (0.61 to 1.22) | 0.40 | ||
| Currently in relationship with study partner at this visit | 369 (85.6) | 7329 (87.5) | 0.91 (0.56 to 1.48) | 0.70 | ||
| Sexual behavior since previous visit | ||||||
| No. of sex acts with study partner | 3 (1–8) | 4 (1–8) | 0.98 (0.96 to 1.01) | 0.19 | ||
| Any condomless sex with study partner | 152 (35.9) | 2547 (30.7) | 1.34 (1.02 to 1.77) | 0.04 | 1.33 (1.01 to 1.77) | 0.04 |
| Any sex with outside partner(s) | 43 (10.0) | 988 (11.8) | 0.95 (0.62 to 1.46) | 0.82 | ||
| STI symptoms | 25 (5.8) | 228 (2.7) | 1.55 (0.74 to 3.24) | 0.24 | ||
| HIV-related characteristics | ||||||
| CD4 count (cells/μL) of partner living with HIV | 471 (293–611) | 511 (344–712) | 1.00 (0.99 to 1.00) | 0.03 | 1.00 (0.99 to 1.00) | 0.54 |
| Plasma HIV RNA (log10 copies/mL) of partner living with HIV | 4.1 (1.6–4.8) | 2.2 (1.5–4.4) | 1.28 (1.15 to 1.42) | <0.001 | 1.16 (1.04 to 1.31) | 0.01 |
| Partner living with HIV on ART at this visit | 256(61.1) | 5975 (73.9) | 0.56 (0.40 to 0.78) | <0.001 | 0.74 (0.51 to 1.07) | 0.11 |
| Psychosocial and behavioral characteristics | ||||||
| HIV-related stigma | 8.0 (6.0–10.0) | 7.0 (4.0–8.0) | 1.13 (1.06 to 1.20) | <0.001 | 1.11 (1.04 to 1.19) | 0.01 |
| Heavy alcohol use | 125 (29.0) | 1465 (17.5) | 1.66 (1.10 to 2.51) | 0.02 | 1.83 (1.18 to 2.82) | 0.01 |
| Social support | 3.5 (2.8–4.0) | 3.8 (3.4–4.0) | 0.48 (0.36 to 0.64) | <0.001 | 0.72 (0.52 to 1.00) | 0.05 |
| Relationship satisfaction with study partner | 27.0(21.0–31.0) | 32.0 (28.0–35.0) | 0.89 (0.87 to 0.91) | <0.001 | 0.91 (0.89 to 0.94) | <0.001 |
| Happy in relationship with study partner§ | 328 (76.6) | 7596 (90.6) | 0.37 (0.25 to 0.56) | <0.001 | 0.70 (0.45 to 1.10) | 0.12 |
| Reported abuse | 11 (2.6) | 39 (0.5) | 4.31 (2.16 to 8.59) | <0.001 | 2.73 (1.48 to 5.02) | 0.01 |
Data are n/N (%) or median (IQR). The number of data points assessed is the total number of visits with each characteristic during study follow-up, stratified by probable depression.
Cognitive, behavioral disengagement, and somatic depressive symptoms were assessed with a Likert response scale ranging from 1 (“Not at all”) to 4 (“Extremely”). A mean score .1.75 was indicative of “probable depression.”
Multivariable models adjusted for study site and all other factors associated with probable depression in the univariable models, as determined by P-value ≤0.10.
Happiness in the relationship was assessed with a single item. Responses of “happy,” “very happy,” “extremely happy,” and “perfect” were coded as “Happy”; responses of “a little unhappy,” “fairly unhappy,” and “extremely unhappy” were coded as “Unhappy.”
OR, odds ratio; STI, sexually transmitted infection.
Depression and PrEP Adherence
The analysis of depression and PrEP adherence included 903 participants, after excluding those who did not initiate PrEP (N = 28), did not have a follow-up visit during the analysis period (N = 23), or did not have a visit where adherence could be calculated due to PrEP discontinuation, missing MEMS data, and MEMS adherence data >120% for all visits when measured (N = 59).
Among women, low (<80%) PrEP adherence was detected at 26.9% of visits and occurred more often during periods when probable depression was reported (41.1% of visits) relative to periods without depression [25.9% of visits; adjusted risk ratio (aRR) 1.77, 95% CI: 1.14 to 2.77, Table 3]. These results were in agreement with marginal structural model findings (aRR 1.53, 95% CI: 1.05 to 2.23) and GEE model findings using continuous HSCL-D mean score (aRR1.80, 95% CI: 1.03 to 3.14). The strength of the association between depression and PrEP adherence decreased when the HSCL-D score cutoff was changed to 1.60 in the GEE model (aRR: 1.61, 95% CI: 1.05 to 2.48) and was no longer statistically significant when the HSCL-D cutoff was changed to 1.50. Our findings were robust to changes in the definition of high adherence and remained statistically significant for adherence cutoffs of >70%, >85%, and >90% (aRR estimates ranged from 1.53 to 1.87; P-values all <0.05). In the model with plasma tenofovir levels, the direction of the association between depression and adherence remained (aRR: 1.21, 95% CI: 0.93 to 1.58), but the magnitude of the estimate was attenuated likely due to the small number of observations (n = 523).
TABLE 3.
Associations Between Depression and Low PrEP Adherence* During Study Follow-up (n = 903 Participants)
|
HIV-Uninfected Women (N = 312) |
Visits With Low PrEP Adherence† (N = 386/1433 Visits) |
Unadjusted GEE Analysis | Adjusted GEE Analysis‡ | Adjusted MSM Analysis§ | |||
| RR (95% CI) | P | aRR (95% CI) | P | aRR (95% CI) | P | ||
| Probable depression║ | 39 (41.1) | 1.46 (1.05 to 2.03) | 0.02 | 1.77 (1.14 to 2.77) | 0.01 | 1.53 (1.05 to 2.23) | 0.03 |
| No probable depression | 347 (25.9) | REF | REF | REF | REF | REF | REF |
| HSCL-D mean score¶ | 1.12 (1.03–1.28) | 1.21 (0.82 to 1.80) | 0.33 | 1.80(1.03 to 3.14) | 0.03 | — | — |
|
HIV-Uninfected Men (N = 591) |
Visits With Low PrEP Adherence† (N = 890/2865 Visits) |
Unadjusted GEE Analysis | Adjusted GEE Analysis‡ | Adjusted MSM Analysis§ | |||
| RR (95% CI) | P | aRR (95% CI) | P | aRR (95% CI) | P | ||
| Probable depression║ | 29 (35.8) | 1.16 (0.75 to 1.81) | 0.49 | 1.18 (0.73 to 1.91) | 0.50 | 1.28 (0.78 to 2.12) | 0.32 |
| No probable depression | 861 (30.9) | REF | REF | REF | REF | REF | REF |
| HSCL-D mean score¶ | 1.11 (1.01–1.23) | 1.05 (0.87 to 1.27) | 0.63 | 1.05 (0.85 to 1.30) | 0.65 | — | — |
Participants were considered to have low adherence if they opened their pill bottles ,80% of the expected days between study visits, based on MEMS caps data.
Data are n/N (%) or median (IQR).
GEE models adjusted for study site, age, stigma, social support, fertility intentions, and condomless sex with their study partner since the previous visit, which were selected a priori, as well as relationship satisfaction with study partner, which resulted in a >10% change in the unadjusted RR in models for both men and women.
Weighted marginal structural model is adjusted for marital status, age, heavy alcohol use, relationship satisfaction, plasma HIV RNA concentration in the partner living with HIV, and depression history. We did not calculate the effect of mean HSCL-D score on PrEP adherence using marginal structural models because inverse probability weights generally perform poorly with continuous exposure variables.
Defined as HSCL-D mean score >1.75.
Defined as continuous HSCL-D score (range 0–4).
MSM, marginal structural models; RR, risk ratio.
Among men, low adherence to PrEP was detected at 35.8% of visits when probable depression was reported and30.9% of visits without depression. The frequency of low PrEP adherence was not associated with depression in the GEE model with probable depression as the exposure (P = 0.50), the GEE model with continuous HSCL-D score (P = 0.65), or the marginal structural model analyses (P = 0.32, Table 3). We also did not detect significant associations between depression and adherence in any sensitivity analyses.
DISCUSSION
In this PrEP demonstration project with HIV-uninfected individuals in mutually disclosed HIV serodiscordant partnerships in East Africa, depression was significantly associated with low PrEP adherence among women, but was not related to PrEP adherence among men. This finding is particularly important, given that in this cohort, as well as other sub-Saharan African settings, the burden of depression is greater among women than men and women may have more difficulty adhering to oral PrEP than men.7,8,43,44 However, overall adherence was high in this sample even among participants with probable depression, and only 4 participants seroconverted during follow-up, suggesting that depressive symptoms may not be sufficient to eliminate the benefit of PrEP in the cohort.25
Overall, few participants (10.2%) endorsed symptoms consistent with depression at enrollment and the prevalence of probable depression decreased over time for men and women. During follow-up, probable depression was associated with gender, marital status, viral load, condomless sex, stigma, alcohol use, social support, relationship satisfaction, and abuse. Other studies in sub-Saharan Africa have found similar associations, and the relationships between depression, risky sexual behavior, abuse, and other psychosocial and behavioral variables are likely bidirectional and dynamic.16,17,45
Consistent with our findings, an analysis with the iPrEx OLE cohort found that the effect of depressive symptoms on PrEP adherence differed between men and transgender women.24 Moreover, research on the relationship between depression and ART adherence among adults living with HIV in sub-Saharan Africa has shown that depression is associated with poorer ART adherence and this relationship is stronger for women than for men.46 Findings from these studies and ours could be explained in part by traditional gender roles in these settings. Women in sub-Saharan Africa are typically viewed as caregivers with primary responsibilities for household tasks, which are often prioritized above their self-care.47,48 Other qualitative research with HIV serodiscordant couples has shown that men may feel their masculinity is threatened by their female partners’ use of ART or PrEP and, as a result, may attempt to control their partners’ medication use.47 Traditional gender roles and relationship power dynamics could influence access to mental health care and severity of depression, thereby strengthening an association between depression and low PrEP adherence over time, particularly for women. The potential influence of gendered power dynamics on health and HIV prevention behaviors also supports our finding that greater relationship satisfaction was strongly associated with reduced depression and better PrEP adherence in this sample.
We classified PrEP adherence using MEMS data and participants were considered to have low adherence for a given visit if MEMS data indicated that <80% of bottle openings occurred. This 80% threshold corresponds to approximately 5–6 PrEP doses per week, which we assumed was sufficient to protect against HIV acquisition in this cohort. However, pharmacokinetic minimum levels of PrEP adherence have not yet been established for women or heterosexual men and, although previous studies have found associations between high levels of adherence and low HIV incidence, recent data suggest that PrEP may be less forgiving to missed doses in vaginal than rectal exposure.35,49,50 To address these uncertainties about effective levels of PrEP, we repeated our analyses using various PrEP adherence cutoffs and plasma tenofovir levels and found similar results.
The strengths of this study included the large, prospective cohort with participants from urban and rural African settings and the use of a validated depression screening tool and electronic monitoring devices to monitor medication use. Overall retention rates were high in the sample, which minimized bias due to differential attrition by depressive symptom severity. Limitations of this study included annual depression measurement, which reduced our ability to analyze more frequent changes in depression status. Depressive symptoms could have been influenced by social desirability bias, but interviews were conducted with trained counselors to minimize this issue. In addition, there may be nondifferential misclassification of probable depression status with respect to PrEP adherence, which would be expected to attenuate our model estimates. Our study was not adequately powered to measure statistical differences in whether gender modified the association between depression and PrEP adherence. Finally, participants were all in stable HIV serodiscordant relationships at enrollment and our findings may not be generalizable to other high-risk populations.
Future research on links between depression and PrEP adherence should consider potential intervention opportunities to incorporate mental health services with PrEP delivery, particularly for women. Several models that integrate depression interventions into existing health care programs are being implemented in sub-Saharan Africa. For example, a “measurement-based care model” for antidepressant delivery, which encourages case managers to screen individuals for depression using brief tools and track their antidepressant adherence during regular clinic visits, has been successfully deployed in HIV clinics in Cameroon and Uganda.51,52 Psychotherapy interventions, such as cognitive behavioral therapy, have also been adapted for lay health care worker delivery in HIV, antenatal, and primary health care clinics throughout sub-Saharan Africa and have been shown to reduce depression and improve ART adherence after 6–8 months among adults living with HIV.53 The successes of these programs suggest that depression screening tools and adapted interventions can be implemented in busy HIV and antenatal care settings and family planning clinics, and similar intervention approaches could be tailored to HIV-uninfected adults seeking PrEP to potentially improve PrEP adherence in the subset with depressive symptoms.
In conclusion, depressive symptoms were relatively uncommon in our study and seem to have negatively impacted PrEP adherence among high-risk, HIV-uninfected women, but not among men. These findings highlight a need for future studies to continue exploring psychosocial barriers to PrEP adherence by gender. Additional research is also necessary to understand the potential mechanisms by which depression influences adherence to preventive therapies and whether there are modifiable factors mediating this relationship. Our study supports the integration of regular depression screening and treatment into PrEP delivery programs, which may reduce the burden of depression and improve PrEP effectiveness among African women.
ACKNOWLEDGMENTS
The authors thank the couples who participated in the study and the teams at the 4 study sites and the University of Washington that supported data collection and management for this work.
Supported by the National Institute of Mental Health of the US National Institutes of Health (Grant R01 MH095507), the Bill & Melinda Gates Foundation (Grant OPP1056051), and through the US Agency for International Development (cooperative agreement AID-OAA-A-12–00023). J.V. was supported by the National Institute of Mental Health of the US National Institutes of Health (Grant F31 MH113420). Gilead Sciences donated the PrEP medication but had no role in data collection or analysis. The results and interpretation presented here do not necessarily reflect the views of the study funders.
APPENDIX 1. Partners Demonstration Project Team
Coordinating Center (University of Washington) and collaborating investigators (Harvard Medical School, Johns Hopkins University, and Massachusetts General Hospital): J.M.B. (protocol chair), C.C. (protocol co-chair), R.H. (project director), Deborah Donnell (statistician), Ruanne Barnabas, J.H., Harald Haugen, Craig Hendrix, Lara Kidoguchi, Mark Marzinke, Susan Morrison, Jennifer Morton, Norma Ware, Monique Wyatt. Project sites: Kabwohe, Uganda (Kabwohe Clinical Research Centre): Stephen Asiimwe and Edna Tindimwebwa; Kampala, Uganda (Makerere University): Elly Katabira and Nulu Bulya; Kisumu, Kenya (Kenya Medical Research Institute): Elizabeth Bukusi, Josephine Odoyo; Thika, Kenya (Kenya Medical Research Institute, University of Washington): Nelly Rwamba Mugo and K.N. Data Management was provided by DF/Net Research, Inc. (Seattle, WA). PrEP medication was donated by Gilead Sciences.
Footnotes
Presented in part at the Conference on Retroviruses and Opportunistic Infections 2018 (CROI); March 4–7, 2018; Boston, MA.
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237–2246. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. [DOI] [PubMed] [Google Scholar]
- 5.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. [DOI] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010; 363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial. Lancet HIV. 2018;5:e68–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geary CW, Bukusi EA. Women and ARV-based HIV prevention—challenges and opportunities. J Int AIDS Soc. 2014;17(3 suppl 2):19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson KA, Baeten JM, Mugo NR, et al. Tenofovir-based oral preexposure prophylaxis prevents HIV infection among women. Curr Opin HIV AIDS. 2016;11:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS One. 2014;9:e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amico KR, Wallace M, Bekker LG, et al. Experiences with HPTN 067/ADAPT study-provided open-label PrEP among women in Cape Town: facilitators and barriers within a mutuality framework. AIDS Behav. 2017;21:1361–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant RM, Koester KA. What people want from sex and preexposure prophylaxis. Curr Opin HIV AIDS. 2016;11:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennon CA, Huedo-Medina TB, Gerwien DP, et al. A role for depression in sexual risk reduction for women? A meta-analysis of HIV prevention trials with depression outcomes. Soc Sci Med. 2012;75:688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao D, Feldman BJ, Fredericksen RJ, et al. A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS Behav. 2012;16:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gore-Felton C, Koopman C. Behavioral mediation of the relationship between psychosocial factors and HIV disease progression. Psychosom Med. 2008;70:569–574. [DOI] [PubMed] [Google Scholar]
- 18.Kuehner C Why is depression more common among women than among men? Lancet Psychiatry. 2017;4:146–158. [DOI] [PubMed] [Google Scholar]
- 19.Nakimuli-Mpungu E, Bass JK, Alexandre P, et al. Depression, alcohol use and adherence to antiretroviral therapy in sub-Saharan Africa: a systematic review. AIDS Behav. 2012;16:2101–2118. [DOI] [PubMed] [Google Scholar]
- 20.Mayston R, Kinyanda E, Chishinga N, et al. Mental disorder and the outcome of HIV/AIDS in low-income and middle-income countries: a systematic review. AIDS. 2012;26(suppl 2):S117–S135. [DOI] [PubMed] [Google Scholar]
- 21.Hall KS, White KO, Rickert VI, et al. Influence of depressed mood and psychological stress symptoms on perceived oral contraceptive side effects and discontinuation in young minority women. Contraception. 2012;86:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garbers S, Correa N, Tobier N, et al. Association between symptoms of depression and contraceptive method choices among low-income women at urban reproductive health centers. Matern Child Health J. 2010;14: 102–109. [DOI] [PubMed] [Google Scholar]
- 23.Joyce K, Diffenbacher G, Greene J, et al. Internal and external barriers to obtaining prenatal care. Soc Work Health Care. 2008;9:89–96. [DOI] [PubMed] [Google Scholar]
- 24.Mehrotra ML, Glidden DV, McMahan V, et al. The effect of depressive symptoms on adherence to daily oral PrEP in men who have sex with men and transgender women: a marginal structural model analysis of the iPrEx OLE study. AIDS Behav. 2016;20:1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heffron R, Ngure K, Odoyo J, et al. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa [version 2; referees: 2 approved]. Gates Open Res. 2018;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Psaros C, Haberer JE, Boum Y, et al. The factor structure and presentation of depression among HIV-positive adults in Uganda. AIDS Behav. 2015;19:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolton P Cross-cultural validity and reliability testing of a standard psychiatric assessment instrument without a gold standard. J Nerv Ment Dis. 2001;189:238–242. [DOI] [PubMed] [Google Scholar]
- 28.Ashaba S, Kakuhikire B, Vořechovská D, et al. Reliability, validity, and factor structure of the Hopkins Symptom Checklist-25: population-based study of persons living with HIV in rural Uganda. AIDS Behav. 2018;22: 1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vaus D Analyzing Social Science Date: 50 Key Problems in Data Analysis. London, United Kingdom: SAGE Publications; 2002. [Google Scholar]
- 30.Simon GE, Katon WJ, VonKorff M, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry. 2001;158:1638–1644. [DOI] [PubMed] [Google Scholar]
- 31.Kalichman SC, Simbayi LC, Cloete A, et al. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-Related Stigma Scale. AIDS Care. 2009;21:87–93. [DOI] [PubMed] [Google Scholar]
- 32.Cherpitel CJ, Ye Y, Bond J, et al. Cross-national performance of the RAPS4/RAPS4-QF for tolerance and heavy drinking: data from 13 countries. J Stud Alcohol. 2005;66:428–432. [DOI] [PubMed] [Google Scholar]
- 33.Broadhead WE, Gehlbach SH, de Gruy FV, et al. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Med Care. 1988;26:709–723. [DOI] [PubMed] [Google Scholar]
- 34.Spanier G Measuring dyadic adjustment: new scale for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976;38:15–28. [Google Scholar]
- 35.Cottrell ML, Yang KH, Prince HMA, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Defechereux PA, Mehrotra M, Liu AY, et al. Depression and oral FTC/TDF pre-exposure prophylaxis (PrEP) among men and transgender women who have sex with men (MSM/TGW). AIDS Behav. 2015;20: 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez P, Andia I, Emenyonu N, et al. Alcohol use, depressive symptoms and the receipt of antiretroviral therapy in Southwest Uganda. AIDS Behav. 2008;12:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomlinson M, O’Connor MJ, le Roux IM, et al. Multiple risk factors during pregnancy in South Africa: the need for a horizontal approach to perinatal care. Prev Sci. 2014;15:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 42.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomlinson M, Grimsrud AT, Stein DJ, et al. The epidemiology of major depression in South Africa: results from the South African Stress and Health study. South Afr Med J. 2009;99(5 pt 2):367–373. [PMC free article] [PubMed] [Google Scholar]
- 44.Haberer JE, Kidoguchi L, Heffron R, et al. Alignment of adherence and risk for HIV acquisition in a demonstration project of pre-exposure prophylaxis among HIV serodiscordant couples in Kenya and Uganda: a prospective analysis of prevention-effective adherence. J Int AIDS Soc. 2017;20:21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machisa MT, Christofides N, Jewkes R. Mental ill health in structural pathways to women’s experiences of intimate partner violence. PLoS One. 2017;12:e0175240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakimuli-Mpungu E, Mojtabai R, Alexandre PK, et al. Lifetime depressive disorders and adherence to anti-retroviral therapy in HIV-infected Ugandan adults: a case-control study. J Affect Disord. 2013;145: 221–226. [DOI] [PubMed] [Google Scholar]
- 47.Carroll JJ, Ngure K, Heffron R, et al. Gendered differences in the perceived risks and benefits of oral PrEP among HIV-serodiscordant couples in Kenya. AIDS Care. 2016;28:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montgomery ET, Chidanyika A, Chipato T, et al. Sharing the trousers: gender roles and relationships in an HIV-prevention trial in Zimbabwe. Cult Health Sex. 2012;14:795–810. [DOI] [PubMed] [Google Scholar]
- 49.Hanscom B, Janes HE, Guarino PD, et al. Preventing HIV-1 Infection in women using oral pre-exposure prophylaxis: a meta-analysis of current evidence. J Acquir Immune Defic Syndr. 2016;73:606–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson PL, Kiser JJ, Gardner EM, et al. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011;66:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams JL, Gaynes BN, McGuinness T, et al. Treating depression within the HIV “medical home”: a guided algorithm for antidepressant management by HIV clinicians. AIDS Patient Care STDs. 2012;26: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pence BW, Gaynes BN, Atashili J, et al. Feasibility, safety, acceptability, and preliminary efficacy of measurement-based care depression treatment for HIV patients in Bamenda, Cameroon. AIDS Behav. 2014;18:1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sikkema KJ, Dennis AC, Watt MH, et al. Improving mental health among people living with HIV: a review of intervention trials in low- and middle-income countries. Glob Ment Health. 2015;2:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]