Abstract
Circadian rhythms, meals, and exercise modulate energy metabolism. This review will explore the novel hypothesis that there is an optimal time of day to exercise to improve 24 h glycemia and lipemia in individuals with type 2 diabetes (T2D).
Keywords: Exercise timing, glucose excursions, triglyceride, postprandial, type 2 diabetes, metabolism
Summary for Table of Contents
Circadian rhythms may make postdinner exercise the most effective time to exercise to improve glycemia and lipemia in individuals with type 2 diabetes.
INTRODUCTION
Individuals with type 2 diabetes (T2D) are characterized by impaired fasting glucose and lipid levels, postprandial hyperglycemia and hypertriglyceridemia, and other complications that lead to cardiovascular diseases. Hyperglycemia and hypertriglyceridemia in T2D cause cardiovascular disease by promoting endothelial dysfunction through several known mechanisms including increased oxidative stress (1), advanced glycosylation end products (2), endothelial progenitor cell senescence (3), and inflammation (3). Following meals, glucose and lipid concentrations can fluctuate dramatically depending on the meal composition and the metabolic health status of the individual. Minimizing these excursions is necessary to prevent diabetes and atherosclerosis-associated disorders in healthy individuals, and to provide protection against diabetes progression and the development of its associated cardiovascular complications. Exercise is critical in controlling postprandial levels because of its ability to alter energy metabolism and insulin sensitivity. Thus, the interplay between exercise and meals can be manipulated to maintain metabolic control.
Glucose and lipid metabolism are also impacted by circadian rhythms. The circadian system consists of a central brain pacemaker (suprachiasmatic nucleus) along with cell-autonomous circadian rhythms in liver, muscle, and adipose tissue that work in synergy to modulate a range of metabolic processes including insulin sensitivity and energy metabolism (4). Metabolically healthy individuals have the most optimal liver and peripheral insulin sensitivity and glycemic control in the morning, which gradually worsens through the remainder of the day (5, 6). Intriguingly, individuals with diabetes have an inverted circadian rhythm where insulin sensitivity and glycemia are relatively better in the evening but worsen during the overnight and early morning period resulting in morning hyperglycemia and hyperlipidemia (i.e. dawn phenomenon) (7–9). Thus, disruptions in the chronobiology in T2D is a major underlying factor contributing to hyperglycemia and hypertriglyceridemia.
Improvements in peripheral insulin sensitivity and realignment of the circadian cycle can be achieved by sleep, diet and physical activity/exercise, which is a fundamental component of improving hyperglycemia and hypertriglyceridemia in T2D. The exercise recommendations set by the American College of Sports Medicine recommends the prescription of exercise with considerations of frequency, duration, type and mode of exercise, but do not consider exercise timing which may be a critical component particularly in those affected by chronic disease. We now know that metabolism clearly shows circadian rhythms both at a central level and peripheral level; thus we speculate that there may be a temporal optimization of exercise to ideally modify endogenous glucose production, β-cell function and tissue insulin sensitivity. Potentially prescribing exercise based on time of day may attenuate postprandial glycemia and lipemia, which in turn may modulate the dawn phenomena by lowering morning fasting glucose and triglyceride levels. This review will explore the novel hypothesis that evening postprandial exercise is the optimal time to exercise to improve glycemia and lipemia in T2D. We will also discuss potential mechanisms for the superior benefits of postdinner evening exercise in this population.
EXERCISE TIMING AND POSTPRANDIAL METABOLISM
Pre Vs. Postprandial Exercise and Glycemia
Postprandial glycemia is a major risk factor for heart disease in T2D. Most (10–18), but not all (19), studies show that exercising after meals has a greater benefit on postprandial glycemia compared to premeal exercise in individuals with T2D. Early work in T2D by Larsen et al. (12, 13) showed that both moderate (45 min at 53% O2max) and high intensity interval cycling exercise (4 bouts, each bout consisting of 3 min at 56% O2max, 4 min at 98%, and 6 min of rest) beginning 45 min after breakfast reduced postprandial glucose levels after breakfast, but had no impact on glycemia after a subsequent lunch meal. Improvements in postprandial glucose responses were due to an increased rate of glucose disappearance (i.e. enhanced muscle glucose uptake). Subsequent studies in T2D revealed that moderate intensity cycling exercise (1 h at 60% O2max) in the fasted (preprandial) state has little impact on glucose levels during exercise, whereas postprandial exercise (0–8 h postprandial) reduces glucose levels during exercise between 28–43%, with the largest reductions occurring when exercise was performed within 5 h after eating (14, 15, 18). Colberg et al. (11) showed that 20 min of self-paced treadmill walking 15 to 20 min after eating a dinner meal reduced postprandial blood glucose levels in individuals with T2D, while the same bout of exercise performed immediately prior to dinner had no impact on postprandial blood glucose levels.
Likewise, work from our lab (10) in individuals with T2D has shown that ~45 min of resistance training (3 sets of 10 repetitions using 10 repetition maximum weight, eight different exercises) either prior to (finished ~30 min prior to dinner) or beginning 45 min after eating dinner lowers postprandial glucose levels, but that postdinner exercise tended to reduce postprandial glycemia to a greater extent. Recent work (20) has also shown that 60 min of moderate intensity (12 on Borg 6–20 scale) treadmill walking after breakfast, but not before breakfast, attenuated glycemia over the next 22 h in hyperglycemic individuals. In contrast to these studies, one study has shown that moderate (55% O2max) or high intensity cycling exercise (15 bouts of 4 min at 40% O2max, 1 min at 100 O2max) performed prior to breakfast had a greater benefit on most aspects of glycemia compared to exercise after breakfast, although both exercise times improved glycemia to some extent in individuals with T2D (19). Taken together, most studies indicate that postprandial exercise, compared to premeal exercise, has a greater benefit on postprandial glycemia in individuals with T2D.
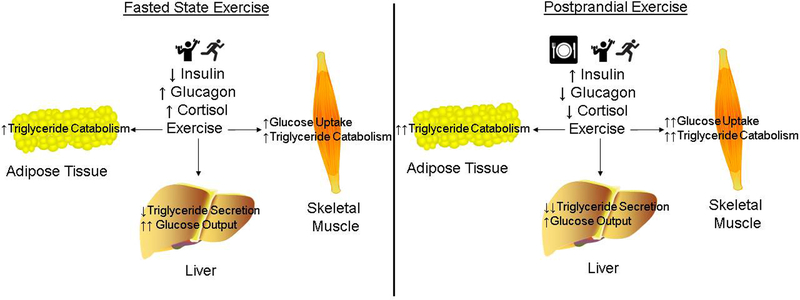
The mechanism for the superior benefit of postmeal exercise on postprandial glycemia may be multifactorial. Skeletal muscle accounts for 50–75% of insulin stimulated glucose uptake (21, 22), and while insulin stimulates GLUT 4 translocation and glucose uptake through an insulin receptor/insulin receptor substrate/phosphatidylinositol-3-kinase/Akt axis (23), skeletal muscle contraction increases GLUT 4 translocation and glucose uptake via activation of a sucrose nonfermenting adenosine monophosphate kinase (AMPK) related kinase/AMPK/TBC1D1/TBC1D4 signaling node (24, 25). When combined, both insulin and muscle contractions stimulate glucose uptake to a greater extent than either stimulus alone (26). Thus, the greater benefit of postprandial exercise on glycemia is due to the synergy between insulin and muscular contraction to increase glucose uptake (Figure 1). In addition to its direct effects on skeletal muscle, insulin also reduces hepatic glucose output. Therefore, the elevation in postprandial insulin levels and subsequent reduction in hepatic glucose output immediately prior to exercise in the postprandial state may also contribute to the reduction in glucose excursions at a time during the day when glucose tolerance is at its lowest due to circadian variation. Further combining this mechanism with what is known about the circadian shifts in glucose metabolism support our novel hypothesis that there may be temporal optimization of exercise. It is important to note that this hypothesis is based on the acute effects of exercise, but longer-term exercise training of varying modes, volumes, and intensities that results in differential adaptations in skeletal muscle and liver metabolism may have different effects. In addition, modified β-cell function could potentially alter this mechanism.
Figure 1:
The muscle, liver and adipose tissue metabolic responses to moderate exercise in individuals with type 2 diabetes in the fasted state versus exercise in the postprandial state.
Pre vs. Postprandial Exercise and Lipemia
In addition to hyperglycemia, hypertriglyceridemia is also a major risk factor for heart disease in individuals with T2D (27). Compared to glycemia, fewer studies have examined the impact of pre vs. postprandial exercise on lipemia in individuals with T2D, but the available research suggests postprandial exercise is more beneficial. For instance, Gill et al. (28) reported that 90 min of moderate intensity (50% O2max) treadmill walking the day prior to a high fat meal (~16–18 h prior) did not impact postprandial triglyceride responses in individuals with T2D; whereas, Tobin et al. (29) reported that 60 min of moderate intensity (57% O2max) cycling exercise beginning 90 min after a high-fat meal reduced postprandial triglyceride concentrations in individuals with T2D. Interestingly, Dalgaard et al. (30) found that 40 min of cycling exercise (40% O2max) either the day before a high-fat test meal or 3.5 h after a high-fat test meal did not significantly alter postprandial triglyceride levels in individuals with T2D. It should be noted that subjects in this study consumed a considerable amount of alcohol (40 g), which may counteract the impact of exercise on triglyceride levels. Additionally, discrepancies in the findings between studies may be due to the timing of exercise relative to the meal. Tobin et al. (29) found that exercise 90 min after the meal reduced postprandial triglyceride levels, whereas, Dalgaard et al. (30) found exercise 3.5 h after eating did not impact postprandial triglyceride levels in T2D. Data from our lab shows that resistance exercise 45 min after a dinner meal (3 sets of 10 repetitions using 10 repetition maximum weight, eight different exercises), but not prior to a dinner meal (ending ~30 min prior to the meal), reduced postprandial triglyceride responses in T2D, which was due to a reduction in very low density lipoprotein (VLDL) triglyceride levels (10). Thus, evidence is accumulating that postprandial exercise should be performed close to the meal to reduce subsequent postprandial triglyceride responses, but the exact timing for exercise has not been elucidated.
The two predominant forms of triglyceride rich lipoproteins in the blood are chylomicrons and VLDL lipoproteins. Chylomicrons and VLDL particles are cleared via the same lipolytic pathway by lipoprotein lipase, although lipoprotein lipase has a much greater affinity for chylomicron than VLDL (31). Since during acute insulin infusion (32) or exercise (33) VLDL secretion is reduced, we hypothesize that the superior benefit of postprandial exercise on reducing postprandial VLDL concentrations is due to the synergy of insulin and exercise to reduce hepatic VLDL secretion. Additionally, insulin increases lipoprotein lipase activity in adipose tissue while exercise increases the activity of lipoprotein lipase in skeletal muscle (34), possibly contributing to reductions in VLDL with postprandial exercise. Taken together, we speculate that acute postprandial exercise reduces triglyceride excursions more effectively than preprandial exercise in T2D because of the synergy of elevated insulin levels and exercise to reduce hepatic VLDL secretion and enhance catabolism of VLDL by lipoprotein lipase. It is well established that long-term exercise training increases skeletal muscle and liver mitochondrial biogenesis, as well as lipoprotein lipase levels in skeletal muscle. More research needs to be conducted in this area on individuals with metabolic dysruptions.
CIRCADIAN MODULATION OF METABOLISM: ROLE OF EXERCISE
Endogenous central and peripheral circadian rhythms regulate endocrinology and metabolism in alignment with the sleep/wake cycle across a typical 24 h day (4). Most studies that have advocated either preprandial or postprandial exercise in controlling postprandial glycemia or lipemia in T2D have primarily focused on early morning hours (breakfast meal) when the meal is preceded by an overnight fast. Only a few studies have examined these changes at meals other than breakfast or across an entire day of eating, and these studies do not consider the diurnal variation in glucose and lipid tolerance or insulin sensitivity when being reported.
In healthy non-diabetic individuals diurnal variations in glucose tolerance have been reported which usually show a peak in the morning (6, 35–39) with impairments in glucose tolerance in the afternoon and evening; these time-of-day effects are independent of the fasting duration (40). These variations are attributed to the insulin secretory response and β-cell responsiveness which are higher in the morning than at other times of day, as well as alterations in hepatic insulin extraction which is lower in the morning than evening indicating diurnal differences in insulin clearance (5, 6, 41, 42). For example, Van Cauter et al (6) demonstrated that in the evening there is a failure of insulin secretion to increase in proportion to changes in postprandial glucose responses as is seen with meals earlier in the day, implying that there may be a circadian variation in the responsiveness of β-cells to glucose or circadian changes in insulin sensitivity in healthy, non-diabetic individuals. Lee reported that in normal weight non-diabetic individuals glucose tolerance diminished by ~40% from morning to evening and insulin sensitivity was impaired ~ 35%, while in obese individuals glucose tolerance was not reduced (41). Likewise, Saad et al. (5) further confirmed these findings in healthy volunteers with normal fasting glucose levels. These subjects ingested identical mixed meals at 0700, 1300, and 1900 h with similar physical activity. The postprandial glucose excursion was lower at 0700 h than 1300 and 1900 h, while β-cell responsivity to glucose and the disposition index was higher. Using a triple-tracer technique they showed that meal glucose rate of appearance did not differ between meals, but they observed the presence of a diurnal pattern of glucose tolerance with a lower postprandial glucose excursion at breakfast than at lunch and dinner. Reduced β-cell responsiveness and insulin action with increased hepatic insulin extraction after lunch and dinner than breakfast led to the appearance of the diurnal pattern. Additionally, preprandial glucagon concentrations were significantly lower at 0700 h than 1300 and 1900 h, while the postprandial glucagon excursion was significantly higher at 0700 h than at 1300 and 1900 h. More recently, Leung et al. (43) showed that the postprandial glucose integrated area under the curve (iAUC) was higher in the evening (2000 h) compared to the morning (0800 h), and that following a low glycemic index meal trial, postprandial glucose and insulin iAUC at evening (2000 h) and midnight (0000 h) were higher than the morning but not significantly different between evening and midnight. Thus, meal intake at night, even when comprised of low glycemic ingredients, contributes to higher glucose excursions and concomitantly greater insulin levels, compared with an equivalent meal in the morning. Thus, exercise in the evening may be more beneficial to improve insulin sensitivity and glucose or lipid tolerance at a time of day when they are typically worst.
In T2D, disrupted chronobiology is suggested to be a major factor contributing to hyperglycemia and hypertriglyceridemia. For instance, disruptions in circadian rhythmicity through genetic modifications of the molecular clock enzymes CLOCK and BMAL1 in pancreatic islets promotes the development of diabetes in animal models (44). In healthy humans, using a 9-hour phase advance to mistime sleep and simulate night shift work worsens glycemia and lipemia (45, 46). In obese individuals, diurnal variations have been reported to be attenuated, phase delayed or absent (36, 41) while in individuals with T2D the diurnal rhythm may be absent or phase delayed by several hours. Boden et al. (7) utilized a 24 h hyperglycemic clamping procedure and found that the glucose infusion rate (i.e. insulin sensitivity), but not the glucose disappearance rate, changed rhythmically over 24 h and these changes were accounted for by circadian changes in hepatic glucose production. Thus, they observed an inverted circadian rhythm where insulin sensitivity was highest at night (1904 h) and then gradually worsened during the overnight period and was worst in the morning (0831 h). Despite this, individuals with T2D exhibit clear rhythms in hepatic glycogen storage (47) and hepatic insulin sensitivity (7); thus in these individuals whole-body insulin sensitivity in type 2 diabetic adults is highest at ~19:00 h and lowest in the morning. These circadian changes in hepatic glucose production in individuals with T2D likely contributes to the early morning rise in glucose and triglycerides, which is known as the dawn phenomenon (7).
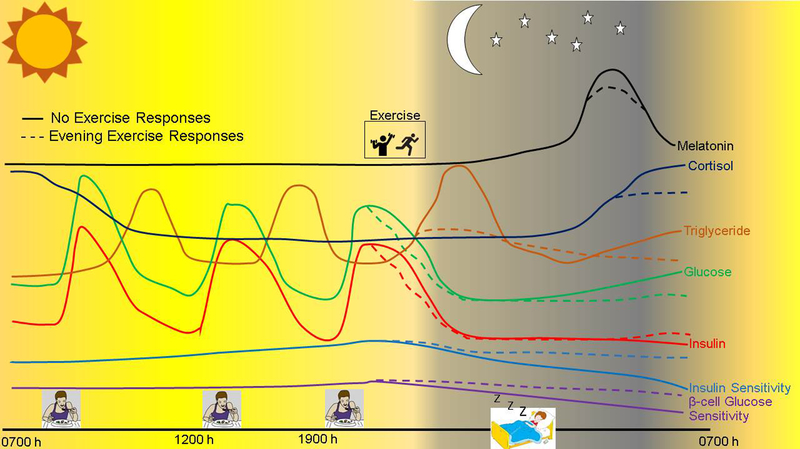
Subsequent work showed that the increase in nocturnal endogenous glucose production was mediated by an increase in liver gluconeogenesis and β-cell glucose insensitivity during the night (i.e. lack of insulin secretion despite rise in glucose levels) (8). Melatonin levels during the night were closely related to endogenous glucose production, and compared to healthy controls, peaked much later in the night in T2D. Furthermore, the dinner meal is typically the biggest meal of the day for many individuals and prior work has shown that a moderate to large size dinner meal (> 30% total daily caloric intake) causes a greater early morning rise in blood glucose (48). Given the known role of exercise to improve insulin sensitivity and glycemia or lipemia, we propose that exercising after dinner (the biggest meal of the day in Western societies) may be the most optimal time of day to exercise to not only improve glycemia and lipemia after dinner, but during the overnight period and into the next day (Figure 2).
Figure 2:
The circadian changes in the hormonal and metabolic responses to meals and exercise in an individual with type 2 diabetes throughout a 24 h period. Changes in these profiles are illustrated for a day with no exercise or a day with evening exercise.
Time of Day of Exercise
The impact of exercise at various times of the day has been well studied in healthy individuals with the outcomes being athletic performance (49). Recently, it was shown that 24 weeks of combined strength and endurance training in the evening leads to greater gains in muscle mass compared to exercise in the morning in young healthy men (50). It would be predicted that these greater gains in muscle mass with evening exercise potentially leads to greater improvements in glycemia given that muscle mass is the largest sink for glucose disposal. However, to our knowledge the impact of exercise at specific times of the day on glycemia and lipemia has not been directly investigated in individuals with or without T2D.
During the overnight period in individuals with T2D, a dysynchrony between endogenous hepatic insulin sensitivity, circulating hormones, and metabolites occur resulting in a rise in endogenous glucose output ultimately causing fasting hyperglycemia in the morning. Thus, performing exercise or physical activity in the evening, when postprandial glycemia is the worst and hepatic insulin sensitivity begins to worsen due to circadian rhythms, may provide the most benefit not only to improve postprandial glycemia after dinner, but also to enhance insulin sensitivity into the nocturnal period and lower fasting morning blood glucose levels in individuals with T2D. Indirect evidence from different studies support our hypothesis that exercise in the evening may be the most effective time to exercise for individuals with T2D. For instance, exercising after lunch had a minimal impact on glycemia over the following 24 hours (17), while work from our lab (10) and Colberg et al. (51) have shown that exercise in the evening reduces glycemia during exercise and the overnight period, when glycemic control is poorest. Another study (16) found that simply providing advice to walk for 10 min after meals was more effective at improving glycemia, especially when walking was performed after the dinner meal, compared to advice to exercise without specifying timing. Although these studies do not directly compare the effects of exercise at various times of the day on glycemia or lipemia in individuals with T2D, when observed together they provide some evidence that exercise after dinner may be the most optimal time to exercise for individuals with T2D to improve glycemia and lipemia.
Given that glycemic excursions are large after every meal in individuals with T2D, another strategy may be to perform exercise/physical activity after each main meal throughout the day. Indeed, several investigations have found that breaking up prolonged sitting with bouts of standing/physical activity improves postprandial glycemia and lipemia, and some show that this strategy is more effective than morning exercise alone. For example, work from our group has shown that in obese, insulin resistant individuals short, frequent periods of exercise throughout the day and evening attenuate glycemia to a greater degree than an equal amount of exercise performed continuously in the morning (52). Duvivier et al. (53) showed that breaking up prolonged sitting with standing and light-intensity walking throughout the day and evening improved glycemic control and insulin sensitivity more effectively than a single exercise session performed ~2 h after breakfast, suggesting that exercise in the morning and evening is more effective than morning exercise alone. Likewise, Dempsey et al. (54) showed that interrupting prolonged sitting with short bouts of walking or simple resistance activities every 30 min throughout a 7 h period improved glycemic control during the day and overnight period, while Grace et al. (55) showed that breaking up prolonged sitting over a 7 h postmeal period with light walking or resistance exercise reduced many different triglyceride species. These studies further support our hypothesis that adding exercise to the evening period is most effective at improving glycemia and lipemia in T2D.
The mechanism by which evening exercise is superior to exercise at other times of the day may be due to alterations in melatonin signaling. Individuals at risk of T2D have increased expression of melatonin receptors in the pancreas, and in cell culture experiments melatonin has been shown to inhibit β-cell glucose stimulated insulin secretion (56), which may contribute to the dawn phenomenon. Buxton et al. (57) demonstrated that exercise in the evening phase advanced the onset of the melatonin peak in healthy non-diabetic individuals, as compared to morning, afternoon and nighttime exercise, while nighttime exercise (after midnight) robustly increased melatonin concentrations. In contrast, Monteleone et al (58) reported that exercise at night (~11 pm) blunts the nocturnal increase in plasma melatonin levels in healthy individuals (58). It is not known how exercise at night impacts melatonin levels in individuals with T2D, but we speculate that evening exercise but not nighttime exercise may potentially relieving melatonin-induced inhibition in β-cell glucose stimulated insulin secretion. This would allow more insulin to be secreted when nocturnal endogenous glucose production and plasma glucose levels rise during the night and ultimately prevent the dawn phenomenon. Furthermore, exercise could reduce melatonin receptor expression in the pancreas in individuals with T2D, which would also improve nocturnal β-cell glucose stimulated insulin secretion and lower fasting plasma glucose levels the following morning. Additionally, exercise prior to fasting overnight will improve hepatic insulin sensitivity, which will attenuate the early morning rise in endogenous glucose production and triglyceride secretion and prevent the dawn phenomenon in T2D.
CONSIDERATIONS
Complicating the interpretation and implementation of many of the above studies is the variability in human physiology such that disease progression as well as individual behaviors can dampen or accentuate many of the responses reported in these studies. This results in the observations within data sets of responders and non-responders to studied interventions. To date, the research on the time of day of exercise is limited and the effects of evening exercise and postprandial activity needs to be studied considerably more in individuals with T2D. The work that has been done has focused on acute bouts of exercise at different times of day when the longer-term effects may be very different and due to different mechanisms. Exercise following long periods of fasting are taking place when lipolysis is high, gluconeogenesis could be elevated resulting in increased endogenous glucose production, which might further worsen postprandial glycemia and lipemia. Research examining the first and second meal on glucose levels show that in healthy individuals the glucose levels at breakfast are often higher than at lunch when given the same meal composition. It is speculated that endogenous glucose production is high at breakfast and eating a meal acts as a primer to the tissues either altering their sensitivity to insulin or altering the pancreatic response to the glucose signal. This would be particularly critical in an insulin insensitive, T2D individual with metabolic inflexibility. Superimposing exercise on these metabolic conditions results in dramatic shifts in metabolism that can vary with different types of exercise (aerobic vs resistance), duration and intensity of exercise, and acute verses chronic effects of exercise. Although not as practical, exercise can be split into multiple bouts during the day (including fasting and postprandial state exercise) and result in different responses in glucose and lipid excursions. Lastly, animal data has supported the idea for temporal coordination of metabolism in the context of health and disease (59) as disruption of metabolism and circadian clock function are observed with behavior misalignment, or high-fat diet). Sleep restriction has also been linked with perturbations in the circadian and metabolic system.
SUMMARY AND FUTURE DIRECTIONS
The impact of dose, frequency, and intensity of exercise on cardiovascular fitness, metabolism, and strength have been well characterized, and are included in most exercise prescriptions. Although we know that circadian rhythms are controlled centrally with peripheral tissues imposing their own rhythms, there is a paucity of exercise research in humans directly comparing exercise at various times relative to meal ingestion and/or time of day and the impact on glycemia and lipemia in T2D. Herein, we provide evidence that acute postprandial exercise, compared to premeal exercise, has a greater benefit on postprandial glucose and triglyceride levels in individuals with T2D, as well as demonstrate that evening exercise appears to be more beneficial to improve these important clinical outcomes
The link between chronobiology, exercise, and energy metabolism in the setting of T2D is not well understood and represents an emerging and ripe area for future study. Future research should address the acute and long-term impact of aerobic, resistance, or combined exercise training regimens at various times relative to a meal, at separate times of the day, and at various times relative to medication use, and varying modes, volumes, and intensities of exercise on metabolic health and lifespan in individuals with T2D. Like pharmacological prescriptions we should start to think about temporal optimization of exercise in relation to meals as well as with time of day effects particularly in individuals in various disease states. The inclusion of consideration of exercise timing allows more diversity in our exercise prescription instead of a one size fits all prescription.
Key Points.
Energy metabolism and insulin sensitivity are influenced by central (i.e., brain) and peripheral (i.e., skeletal muscle, liver, adipose tissue) circadian rhythms.
Diurnal variations in energy metabolism and insulin secretion are found in healthy non-diabetic individuals with greater insulin sensitivity and glucose or lipid tolerance in the morning, which all gradually worsen in the afternoon and evening.
Individuals with type 2 diabetes (T2D) have an inverted circadian rhythm in insulin sensitivity, which is relatively improved in the evening, which thereafter begins to gradually worsen overnight and into the morning ultimately resulting in abnormally elevated morning glycemia and lipemia (i.e., dawn phenomena).
Exercise after meals, compared to exercise before meals, reduces postprandial glycemia and lipemia most effectively in individuals with T2D.
Exercise in the evening, compared to exercise in the morning, has a more beneficial impact on glycemia and lipemia in individuals with T2D.
Acknowledgments
Disclosure of Funding: The authors were funded by the following grants while writing this manuscript: NIH DK109556 (TDH), DK110338 (TDH), and DK101513 (JAK).
Footnotes
Conflicts of Interest: The authors have no conflict of interest to report.
REFERENCES
- 1.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soro-Paavonen A, Zhang WZ, Venardos K et al. Advanced glycation end-products induce vascular dysfunction via resistance to nitric oxide and suppression of endothelial nitric oxide synthase. J Hypertens. 2010;28(4):780–8. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Wen T, Zheng XY et al. Remnant-like particles accelerate endothelial progenitor cells senescence and induce cellular dysfunction via an oxidative mechanism. Atherosclerosis. 2009;202(2):405–14. [DOI] [PubMed] [Google Scholar]
- 4.Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad A, Dalla Man C, Nandy DK et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61(11):2691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262(4 Pt 1):E467–75. [DOI] [PubMed] [Google Scholar]
- 7.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes. 1996;45(8):1044–50. [DOI] [PubMed] [Google Scholar]
- 8.Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia. 2006;49(7):1619–28. [DOI] [PubMed] [Google Scholar]
- 9.Taskinen MR, Sane T, Helve E, Karonen SL, Nikkila EA, Yki-Jarvinen H. Bedtime insulin for suppression of overnight free-fatty acid, blood glucose, and glucose production in NIDDM. Diabetes. 1989;38(5):580–8. [DOI] [PubMed] [Google Scholar]
- 10.Heden TD, Winn NC, Mari A et al. Postdinner resistance exercise improves postprandial risk factors more effectively than predinner resistance exercise in patients with type 2 diabetes. J Appl Physiol (1985). 2015;118(5):624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colberg SR, Zarrabi L, Bennington L et al. Postprandial walking is better for lowering the glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J Am Med Dir Assoc. 2009;10(6):394–7. [DOI] [PubMed] [Google Scholar]
- 12.Larsen JJ, Dela F, Kjaer M, Galbo H. The effect of moderate exercise on postprandial glucose homeostasis in NIDDM patients. Diabetologia. 1997;40(4):447–53. [DOI] [PubMed] [Google Scholar]
- 13.Larsen JJ, Dela F, Madsbad S, Galbo H. The effect of intense exercise on postprandial glucose homeostasis in type II diabetic patients. Diabetologia. 1999;42(11):1282–92. [DOI] [PubMed] [Google Scholar]
- 14.Poirier P, Mawhinney S, Grondin L et al. Prior meal enhances the plasma glucose lowering effect of exercise in type 2 diabetes. Med Sci Sports Exerc. 2001;33(8):1259–64. [DOI] [PubMed] [Google Scholar]
- 15.Poirier P, Tremblay A, Catellier C, Tancrede G, Garneau C, Nadeau A. Impact of time interval from the last meal on glucose response to exercise in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2000;85(8):2860–4. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds AN, Mann JI, Williams S, Venn BJ. Advice to walk after meals is more effective for lowering postprandial glycaemia in type 2 diabetes mellitus than advice that does not specify timing: a randomised crossover study. Diabetologia. 2016;59(12):2572–8. [DOI] [PubMed] [Google Scholar]
- 17.Haxhi J, Leto G, di Palumbo AS et al. Exercise at lunchtime: effect on glycemic control and oxidative stress in middle-aged men with type 2 diabetes. Eur J Appl Physiol. 2016;116(3):573–82. [DOI] [PubMed] [Google Scholar]
- 18.Gaudet-Savard T, Ferland A, Broderick TL et al. Safety and magnitude of changes in blood glucose levels following exercise performed in the fasted and the postprandial state in men with type 2 diabetes. Eur J Cardiovasc Prev Rehabil. 2007;14(6):831–6. [DOI] [PubMed] [Google Scholar]
- 19.Terada T, Wilson BJ, Myette-Comicronte E et al. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism. 2016;65(5):599–608. [DOI] [PubMed] [Google Scholar]
- 20.Nygaard H, Ronnestad BR, Hammarstrom D, Holmboe-Ottesen G, Hostmark AT. Effects of exercise in the fasted and postprandial state on interstitial glucose in hyperglycemic individuals. J Sports Sci Med. 2017;16(2):254–63. [PMC free article] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32 Suppl 2:S157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frosig C, Richter EA. Improved insulin sensitivity after exercise: focus on insulin signaling. Obesity (Silver Spring). 2009;17 Suppl 3:S15–20. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5(4):237–52. [DOI] [PubMed] [Google Scholar]
- 24.Koh HJ, Toyoda T, Fujii N et al. Sucrose nonfermenting AMPK-related kinase (SNARK) mediates contraction-stimulated glucose transport in mouse skeletal muscle. Proc Natl Acad Sci U S A. 2010;107(35):15541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neill HM, Maarbjerg SJ, Crane JD et al. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci U S A. 2011;108(38):16092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest. 1981;68(6):1468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginsberg HN, Illingworth DR. Postprandial dyslipidemia: an atherogenic disorder common in patients with diabetes mellitus. Am J Cardiol. 2001;88(6A):9H–15H. [DOI] [PubMed] [Google Scholar]
- 28.Gill JM, Al-Mamari A, Ferrell WR et al. Effect of prior moderate exercise on postprandial metabolism in men with type 2 diabetes: heterogeneity of responses. Atherosclerosis. 2007;194(1):134–43. [DOI] [PubMed] [Google Scholar]
- 29.Tobin LW, Kiens B, Galbo H. The effect of exercise on postprandial lipidemia in type 2 diabetic patients. Eur J Appl Physiol. 2008;102(3):361–70. [DOI] [PubMed] [Google Scholar]
- 30.Dalgaard M, Thomsen C, Hermansen K. Effects of one single bout of low-intensity exercise on postprandial lipaemia in type 2 diabetic men. Br J Nutr. 2004;92(3):469–76. [DOI] [PubMed] [Google Scholar]
- 31.Bjorkegren J, Packard CJ, Hamsten A et al. Accumulation of large very low density lipoprotein in plasma during intravenous infusion of a chylomicron-like triglyceride emulsion reflects competition for a common lipolytic pathway. J Lipid Res. 1996;37(1):76–86. [PubMed] [Google Scholar]
- 32.Adiels M, Westerbacka J, Soro-Paavonen A et al. Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia. 2007;50(11):2356–65. [DOI] [PubMed] [Google Scholar]
- 33.Sondergaard E, Rahbek I, Sorensen LP et al. Effects of exercise on VLDL-triglyceride oxidation and turnover. Am J Physiol Endocrinol Metab. 2011;300(5):E939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiens B, Lithell H, Mikines KJ, Richter EA. Effects of insulin and exercise on muscle lipoprotein lipase activity in man and its relation to insulin action. J Clin Invest. 1989;84(4):1124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarrett RJ, Baker IA, Keen H, Oakley NW. Diurnal variation in oral glucose tolerance: blood sugar and plasma insulin levels morning, afternoon, and evening. Br Med J. 1972;1(5794):199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmet PZ, Wall JR, Rome R, Stimmler L, Jarrett RJ. Diurnal variation in glucose tolerance: associated changes in plasma insulin, growth hormone, and non-esterified fatty acids. Br Med J. 1974;1(5906):485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarrett RJ, Keen H. Diurnal variation of oral glucose tolerance: a possible pointer to the evolution of diabetes mellitus. Br Med J. 1969;2(5653):341–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll KF, Nestel PJ. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes. 1973;22(5):333–48. [DOI] [PubMed] [Google Scholar]
- 39.Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271(2 Pt 1):E246–52. [DOI] [PubMed] [Google Scholar]
- 40.Hulman A, Faerch K, Vistisen D et al. Effect of time of day and fasting duration on measures of glycaemia: analysis from the Whitehall II Study. Diabetologia. 2013;56(2):294–7. [DOI] [PubMed] [Google Scholar]
- 41.Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes. 1992;41(6):750–9. [DOI] [PubMed] [Google Scholar]
- 42.Morris CJ, Yang JN, Garcia JI et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung GKW, Huggins CE, Bonham MP. Effect of meal timing on postprandial glucose responses to a low glycemic index meal: A crossover trial in healthy volunteers. Clin Nutr. 2017. [DOI] [PubMed] [Google Scholar]
- 44.Marcheva B, Ramsey KM, Buhr ED et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hampton SM, Morgan LM, Lawrence N et al. Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol. 1996;151(2):259–67. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro DC, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. J Endocrinol. 1998;158(3):305–10. [DOI] [PubMed] [Google Scholar]
- 47.Macauley M, Smith FE, Thelwall PE, Hollingsworth KG, Taylor R. Diurnal variation in skeletal muscle and liver glycogen in humans with normal health and Type 2 diabetes. Clin Sci (Lond). 2015;128(10):707–13. [DOI] [PubMed] [Google Scholar]
- 48.Beebe CA, Van Cauter E, Shapiro ET et al. Effect of temporal distribution of calories on diurnal patterns of glucose levels and insulin secretion in NIDDM. Diabetes Care. 1990;13(7):748–55. [DOI] [PubMed] [Google Scholar]
- 49.Chtourou H, Souissi N. The effect of training at a specific time of day: a review. J Strength Cond Res. 2012;26(7):1984–2005. [DOI] [PubMed] [Google Scholar]
- 50.Kuusmaa M, Schumann M, Sedliak M et al. Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations. Appl Physiol Nutr Metab. 2016;41(12):1285–94. [DOI] [PubMed] [Google Scholar]
- 51.Bacchi E, Negri C, Trombetta M et al. Differences in the acute effects of aerobic and resistance exercise in subjects with type 2 diabetes: results from the RAED2 Randomized Trial. PLoS One. 2012;7(12):e49937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmstrup M, Fairchild T, Keslacy S, Weinstock R, Kanaley J. Multiple short bouts of exercise over 12-h period reduce glucose excursions more than an energy-matched single bout of exercise. Metabolism. 2014;63(4):510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duvivier BM, Schaper NC, Hesselink MK et al. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia. 2017;60(3):490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dempsey PC, Blankenship JM, Larsen RN et al. Interrupting prolonged sitting in type 2 diabetes: nocturnal persistence of improved glycaemic control. Diabetologia. 2017;60(3):499–507. [DOI] [PubMed] [Google Scholar]
- 55.Grace MS, Dempsey PC, Sethi P et al. Breaking Up Prolonged Sitting Alters the Postprandial Plasma Lipidomic Profile of Adults With Type 2 Diabetes. J Clin Endocrinol Metab. 2017;102(6):1991–9. [DOI] [PubMed] [Google Scholar]
- 56.Lyssenko V, Nagorny CL, Erdos MR et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol. 2003;284(3):R714–24. [DOI] [PubMed] [Google Scholar]
- 58.Monteleone P, Maj M, Fusco M, Orazzo C, Kemali D. Physical exercise at night blunts the nocturnal increase of plasma melatonin levels in healthy humans. Life Sci. 1990;47(22):1989–95. [DOI] [PubMed] [Google Scholar]
- 59.Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. 2015;277(5):513–27. [DOI] [PubMed] [Google Scholar]