Abstract
Background and aims:
Despite its strong link to cardiovascular outcomes, the association of chronic kidney disease (CKD) with abdominal aortic aneurysm (AAA) has not been explicitly and comprehensively investigated.
Methods:
In 10,724 participants in the Atherosclerosis Risk in Communities Study (aged 53–75 years during 1996–1998), we evaluated the associations of two key CKD measures—estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (ACR) - with incident AAA (AAA diagnosis in outpatient, hospitalization discharge, or death records). Additionally, we performed a cross-sectional analysis for the CKD measures and ultrasound-based abdominal aortic diameter in 4,258 participants during 2011–2013.
Results
During a median follow-up of 13.9 years, 347 participants developed AAA. The demographically-adjusted hazard ratio (HR) was 4.44 (95% CI 1.58–12.49) for eGFR <30, 3.29 (1.89–5.72) for 30–44, 2.03 (1.29–3.19) for 45–59, and 1.62 (1.11–2.35) for 60–74 compared to eGFR ≥90 ml/min/1.73m2 and was 2.49 (1.28–4.87) for ACR ≥300, 1.99 (1.40–2.83) for 30–299, and 1.46 (1.08–1.97) for 10–29 compared to ACR <10 mg/g. The associations were generally similar after accounting for additional confounders, such as smoking (although attenuated), or after stratifying by subgroups, including diabetes. The cross-sectional analysis also showed continuous positive associations of these CKD measures with aortic diameter, particularly at the distal aortic segment assessed.
Conclusions:
Reduced eGFR and elevated albuminuria were independently associated with greater incidence of AAA and greater abdominal aortic diameter. Our results suggest the potential usefulness of CKD measures to identify persons at high risk of AAA and the need to investigate pathophysiological pathways linking CKD to AAA.
Keywords: Glomerular filtration rate, albuminuria, abdominal aortic
Introduction
Chronic kidney disease (CKD), defined as reduced kidney function or kidney damage, is a major global public health problem,1, 2 affecting 10–20% of adults in many countries of the world.3–6 CKD increases risk of various adverse outcomes such as cardiovascular disease (CVD) and infectious diseases.7, 8 The investigation of the link between CKD and CVD is particularly important since up to half of individuals with CKD die from CVD.9 Accordingly, numerous studies have investigated the association of kidney function and/or damage with major CVDs such as CVD mortality, coronary heart disease, stroke, and heart failure.10–14 However, data are sparse for other subtypes of CVD, despite their potential impact on prognosis and quality of life.15
In this context, abdominal aortic aneurysm (AAA) is an important CVD subtype, as it constitutes the 14th leading cause of death in the US (10th in older men).16 Prevention and early detection of AAA is critical given its potential for continued enlargement and catastrophic rupture, which has an estimated mortality risk of 65%−85%.16, 17 The few existing studies of the association of CKD with AAA have all been cross-sectional and yielded conflicting results.18–20 Furthermore, to our knowledge, there are no studies investigating albuminuria, the other key measure of CKD representing kidney damage,21 as a risk factor for AAA.
Therefore, using data from a community-based cohort, the Atherosclerosis Risk in Communities (ARIC) Study, our main aim was to evaluate the prospective associations of both kidney function and albuminuria with future risk of AAA over 15 years of follow-up. The ARIC Study also provided us an opportunity to cross-sectionally evaluate the associations of CKD measures with abdominal aortic diameter assessed by ultrasound at a recent visit.
Materials and methods
Study design and population
The ARIC Study enrolled 15,792 men and women from four US communities (Washington County, Maryland; suburban Minneapolis, Minnesota; Jackson, Mississippi; and Forsyth County, North Carolina) in 1987–1989.22 Participants were aged 45–64 years old at study initiation and predominantly white or black. The current study contained two sets of analyses, a survival analysis with incident clinical AAA as the outcome and a cross-sectional analysis with abdominal aortic diameter as the dependent variable. For the survival analysis, we used ARIC visit 4 (1996–1998) as baseline, when albuminuria was assessed for the first time in the ARIC Study. Of 15,792 ARIC participants, 11,656 participants attended visit 4. After excluding participants who did not self-identify as white or black (n=31), those with missing values of creatinine, cystatin C, or albuminuria (n=440), those with missing covariates (n=396), and those with a clinical history of AAA prior to visit 4 or missing follow-up (n=65), the survival analysis included 10,724 participants. For the cross-sectional analysis, we used data at visit 5, when abdominal ultrasound was performed. Of 6,538 participants at visit 5 (2011–2013), we excluded participants from racial groups other than white or black (n=18), those with missing values of any kidney measures (n=820), those with missing covariates (n=1,086), and those with missing or uninterpretable aortic ultrasound imaging (n=356), leaving the cross-sectional study sample of 4,258 participants.
CKD measures
As a measure of kidney function, estimated glomerular filtration rate (eGFR) was calculated with the CKD-EPI equation using two filtration markers, creatinine and cystatin C, as well as age, sex, and race, since this equation is considered to best estimate measured GFR.23 As a measure of kidney damage, urine albumin-to-creatinine ratio (ACR) was used, as recommended in clinical guidelines.21 At visit 4 for the prospective analysis, plasma creatinine concentration was measured using a modified kinetic Jaffe method and then standardized to isotope dilution mass spectrometry.24 Serum cystatin C was measured by a particle-enhanced immunonephelometric assay with a BNII nephelometer (Siemens Healthcare Diagnostics, Deerfield, Illinois, USA)25. Urine albumin was measured using a nephelometric method while urine creatinine was measured with the Jaffe method.25 At visit 5 for the cross-sectional analysis, urine/serum creatinine and cystatin C were measured using a creatinase enzymatic method with a Roche Modular P Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN) and the Gentian Cystatin C Immunoassay with the Beckman Coulter Olympus AU400 analyzer (Beckman Coulter, Brea, CA), respectively. Urine albumin was measured using an immunoturbidometric method on the ProSpec nephelometric analyzer (Dade Behring GMBH. Marburg, Germany).
Covariates
Covariates for analysis came from the relevant ARIC visit 4 or 5 examination. Information on age, race, sex, smoking, and alcohol intake were based on self-report. Diabetes mellitus was defined as a fasting glucose ≥7.0 mmol/L, non-fasting glucose ≥11.1 mmol/L, self-reported physician diagnosis of diabetes, or use of antidiabetic medications. The use of medications, including antidiabetics and antihypertensives, was based on self-report and inspection of medication bottles brought by participants. Body mass index was calculated as body weight (kg) divided by the square of height (m). Blood pressure was measured twice at visit 4 and thrice at visit 5 in the sitting position after 5 minutes of rest, and the average of the last two values was recorded for the analysis. Total cholesterol and high-density lipoprotein cholesterol were measured using enzymatic methods. History of coronary heart disease, stroke, and heart failure were determined by self-report at visit 1 and subsequent clinical events identified during the follow-up of the ARIC Study.26
Outcome variables: incident clinical AAA and abdominal aortic diameter
The ARIC Study identified incident clinical AAAs through 2011 according to a few strategies, details of which were published elsewhere.27, 28 Briefly, during annual follow-up telephone calls (semi-annual from 2012) ARIC staff asked participants (or their family members when appropriate) about any interim hospitalizations or deaths. In case of such events, the ARIC Study sought to obtain detailed records (e.g., medical records and death certificates). The ARIC Study also performed surveillance of local hospitals to identify additional hospitalizations or deaths. Additionally, the ARIC Study linked participant identifiers to data at the Centers for Medicare and Medicaid Services (CMS) to find any additional clinical events (both inpatient and outpatient) for those aged ≥65 years. We defined clinical AAAs as hospitalizations from any source or two CMS outpatient claims (at least a week apart) with the ICD-9 codes of 441.3 (abdominal aneurysm, ruptured), 441.4 (abdominal aneurysm without mention of rupture), 38.44 (resection of vessel with replacement, aorta, abdominal) or 39.71 (endovascular implantation of other graft in abdominal aorta) or deaths with the ICD-9 or 10 codes of 441.3, 441.4, I71.3 (abdominal aortic aneurysm, ruptured) or I71.4 (abdominal aortic aneurysm, without rupture). AAAs based on the procedure codes were required to be verified by diagnosis codes.
Abdominal ultrasound was performed at visit 5 (2011–2013). Certified technicians obtained images and measured aortic diameter, using a Philips iE33 high resolution duplex scanner and a Philips C5–1 transducer.27, 28 Technicians recorded transverse images at the proximal aorta (just below the superior mesenteric artery), the mid aorta (2 cm below the renal arteries), the distal aorta (1 cm above the bifurcation), and the point of maximal aortic diameter if it was found in a part other than the three points mentioned above. Using these images, technicians measured anterior-posterior and transverse diameters of abdominal aorta. A radiologist with expertise in vascular imaging reviewed images when the technicians reported abdominal aortic diameter >2.8 cm or probable pathology, as well as a 5% random sample of the rest.
Statistical analysis
All analyses were conducted using Stata 13 software (Stata Corp, College Station, TX), and a two-tailed p-value of less than 0.05 was considered statistically significant. Baseline characteristics were summarized by eGFR < vs. ≥60 ml/min/1.73m2 and ACR ≥ vs. <30 mg/g at both visit 4 (baseline for survival analysis of incident clinical AAAs) and visit 5 (for cross-sectional analysis with abdominal aortic diameter). Continuous and categorical variables were compared between these eGFR and ACR categories using ANOVA and chi-square tests, as appropriate.
For survival analysis, we first assessed continuous associations between each CKD measure at visit 4 and subsequent incidence rates of clinical AAA after the adjustment for age, sex, race, and center using Poisson regression models. eGFR and ACR were modeled as linear spline terms with knots at eGFR 45, 60, 75, and 90 ml/min/1.73m2 and ACR 10, 30, and 300 mg/g.
Subsequently, Cox proportional hazards models were used to quantify the association between clinical categories of each CKD measure and incident AAA. eGFR was categorized into ≥90, 75–89, 60–74, 45–59, 30–44, and <30 ml/mm/1.73m2 and ACR into <10, 10–29, 30–299, and ≥300 mg/g.21 We constructed two models to evaluate the impact of potential confounding variables. Model 1 included demographic variables, i.e., age, sex, race, and center. Model 2 further adjusted for traditional CVD risk factors, i.e., alcohol intake, body mass index, systolic/diastolic blood pressure, antihypertensive medication, diabetes, smoking (current vs. never/former), total cholesterol, and high-density lipoprotein cholesterol, prevalent stroke, coronary heart disease, and heart failure, as well as each CKD measure (i.e., accounting for ACR in the eGFR analysis and vice versa). P for trend was evaluated from the models with a linear term of eGFR and ACR. We also performed subgroup analyses (a) modeling log-ACR and eGFR linearly and (b) cross-categorizing ACR and eGFR, with eGFR <30 and 30–44 ml/min/1.73m2 merged into a single category to enhance precision.
For the cross-sectional analysis of CKD measures and ultrasound abdominal aortic diameter using data from visit 5, we ran linear regression models with the same selection of covariates for Models 1 and 2, described above. eGFR and ACR were modeled continuously and categorically as in the survival analysis. One exception was the use of eGFR 75–89 ml/min/1.73 m2 as the reference, given the lower proportion of participants with eGFR ≥90 ml/min/1.73 m2 at visit 5 (when participants were on average 15 years older than at visit 4).29
Results
Baseline characteristics at ARIC visit 4
The mean age of the 10,724 participants at visit 4 for our survival analysis was 63.3 (SD 5.6) years, and 22.0% were black (Table 1). The prevalence of current and former smoking was 14.9% and 43.6% respectively. Compared to their normal counterparts, participants with low eGFR <60 ml/min/1.73m2 and high ACR ≥30 mg/g tended to have poorer risk factor profiles (e.g., older age, higher body mass index, and higher prevalence of diabetes, hypertension, coronary heart disease, stroke, and heart failure). The prevalence of current smoking was higher in participants with ACR ≥30 mg/g compared to ACR <30 mg/g but did not differ between the two eGFR categories. Lipid profiles were not evidently different between eGFR and ACR categories.
Table 1.
Baseline characteristics at visit 4 according to CKD status, ARIC, 1996–1998
| eGFR (mL/min/1.73 m2) | ACR (mg/g) | |||||
|---|---|---|---|---|---|---|
| ≥60 | <60 | p-value | <30 | ≥30 | p-value | |
| Number | 9,522 | 1,202 | 9,837 | 887 | ||
| Age, years (SD) | 63 (6) | 67 (5) | <0.001 | 63 (6) | 65 (6) | <0.001 |
| Black, % | 2136 (22%) | 228 (19%) | 0.006 | 2028 (21%) | 336 (38%) | <0.001 |
| Female, % | 5338 (56%) | 687 (57%) | 0.471 | 5556 (56%) | 469 (53%) | 0.038 |
| Center, % | <0.001 | <0.001 | ||||
| Forsyth County, NC | 2343 (25%) | 303 (25%) | 2482 (25%) | 164 (18%) | ||
| Jackson, MS | 1887 (20%) | 200 (17%) | 1784 (18%) | 303 (34%) | ||
| Suburban Minneapolis, MN | 2780 (29%) | 302 (25%) | 2912 (30%) | 170 (19%) | ||
| Washington County, MD | 2512 (26%) | 397 (33%) | 2659 (27%) | 250 (28%) | ||
| Smoking status, % | 0.611 | <0.001 | ||||
| Non-smoker | 3968 (42%) | 483 (40%) | 4128 (42%) | 323 (36%) | ||
| Former smoker | 4143 (43%) | 535 (45%) | 4304 (44%) | 374 (42%) | ||
| Current smoker | 1411 (15%) | 184 (15%) | 1405 (14%) | 190 (21%) | ||
| Drinking status, % | <0.001 | <0.001 | ||||
| Non-drinker | 1900 (20%) | 308 (26%) | 1987 (20%) | 221 (25%) | ||
| Former drinker | 2782 (29%) | 420 (35%) | 2879 (29%) | 323 (36%) | ||
| Current drinker | 4840 (51%) | 474 (40%) | 4971 (51%) | 343 (39%) | ||
| Anti-HTN medication, % | 3810 (40%) | 818 (68%) | <0.001 | 3992 (41%) | 636 (72%) | <0.001 |
| Diabetes, % | 1486 (16%) | 288 (24%) | <0.001 | 1387 (14%) | 387 (44%) | <0.001 |
| Prevalent stroke, % | 173 (2%) | 58 (5%) | <0.001 | 184 (2%) | 47 (5%) | <0.001 |
| Prevalent HF, % | 426 (4%) | 145 (12%) | <0.001 | 442 (4%) | 129 (15%) | <0.001 |
| Prevalent CHD, % | 700 (7%) | 182 (15%) | <0.001 | 722 (7%) | 160 (18%) | <0.001 |
| BMI, kg/m2 (SD) | 29 (6) | 30 (6) | <0.001 | 29 (6) | 30 (6) | <0.001 |
| SBP, mmHg (SD) | 127 (19) | 131(22) | <0.001 | 126 (18) | 141 (23) | <0.001 |
| DBP, mmHg (SD) | 71 (10) | 70 (11) | <0.001 | 71 (10) | 74 (12) | <0.001 |
| HDL cholesterol, mmol/L (SD) | 1.3 (0.4) | 1.2 (0.4) | <0.001 | 1.3 (0.4) | 1.2 (0.5) | 0.001 |
| Total cholesterol, mmol/L (SD) | 5.2 (0.9) | 5.2 (1.0) | 5.2 (0.9) | 5.2 (1.1) | 0.828 | |
| eGFR, mL/min/1.73 m2 (SD) | 82 (12) | 49 (10) | <0.001 | 79 (15) | 70 (23) | <0.001 |
| ACR, mg/g [IQI] | 3.6 [1.7–7.3] | 5.1 [2.3–19.5] | <0.001 | 3.3 [1.6–6.3] | 83.9 [46.4–252.1] | <0.001 |
| Incident AAA, % | 280 (3%) | 67 (6%) | <0.001 | 302 (3%) | 45 (5%) | 0.001 |
Values are mean (SD), n (%), or median [interquartile interval, IQI]. CKD: chronic kidney disease, CHD: coronary heart disease, HF: heart failure, BMI: body mass index, BP: blood pressure, HDL: high density lipoprotein, eGFR: estimated glomerular filtration rate, ACR: urine albumin-to-creatinine ratio, AAA: abdominal aortic aneurysm.
Survival analysis with CKD measures at visit 4
During a median follow-up of 13.9 years, there were 347 cases of clinical AAA (incidence rate 2.5 per 1,000 person-years). After adjustment for age, race, sex, and center, the incidence rate of clinical AAA was steadily greater across lower eGFR categories (Figure 1A). The incidence rate was largely flat above eGFR 90 ml/min/1.73m2. ACR demonstrated a graded positive association with clinical AAA incidence, although a risk gradient was not evident above ACR 300 mg/g (Figure 1B).
Figure 1.
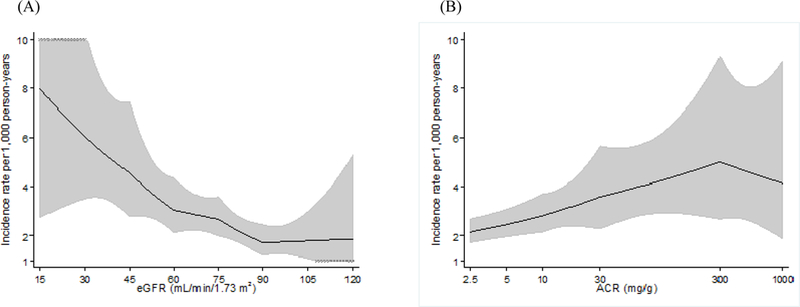
Incidence rate of AAA hospitalizations per 1,000 for CKD measures, adjusted for age, gender, race, and center. (A) eGFR, (B) ACR.
Similar associations were observed when clinical categories of eGFR and ACR were evaluated (Tables 2 and 3). Adjusted for demographic variables (Model 1 in Table 2), the hazard ratio (HR) was 4.44 (95% CI 1.58–12.49) for eGFR <30, 3.29 (1.89–5.72) for 30–44, 2.03 (1.29–3.19) for 45–59, and 62 (1.11–2.35) for 60–74 ml/min/1.73 m2 compared to eGFR ≥90 (p for trend <0.001). Although the associations were attenuated in Model 2, the general pattern was consistent (p for trend 0.035).
Table 2.
Adjusted hazard ratio of incident AAA across eGFR categories, ARIC, 1996–2011
| eGFR (mL/min/1.73 m2) | |||||||
|---|---|---|---|---|---|---|---|
| 90+ n = 2,447 42 AAAs (2%) |
75–89 n = 4,172 117 AAAs (3%) |
60–74 n = 2,903 121 AAAs (4%) |
45–59 n = 838 43 AAAs (5%) |
30–44 n = 291 20 AAAs (7%) |
<30 n=73 4 AAAs (5%) |
p for trend |
|
| Model 1 | Reference | 1.18 (0.82–1.70) | 1.62 (1.11–2.35) | 2.03 (1.29–3.19) | 3.29 (1.89–5.72) | 4.44 (1.58–12.49) |
<0.001 |
| Model 2 | Reference | 1.06 (0.74–1.53) | 1.39 (0.95–2.03) | 1.41 (0.89–2.25) | 1.93 (1.09–3.44) | 1.97 (0.68–5.65) | 0.035 |
Model 1 adjusted for age, gender, race, and center, and Model 2 further adjusted for smoking status, alcohol intake, BMI, systolic and diastolic blood pressures, anti-hypertensive medication, diabetes, total and HDL cholesterols, prevalent stroke, CHD, HF, and ACR. p for trend was based on eGFR as a continuous variable below 90 ml/min/1.73 m2.
Table 3.
Adjusted hazard ratio of incident AAA across ACR categories, ARIC, 1996–2011
| ACR (mg/g) | |||||
|---|---|---|---|---|---|
| <10 n = 8,562 250 cases (3%) |
10–29 n = 1,275 52 cases (4%) |
30–299 n = 699 36 cases (5%) |
300+ n = 188 9 cases (5%) |
p for trend |
|
| Model 1 | Reference | 1.46 (1.08–1.97) | 1.99 (1.40–2.83) | 2.49 (1.28–4.87) | <0.001 |
| Model 2 | Reference | 1.34 (0.99–1.83) | 1.48 (1.02–2.15) | 1.59 (0.76–3.29) | 0.005 |
Model 1 adjusted for age, gender, race, and center, and Model 2 further adjusted for smoking status, alcohol intake, BMI, systolic and diastolic blood pressures, anti-hypertensive medication, diabetes, total and HDL cholesterols, prevalent stroke, CHD, HF, and ACR. p for trend was based on log-ACR as a continuous variable.
In analyses with ACR <10 mg/g as the reference, the demographically adjusted HR was 2.49 (1.28–4.87) for ≥300 and 1.99 (1.40–2.83) for 30–299 mg/g (p for trend <0.001) (Model 1 in Table 3). Of note, even high normal ACR 10–29 mg/g demonstrated a statistically significant HR of AAA (1.46 [1.08–1.97]). The associations were generally similar after further adjusting for other potential confounding variables (p for trend 0.005) (Model 2 in Table 3).
Both lower continuous eGFR and higher continuous ACR demonstrated largely consistent results in key demographic and clinical subgroups without any significant interactions (Figure 2). Although not statistically significant, both CKD measures tended to be more strongly related to AAA risk in current and former smokers compared to never smokers. The analysis with cross-categories of eGFR and ACR confirmed their independent and multiplicative associations with clinical AAA incidence (p for interaction 0.653) (Supplemental Figure 1).
Figure 2.
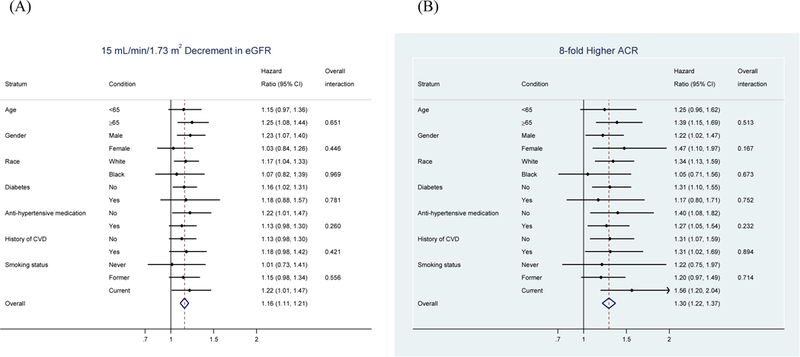
Fully adjusted hazard ratio associating CKD measures with incident AAA within subgroups. Adjusted for age, gender, race, center, smoking status, alcohol intake, BMI, systolic and diastolic blood pressures, anti-hypertensive medication, diabetes, total and HDL cholesterols, prevalent stroke, CHD, HF, and each of CKD measures as appropriate. “Overall” indicates overall study sample. (A) eGFR, (B) ACR.
Cross-sectional analysis at visit 5
At visit 5, the mean age of the 4,258 participants with available ultrasound measures was 76.1 (SD 5.1) years, and 22.4% were black. Similar to visit 4 (Table 1), participants with eGFR <60 ml/min/1.73m2 and ACR ≥30 mg/g showed poorer risk factor profiles compared to their counterparts, particularly, in terms of the prevalence of diabetes, hypertension, and a history of other CVDs (Supplemental Table 1).
When the maximum diameter at any part of the imaged abdominal aorta was modeled as the dependent variable with adjustment for demographic variables, both lower eGFR and higher ACR were modestly but continuously associated with greater diameter (Supplemental Figure 2). The associations were similar for clinical categories of eGFR and ACR (Supplemental Tables 2–3), although none of the eGFR categorical associations reached statistical significance in Model 2. In terms of each imaged segment of the aorta (i.e., proximal, mid, and distal), for both eGFR and ACR, the associations appeared strongest for the distal aorta (e.g., adjusted mean difference in diameter between ACR ≥300 vs. <10 mg/g +0.15 cm [95% CI +0.08 to +0.22] in the distal aorta versus +0.01 cm [−0.05 to +0.07] in the proximal aorta).
We observed generally consistent results in most demographic and clinical subgroups (Supplemental Figure 3). An exception was the consistently stronger associations for both eGFR and ACR in current and former smokers compared to never smokers. Another significant interaction was observed between eGFR, but not ACR, and sex, with a stronger inverse association of eGFR and aortic diameter in men than in women.
Discussion
This community-based study demonstrated that the two key measures of CKD, eGFR and albuminuria, were associated with incident clinical AAA. The associations were generally independent of each other as well as measured risk factors for AAA and were largely consistent across key subgroups, although the associations appeared stronger in current and former smokers than in never smokers. Moreover, using ultrasound we confirmed cross-sectional associations of eGFR and albuminuria with greater abdominal aortic diameter, particularly for the distal aorta.
To the best of our knowledge, this is the first prospective study quantifying the association of reduced kidney function with greater incidence of AAA. This is important since previous cross-sectional studies reported conflicting results.18–20 In addition to the prospective design, another potential strength of our study is that we used a robust equation for estimating eGFR using two filtration markers, creatinine and cystatin C, which has been shown to better estimate measured GFR and predict CKD complications than eGFR based on creatinine alone.23
In addition, our study uniquely found that albuminuria was independently and positively associated with incident AAA and abdominal aortic diameter. This finding is consistent with the robust associations between albuminuria and other forms of CVD reported in previous studies.11–13, 26 On the other hand, this result is not entirely consistent with the inverse association between diabetes and AAA,30, 31 since diabetes is a major cause of albuminuria.21 Although it is still under debate whether diabetes is actually protective against AAA,30, 31 in our study, we did observe that participants with diabetes had lower risk of incident AAA compared to those without diabetes (data not shown). In this context, it is noteworthy that we did not observe that diabetes status modified the association between albuminuria and risk of incident AAA or abdominal aorta diameter.
There are several plausible mechanisms linking CKD to the risk of AAA. First, CKD and AAA share traditional risk factors such as hypertension and smoking.32 However, the association between CKD and AAA remained significant even after accounting for these factors, in addition to a wide range of other potential confounders. Second, matrix metalloproteinases may mediate the CKD-AAA relationship since they play key roles in the pathogenesis of AAA,33 as well as kidney fibrosis34 and albuminuria.35 Third, CKD induces systemic inflammation, a condition considered to increase the risk of AAA.27, 36 Finally, neovascularization has been reported to play an important role in the pathophysiology of AAA,37 and given that CKD is a clinical phenotype of microvascular disease, it is possible that those with CKD are more likely to have abnormal aortic neovascularization compared to those without CKD.38 Nonetheless, future studies are needed to explore these and other pathophysiological mechanisms, which may link CKD, particularly albuminuria, to AAA.
There are a few clinical and research implications from our study. CKD measures may be useful to identify persons at particularly high risk of developing or having AAA. Given that serum creatinine is routinely measured in clinical practice 39 and evaluation of albuminuria is recommended in patients with diabetes and hypertension,40, 41 CKD measures may guide targeted screening of AAA, particularly when these are already measured for some clinical indications. This aspect is important since who should undergo ultrasound for screening AAA is still controversial, particularly screening for never smokers and women.42 In addition, future research will be needed to investigate pathophysiological pathways linking CKD to AAA. In this context, the potentially tangled relationship among albuminuria, diabetes, and AAA will be of particular interest.
Although our study had a number of strengths, several limitations should be mentioned. First, clinical AAA identification relied on ICD codes. Nonetheless, we confirmed similar results using ultrasound assessed abdominal aortic diameters. Second, ultrasound was not performed at the study baseline. Thus some participants might have had AAAs at the start of our survival analysis. Nonetheless, such prevalent cases should be few, because the rate of AAA is relatively low in subjects less than 75 years and we excluded participants who had AAA repair before visit 4. Third, the lack of baseline abdominal ultrasound also precluded us from assessing whether CKD measures are associated with changes in abdominal aortic diameter over time. Finally, although we adjusted for a number of potential confounders, our results may still experience residual confounding (e.g., family history of AAA).
In conclusion, both eGFR and albuminuria were prospectively associated with incident clinical AAA, independently of each other and potential confounding variables. We confirmed similar associations using cross-sectional data on aortic diameter based on ultrasound. Taken altogether, our results suggest the potential usefulness of CKD measures to identify persons at high risk of AAA and the need to investigate pathophysiological pathways linking CKD (particularly albuminuria) to AAA.
Supplementary Material
Highlights:
Chronic kidney disease was positively related to incident clinical abdominal aortic aneurysm.
Both kidney function and damage showed independent associations.
We also confirmed greater ultrasound-based aortic diameter related to chronic kidney disease.
Acknowledgements
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This specific study was supported by NHLBI grant R01HL103695. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–259. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 3.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371(9631):2173–2182. [DOI] [PubMed] [Google Scholar]
- 4.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol. 2003;14(7 Suppl 2):S131–138. [DOI] [PubMed] [Google Scholar]
- 5.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J. Am. Soc. Nephrol. 2006;17(8):2275–2284. [DOI] [PubMed] [Google Scholar]
- 6.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. [DOI] [PubMed] [Google Scholar]
- 7.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–2757. [DOI] [PubMed] [Google Scholar]
- 10.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. The lancet. Diabetes & endocrinology. 2015;3(7):514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of Kidney Function and Albuminuria With Cardiovascular Mortality in Older vs Younger Individuals: The HUNT II Study. Arch Intern Med. 2007;167(22):2490–2496. [DOI] [PubMed] [Google Scholar]
- 13.Hui X, Matsushita K, Sang Y, Ballew SH, Fulop T, Coresh J. CKD and cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study: interactions with age, sex, and race. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62(4):691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics−−2014 Update: A Report From the American Heart Association. Circulation. 2013;129(3):e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lederle FA. In the clinic. Abdominal aortic aneurysm. Ann Intern Med. 2009;150(9):ITC5–1-15; quiz ITC15–16. [DOI] [PubMed] [Google Scholar]
- 17.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577–1589. [DOI] [PubMed] [Google Scholar]
- 18.Chun KC, Teng KY, Chavez LA, et al. Risk Factors Associated with the Diagnosis of Abdominal Aortic Aneurysm in Patients Screened at a Regional Veterans Affairs Health Care System. Annals of Vascular Surgery. 2014;28(1):87–92. [DOI] [PubMed] [Google Scholar]
- 19.Svensjo S, Bjorck M, Gurtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low Prevalence of Abdominal Aortic Aneurysm Among 65-Year-Old Swedish Men Indicates a Change in the Epidemiology of the Disease. Circulation. 2011;124(10):1118–1123. [DOI] [PubMed] [Google Scholar]
- 20.Alcorn HG, Wolfson SK, Sutton-Tyrrell K Jr., Kuller LH, O’Leary D, Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16(8):963–970. [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International. Supplement. 2013;3(1):1–150. [Google Scholar]
- 22.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. . Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waheed S Combined association of Creatinine, Albuminuria and Cystatin C with All-Cause Mortality Cardiovascular and Kidney Outcomes: The Atherosclerosis Risk in Communities (ARIC) Study. Clin. J. Am. Soc. Nephrol. 2013;8(3):434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astor BC, Shafi T, Hoogeveen RC, et al. Novel Markers of Kidney Function as Predictors of ESRD, Cardiovascular Disease, and Mortality in the General Population. Am J Kidney Dis. 2012;59(5):653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita K, Sang Y, Ballew SH, et al. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the atherosclerosis risk in communities study. Arterioscler. Thromb. Vasc. Biol. 2014;34(8):1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folsom AR, Yao L, Alonso A, et al. Circulating Biomarkers and Abdominal Aortic Aneurysm Incidence: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;132(7):578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, Yao L, Roetker NS, et al. Lifetime Risk and Risk Factors for Abdominal Aortic Aneurysm in a 24-Year Prospective Study: The ARIC Study (Atherosclerosis Risk in Communities). Arterioscler Thromb Vasc Biol. 2016;36(12):2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. Jama. 2012;308(22):2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Rango P, Farchioni L, Fiorucci B, Lenti M. Diabetes and abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47(3):243–261. [DOI] [PubMed] [Google Scholar]
- 31.Lederle FA. The strange relationship between diabetes and abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2012;43(3):254–256. [DOI] [PubMed] [Google Scholar]
- 32.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klaus V, Tanios-Schmies F, Reeps C, et al. Association of Matrix Metalloproteinase Levels with Collagen Degradation in the Context of Abdominal Aortic Aneurysm. European Journal of Vascular and Endovascular Surgery. 2017;53(4):549–558. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Qiao X, Tan TK, et al. Matrix metalloproteinase 9-dependent Notch signaling contributes to kidney fibrosis through peritubular endothelial-mesenchymal transition. Nephrol Dial Transplant. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulido-Olmo H, Garcia-Prieto CF, Alvarez-Llamas G, et al. Role of matrix metalloproteinase-9 in chronic kidney disease: a new biomarker of resistant albuminuria. Clin Sci (Lond). 2016;130(7):525–538. [DOI] [PubMed] [Google Scholar]
- 36.Kisic B, Miric D, Dragojevic I, Rasic J, Popovic L. Role of Myeloperoxidase in Patients with Chronic Kidney Disease. Oxid Med Cell Longev. 2016;2016:1069743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paik DC, Fu C, Bhattacharya J, Tilson MD. Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exp Mol Med. 2004;36(6):524–533. [DOI] [PubMed] [Google Scholar]
- 38.Wu CC, Hung SC, Kuo KL, Tarng DC. Impact of Indoxyl Sulfate on Progenitor Cell-Related Neovascularization of Peripheral Arterial Disease and Post-Angioplasty Thrombosis of Dialysis Vascular Access. Toxins (Basel). 2017;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inker LA, Levey AS. Pro: Estimating GFR using the chronic kidney disease epidemiology collaboration (CKD-EPI) 2009 creatinine equation: the time for change is now. Nephrol Dial Transplant. 2013;28(6):1390–1396. [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes A Executive summary: Standards of medical care in diabetes−−2012. Diabetes Care. 2012;35 Suppl 1:S4–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289(19):2560–2571. [DOI] [PubMed] [Google Scholar]
- 42.LeFevre ML, Force USPST. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(4):281–290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


