Abstract
Prebiotic phosphorylation of (pre)biological substrates under aqueous conditions is a critical step in the origins of life. Previous investigations have had limited success and/or require unique environments which are incompatible with the subsequent generation of the corresponding oligomers or higher order structures. Here we demonstrate that diamidophosphate (DAP), a plausible prebiotic agent produced from trimetaphosphate, efficiently (amido)phosphorylates a wide variety of (pre)biological building blocks (nucleosides/tides, amino acids, and lipid precursors) under aqueous (solution/paste) conditions, without the need of a condensing agent. Significantly, higher order structures (oligonucleotides, peptides and liposomes) are formed under the same phosphorylation reaction conditions. This plausible prebiotic phosphorylation process under similar reaction conditions could enable the systems chemistry of the three classes of (pre)biologically relevant molecules, and their oligomers, in a single-pot aqueous environment.
Graphical Abstract
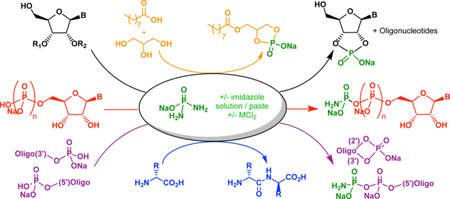
Phosphorylation of (pre)biological molecules in water has been a long-sought goal in prebiotic chemistry. Here, it is demonstrated that diamidophosphate phosphorylates nucleosides, amino acids and glycerol/fatty acids in aqueous medium, while simultaneously leading to higher-order structures (oligonucleotides, peptides and liposomes) in the same pot.
Phosphorylation of biologically relevant molecules under potential prebiotic conditions is an important step in the origins of life and has been investigated (especially for nucleosides) over a wide variety of conditions1–4 with more recent variations5,6. All of these approaches use differing phosphorylation sources and are limited in substrate scope due to their reaction conditions, or require unique non-aqueous environments to enable the phosphorylation process7,8. Many of these approaches are, therefore, incompatible with next step of generating the corresponding oligomers and higher order structures (towards RNA or pre-RNA worlds) from these phosphorylated substrates, necessitating spatially separated processes and other discrete mechanisms and chemistries. A universal and efficient phosphorylating agent which would phosphorylate a wide class of (pre)biological molecules in water under similar reaction conditions, and enable formation of higher order structures, would be of significance in the context of prebiotic systems chemistry9.
We have previously shown that diamidophosphate (DAP, Fig. 1) efficiently phosphorylates a wide variety of prebiotically relevant sugar molecules and their building blocks10. A prebiotic synthesis of phosphoenol pyruvate has been recently demonstrated using DAP as the prebiotic phosphorylating reagent11. In all cases, the mechanism involves a nucleophilic attack of NH2-group of DAP on the free aldehyde moiety to facilitate an intramolecular phosphorylation of the α-hydroxy-aldehydes (Supplementary Fig. 1). Such a mechanism precludes the phosphorylation of hydroxyl groups which are not next to a carbonyl moiety (e.g. nucleosides and glycerol). However, when we observed that DAP alone in water at pH 6, or in the presence of ortho- or pyro-phosphates, produced trimetaphosphate (Supplementary Fig. 2–5), it suggested that direct phosphorylation of other nucleophilic groups in aqueous medium should be possible by a direct attack on the phosphorous center with the protonated NH2-group of the DAP acting as the leaving group12.
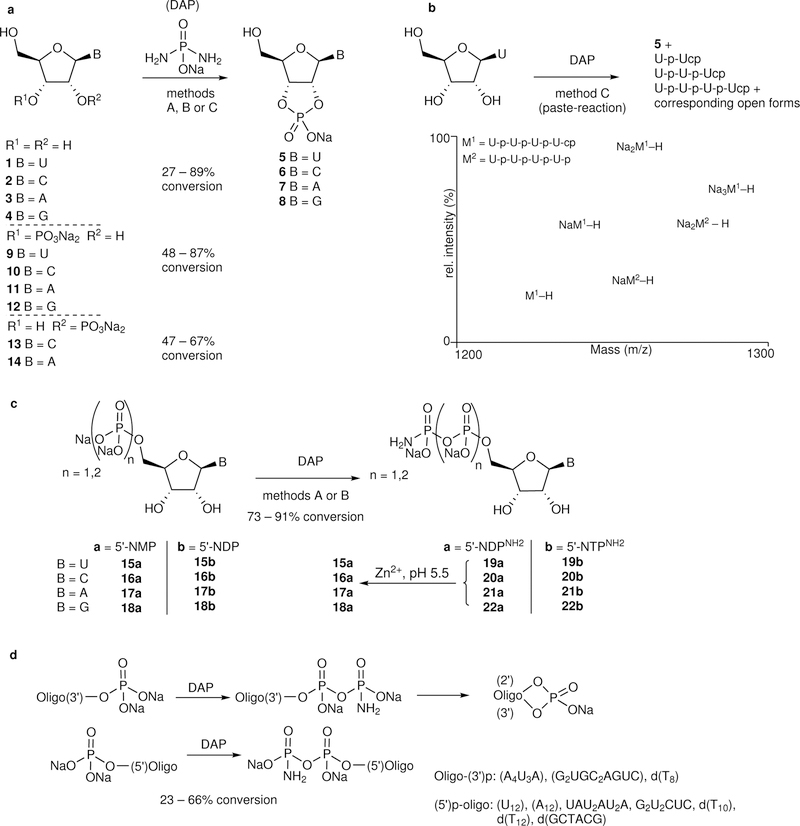
Figure 1: DAP mediated phosphorylation of nucleosides/tides demonstrating the potential to generate and progress through the successive levels of nucleotides and oligonucleotides under similar conditions.
Phosphorylation of (a) nucleosides, and 2’- and 3’-mononucleotides to give cyclophosphates, (b) uridine under paste-reaction conditions forming also oligonucleotides, (c) 5’-nucleotides, and (d) 5’- and 3’-phosphorylated oligonucleotides by diamidophosphate (DAP). The reactions were carried out by method A (aqueous, pH 5.5), by method B (aqueous, pH ≈ 7) with or without Mg2+ or Zn2+ or imidazole, or by method C (paste-reaction). For details see supplementary information and supplementary Tables 1–11.
Results
Phosphorylation of nucleosides/tides and oligonucleotides by DAP.
We began by investigating the phosphorylation of nucleosides with DAP. Reacting 0.1 M uridine 1 with DAP in aqueous solution, under a range of conditions (1–25 equiv. DAP, pH 5.5–10, r.t.–50 °C, with and without Zn2+ or Mg2+ or imidazole, and slowly over days-weeks, Supplementary Table 1), we observed the formation of uridine-2’,3’-cyclophosphate (5, c-UMP, Fig. 1a) and traces of the 5’-amidophosphate of 5. While Zn2+ or Mg2+ accelerated the reaction, Zn2+ also lowered the pH, leading to the hydrolysis13/condensation of DAP, necessitating its continuous addition. The addition of imidazole (conjugate acid pKa = 7.05) circumvented this problem at pH 7, affording similar conversion while decelerating the hydrolysis/condensation of DAP. Cytidine 2 was converted to 2’,3’-c-CMP (6, 27%) under similar conditions, while purines 3 and 4, reacted equally well forming 2’,3’-c-AMP (7, 31%), and 2’,3’-c-GMP (8, 27%), respectively. Further addition of DAP, over days/weeks, facilitated the ongoing conversions to the respective c-NMPs.
Dry/solid-state and low water-activity prebiotic phosphorylation reactions have been widely investigated4,6,14–16. Mixing DAP, imidazole and 1 in the solid-state at room temperature with drops of water led to a paste-like material (“paste-reaction” conditions) and efficiently produced 5 (≈ 80%, 30 days, Supplementary Table 1). A preparative experiment on a 1-gram scale afforded 5 in 65% isolated yield (Supplementary Figs. 18–21). To our delight, oligouridylate (up to tetramer, Fig. 1b) with terminal 2’,3’-cyclophosphate was also observed, indicating that oligomerization is taking place under the same mild phosphorylation conditions. Cytidine 2 was cyclo-phosphorylated under similar conditions (42%, 10 days). However, phosphorylation of purine-nucleosides 3 (<5%) and 4 (17%) was less efficient. Further investigations are underway to optimize the efficacy of the paste-reactions and the oligomerization process, and to analyze the nature of the phosphodiester linkages (2’,5’ vs 3’,5’) of oligomers.
The phosphorylation proceeds through nucleophilic attack of the 2’,3’-cis-hydroxyl groups on the protonated DAP leading to a putative monophosphoramidate (2’-NMPNH2 or 3’- NMPNH2) intermediate, which then cyclizes to give the 2’,3’-cyclophosphate derivatives 5-8. When imidazole is present, the protonated DAP is converted to the amidophosphoimidazolide (Supplementary Fig. 7), which reacts further. Support for these intermediates was obtained from 31P-NMR using 15N-labeled reactants (Supplementary Figs. 7–13). This phosphorylation chemistry, in principle, can be extended to other alternative (prebiotically plausible) nucleosides that have the adjacent cis-hydroxyl configuration,17 a work that is ongoing.
The hydrolysis of nucleoside-2’,3’-cyclophosphates to the 2’/3’-phosphates, and the reactivation to regenerate 2’,3’-cyclophosphates is of importance in prebiotic formation of oligonucleotides18. The reaction of 3’-nucleoside monophosphates (3’-NMPs, 9-12) and 2’-monophosphates (2’-CMP and 2’-AMP, 13-14) with DAP in water formed the corresponding 2’,3’-cyclophosphates efficiently under various conditions (Fig. 1a). The corresponding 3’- and 2’-amidodiphosphate derivatives are observed as intermediates, on the pathway to c-NMPs formation.
The 5’-nucleoside monophosphates (5’-NMPs, 15a-18a) were amidophosphorylated in water at pH 7 by DAP (Fig. 1c, Supplementary Tables 7–8), with 77–90% conversions to the corresponding 5’-nucleoside-amidodiphosphates (5’-NDPNH2, 19a-22a) within 5 days. The 5’-nucleoside diphosphates (5’-NDPs, 15b-18b) were similarly converted to the nucleoside-amidotriphosphates (5’-NTPNH2, 19b-22b) efficiently (Fig. 1c, Supplementary Table 9), with up to 10% of the corresponding 2’,3’-cyclophosphates. At pH 5.5 with Zn2+, the 5’-NDPNH2 (19a-22a) hydrolyze back to the starting 5’-NMP (15a-18a).
The phosphorylation-followed-by-hydrolysis raises the possibility of using DAP as a (re)activating agent for 5’-, 2’-, and 3’-nucleoside phosphates in water for ligation/oligomerization reactions, providing an alternative to the current methods of activation in prebiotic oligomerization and replication studies19. Phosphorylation of selected oligonucleotides with 5’-phosphate and 3’-phosphate terminal ends were briefly investigated, and found to produce the respective oligo-5’-DPNH2 and the corresponding oligo-2’,3’-cyclophosphate (via the oligo-3’-DPNH2), paralleling the observations made in the mononucleotides (Fig. 1d, Supplementary Tables 10–11). Investigations probing the scope of DAP for template-mediated ligation/oligomerization are ongoing.
Phosphorylation of glycerol and fatty acids by DAP leading to (self-assembly) vesicles.
Since cis-disposed 2’,3’-hydroxyl groups of nucleosides are converted to cyclophosphates, glycerol 23 was reacted with DAP with the purpose of accessing the building blocks of phospholipids (Fig. 2a). Mixing 23 with DAP and imidazole in water at r.t. led to the formation of glycerol-1,2-cyclophosphate 24 (≈ 12%); with the paste-reaction demonstrating efficient conversion (≈ 60%) via the amidophosphorylated intermediate (24i, Supplementary Table 12). Heating the paste reaction (50 °C) accelerated the reaction, but also led to the formation of the diglycerol- phosphodiester, indicating that the cyclophosphate is attacked by another molecule of glycerol. Based on the studies of Deamer20, we probed the ability for DAP to mediate ester bond formation by reacting nonanol with ammonium acetate under paste-reaction conditions, and found that DAP increased the efficiency of ester bond formation when compared to the control (Supplementary Fig. 117, Table 13). Encouraged by these observations, we performed a one-pot paste-reaction with nonanoic acid 2520 and glycerol in the presence of DAP and imidazole (Fig. 2b). Analysis of the crude reaction mixture suggested the formation of a cyclophospholipid 26, the structure of which was confirmed by comparison with synthesized authentic 26, (Fig. 2c, Supplementary Figs. 118–123). Such cyclophospholipid structures (e.g. cyclophosphatidic acid) are known in extant biology, but in a diagnostic and pharmacological context21.
Figure 2: DAP induced phosphorylation and esterification of glycerol with short chain fatty acids gives rise to simple mimics of phospholipids leading to formation of protocell like structures under the same reaction conditions signifying the single pot transition of simple building blocks to higher order self-assemblies.

Phosphorylation reaction of DAP (a) with glycerol, and (b) with nonanoic acid and glycerol under the paste-reaction conditions. (c) Synthesis of authentic cyclophospholipid, 26. (d) Transmission electron microscope (TEM) image of a sample (15 mg in 1 mL water) of crude reaction from Fig. 2b showing the formation of vesicle-like structures with a diameter ca. 9.2 μm. (e) TEM image of a sample (1 mg in 1 mL water) of authentic phospholipid 26 from Fig. 2c showing the formation of vesicle-like structures with a diameter ca. 3.5 μm. (F-H) Confocal laser scanning microscopy fluorescence images of vesicles prepared with authentic phospholipid 26 (1 mg in 0.1 mL water) with dye-encapsulation. (f) Green fluorescence indicates hydrophilic pyranine dye encapsulated within the cavity of the liposome. (g) Red fluorescence indicates rhodamine b dye labelling the bilayer phospholipid membrane of the liposome. (h) Fluorescence merged image of phospholipid vesicle prepared with both rhodamine b dye and pyranine dye. For details see supplementary information.
Mixing a portion of this crude paste-reaction containing 26 in water (pH ≈ 8.5) resulted in an opaque solution, which became clear upon sonication. It was filtered (0.2 µm) and analyzed by dynamic light scattering (DLS) and transmission electron microscope (TEM) techniques, which revealed formation of micelles and vesicle-like bilayer and multi-lamellar structures in the range of 30–110 nm (Supplementary Figs. 124 & 137). TEM analysis of the opaque solution itself (without sonication and filtration) displayed liposome-like structures with diameters around 280 nm (Supplementary Fig. 125). In addition, we were pleasantly surprised to find also TEM images implying the formation of giant bilayer vesicle-like structures with diameters on the average of 9.2 µm (Fig. 2d) with some structures nearing 20 µm (Supplementary Fig. 126). The control reaction lacking DAP, but containing only nonanoic acid and glycerol (Supplementary Figure 133), or each of the components alone (for example, nonanoic acid alone), did not show the formation of these “vesicle-like” structures (Supplementary Figs. 132–135). The opaque solution generated from the synthetic cyclophospholipid 26 in water was analyzed by TEM which showed “vesicle-like” structures (Fig. 2e) comparable to those obtained from the crude paste-reaction mixture. This demonstrates that cyclophospholipid 26, generated in the crude paste-reaction mixture, is indeed capable of forming these vesicle-like structures – perhaps even better in heterogeneous mixtures with glycerol20. To prove that the structures are indeed liposomes, we performed the dye-inclusion experiments20, wherein the confocal microscopy images showed that the red-rhodamine dye was retained within the bilayer, while the green-pyranine dye was encapsulated within the cavity of the liposome (Fig. 2g and 2f). Preparing the liposomes with both dyes afforded a merged-image suggesting an “uneven-enclosed” structure (Fig. 2h), probably from the changes in internal osmotic pressure22 and interlayer-distances23 when both dyes are present. Preliminary reactions of octanoic acid with DAP under the paste-reaction conditions gave rise to smaller vesicle-like structures (Supplementary Fig. 131). That a library of prebiotically plausible fatty acids, available through meteoritic delivery24 or other prebiotic means20 could form stable phospholipid liposomes such as giant-vesicles by phosphorylation in the presence of glycerol, while unprecedented, does align with the advantage provided by the physical and chemical heterogeneity in vesicles in the context of protocells25.
Phosphorylation of amino acids by DAP leading to oligopeptides.
Motivated by these observations we briefly explored – as a proof of principle – the phosphorylation of carboxylic acids of three representative prebiotic amino acids 27-29 (Fig. 3a) under aqueous conditions3. When glycine 27 was reacted with DAP in water, quantitative phosphorylation of the α-amino and the α-carboxyl groups was observed (Figs. 3b1–b6, Supplementary Figs. 138–140). Concomitant formation of peptides up to an octamer was observed by mass spectral analysis (Supplementary Figs. 141–143). Controls lacking DAP did not show any oligo-glycine formation (Fig. 3b1). The acyl-phosphoramidate of glycine 27i observed by NMR (Figs. 3b3 & 3b6) is proposed to be the active intermediate26 which leads to amide bond formation. There may be other possible mechanistic pathways,27−28 the establishment of which need further detailed investigations. Diketopiperazine (DKP) was also detected. With Gly-Gly 30, the formation of oligomers up to an octamer was observed by mass spectrometry (Supplementary Fig. 144). The reaction of DAP with aspartic acid 28 and glutamic acid 29 (Fig. 3a) also showed the formation of oligopeptides up to tetramers (Supplementary Figs. 145 & 147). In the case of 28, 1H-NMR shows ca. 23% conversion to higher order products with minimal DKP (Supplementary Fig. 146), while for 29, it was ca. 15% conversion to higher order products (DKP + peptides, Supplementary Fig. 148). Accurate determination of oligopeptide yields (currently complicated by the interference from the various phosphorylated species), optimization of reaction conditions for increasing oligopeptides yields, the nature of mechanistic pathways, and elucidation of the nature of connectivity (α- versus β-) are some of the issues that need to be addressed, and are underway. The promising preliminary results of oligopeptide formation in water warrant in-depth and systematic investigations to determine the scope and selectivity of the oligopeptide forming process, and to address the issues associated with random peptide synthesis such as the rapid increase in diversity of even short peptide sequences29.
Figure 3: DAP phosphorylates amino acids in water while activating them towards formation of oligopeptides in the same reaction setting.
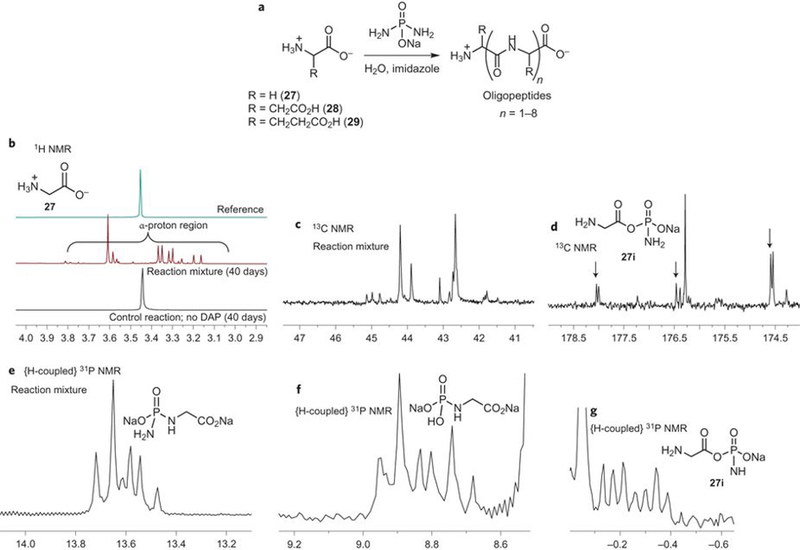
(a) Reaction of DAP with glycine, aspartate or glutamate leading to oligopeptides. (b) Reaction of glycine with DAP leading to (quantitatively) phosphorylated species which are intermediates on the way to oligopeptide formation, as seen by 1H-NMR (b1), 13C-NMR (b2-b3) and 1H-coupled-31P-NMR (b4-b6) of the reaction mixture. For conditions and details see supplementary information.
Discussion
The similar phosphorylation-conditions for all three classes of prebiological molecules (nucleosides, fatty acids and glycerol, and amino acids) suggest that they could be combined and conducted in a single-pot. Moreover, the commonality of conditions for the oligomerization of different building blocks suggests that productive and mixed chemistries might be possible,30–31 such as cross-catalyzing the oligomerization and self-assembly process, and the resulting higher order structures could, in turn, increase the efficiency of phosphorylation. This approach could create opportunities which go beyond the generation of phosphorylated building blocks, leading to the co-formation and co-existence of building blocks and their oligomers in the same locality, conducive for the emergence of primordial synergistic systems within confined (aqueous) environments– towards an RNA or pre-RNA world(s). Though there are similarities with extant biochemical pathways using such P-N activation chemistries32 (such as N-phosphoryl transfers 27–28), any comparison must be viewed with caution given the pitfalls of extrapolating extant biochemical pathways backwards all the way to prebiotic chemistry and vice-versa.33
It has been conjectured that combining the nitrogenous and oxygenous versions of prebiotic molecules (as in TNA and its nitrogenous versions34), could provide a library of alternative opportunities32,. The results from this work and previous DAP-phosphorylation investigations10,11,35, demonstrate that such an expansion of the phosphorylation scenario to include the prebiotically plausible nitrogenous version of phosphate (such as DAP) provides an efficient and alternative solution to the prebiotic-phosphorylation-problem. While DAP has been used as a prebiotically plausible reagent11,35, it has been produced from the prebiotically available trimetaphosphate36 by reaction with ammonia,37–38 at relatively high pH. Since the results demonstrated in this work proceed at lower pH, investigation of additional, prebiotically plausible and alternative pathways at milder pH, such as corrosion of phosphide minerals (schreibersite)39–40, towards the formation of DAP would be desirable. In that context, the detection of PN containing compounds in interstellar medium41–42 and star forming regions43 suggests that nitrogenous versions (or forerunners) of phosphate may not be unreasonable to contemplate32,44. The phosphorylation of small molecules by DAP and its analogs, may also prove to be useful in synthetic chemistry.32
Methods Summary (full details are given in the Supplementary Information)
General phosphorylation protocols:
DAP in water:
To the substrates (nucleosides, nucleotides, oligonucleotides, glycerol, nonanoic acid or amino acids) in water, DAP, with or without imidazole (and/or zinc chloride and/or magnesium chloride), was added. The pH was adjusted and maintained between 5.5–8 by the addition of aq. 4M HCl and was agitated at room temperature. Additional DAP was added based on the progress of the reaction and its consumption as monitored by NMR analyses.
DAP in “paste-reaction”:
The substrates (nucleosides, nucleotides, oligonucleotides and glycerol) and DAP, with or without imidazole, were ground together with few drops of water. Additional DAP was added based on the progress of the reaction and its consumption as monitored by NMR analyses.
Phosphorylation reaction analyses
The progress of the reactions was monitored by the following techniques: 1H, 13C and 31P NMR spectroscopy (nucleosides, nucleotides, glycerol, fatty acids and amino acids); FPLC (oligonucleotides); LC-MS (glycerol and amino acids); MALDI-Tof (oligouridylate, oligonucleotides and amino acids)
Vesicle preparation
Fatty acid (1 equiv.), glycerol (1 equiv.), DAP (5 equiv.), imidazole (5 equiv.) were mixed together with few drops of water and left at room temperature. An aliquot of this crude reaction mixture was mixed with water, vortexed (with and without sonication and filtration) to allow for the formation of micelles/vesicles.
Vesicle characterization
The structures obtained from the vesicle preparation described above (and also from synthetic phospholipid 26) were characterized by dynamic light scattering and visualized by transmission electron microscopy with negative staining, and confocal laser scanning microscopy with dye incorporation.
The data that support the findings if this study are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgements
The work was supported by a grant from the Simons Foundation to R. K. (327124) and NASA (NNX14AP59G). This is manuscript #29523 from The Scripps Research Institute. We thank Dr. M. Wood, Dr. T. Fassel and Dr. W. B. Kiosses of the Core Microscope Facility of TSRI, Dr. M. Janssen for initial TEM measurements, Dr. J. Kelly’s laboratory for DLS measurements, Dr. L. Leman for help with analysis of peptides, Dr. S. F. Dowdy laboratory for MALDI-Tof analysis. We are grateful to Dr. R. Ghadiri, Dr. D. Deamer, Dr. S. Mansy, Dr. P. Banerjee, Dr. Jack Szostak, Dr. Gerald Joyce, and our lab members for helpful discussions.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Lohrmann R & Orgel LE Prebiotic synthesis: phosphorylation in aqueous solution. Science 161, 64–66 (1968). [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AW Specific phosphorylation of the 2’- and 3’-positions in ribonucleosides. J. Chem. Soc. D 1393a (1969).
- 3.Leman LJ, Orgel LE & Ghadiri MR Amino acid dependent formation of phosphate anhydrides in water mediated by carbonyl sulfide. J. Am. Chem. Soc 128, 20–21 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Powner MW, Gerland B & Sutherland JD Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Burcar B et al. Darwin’s warm little pond: a one-pot reaction for prebiotic phosphorylation and the mobilization of phosphate from minerals in a urea-based solvent. Angew. Chem. Int. Ed 55, 13249–13253 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ et al. Evaporite borate-containing mineral ensembles make phosphate available and regiospecifically phosphorylate ribonucleosides: borate as a multifaceted problem solver in prebiotic chemistry. Angew. Chem. Int. Ed 55, 15816–15820 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Pasek MA & Kee TP On the origin of phosphorylated biomolecules. Origins of Life: The Primal Self-Organization, eds Egel Richard, Lankenau Dirk-Henner, & Mulkidjanian Armen Y.) 57–84 (Springer Berlin; Heidelberg, 2011). [Google Scholar]
- 8.Gull M Prebiotic phosphorylation reactions on the early Earth. Challenges 5, 193–212 (2014). [Google Scholar]
- 9.Ruiz-Mirazo K, Briones C & de la Escosura A Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev 114, 285–366 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy R, Guntha S & Eschenmoser A Regioselective α-phosphorylation of aldoses in aqueous solution. Angew. Chem. Int. Ed 39, 2281–2285 (2000). [PubMed] [Google Scholar]
- 11.Coggins AJ & Powner MW Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis. Nat. Chem 9, 310–317 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Meznik L, Thomas B & Töpelmann W Zum Reaktionsverhalten von phosphoroxidtriamid in alkalischen lösungen. Zeit. Chemie 22, 211–211 (1982). [Google Scholar]
- 13.Richter S, Töpelmann W & Lehmann H-A Zur Hydrolyse der Phosphorsäureamide. Zeit. anorg. allgem. Chemie 424, 133–143 (1976). [Google Scholar]
- 14.Bishop MJ, Lohrmann R & Orgel LE Prebiotic phosphorylation of thymidine at 65° C in simulated desert conditions. Nature 237, 162–164 (1972). [DOI] [PubMed] [Google Scholar]
- 15.Lohrmann R & Orgel LE Urea-inorganic phosphate mixtures as prebiotic phosphorylating agents. Science 171, 490–494 (1971). [DOI] [PubMed] [Google Scholar]
- 16.Schoffstall AM Prebiotic phosphorylation of nucleosides in formamide. Origins Life Evol. Biosph 7, 399–412 (1976). [DOI] [PubMed] [Google Scholar]
- 17.Cafferty BJ, Fialho DM, Khanam J, Krishnamurthy R & Hud NV Spontaneous formation and base pairing of plausible prebiotic nucleotides in water. Nat. Commun 7:11328 doi: 10.1038/ncomms11328 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowler FR et al. Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation. Nat. Chem 5, 383–389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kervio E, Sosson M & Richert C The effect of leaving groups on binding and reactivity in enzyme-free copying of DNA and RNA. Nucleic Acids Res 44, 5504–5514 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apel CL, Deamer DW & Mautner MN Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim. Biophys. Acta 1559, 1–9 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Gendaszewska-Darmach E & Drzazga A Biological relevance of lysophospholipids and green solutions for their synthesis. Curr. Org. Chem 18, 2928–2949 (2014). [Google Scholar]
- 22.Dubois M & Zemb Th. Swelling limits for bilayer microstructures: the implosion of lamellar structure versus ordered lamellae. Curr. Opin. Colloid Interface Sci 5, 27–37 (2000). [Google Scholar]
- 23.Zhu TF & Szostak JW Exploding vesicles. J. Sys. Chem 2, 4 (2011). [Google Scholar]
- 24.Lawless JG & Yuen GU Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 282, 396–398 (1979). [Google Scholar]
- 25.Szostak JW An optimal degree of physical and chemical heterogeneity for the origin of life? Phil. Trans. Royal Soc. Lond. B: Biol. Sci 366, 2894–2901 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhiman RS, Opinska LG & Kluger R Biomimetic peptide bond formation in water with aminoacyl phosphate esters. Org. Biomol. Chem 9, 5645–5647 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Knowles J Enzyme-catalyzed phosphoryl transfer reactions. Ann. Rev. Biochem 49, 877–919 (1980). [DOI] [PubMed] [Google Scholar]
- 28.Lassila JK, Zalatan JG & Herschlag D Biological phosphoryl-transfer reactions: Understanding mechanism and catalysis. Ann. Rev. Biochem 80, 669–702 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frederix PWJM et al. Exploring the sequence space for (tri-)peptide self-assembly to design and discover new hydrogels. Nat. Chem 7, 30–37 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Griesser H et al. Ribonucleotides and RNA promote peptide chain growth. Angew. Chem. Int. Ed 56, 1219–1223 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Adamala K & Szostak JW Competition between model protocells driven by an encapsulated catalyst. Nat. Chem 5, 495–501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karki M, Gibard C, Bhowmik S & Krishnamurthy R Nitrogenous derivatives of phosphorus and the origins of life: plausible prebiotic phosphorylating agents in water. Life, 7, 32 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazcano A Complexity, self-organization and the origin of life. The happy liaison? In Origins of Life: Self-Organization and/or Biological Evolution? 13,13–22 (2009). [Google Scholar]
- 34.Eschenmoser A The TNA-family of nucleic acid systems: properties and prospects. Orig. Life Evol. Biosph 34, 277–306 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Anastasi C et al. The Search for a potentially prebiotic synthesis of nucleotides via arabinose-3-phosphate and its cyanamide derivative. Chem. Eur. J 14, 2375–2388 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Yamagata Y, Watanabe H, Saitoh M & Namba T Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature 352, 516–519 (1991). [DOI] [PubMed] [Google Scholar]
- 37.Feldmann W & Thilo E Zur Chemie der kondensierten Phosphate und Arsenate. XXXVIII. Amidotriphosphat. Zeit. anorg. allgem. Chemie 328, 113–126 (1964). [Google Scholar]
- 38.Thilo E Zur Strukturchemie der kondensierten anorganischen Phosphate. Angew. Chem 77, 1056–1066 (1965). [Google Scholar]
- 39.Pasek MA & Lauretta DS Aqueous corrosion of phosphide minerals from iron meteorites: A highly reactive source of prebiotic phosphorus on the surface of the early earth. Astrobiology 5, 515–535 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Bryant DE & Kee TP Direct evidence for the availability of reactive, water soluble phosphorus on the early Earth. H-Phosphinic acid from the Nantan meteorite. Chem. Commun 2344–2346 (2006). [DOI] [PubMed]
- 41.Turner BE & Bally J Detection of interstellar PN: The first identified phosphorus compound in the interstellar medium. Astrophys. J 321, L75–79 (1987). [DOI] [PubMed] [Google Scholar]
- 42.Ziurys LM Detection of interstellar PN: The first phosphorous-bearing species observed in molecular clouds. Astrophys. J 321, L81–85 (1987). [DOI] [PubMed] [Google Scholar]
- 43.Rivilla VM et al. The first detections of the key prebiotic molecule PO in star-forming regions. Astrophys. J 826, 161 (2016). [Google Scholar]
- 44.Oró J, Miller SL & Lazcano A The origin and early evolution of life on earth. Ann. Rev. Earth Planetary Sci 18, 317–356 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.