Abstract
Objective:
To evaluate the performance of a rapid, low cost, non-contrast MRI examination as a secondary screening tool in detection of clinically significant prostate cancer.
Methods:
In this prospective single institution study 129 patients with elevated PSA levels or abnormal digital rectal examination findings underwent MRI with an abbreviated biparamatric MRI protocol consisting of high resolution axial T2- and diffusion weighted images. Index lesions were classified according to PI-RADS v2.0. All patients underwent standard transrectal ultrasound guided (TRUS) biopsy after MRI with the urologist being blinded to MRI results. Subsequently all patients with suspicious lesions (PI-RADS 3, 4 or 5) underwent cognitively guided targeted biopsy after discussion of MRI results with the urologist. Sensitivity and negative predictive value for identification of clinically significant prostate cancer (Gleason 3+4 and above) were determined.
Results:
Rapid biparametric MRI discovered 176 lesions identified in 129 patients. Rapid MRI detected clinically significant cancers with a sensitivity of 95.1% with a negative predictive value of 95.1% and positive predictive value of 53.2%, leading to a change in management in 10.8% of the patients. False negative rate of bp-MRI was 4.7%.
Conclusions:
We found that a bp-MRI examination can detect clinically significant lesions and changed patient management in 10.8% of the patients. A rapid MRI protocol can be used as a useful secondary screening tool in men presenting with suspicion of prostate cancer.
Original Research, submission category:
Oncology
Keywords: abbreviated MRI, prostate cancer, biparametric magnetic resonance imaging, prostate MRI, rapid MRI protocol, PI-RADS
Introduction
An ongoing challenge in the diagnosis and management of prostate cancer is to identify biologically significant disease at a stage at which it is still of a curative size and grade, while minimizing the detection of ‘insignificant’ cancers (1). The widespread adoption of prostate specific antigen (PSA) as a screening tool led to a decrease in prostate cancer mortality (2). However, there is concern that PSA screening has led to overdiagnosis and overtreatment of clinically insignificant prostate cancers. There is an ongoing need for improved prostate cancer screening tools to prevent both death related to aggressive prostate cancer and morbidity due to unnecessary treatment. Multiparametric magnetic resonance imaging (mp-MRI) has emerged as an important tool in prostate cancer diagnosis and risk stratification with regard to the cancer aggressiveness (3,4). Unfortunately, the use of mp-MRI as a screening tool for prostate cancer is cost- and time-prohibitive due to the long scanning time necessary to acquire multiple sequences, added cost of IV contrast, and patient discomfort associated with long MRI scanning protocols.
Currently, prostate MRI exams are evaluated and reported according to the standards of the Prostate Imaging Reporting and Data System (PI-RADS) version 2 (5). In PI-RADS v2, T2-weighted (T2w) and diffusion weighted imaging (DWI) sequences are key in determining the appropriate score. Positive early enhancement on dynamic contrast enhanced (DCE) imaging may serve as a modifier to upgrade the score from PI-RADS 3 to 4 in the peripheral zone. Thus DCE only plays a supplementary role in the peripheral zone and has no role in the transition zone. It may be argued that both PI-RADS 3 and 4 lesions require further attention regardless, and many urologists (including those at our institution) prefer to biopsy all lesions with PIRADs scores ≥3. Hence, the effect of DCE is not critical and may not warrant its drawbacks.
Thus, there is potential benefit in creating a biparametric (T2w and DWI based) protocol using a limited number of sequences and omitting DCE acquisition for prostate cancer screening as shown also by Boesen et al. (6). Other prior evaluations of a biparametric protocol were either retrospective (7) or if prospective, were part of a two-step assessment where patients were scanned with a complete mp-MRI protocol while a “hypothetical biparametric protocol” consisting of T2w and DWI sequences were evaluated first and then compared with the full multiparametric protocol (8,9). Although diagnostic accuracy of bp-MRI can be determined in this setting, it remains unclear whether clinical decisions were based on mp-MRI or bp-MRI findings. Here we provide another proof of concept study to add to the experience of Boesen et al. (6), exploring the use of a limited, rapid biparametric MRI exam in conjunction with a modified PIRADs v2 assessment and a cognitively targeted biopsy to diagnose prostate cancer.
The aim of the present study was to prospectively evaluate a non-contrast biparametric screening MRI protocol including only T2w and DWI images for its effect on patient throughput time and diagnostic accuracy using histopathology from systematic and targeted prostate biopsy as a gold standard.
Materials and Methods
Study design
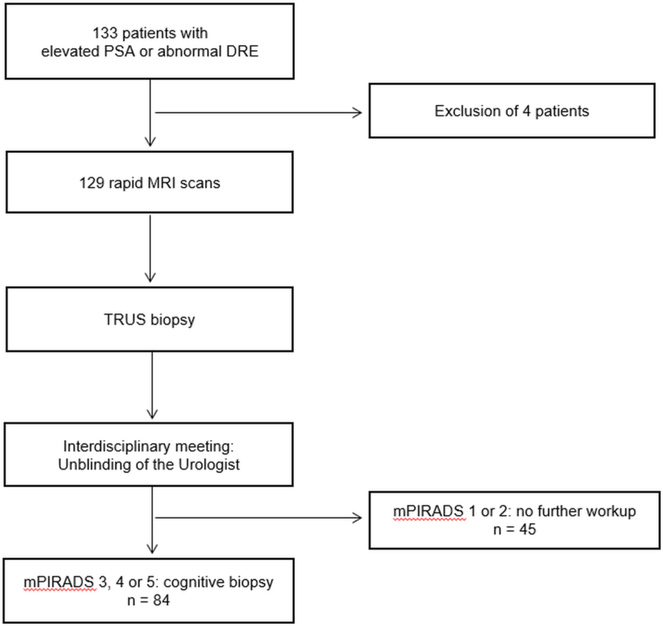
This study was approved by the local institutional review board (IRB). Between September 2015 and April 2017, 133 biopsy naïve men were referred to the Urology Department of our tertiary care center for evaluation of elevated prostate specific antigen (PSA) or abnormal digital rectal examination (DRE) and provided informed consent for participation in this prospective study (Figure 1). Abnormal PSA blood test was defined as >2.5 ng/mL for men <50 years of age, >3.5 ng/mL for men <60 years of age, and >4.5 ng/mL for men >60 years of age. Abnormal digital rectal exam was defined as a palpable nodule, induration, or firm area within the prostate. The current standard for these patients is to perform a transrectal ultrasound (TRUS) guided biopsy. In order to avoid any bias due to post biopsy changes in MRI, imaging was performed prior to biopsy in all patients (Figure 1). MRI was successfully performed for 129 patients and these were included in the analysis. Four patients were excluded due to extensive artefacts from hip implants strongly degrading the study (n=2), degradation of diffusion weighted images due to excess bowel gas (n=1) and a technical issue in the MRI scanner at the time of examination (n=1) (Figure 1).
Figure 1. Study participant workflow.
A total of 133 patients with elevated PSA were enrolled in this study. Four patients were excluded; three because of extensive artefacts in the prostate region due to total endoprosthetic hip replacement (n=2) or due to excessive bowel gas causing sever distortion artefacts on diffusion weighted images (n=1). Another patient was excluded due to technical problems of the MRI scanner and the inability to obtain a full diagnostic MRI exam. The MRI exams of 129 patients scanned with the biparametric rapid MRI protocol were included in the statistical analysis. All 129 patients subsequently underwent TRUS biopsy. After systematic biopsy the Radiologists unblinded the Urologist to MRI findings. 84 Patients with a mPI-RADS lesion 3, 4 or 5 underwent further cognitive targeted biopsy in the same biopsy session. PSA = Prostate specific antigen; DRE = Digital rectal examination; MRI = Magnetic resonance imaging, TRUS = Transrectal ultrasound guided 12 quadrant prostate biopsy, mPI-RADS = modified Prostate Imaging Reporting and Data System
MR Imaging
MRI examinations were performed on 3T scanners (Magnetom Verio® and Magnetom Skyra®, Siemens Healthinieers, Erlangen, Germany). The rapid MRI protocol contained a single shot T2w sequence in axial, coronal and sagittal planes, an axial high resolution T2w turbo spin echo (TSE) and axial diffusion weighted imaging (DWI) with apparent diffusion coefficient (ADC) mapping. The detailed sequence parameters are summarized in Supplementary Table 1.
Suspicious lesions were categorized on a 5-point scale according to a modified PI-RADS v2.0 (mPI-RADS) by a fellowship trained radiologist with 14 years of experience in clinical MR imaging at the time of study beginning (***). We refer to this as a mPIRADS system from here forward, as DCE, which is part of formal PIRADs classification, is not included. Since the bp-MRI protocol does not include dynamic contrast-enhanced imaging, scoring of lesions in the peripheral zone relied on diffusion-weighted image findings (dominant sequence) only. An equivocal score of 3 in the peripheral zone was not considered for upgrading to a score of 4 due to the lack of positive dynamic contrast-enhanced images. Scoring of transition zone lesions was unaffected. Although all patients underwent 12-core TRUS biopsy after MRI, lesions diagnosed as mPI-RADS 3, 4 or 5 were recommended for additional targeted biopsy. Studies with a mPI-RADS score of 1 or 2 were not recommended for biopsy based on MRI results but all patients still underwent a standard TRUS biopsy as a part of the study protocol. Therefore, the results of these biopsy examinations were compared to MRI findings. The table time (i.e. the time elapsed from when the patient entered the scanner to when they exited the scanner) and scan time (the time from beginning to end of image acquisition) was recorded for every patient.
Prostate Biopsy
All patients underwent standard 12 core TRUS guided sextant biopsy following MRI, performed by two urologists (*** or ***) with 13 and 18 years of experience at the time of study initiation, who were initially blinded to the MRI results. After systematic biopsy, MRI data were revealed to the urologist and suspicious lesions were cognitively targeted with 2 cores per lesion during the same session. All cores were evaluated on histopathology and classified according to the Gleason scoring system. The lesions with Gleason score 3 + 4 and above were classified as clinically significant cancer. The locations of the suspicious lesions recommended for additional targeted biopsy were then correlated with the histological locations listed on the pathology report to determine correspondence between MRI and pathology locations.
Statistical Analysis
Using histopathology as the gold standard, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the rapid MRI protocol were calculated using SPSS statistics software (Version 25, IBM, Armonk, New York, USA). The area under the curve (AUC) of the receiver operating characteristics (ROC) was assessed. On MRI, a mPI-RADS score of 1 or 2 was considered as negative and a mPI-RADS 3 or above as positive. On pathology, a Gleason score 3 + 4 or higher was considered to represent positive for clinically significant cancer, while benign prostate tissue, prostatitis, high grade prostate intraepithelial neoplasia, small acinar cell proliferation or Gleason 3+3 disease was classified as true negatives.
The lesions missed by standard TRUS biopsy and by the MRI exam were separately described. We further evaluated the instances in which MRI changed the management for the patients compared to the systematic TRUS-guided biopsy. Patients who had histological findings that differed between TRUS and targeted biopsy were analyzed, and cases where targeted biopsy identified an otherwise undetected clinically significant cancer or upgraded the Gleason score were considered a change in management over TRUS. The specific cases were reported and the percentage of cases with this occurrence was calculated.
Results
Examination time
A total of 129 patients (age range: 47–77 years; mean age 61.8 years) were successfully scanned with the rapid MRI protocol. A summary of patient baseline characteristics is shown in supplementary Table 2. The average scan time for running this protocol was 11.9 minutes. The average table time was 15.7 minutes. A total of 184 lesions were identified and assigned a mPI-RADS score: 111 lesions were assigned a score of mPI-RADS 3, 4 or 5 and 65 lesions were assigned a mPI-RADS score of 2. Eight studies were read as entirely normal and were assigned as mPI-RADS 1 (supplementary Table 3).
MRI pathology correlation
All 23 of 23 (100%) lesions categorized as mPI-RADS 5 turned out to be clinically significant prostate cancer. A total of 25 out of 39 (64.1%) mPI-RADS 4 lesions and 11 out of 49 (22.4%) mPI-RADS 3 lesions were proven to be clinically significant cancer. In 3 patients with a PI-RADS 2 lesion (4.7%), TRUS biopsy revealed clinically significant cancer. No cancer was found on biopsy on MRI scans categorized as mPI-RADS 1 (0 out of 8) (Table 1). MRI detected clinically significant cancers with a sensitivity of 95.1% (95% CI 86.5%, 98.99%), specificity of 57.3% (95% CI 48.1%, 66.26%), NPV of 95.1% (95% CI 88.45%, 98.61%) and PPV of 53.2% (95% CI 47.82%, 58.41%). This results in an area under the ROC curve (AUC) of 0.76 (Table 3).
Table 1.
Pathologic correlation of MRI findings in 129 patients with 184 lesions
| MRI findings | Pathological correlation | N |
|---|---|---|
| mPIRADS 1 (n=8) | Benign prostatic tissue | 4 |
| Prostatitis | 4 | |
| mPIRADS 2 (n=65) | Benign prostatic tissue | 17 |
| High grade prostatic intraepithelial neoplasia(HGPIN) | 6 | |
| Small acinar proliferation | 3 | |
| Prostatitis | 29 | |
| Gleason 3+3 = 6 | 7 | |
| Gleason 3+4 = 7 | 2 | |
| Gleason 4+3 = 7 | 1 | |
| mPIRADS 3 (n=49) | Benign prostatic tissue | 11 |
| High grade prostatic intraepithelial neoplasia (HGPIN) | 2 | |
| Small acinar proliferation | 1 | |
| Prostatitis | 16 | |
| Gleason 3+3 = 6 | 8 | |
| Gleason 3+4 = 7 | 7 | |
| Gleason 4+3 = 7 | 3 | |
| Gleason 4+4 = 8 | 1 | |
| mPIRADS 4 (n=39) | Benign prostatic tissue | 4 |
| High grade prostatic intraepithelial neoplasia (HGPIN) | 2 | |
| Prostatitis | 3 | |
| Gleason 3+3 = 6 | 5 | |
| Gleason 3+4 = 7 | 10 | |
| Gleason 4+3 = 7 | 11 | |
| Gleason 4+4 = 8 | 2 | |
| Gleason 4+5 = 9 | 2 | |
| mPIRADS 5 (n=23) Gleason | 3+4 = 7 | 9 |
| Gleason 4+3 = 7 | 8 | |
| Gleason 4+4 = 8 | 2 | |
| Gleason 4+5 = 9 | 3 | |
| Gleason 5+5 = 10 | 1 |
Table 3.
Diagnostic performance of rapid MRI protocol
| Biopsy proven pathology | |||
|---|---|---|---|
| MRI | Cancer | Benign | |
| Positive | 59 (TP) | 52 (FP) | 0.53 PPV |
| Negative | 3 (FN) | 70 (TN) | 0.96 NPV |
| 0.95 Sensitivity | 0.573 Specificity | 70.11% Accuracy | |
Rapid biparametric MRI resulted in a sensitivity and specificity of 95% and 57.3% and a positive predictive value (PPV) of 53.1% and negative predictive value (NPV) of 95.8% for the detection of clinically significant cancer (Gleason ≥3+4). This results in a diagnostic accuracy calculated as (TN+TP)/(TN+TP+FN+FP) of 70.11%
A positive MRI includes lesions scored PIRADS 3 and above, while a negative MRI includes PIRADS 1 and 2 lesions.
MRI = Magnetic resonance imaging, TP = true positive, FP = false positive, FN = false negative, TN = true negative, PPV = positive predictive value, NPV = Negative predictive value
Clinical management/Impact of MRI
The rapid MRI protocol and subsequent cognitive guided biopsy changed management in 14 out of 129 (10.8%) of the patients (Table 2). In 13 patients, the rapid MRI detected tumors (located anteriorly, apically or in the transitional zone) that were diagnosed as benign prostatic tissue, inflammation or Gleason 3+3 tumor based on TRUS biopsy. Further, a Gleason 4+3 tumor was upgraded to a Gleason 4+4 tumor in one patient due to targeted biopsy based on MRI guidance compared to TRUS biopsy results.
Table 2.
Change of management due to rapid MRI protocol or TRUS biopsy
| Management changed by MRI | ||
|---|---|---|
| Diagnosis on targeted biopsy | Diagnosis on TRUS biopsy | N (%) |
| Gleason 3+4 | Benign prostatic tissue | 8 (6.2%) |
| Gleason 3+4 | Gleason 3+3 | 2 (1.6%) |
| Gleason 4+3 | Gleason 3+3 | 2 (1.6%) |
| Gleason 4+3 | Benign prostatic tissue | 1 (0.8%) |
| Gleason 4+4 | Benign prostatic tissue | 1 (0.8%) |
| Gleason 4+4 | Gleason 4+3 | 1 0.8(%) |
| Management changed by 12-quadrant TRUS biopsy | ||
| Diagnosis on targeted biopsy | Diagnosis on TRUS biopsy | N |
| No suspicious lesion to target | Gleason 3+4 | 2 (1.6%) |
| No suspicious lesion to target | Gleason 4+3 | 1 (0.8%) |
| Gleason 3+3 | Gleason 3+4 | 1 (0.8%) |
| Gleason 3+3 | Gleason 4+3 | 1 (0.8%) |
| Gleason 3+4 | Gleason 4+3 | 2 (1.6%) |
| Gleason 4+3 | Gleason 4+4 | 1 (0.8%) |
| Gleason 5+4 | Gleason 5+5 | 1 (0.8%) |
In 15 out of 129 (%) patients targeted biopsy based on findings from the rapid biparametric MRI protocol revealed an increase of histological tumor grade compared to the diagnosis based on TRUS biopsy. In 3 out of 129 (2.3%) patients there was no targetable lesion identifiable on MRI Images although TRUS biopsy diagnosed a Gleason 3+4 (n=2) and 4+3 (n=1) cancer. In 6 out of 129 (4.7%) histological tumor grade increased from targeted to TRUS biopsy. However, diagnosis changed from clinically insignificant to significant cancer in 2 patients (1.6%).
MRI = Magnetic resonance imaging, TRUS = Transrectal ultrasound
MRI was falsely negative for clinically significant cancer in 4.7% of patients as TRUS biopsy revealed a Gleason 3+4 tumor in two of these patients (tumor volume 20% of the core in one patient and 40% of the core in second patient) and a Gleason 4+3 tumor in one patient (tumor volume of 5% of the core) while MRI did not show a suspicious lesion (Table 2). The MRI scans for these three patients were reevaluated but no suspicious lesion was identified even on retrospective evaluation.
Discussion
This study prospectively demonstrated the clinical feasibility and utility of a rapid MRI protocol for detection of clinically significant prostate cancer. The rapid MRI protocol detected cancers proven to be Gleason 3+4 grade or above on pathology with a high negative predictive value and high sensitivity without the need for contrast, while also improving clinical throughput compared to a traditional multi-parametric contrast enhanced MRI exam. Our results demonstrating a NPV of the bp-MRI protocol of 96% for a modified PI-RADS score of ≥3 for clinically significant prostate cancer are concordant with Boesen et al. (97%) The sensitivity reported in their protocol was also similar to ours (95% and 98%, respectively) (6). Current state of the art for this examined patient cohort is systematic TRUS biopsy. The bp-MRI protocol allowed detection of more cases of clinically significant cancer than TRUS biopsy and thus resulted more often in appropriate workup and treatment.
This concept of bp-MRI has been hypothesized in meta-analyses (11,12), and was retrospectively evaluated by analyzing a subset of sequences acquired as a part of mp-MRI protocol by Kuhl et al. (13). They also stated that a rapid bp-MRI protocol can serve as a quick noninvasive test to sort out the multitude of men who do not require biopsy and help identify and consolidate the ones at risk of having significant prostate cancer. However, slightly differently from Kuhl et al., we included coronal and sagittal plane single shot T2w sequences, which allows better anatomic localization of suspicious cancer lesions within the gland and gives the urologist more information for biopsy planning. A biparametric MRI examination was evaluated prospectively by Jambor et al. as a prospective clinical trial (IMPROD trial) (14). However, this study from Jambor et al. did not use PI-RADS for lesion classification on MRI and the urologists performed cognitive biopsy based on MRI findings followed by a transrectal ultrasound guided biopsy. In our study, the urologists were initially blinded to the MRI findings and a TRUS-guided biopsy was performed as per the standards of clinical care. Later, MRI findings were revealed to the urologist and additional targeted biopsy was performed based on the PI-RADS score assigned to the lesions.
Several other studies have compared a “biparametric reading session” out of a full mp-MRI protocol with the results of the latter in biopsy-naïve men (7–9) as well as in the setting of tumor recurrence assessment after therapy (15). All showed that bp-MRI and mp-MRI had similar or even higher (16) diagnostic accuracy as the full exam, but that bp-MRI has the advantage of being shorter and cheaper. The present study shows that effective patient care is possible in patients with suspicion of prostate cancer with use of a rapid MRI protocol that can act as a secondary triaging tool without placing undue burden on clinical scanners in terms of scanning time or costs. The table time for traditional multiparametric prostate MRI exam is approximately 45 minutes while this protocol achieved a high diagnostic accuracy in approximately a third of the table time without the use of a contrast agent. Many of these patients may have repeated MRIs over their lifetimes. Given concerns about gadolinium deposition, current recommendations suggest that gadolinium use should be limited to settings where it is necessary for diagnosis, and this consideration may also factor into the use of this non-contrast protocol (17). It has also been shown through decision analysis models that such a protocol can be cost effective in clinical practice in a variety of settings (18–20).
An implicit trade-off in the biparametric approach is the loss of ability to potentially upgrade a Category 3 lesion to a Category 4 in the peripheral zone. The importance of this tradeoff depends greatly on how the management of these lesions is approached at an institution. If the adopted approach is that all Category 3 or higher lesions are to be biopsied (the approach at our Institution), then there is no clinical consequence to a biparametric approach, as Category 3 and Category 4 lesions are both considered “biopsy” lesions. However, if the adopted approach involves not biopsying some or all of Category 3 lesions based on risk stratification, then the distinction between Category 3 and 4 has the potential to be clinically significant. Transition zone lesions would still not be affected by this biparametric approach, as DCE plays no role in categorizing these lesions. Similarly, patients with a higher suspicion synchronous lesion would also still go to biopsy. If a decision is made to call back patients needing a DCE stratification to make a biopsy decision, per our data, 26 patients (20% of the total population screened in this manner) could require additional DCE evaluation if an institution chooses to not biopsy Category 3 lesions. Of course, if the pretest suspicion of cancer is high in a subset of this group and thus a biopsy would be indicated regardless of DCE score, then the fraction needing to be recalled would be lower.
We used a cognitively guided biopsy technique. Our results are in line with previous studies that used cognitive MRI guidance for prostate biopsy (21,22). We were able to diagnose more anteriorly located, transitional zone and apical tumors on MRI-targeted biopsy compared to TRUS biopsy. Previous studies have shown that these are the tumors that are most frequently missed by standard 12-quadrant ultrasound guided biopsy (23). Moreover, there has been rising concern over the morbidity and cost of over-diagnosis and overtreatment in men diagnosed with low risk, clinically nonsignificant disease (Gleason grade ≤3+4) based on abnormal PSA level or abnormal DRE findings (24,25). Hence, a secondary screening diagnostic tool with a high negative predictive value for clinically significant cancer is desirable. Our results are in line with previous studies that MRI followed by a targeted biopsy has a high negative predictive value for detection of clinically significant prostate cancers (26,27). In addition, the false negative rate was lower than 5%, which is in the same range as for mpMRI (6,28,29).
Limitations
There are several limitations to our study. First, there is no control group of patients who received a full multiparametric MRI protocol and thus this is a non-randomized study design. It has been demonstrated that the sensitivity of bp-MRI at 3T in the detection prostate cancer is similar to that of mp-MRI (8,16,30,31), and some investigators have found that DCE could potentially add to the false positive exams (8). Kuhl et al. showed that bp-MRI missed 1 out of 139 clinically significant cancer while mp-MRI misclassified 11 cases as false positive. This study focused on the correlation of bp-MRI with biopsy pathology only and not prostatectomy, which is the ultimate gold standard. While it is obviously not ethical to add prostatectomy for research purposes only, as experience with this exam grows and patients who have undergone the exam have prostatectomies, future work can explore this more direct correlation to the gold standard. Another limitation of this study was that all patients were grouped into mPI-RADS 1 or 2 vs. mPI-RADS 3–5 and no further distinction in the treatment was made between P3 and 4, the clinical implications of which have been discussed above. An additional limitation is that we used cognitive targeting rather than ultrasound-MR fusion or direct in-bore biopsy, and this may be less accurate. As this study was initiated in 2015, at that time we used cognitive targeting at our institution. Although it seems intuitive that cognitive targeting should be less accurate than MRI-ultrasound fusion or in-gantry biopsy techniques (32), recent studies from Monda et al and Lee et al indicate that there is still a lack of consensus regarding the superiority of ultrasound-MR fusion over cognitive targeting (33,34). Future work can explore additional forms of targeting. Finally, this is a proof of concept study with a relatively small sample size. Future studies with larger cohorts are needed to continue to explore this important topic.
Conclusions
This study demonstrates that a rapid MRI protocol can be implemented in clinical practice and utilized for triaging patients that need further workup based on a modified PIRADs scoring system, from those with a very low probability of clinically significant cancer.
Supplementary Material
Acknowledgements:
This study is supported by Siemens Healthineers and NIH grants - NIH 1R01EB01672801A1 and NIH 5R01EB017219–02. Siemens Healthineers did not had influence on the study design, sequence selection or interpretation of the results. We are grateful for the support provided by Ananya Panda, MD, Department of Radiology, Christina Buzzy, PhD and Amr Mahran, MD, MS, Department of Urology, Case Western Reserve University and University Hospitals Cleveland Medical Center, Cleveland, Ohio.
Abbreviations
- ADC
Apparent Diffusion Coefficient
- AUC
Area under the Curve
- Bp
Biparametric
- DCE
Dynamic contrast enhanced
- DRE
Digital rectal examination
- DWI
Diffusion weighted imaging
- Mp
Multiparametric
- MRI
Magnetic Resonance Imaging
- NPV
Negative Predictive Value
- PI-RADS
Prostate Imaging – Reporting and Data System
- PPV
Positive predictive value
- PSA
Prostate specific antigen
- ROC
Receiver operating characteristics
- TRUS
Transrectal ultrasound
- TSE
Turbo spin echo
Footnotes
Conflict of interest: None of the authors declares a conflict of interest related to this manuscript.
Submission declaration and verification: None of the content has been published yet or is under consideration elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kozlowski P, Chang SD, Jones EC, Goldenberg SL. Assessment of the need for DCE MRI in the detection of dominant lesions in the whole gland: Correlation between histology and MRI of prostate cancer. NMR in biomedicine 2018;31(3). [DOI] [PubMed] [Google Scholar]
- 2.Merrill RM, Stephenson RA. Trends in mortality rates in patients with prostate cancer during the era of prostate specific antigen screening. The Journal of urology 2000;163(2):503–510. [PubMed] [Google Scholar]
- 3.Abd-Alazeez M, Ahmed HU, Arya M, et al. The accuracy of multiparametric MRI in men with negative biopsy and elevated PSA level--can it rule out clinically significant prostate cancer? Urologic oncology 2014;32(1):45.e17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet (London, England) 2017;389(10071):815–822. [DOI] [PubMed] [Google Scholar]
- 5.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. European urology 2016;69(1):16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boesen L, Nørgaard N, Løgager V, et al. Assessment of the diagnostic accuracy of biparametric magnetic resonance imaging for prostate cancer in biopsy-naive men: The biparametric mri for detection of prostate cancer (bidoc) study. JAMA Network Open 2018;1(2):e180219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Campli E, Delli Pizzi A, Seccia B, et al. Diagnostic accuracy of biparametric vs multiparametric MRI in clinically significant prostate cancer: Comparison between readers with different experience. European journal of radiology 2018;101:17–23. [DOI] [PubMed] [Google Scholar]
- 8.Kuhl CK, Bruhn R, Kramer N, Nebelung S, Heidenreich A, Schrading S. Abbreviated Biparametric Prostate MR Imaging in Men with Elevated Prostate-specific Antigen. Radiology 2017;285(2):493505. [DOI] [PubMed] [Google Scholar]
- 9.Weiss J, Martirosian P, Notohamiprodjo M, et al. Implementation of a 5-Minute Magnetic Resonance Imaging Screening Protocol for Prostate Cancer in Men With Elevated ProstateSpecific Antigen Before Biopsy. Investigative radiology 2018;53(3):186–190. [DOI] [PubMed] [Google Scholar]
- 10.Barentsz JO, Choyke PL, Cornud F, et al. Reply to Erik Rud and Eduard Baco’s Letter to the Editor re: Re: Jeffrey C. Weinreb, Jelle O. Barentsz, Peter L. Choyke, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16–40. European urology 2016;70(5):e137-e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR American journal of roentgenology 2012;199(1):103–110. [DOI] [PubMed] [Google Scholar]
- 12.Rhudd A, McDonald J, Emberton M, Kasivisvanathan V. The role of the multiparametric MRI in the diagnosis of prostate cancer in biopsy-naive men. Current opinion in urology 2017;27(5):488494. [DOI] [PubMed] [Google Scholar]
- 13.Kuhl CK, Bruhn R, Krämer N, Nebelung S, Heidenreich A, Schrading S. Abbreviated Biparametric Prostate MR Imaging in Men with Elevated Prostate-specific Antigen. https://doiorg/101148/radiol2017170129 2017. [DOI] [PubMed]
- 14.Jambor I, Bostrom PJ, Taimen P, et al. Novel biparametric MRI and targeted biopsy improves risk stratification in men with a clinical suspicion of prostate cancer (IMPROD Trial). Journal of magnetic resonance imaging : JMRI 2017;46(4):1089–1095. [DOI] [PubMed] [Google Scholar]
- 15.Lotte R, Lafourcade A, Mozer P, et al. Multiparametric MRI for Suspected Recurrent Prostate Cancer after HIFU:Is DCE still needed? Eur Radiol 2018. [DOI] [PubMed] [Google Scholar]
- 16.De Visschere P, Lumen N, Ost P, Decaestecker K, Pattyn E, Villeirs G. Dynamic contrast-enhanced imaging has limited added value over T2-weighted imaging and diffusion-weighted imaging when using PI-RADSv2 for diagnosis of clinically significant prostate cancer in patients with elevated PSA. Clinical Radiology 2017;72(1):23–32. [DOI] [PubMed] [Google Scholar]
- 17.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. Gadolinium deposition in the brain: summary of evidence and recommendations. The Lancet Neurology 2017;16(7):564–570. [DOI] [PubMed] [Google Scholar]
- 18.Cerantola Y, Dragomir A, Tanguay S, Bladou F, Aprikian A, Kassouf W. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy in diagnosing prostate cancer. Urologic oncology 2016;34(3):119.e111–119. [DOI] [PubMed] [Google Scholar]
- 19.Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR Imagingguided Strategies for Detection of Prostate Cancer in Biopsy-Naive Men. Radiology 2017;285(1):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faria R, Soares MO, Spackman E, et al. Optimising the Diagnosis of Prostate Cancer in the Era of Multiparametric Magnetic Resonance Imaging: A Cost-effectiveness Analysis Based on the Prostate MR Imaging Study (PROMIS). European urology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU international 2011;108(8 Pt 2):E171–178. [DOI] [PubMed] [Google Scholar]
- 22.Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. European urology 2015;68(3):438–450. [DOI] [PubMed] [Google Scholar]
- 23.Petralia G, Alessi S, Alconchel A, et al. Anterior prostatic tumours are difficult to diagnose without MRI. Ecancermedicalscience. Volume 6; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. European urology 2014;65(6):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the Cost of Cancer Care in the United States: 2010–2020. J Natl Cancer Inst 2011;103(2):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen NL, Kesch C, Barrett T, et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU international 2017;120(5):631–638. [DOI] [PubMed] [Google Scholar]
- 27.Wegelin O, van Melick HHE, Hooft L, et al. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? European urology 2017;71(4):517–531. [DOI] [PubMed] [Google Scholar]
- 28.Porpiglia F, De Luca S. Prostate cancer biomarkers: new scenarios in the multi-parametric magnetic resonance imaging era. BJU international 2017;120(6):745–746. [DOI] [PubMed] [Google Scholar]
- 29.Washino S, Kobayashi S, Okochi T, et al. Cancer detection rate of prebiopsy MRI with subsequent systematic and targeted biopsy are superior to non-targeting systematic biopsy without MRI in biopsy naive patients: a retrospective cohort study. BMC urology 2018;18(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fascelli M, Rais-Bahrami S, Sankineni S, et al. Combined Biparametric Prostate Magnetic Resonance Imaging and Prostate-specific Antigen in the Detection of Prostate Cancer: A Validation Study in a Biopsy-naive Patient Population. Urology 2016;88:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanzione A, Imbriaco M, Cocozza S, et al. Biparametric 3T Magnetic Resonance Imaging for prostatic cancer detection in a biopsy-naive patient population: a further improvement of PIRADS v2? European journal of radiology 2016;85(12):2269–2274. [DOI] [PubMed] [Google Scholar]
- 32.Cool DW, Zhang X, Romagnoli C, Izawa JI, Romano WM, Fenster A. Evaluation of MRI-TRUS Fusion Versus Cognitive Registration Accuracy for MRI-Targeted, TRUS-Guided Prostate Biopsy. American Journal of Roentgenology 2014;204(1):83–91. [DOI] [PubMed] [Google Scholar]
- 33.Lee H, Hwang SI, Lee HJ, Byun SS, Lee SE, Hong SK. Diagnostic performance of diffusion-weighted imaging for prostate cancer: Peripheral zone versus transition zone. PloS one 2018;13(6):e0199636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monda SM, Vetter JM, Andriole GL, et al. Cognitive Versus Software Fusion for MRI Targeted Biopsy: Experience Before and After Implementation of Fusion. Urology 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.