Introduction
Autism spectrum disorder (ASD) is a group of heterogeneous childhood onset conditions characterized by social communication deficits, restricted interests and repetitive behaviors1. The estimated worldwide prevalence ranges from 6 to 14 per 1,000 in children with a 4 to 5 fold higher rate in boys than girls2. Because ASD has an early onset and chronic course, many affected individuals require lifelong care3. The core features of ASD can cause substantial impairments that may be amplified by behavioral and emotional problems4, 5. These cooccurring problems are common complaints from parents of children with ASD and the focus of psychotropic medication and behavioral intervention in this population6, 7.
Concomitant psychiatric disorders are common in children with ASD 8,9, 10. In a sample of 109 children with autism spectrum disorders, Leyfer et al. (2006)11 reported that 72% of the children (average age = 9 years; 68% IQ > 70) had at least one Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) Axis I disorder. The median number of cooccurring psychiatric disorders was three. Children in this study were consecutive cases recruited from longitudinal and neuroimaging studies, unselected for psychiatric problems. Using an epidemiological sample of 10 to 14-year olds with DSM-IV ASD (N=112), Simonoff et al. (2008) 10 reported a 3-month prevalence of 70% for concomitant psychiatric disorders with 41% having had two or more diagnoses. The most common were Social Anxiety Disorder (29%), ODD (30%), and ADHD (28%). Brookman-Frazee et al. (2017)12 reported that 92% of children with ASD (N = 201; mean age 9 years, range 4-14) receiving publicly funded mental health services in Southern California met criteria for another psychiatric disorder. The most frequently reported psychiatric disorders were ADHD (78%), ODD (58%), and Anxiety disorders (56%).
In contrast, a randomly selected community sample of 986 children and adolescents with intellectual disability from a Dutch province reported much lower 1-year prevalence rates, with only 41% of those screening positive for an ASD meeting criteria for any impairing DSM-IV diagnosis13. Therefore, although psychiatric disorders appear common in youth with ASD, the range and types of disorders common to this population merits further study. There is also a need to understand the demographic and clinical characteristics that are associated with these concomitant disorders.
The high likelihood of a second psychiatric disorder in children with one disorder is well established14. This observation raises questions about uncertainty of the boundaries between psychiatric diagnoses in children. The overlap and shared symptomatology across diagnostic categories is not unique to children and has led to growing interest in transdiagnostic constructs15,16,17.
In this report, we explore rates, patterns, and correlates of concomitant psychiatric disorders in a large sample of well-characterized, treatment-seeking children with ASD who participated in one of six federally-funded, multisite RCTs. The children in all six studies were recruited for disruptive behavior such as tantrums, aggression, self-injury, impulsive behavior or hyperactivity. We expected to find (a) high rates of concomitant psychiatric disorders, (b) higher rates of anxiety disorders in children with an IQ ≥ 70, and (c) poorer response to the study intervention in children with more concomitant psychiatric disorders.
Methods
Participants
The sample of 658 children (585 males and 97 females, aged 3-17 years) with ASD were participants in one of six multisite RCT18, 19, 20, 21, 22, 23. Table 1 shows key characteristics of the six studies, including treatment targets, entry criteria, and key outcome measures. All studies were approved by each site’s institutional review board and written informed consent was obtained from parents or legal guardian prior to data collection. Participants were recruited from a number of sources across sites and studies, including schools, the internet, the radio, outpatient clinics, and word of mouth.
Table 1.
Key Characteristics of the Six Multisite RCT
| Study | Aim | N | Age Range |
IQ evaluation | Treatment Target | Outcome Measures |
Entry Criteria | Primary finding |
|---|---|---|---|---|---|---|---|---|
| Aman et al., 200918 | RIS alone vs. RIS + PT | 124 | 4-14 | ASB Leiter MSEL | Irritability | ABC–I HSQ CGI-I | ABC-I ≥ 18 CGI-S ≥ 4 | RIS + PT > RIS |
| Bearss et al., 201519 | PT vs PEP | 180 | 3-6 | MSEL | Irritability | ABC-I HSQ-ASD CGI-I | ABC-I ≥ 15 CGI-S ≥ 4 | PT > PEP |
| Handen et al., 201520 | ATX vs placebo and PT vs no PT | 128 | 5-14 | ASB MSEL | Hyperactivity and Inattention | CGI-I SNAP HSQ ABC | SNAP-IV M ≥ 1.5 CGI-S ≥ 4 | ATX & PT > placebo |
| RUPP Autism Network, 200221 | RIS vs. Placebo | 101 | 5-17 | NR | Irritability | ABC-I CGI-I | ABC-I ≥ 18 CGI-S ≥ 4 | RIS > Placebo |
| RUPP Autism Network, 200522 | MPH vs. Placebo | 72 | 5-17 | SIT | Hyperactivity | ABC-H CGI-I | SNAP-IV ADHD ≥ 27 SNAP-IV H-I ≥ 10 CGI-S ≥ 4 |
MPH > Placebo |
| Scahill et al., 201523 | GUAN vs Placebo | 62 | 5-14 | ASB MSEL | Hyperactivity | ABC-H ADHD Rating Scale CGI-I | ABC-H ≥ 24 CGI-S ≥ 4 | GUAN > Placebo |
Abbreviations: GUAN=Guanfacine; MPH=Methylphenidate; RIS=Risperidone; PEP=Parent education; PT=Parent Training; ASB= Abbreviated Stanford-Binet Intelligence Scales, fifth edition (Roid, 2003); Leiter= Leiter International Performance Scale (Roid and Miller, 1997); MSEL= Mullen Scale of Early Learning (Mullen, 1995); NR=Not reported; SIT= Slosson Intelligence Test (Slosson, 1983); ABC-I= Aberrant Behavior Checklist-Irritability subscale; HSQ=Home Situation Questionnaire; CGI-I=Clinical Global Impression-Improvement; HSQ-ASD= Home situation Questionnaire-Autism Spectrum Disorder; CYBOCS-PDD= Children’s Yale-Brown Obsessive-Compulsive Scale-Modified for Pervasive Developmental Disorders; RBS-R=Repetitive Behavior Scale-Revised; ABC-H= Aberrant Behavior Checklist-hyperactivity subscale; SNAP-IV ADHD= Swanson, Nolan, and Pelham– version IV ADHD subscale; SNAP-IV H/I= Swanson, Nolan, and Pelham– version IV Hyperactivity/Impulsivity subscale; M = mean
Procedure
An experienced multidisciplinary team conducted a pretreatment evaluation that included medical and developmental histories, as well as behavioral and diagnostic assessments. The diagnosis of ASD was based on the current version of the DSM at the time of the study24, 25. In addition to clinical assessment, ASD diagnoses were supported by either the Autism Diagnostic Interview-Revised26 or the Autism Diagnostic Observation Schedule27. The pretreatment assessment also included parent and clinician ratings (described below). Participants had to be healthy, meet study-specific symptom severity thresholds, and have a minimum mental age (e.g., receptive language ≥ 18 months). All drug studies required participants to be medication-free at baseline (with the exception of stable anticonvulsant treatment for seizure disorder). The parent training study19 permitted children to be on psychotropic medication if stable with no planned changes for the duration of the six-month study.
Measures
Early Childhood Inventory (ECI)/ Child and Adolescent Symptom Inventory (CASI)
The pretreatment assessment also included the ECI or CASI to screen for concomitant psychiatric conditions. These DSM-IV-referenced, parent-rated scales are designed to screen for child psychiatric disorders28, 29, 30, 31. The ECI and CASI are identical except for minor differences based on age. Here we used the subscales for ADHD (18 items), ODD (8 items), CD (10 items for ECI and 15 items for CASI), major depressive disorder and dysthymia (11 items for ECI and 13 items for CASI), and ASD (12 items). The subscales for anxiety disorders (21 items for ECI and 20 items for CASI) included social phobia, generalized anxiety, and separation anxiety. We did not include specific phobia as it only includes one item. Previous studies have supported the validity of ECI and CASI subscales in children with ASD32, 33. Items are rated 0 (never) to 3 (very often) and can be scored in two different ways: symptom severity (total of scores within diagnostic category) or symptom count (number of items rated 2 or 3 within category). In the current study, symptom counts that met or exceeded DSM-IV criteria were used to define a positive screen for each diagnostic category.
Aberrant Behavior Checklist (ABC)
The ABC is a 58-item parent rating comprised of five subscales: Irritability (tantrums, aggression, and self-injurious behaviors, 15 items); Social Withdrawal (response to others, initiation of interaction, 16 items); Stereotypy (mannerisms and repetitive movements, 7 items); Hyperactivity and noncompliance (16 items); and Inappropriate Speech (repetitive vocalizations, 4 items)34. Each item is rated 0 to 3 with higher scores indicating greater severity. Kaat et al. (2014)35 provided evidence of validity and normative data for children with ASD.
Clinical Global Impression-Scale
The Clinical Global Impression – Severity (CGI-S) subscale is a 7-point scale ranging from 1 (normal) to 7 (among the most extremely ill patients)36. By convention, a score of 3 (Mild) was used to describe a child who met criteria for ASD without associated behavioral problems. A score of 4 (Moderate) was required for entry in all trials. Although the pre-treatment CGI-S score was weighted by the specific treatment target (e.g., irritability, hyperactivity), the evaluating clinician incorporated all available information to assign the score.
The Clinical Global Impression-Improvement (CGI-I) subscale is also a 7-point scale designed to measure overall change from baseline. Scores on the CGI-I range from 1 (Very Much Improved) through 4 (Unchanged) to 7 (Very Much Worse). In all six trials, scores of Much Improved or Very Much Improved defined positive treatment response. The CGI-I was rated by an independent evaluator, who was blind to treatment assignment. In this report, we used the CGI-I to classify treatment response at the end of the acute phase. The Handen et al. (2015)20 study used separate CGIs for Hyperactivity/Inattention and Noncompliance, as this was a study for youth selected for ADHD. We used the CGI Hyperactivity/Inattention in this report.
Measures of Intellectual functioning
Different tests were used across the studies to measure intellectual functioning. They included the Stanford-Binet Intelligence Scales: Fifth edition37; Leiter International Performance Scale-Revised38, Mullen Scales of Early Learning39, and Slosson Intelligence Test40. Because several different tests were used, children were classified as ≥ 70 or < 70 IQ.
Statistical Methods
Each of the six data sets was examined for missing data across common measures. Minor differences in the documentation of demographic data (e.g., school placement) were resolved by consensus (LL, CMcC) and by collapsing across levels to allow for aggregating data across studies. Descriptive statistics were calculated for all variables of interest and included means and standard deviations for continuous measures or counts and percentages for categorical data. The association of concomitant diagnoses was examined using generalized estimating equations (GEE) with a binomial distribution and a logit link41. The GEE approach was used to account for the correlation between participants nested within a study. Resulting association between diagnoses are presented as adjusted odds ratios with corresponding 95% confidence intervals.
The frequency of concomitant psychiatric disorders was compared across demographic and clinical subgroups using Chi-square tests. In analyses with the CGI-I, only children receiving the active treatment were included (n = 478). When a demographic or clinical variable was ordinal (e.g., CGI-Severity: Moderate, Marked, Severe), associations with diagnosis were tested using the Cochran Armitage test for trend. For continuous variables, such as ASD severity score and age, subjects were stratified by the median value of the sample (e.g., age ≤ 6 years vs > 6 years).
Uniform criteria were used to collapse ECI/CASI subscales for analyses. For example, the presence of any anxiety disorder was based on a positive screen for generalized anxiety, separation anxiety, or social anxiety. Similarly, the presence of any mood disorder was based on a positive screen for major depressive disorder or dysthymia. The presence of any mood or anxiety disorder was classified as internalizing disorders; and externalizing disorders comprised ADHD, ODD, or CD.
Statistical analyses were performed using SAS v. 9.4. Given the sample size and number of comparisons, statistical significance was assessed at the 0.01 level to control for false discovery rate.
Results
Demographics
Table 2 shows demographic and clinical characteristics of children with ASD and their caregivers. In all, 73.1% of children were non-Hispanic White and 59.4% had an IQ ≥ 70. Altogether, 83.5% of mothers had attended some college/trade school.
Table 2.
Demographic and Clinical Characteristics of Children with ASD
| Characteristics N (%), unless otherwise noted |
N = 658 |
|---|---|
| Age (years), Mean ± SD | 7.2 ±2.6 |
| Race | |
| Non-Hispanic White | 481 (73.1%) |
| Non-Hispanic Black | 65 (9.9%) |
| Asian | 29 (4.4%) |
| Hispanic/Latino | 54 (8.2%) |
| Other | 29 (4.4%) |
| Maternal Education (n = 557)a | |
| High School Graduate or Less | 91 (16.3%) |
| Some College/Trade school or 4 year degree | 385 (69.1%) |
| Graduate or Professional school | 80 (14.4%) |
| Not in household | 1 (0.2%) |
| Intelligence Quotient (n = 633)b | |
| < 70 | 257 (40.6%) |
| ≥ 70 | 376 (59.4%) |
| CGI-Severity (n = 656)c | |
| Moderate (4) | 199 (30.3%) |
| Marked (5) | 327 (49.9%) |
| Severe or extreme (6 or 7) | 130 (19.8%) |
| ABC, Mean ± SD | |
| Irritability (n = 656) | 22.7 ± 9.1 |
| Social Withdrawal (n = 656) | 13.6 ± 8.6 |
| Stereotypic Behavior (n = 655) | 7.6 ± 5.4 |
| Hyperactivity/Noncompliance (n = 655) | 32.3 ± 9.0 |
| Inappropriate Speech (n = 654) | 5.9 ± 3.5 |
| CASI ASD Total Scoree Mean ± SD (n = 654) | 20.6 ± 7.3 |
RUPP (2002) did not collect maternal education
IQ not available in 25 subjects
CGI not available in 2 subjects
CGI-Improvement summarized only for patients receiving active study treatment
Summed score across 12 PDD items. Scores range 0 – 36.
Rates and patterns of concomitant disorders
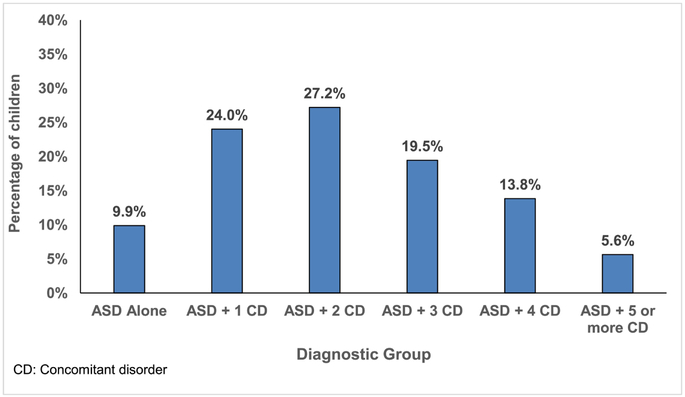
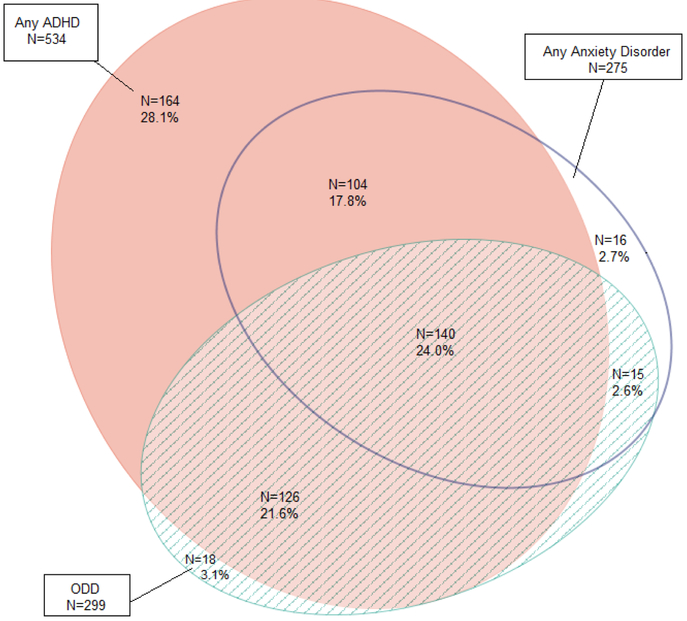
Table 3 shows positive screen rates for all diagnostic categories. With a few exceptions, rates were quite consistent across studies. 81.2% of the total sample met criteria for any type of ADHD, with most children meeting criteria for the combined subtype (49.1%). Rates for ODD and any anxiety disorder were 45.5% and 41.9%, respectively. CD and any mood disorders occurred at 11.7% and 7.5%, respectively. As shown in Figure 1, 66.1% of the sample had two or more concomitant psychiatric disorders. Figure 2 depicts the diagnostic overlap in a proportional Venn Diagram for the three most frequently endorsed diagnostic categories of ADHD, ODD, and any anxiety disorder. Among children who met criteria for ADHD only 28.1% did not meet criteria for ODD or an anxiety disorder. In the full sample, 24% met criteria for all three diagnostic categories.
Table 3.
Rates of Diagnosis in Each Study
| RUPP, 2002 (n = 101) |
RUPP, 2005 (n = 66) |
Aman etal., 2009 (n = 124) |
Bearss etal., 2015 (n =177) |
Scahill etal., 2015 (n = 62) |
Handen etal., 2015 (n = 128) |
Total (n = 658) |
|
|---|---|---|---|---|---|---|---|
| ADHD | 72 (71.2%) | 57 (86.4%) | 105 (84.7%) | 132 (74.6%) | 59 (95.2%) | 109 (85.1%) | 534 (81.1%) |
| ADHD combined | 44 (43.6%) | 40 (60.6%) | 79 (63.7%) | 72 (40.7%) | 34 (54.8%) | 54 (42.2%) | 323 (49.1%) |
| ADHD hyperactive | 11 (10.9%) | 5 (7.6%) | 7 (1.1%) | 38 (21.5%) | 8 (12.9%) | 9 (7.0%) | 78 (11.9%) |
| ADHD inattentive | 17 (16.8%) | 12 (18.2%) | 19 (15.3%) | 22 (12.4%) | 17 (27.4%) | 46 (35.9%) | 133 (20.2%) |
| ODD (n = 657) | 42 (41.6%) | 23 (35.4%) | 78 (62.9%) | 95 (53.7%) | 20 (32.3%) | 41 (32.0%) | 299 (45.5%) |
| CD (n = 656) | 19 (19.0%) | 4 (6.2%) | 27 (21.8%) | 18 (10.2%) | 4 (6.5%) | 5 (3.9%) | 77 (11.7%) |
| Any Anxiety Disorder (n = 657) | 34 (33.7%) | 30 (46.2%) | 54 (43.6%) | 71 (40.1%) | 28 (45.3%) | 58 (45.3%) | 275 (41.9%) |
| Generalized | 18 (17.8%) | 19 (29.2%) | 30 (24.2%) | 35 (19.8%) | 18 (29.0%) | 40 (31.3%) | 160 (24.4%) |
| Social | 22 (21.8%) | 11 (16.7%) | 31 (25.0%) | 27 (15.3%) | 18 (29.0%) | 32 (25.0%) | 141 (21.4%) |
| Separation | 9 (8.9%) | 10 (15.2%) | 21 (16.9%) | 22 (12.4%) | 10 (15.2%) | 7 (5.5%) | 74 (11.3%) |
| Any Mood Disorder | 7 (6.9%) | 4 (6.1%) | 13 (10.5%) | 19 (10.7%) | 4 (6.5%) | 2 (1.6%) | 49 (7.5%) |
| Major Depression | 3 (3.0%) | 1 (1.5%) | 4 (3.2%) | 7 (4.0%) | 0 (0%) | 1 (0.8%) | 16 (2.4%) |
| Dysthymia | 7 (6.9%) | 4 (6.1%) | 11 (8.9%) | 19 (10.7%) | 4 (6.5%) | 1 (0.8%) | 46 (7.0%) |
| Any Externalizing Disorder | 75 (76.3%) | 58 (89.2%) | 116 (93.6%) | 148 (83.6%) | 59 (95.2%) | 112 (87.5%) | 568 (86.5%) |
| Any Internalizing Disorder | 36 (35.6%) | 31 (47.7%) | 57 (46.0%) | 78 (44.1%) | 29 (46.8%) | 59 (46.1%) | 290 (44.1%) |
Fig. 1.
Number of concomitant psychiatric diagnoses.
Fig. 2.
Proportional Venn Diagram illustrating patterns of comorbidity.
Table 4a presents associations between ADHD and other diagnostic conditions. It shows significant associations with most categories. All adjusted odds ratios were above 2.0, with the exception of mood disorders. The odds ratios indicated that the odds of children with ADHD also having ODD were 2.7 times higher than children without ADHD. Similarily, the odds of having an internalizing disorder were 2.5 times higher in children with ADHD compared to those without ADHD. The odds of having CD were 3 times higher in children with ADHD compared to children without ADHD; however the p value = .025 and the confidence interval was quite large.
Table 4a.
Frequency of Concomitant Psychiatric Diagnoses in Children with and without ADHD1
| No ADHD (N = 123) |
ADHD (N = 534) |
Adjusted Odds Ratio (95% CI)2 |
p-value | |
|---|---|---|---|---|
| ODD | 33 (26.8%) | 266 (49.8%) | 2.7 (1.66 – 4.43) | <0.001 |
| CD | 6 (4.9%) | 71 (13.3%) | 3.0 (1.15 – 7.83) | 0.025 |
| Anxiety Disorder | 31 (25.2%) | 244 (45.7%) | 2.5 (1.37 – 4.55) | 0.003 |
| Mood Disorder | 7 (5.7%) | 42 (7.9%) | 1.4 (0.76 – 2.69) | 0.272 |
|
Internalizing Disorder |
33 (26.8%) | 257 (48.1%) | 2.5 (1.35 – 4.74) | 0.004 |
Percents are percent of the column with the indicated diagnosis
Adjusted for correlation of subjects nested within study.
Table 4b presents associations between anxiety disorders and other diagnostic conditions. All values are statistically significant (p ≤ .003) with adjusted odds ratios > 2.0. Odds of screening positive for an externalizing disorder were 4.2 times higher in children with an anxiety disorder compared to children without an anxiety disorder.
Table 4b.
Frequency of Concomitant Psychiatric Diagnoses in Children with and without an Anxiety Disorder1
| No Anxiety Disorder (N = 382) |
Anxiety Disorder (N = 275) |
Adjusted Odds Ratio (95% CI)1 |
p-value | |
|---|---|---|---|---|
| Any ADHD | 290 (75.9%) | 244 (88.7%) | 2.5 (1.37 – 4.55) | 0.003 |
| ODD | 144 (37.7%) | 155 (56.4%) | 2.1 (1.39 – 3.28) | <0.001 |
| CD | 28 (7.4%) | 49 (17.8%) | 2.7 (2.13 – 3.51) | <0.001 |
| Mood Disorder | 15 (3.9%) | 34 (12.4%) | 3.5 (2.47 – 4.82) | <0.001 |
| Externalizing Disorder | 308 (80.6%) | 260 (94.6%) | 4.2 (1.86 – 9.34) | <0.001 |
Percents are percent of the column with the indicated diagnosis
Adjusted for correlation of subjects nested within study.
Demographic and Clinical Characteristics of Participants with and without Concomitant Conditions
Tables 5a and 5b show demographic and clinical characteristics of children with ASD and at least one additional psychiatric disorder (note: groups are not mutually exclusive). Table 5a reveals no association between the presence of a concomitant disorder and sex. Children under the age of six years had higher rates of ODD (51.9% vs 41.1%, p = 0.007), but other significant associations with age were not observed. With the exception of CD and ADHD, children with a concomitant disorder and those with two or more disorders were significantly more common in children with IQ ≥ 70.
Table 5a.
| Co-occuring Disorder Group |
Sex | Age | IQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (N = 563) |
Female (N = 94) |
P-value | ≤6 year (N = 268) |
> 6 years (N = 390) |
p-value | < 70 (N = 257) |
≥ 70 (N = 376) |
p-value | |
| ADHD | 464 (82.3%) | 70 (74.5%) | 0.073 | 212 (79.1%) | 322 (82.6%) | 0.265 | 203 (79.0%) | 311 (82.7%) | 0.239 |
| ODD | 260 (46.2%) | 39 (41.5%) | 0.398 | 139 (51.9%) | 160 (41.1%) | 0.007* | 96 (37.4%) | 195 (51.9%) | <0.001* |
| CD | 64 (11.4%) | 13 (13.8%) | 0.496 | 35 (13.1%) | 42 (10.8%) | 0.366 | 35 (13.6%) | 40 (10.7%) | 0.260 |
| Any Anxiety Diosrder | 237 (42.1%) | 38 (40.4%) | 0.761 | 103 (38.4%) | 172 (44.2%) | 0.140 | 82 (31.9%) | 188 (50.0%) | <0.001* |
| Any Mood Disorder | 42 (7.5%) | 7 (7.5%) | 1.00 | 23 (8.6%) | 26 (6.7%) | 0.358 | 10 (3.9%) | 35 (9.3%) | 0.009* |
| Internalizing Disorder | 249 (44.2%) | 41 (43.6%) | 0.912 | 111 (41.4%) | 179 (46.0%) | 0.244 | 85 (33.1%) | 197 (52.4%) | <0.001* |
| Externalizing Disorder | 492 (87.4%) | 76 (80.9%) | 0.086 | 232 (86.6%) | 336 (86.4%) | 0.944 | 212 (82.5%) | 336 (89.4%) | 0.013* |
| ≥ 2 additional disorders * | 373 (66.1%) | 62 (66.0%) | 0.973 | 180 (67.2%) | 255 (65.4%) | 0.636 | 148 (57.6%) | 276 (73.4%) | <0.001* |
Groups are not mutually exclusive
Percents are percent of the column with the indicated diagnosis
Table 5b shows no association at the p <.01 level between diagnostic subtypes and CGI-S. Only one significant association emerged with CGI-I. Children who responded to study interventions were less likely to screen positive for ODD (37.6% positive responder vs 51.2% non responder, p = 0.003). Finally, children with more elevated ASD scores had higher rates of psychiatric problems in all categories, but only ADHD and externalizing disorders reached statistical significance at the p < 0.01 level.
Table 5b.
Clinical Characteristics of Children with ASD by Concomitant Condition1
| Co-occuring Disorder Group |
CGI-Severity | ASD Score | CGI-Improvement (N = 478) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moderate (N = 199) |
Marked (N = 327) |
Severe (N = 129) |
p- value3 |
≤ 20 (N = 333) |
> 20 (N = 321) |
p-value | Non Response (N = 189) |
Positive Responder (N = 289) |
p-value | |
| ADHD | 161 (80.9%) | 262 (80.1%) | 109 (83.9%) | 0.573 | 255 (76.6%) | 277 (86.3%) | 0.001* | 157 (83.1%) | 232 (80.3%) | 0.443 |
| ODD | 84 (42.2%) | 144 (44.0%) | 69 (53.5%) | 0.060 | 146 (43.8%) | 153 (47.7%) | 0.327 | 71 (37.6%) | 148 (51.2%) | 0.003* |
| CD | 20 (10.1%) | 33 (10.1%) | 24 (18.6%) | 0.035* | 30 (9.0%) | 47 (14.7%) | 0.025* | 24 (12.8%) | 31 (10.7%) | 0.496 |
| Any Anxiety Disorder | 80 (40.2%) | 140 (42.8%) | 55 (42.6%) | 0.619 | 124 (37.2%) | 150 (46.7%) | 0.014* | 80 (42.3%) | 120 (41.5%) | 0.861 |
| Any Mood Disorder | 20 (10.1%) | 24 (7.3%) | 5 (3.9%) | 0.037* | 19 (5.7%) | 30 (9.4%) | 0.077 | 9 (4.8%) | 28 (9.7%) | 0.049 |
| Internalizing Disorder | 84 (42.4%) | 149 (45.6%) | 57 (44.2%) | 0.654 | 132 (39.6%) | 157 (48.9%) | 0.017* | 85 (45.0%) | 126 (43.6%) | 0.767 |
| Externalizing Disorder | 173 (86.9%) | 277 (84.7%) | 116 (89.9%) | 0.570 | 276 (82.9%) | 290 (90.3%) | 0.005* | 165 (87.3%) | 252 (87.2%) | 0.973 |
| At least 2 additional dx* | 127 (63.8%) | 217 (66.4%) | 89 (68.5%) | 0.375 | 206 (61.9%) | 228 (71.0%) | 0.013* | 126 (66.7%) | 198 (68.5%) | 0.673 |
Groups are not mutually exclusive
Percents are percent of the column with the indicated diagnosis
Test for trend; Treating CGI as an ordinal outcome
Discussion
This is one of the largest studies of concomitant psychiatric disorders in children with ASD published to date. A unique aspect of the report is the well-characterized sample, with longitudinal data on treatment outcome. The findings suggest that boundaries of co-occurring DSM-defined disorders are blurry in this sample of children with ASD seeking treatment for disruptive behavior. The high frequency of multiple DSM-defined disorders has been reported in several studies of children with ASD using different methodologies12, 8, 9, 10
The rates and patterns of probable concomitant psychiatric disorders observed in this sample were similar to those reported in children with ASD receiving publicly-funded mental health services12. The high rates of ODD and CD in children with ADHD have been observed in the general pediatric population and in children with ASD14, 10. In the current sample of children with ASD, there was also a high co-occurrence of ADHD and anxiety disorders. Almost half (244 of 534) of children with ADHD also screened positive for an anxiety disorder. The prevalence estimates for all anxiety disorders are likely understated because specific phobia was not included. The high co-occurrence of ADHD and anxiety disorders in youth with ASD was also observed by Brookman-Frazee et al. (2017)12. In the general pediatric population, by contrast, the review by Angold et al. (1999)14 reported significantly lower co-occurrence of ADHD and anxiety disorders compared to the association between ADHD and ODD/CD. In their sample of 579 children with ADHD, the Multimodal Treatment Study of ADHD (MTA) observed a 34% rate of anxiety disorders (excluding specific phobia)42. This higher co-occurrence of ADHD and anxiety disorders in our sample of children with ASD compared to the general pediatric population may reflect the difficulties parents have in distinguishing between anxiety symptoms from ADHD symptoms such as restlessness, distractibility and disruptive behavior43. It may also speak to the shared etiology between disorders44, 45.
In the current sample, the rates of concomitant psychiatric disorders were higher in children with IQ ≥ 70 than children < IQ 70. This trend was not observed in studies by Simonoff et al. (2008)10 or Brookman-Frazee et al. (2017)12. The difference across IQ groups in our sample is greatest in anxiety disorders. This is consistent with findings in a prior study in which CASI anxiety scores were significantly lower in children with IQ < 70, presumably because the presence of language is a precondition for endorsing several anxiety items46.
The presence of another psychiatric disorder did not drive the CGI-S ratings. For example, the rates of ADHD and anxiety disorders were similar across CGI-S ratings of Moderate, Marked or Severe. On the CGI-I, children with concomitant ODD were more likely to show a positive response. We note that these trials selected children with disruptive behavior and ADHD. That higher levels of noncompliant and defiant behavior at baseline, the essence of ODD, predicted higher positive response rates is not surprising given that the study treatments were directed at these behaviors and selection criteria would have guaranteed substantial room for participants to show improvements. The rate of positive response was not influenced by any other diagnostic category. The minor difference in the rate of positive response for children with or without a mood disorder fell below our predetermined significance level. Even the presence of two or more concomitant disorders did not reduce the rate of positive response. In the MTA study, Jensen et al. (2001)47 reported that the presence of multiple concomitant psychiatric disorders did affect outcome. Children with ADHD and multiple comorbid psychiatric disorders required combined mediation and behavioral intervention to respond optimally.
The results of this study raise fundamental questions about the meaning of concomitant psychiatric disorders in children with ASD. The introduction of DSM-III was an important milestone for criteria-driven categorical diagnosis. It has also sparked extensive debate whether the co-occuring conditions are separate or somehow etiologically related. In a condition such as ASD involving many aspects of everyday life, it may not be surprising that youth with ASD would exhibit behaviors that fall under various diagnostic categories. Whether the patterns of co-occuring psychiatric disorders in children with ASD observed in this study are distinct conditions or variable phenotypic manifestations of ASD cannot be resolved here. The aim of this study was to describe the patterns of concomitant disorders in children with ASD and to evaluate similarities and differences of identified subgroups. Further work on identifying subgroups of children with ASD using categorical diagnoses, dimensions of symptom severity or biological markers could lead to refinements in the psychopharmacological and behavioral interventions in ASD.
Our findings should be interpreted in light of several limitations. The children described in this report were participants in RCTs focused on either hyperactivity or disruptive behavior such as tantrums, aggression and self-injury. The findings may not apply to all children with ASD. Indeed, RCTs are often criticized for neglecting external validity in favor of internal validity48. The concern is that over emphasis on internal validity leads to narrow entry criteria and limited generalizability. Despite the imposition of relatively strict entry criteria, however, the participants in these multisite studies appear to resemble the complex cases seen in clinic. In addition, study participants were enrolled from 11 cities in the US, which also supports the generalizability of the results. When this limitation on generalizability is appreciated, such a clinical sample can be very informative if the target group we wish to generalize to is similar. Another limitation is the high reliance on parents as informants. Parental biases in reporting may have contributed to the blurring of boundaries between disorders and enhanced the identification of concomitant disorders. There might also be misinterpretation by raters of core features of ASD as psychiatric symptoms. Relatedly, diagnoses were based solely on caregiver-completed CASI/ECI symptom count cutoff scores, which do not include consideration of impairment, differential diagnosis, or multiple sources of information. Therefore, rates may differ to estimates of “caseness” based on alternative evaluative strategies. Finally, our analysis focused on DSM categories. We did not delve into transdiagnostic approaches or research domain criteria, both of which warrant further research in children with ASD. Of course, clinical and research realities necessitate both categorical and dimensional approaches. Categories have dimensions, and dimensions become categories when cutoffs are used.
Conclusions and clinical implications
These data on treatment-seeking children with ASD show that psychiatric problems often occur in multiples. It is further evidence of heterogeneity of clinical presentation in ASD and the need to individualize treatment based on specific pattern of clinical manifestations. Diagnostic assessments of children with suspected ASD also need to be broad-based and include careful screening for internalizing disorders given their substantial presence in ASD. As treatments for the core deficits of ASD are examined, their effects on co-morbid symptomatology should also be carefully assessed. Lastly, comprehensive early intervention programs should be aware of the importance of future psychiatric comorbidity, and consider preventative interventions attempting to reduce the emergence of later psychopathology.
Highlights.
We observed a high frequency of multiple concomittant DSM-defined disorders
50% of children who met criteria for ADHD also met criteria for ODD
46% of children who met criteria for ADHD also met criteria for an anxiety disorder
Findings highlight the importance of improving diagostic practices in ASD
Acknowledgments
Grant information
This work was funded by the following grants and contracts: National Institute of Mental Health, NO1 MH070001, UO1 MH066766, RO1 MH083739, R01MH08096, N01MH70009, N01MH70010, N01MH70001, N01MH70070; N01MH80011, U10MH66768, U10MH66766, 5R01MH081221-02; R01MH079080; U10MH66764; M01 RR00750, M01 RR06022, M01 RR00034, M01 RR00052, MH01805; R01MH079082-05, R01 MH083247. Johnson &Johnson Pharmaceutical Research & Development (provided medication); Korczak Foundation (financial support); Marcus Foundation (financial support).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures/Conflicts of Interest
Dr. Aman has received research contracts, consulted with, served on advisory boards, or done investigator training for Aevi Genomic Medicine; AMO Pharma; Bracket Global; CogState, Inc.; CogState Clinical Trials, Ltd.; Confluence Pharmaceutica; Coronado Biosciences; Hoffman-La Roche; Johnson and Johnson; Lumos Pharma; MedAvante, Inc.; MedAvante-Prophase; Ovid Therapeutics; ProPhase LLC; Supernus Pharmaceuticals, and Zynerba Pharmaceuticals. He receives royalties from Slosson Educational Publications. Dr. Arnold has received research funding from Forest, Lilly, Noven, Shire, Supernus, Roche, and YoungLiving; has consulted with Pfizer, Tris Pharma, and Waypoint; and been on advisory boards for Arbor, Ironshore, Otsuka, Pfizer, Roche, Seaside Therapeutics, Shire. Over the past two years, Dr. Scahill has served as a consultant to Roche, Shire, Supernus, Bracket and the Tourette Association of America. Dr. Handen has received research support from Roche, Eli Lilly, Curemark, and Autism Speaks; Dr. James McCracken has received research funding from Roche, Psyadon, and Think Now, Inc, consultant payments from Roche, payment for DSMB service from Alcobra, and expert testimony payment from Lannett. No conflicts for Drs. Lecavalier, CE McCracken; McDougle, Johnson, Swiezy, Tierney, King, Smith, Vitiello, Bearss.
References
- 1.American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revised Washington, DC: American Psychiatric Association. [Google Scholar]
- 2.Elsabbagh M, Divan G, Yun-Joo Koh YJ, et al. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research, 5, 160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croen LA, Najjar DV, Ray GV, Lotspeich L, Bernal P (2006). A comparison of health care utilization and costs for children with and without autism spectrum disorder in a large group-model health plan. Pediatrics, 118, e1203–1211. [DOI] [PubMed] [Google Scholar]
- 4.Lecavalier L (2006) Behavior and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders, 36, 1101–1114. [DOI] [PubMed] [Google Scholar]
- 5.Maskey M, Warnell F, Parr JR Le Couteur A, & McConachie H (2013). Emotional and behavioural problems in children with autism spectrum disorder. Journal of Autism aand Developmental Disorders, 43, 851–859. [DOI] [PubMed] [Google Scholar]
- 6.Mandell DS, Morales KH, Marcus SC, Stahmer AC, Doshi J, & Polsky DE (2008). Psychotropic medication use among Medicid-enrolled children with autism spectrum disorder. Pediatrics, 121, e441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postorino V, Sharp W. G.m McCracken CE, Bearss K, Burrell TL, Evans AN, & Scahill L (2017). A systematic review and meta-analysis of parent training for disruptive behavior in children with autism spectrum disorder. Clinical Child Family Psychological Review, 20, 391–402. [DOI] [PubMed] [Google Scholar]
- 8.Gadow KD, DeVincent CJ, Pomeroy J, & Azizian A (2005). Comparison of DSM-IV symptoms in elementary school-aged children with PDD versus clinic and community samples. Autism, 9, 392–415. [DOI] [PubMed] [Google Scholar]
- 9.Joshi G, Petty C, Wozniak J, Henin A, Fried R, .....Biederman J (2010). The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders, 40, 1361–1370. [DOI] [PubMed] [Google Scholar]
- 10.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity and associated factors in a population-driven sample. Journal of the American Academy Child and Adolescent Psychiatry, 47, 921–929. [DOI] [PubMed] [Google Scholar]
- 11.Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, & Morgan J (2006). Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders, 36, 849–861. [DOI] [PubMed] [Google Scholar]
- 12.Brookman-Frazee L, Stadnick N, Chlebowski C, Baker-Ericzen M, & Granger W (2017). Characterizing psychiatric comorbidity in children with autism spectrum disorder receiving publicly funded mental health services. Autism. DOI: 10.1177/1362361317712650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekker MC, & Koot HM (2003). DSM-IV disorders in children with borderline to moderate intellectual disability. I: Prevalence and impact. Journal of the American Academy of Child and Adolescent Psychiatry, 42, 915–922. [DOI] [PubMed] [Google Scholar]
- 14.Angold AA, Costello J, & Erkanli A (1999). Comorbidity. Journal of Child Psychology and Psychiatry, 40, 57–87. [PubMed] [Google Scholar]
- 15.Clark LA, Cuthbert B, Lewis-Fernandez R, Narrow WE, Reed GM (2017). Three Approaches to Understanding and Classifying Mental Disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychological Science in the Public Interest, 18, 72–145. [DOI] [PubMed] [Google Scholar]
- 16.Beauchaine TP & Ciccetthi D (2016). A new generation of comorbidity research in the era of neuroscience and Research Domain Criteria. Development and Psychopathology, 28, 891–894. [DOI] [PubMed] [Google Scholar]
- 17.Mazefsky CA, Herrington J, Siegel M, Scarpa A, .... White SW (2013). The role of emotion regulation in autism spectrum disorder. Journal of the Amercian Academy of Child and Adolescent Psychiatry, 52, 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aman MG, McDougle CJ, Scahill L, et al. (2009). Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bearss K, Johnson C, Smith T, Lecavalier L et al. (2015). Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: a randomized clinical trial. Journal of the American Medical Association, 313, 1524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handen BL, Aman MG, Arnold LE, Hyman SL … (2015). Effects of Atomoxetine, parent training, and their combination in children with autism spectrum disorder and ADHD symptoms. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Research Units on Pediatric Psychopharmacology Autism Network. (2002). Risperidone in children with autism and serious behavioral problems. New England Journal of Medicine, 347, 314–21. [DOI] [PubMed] [Google Scholar]
- 22.Research Units on Pediatric Psychopharmacology Autism Network. (2005). Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archives of General Psychiatry, 62, 1266–74. [DOI] [PubMed] [Google Scholar]
- 23.Scahill L, McCracken JT, King BH, Rockhill C, Shah B, .... McDougle C (2015). Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. American Journal of Psychiatry, 172, 1197–206. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- 25.American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revised Washington, DC: American Psychiatric Association. [Google Scholar]
- 26.Lord C, Rutter M, & Le Couteur A (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- 27.Lord C, Risi S, Lambrecht L, et al. (2000) The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- 28.Gadow KD, & Sprafkin J (1997). Early Childhood Inventory-4 norms manual. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- 29.Gadow KD, & Sprafkin J (2000). Early Childhood Inventory-4 screening manual. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- 30.Gadow KD, & Sprafkin J (2002). Child Symptom Inventory-4 screening and norms manual. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- 31.Gadow KD, & Sprafkin J, (2005). Child and Adolescent Symptom Inventory-4R Parent Checklist. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- 32.Lecavalier L, Gadow K, DeVincent CJ, & Edwards MC (2009). Validation of DSM-IV model of psychiatric syndromes in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 278–289. [DOI] [PubMed] [Google Scholar]
- 33.Lecavalier L, Gadow KD, DeVincent CJ, Houts C, & Edwards MC (2011). Validity of DSM-IV syndromes in preschoolers with autism spectrum disorders. Autism, 15, 527–543. [DOI] [PubMed] [Google Scholar]
- 34.Aman MG, & Singh NN (2017). Aberrant Behavior Checklist Manual, Second Edition East Aurora, NY: Slosson Educational Publications, Inc. [Google Scholar]
- 35.Kaat AJ, Lecavalier L, & Aman MG (2014). Validity of the aberrant behavior checklist in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 44, 1103–1116. [DOI] [PubMed] [Google Scholar]
- 36.Guy W Clinical Global Impressions (1976). In ECDEU Assessment Manual for Psychopharmacology, revised. Rockville, MD, National Institute of Mental Health. [Google Scholar]
- 37.Roid GH (2003) Stanford-Binet Intelligence Scales, 5th ed Rolling Meadows, III, Riverside. [Google Scholar]
- 38.Roid GM, & Miller LJ (1997). Leiter International Performance Scale-Revised: Examiners Manual. Wood Dale, IL, Stoelting Co. [Google Scholar]
- 39.Mullen EJ (1995). Mullen Scales of Early Learning. Bloomington, MN, Pearson Assessments. [Google Scholar]
- 40.Slosson RL (1983). Slosson Intelligence Test. East Aurora, NY: Slosson Educational Publications. [Google Scholar]
- 41.Fleiss JL, Levin B, & Paik MC (2003) Statistical methods for rates and proportions – Third Edition Wiley, Hoboken. [Google Scholar]
- 42.The MTA Cooperative Group. (1999). A 14-Month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry, 56,1073–1086. [DOI] [PubMed] [Google Scholar]
- 43.Bearss K, Taylor CA, Aman MG, Whittemore R, Lecavalier L, Miller J, Pritchett J, May B Scahill L (2016). The application of qualitative methods in instrument development for anxiety in children with autism spectrum disorder. Autism, 20, 663–672. [DOI] [PubMed] [Google Scholar]
- 44.Rommelse NNJ, Franke B, Geurts HM, Hatman CA, & Buitelaar JK (2010). Shared heritability of attention deficit/hyperactivity disorder and autism spectrum disorder. European Child and Adolescent Psychiatry, 19, 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leitner Y (2014). The Co-Occurence of autism and Attention Deficit Hyperactivity Disorder in Children – What do we know? Frontiers of Human Neuroscience, 8, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallett V, Lecavalier L, Sukhodolsky DG, Cipriano N, ... & Scahill L (2013). Exploring the manifestations of anxiety in children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 43, 2341–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen P, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH Vitiello B (2001). ADHD comorbidity findings from the MTA study: Comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry, 40, 147–158. [DOI] [PubMed] [Google Scholar]
- 48.Rothwell PM (2005). External validity of randomized controlled: “to whom do the results of this trial apply?” Lancet, 365 (9453), 82–93. [DOI] [PubMed] [Google Scholar]