Abstract
Purpose:
EGFR exon 20 insertions account for up to 10% of all EGFR mutations in lung adenocarcinomas, representing the third most common cluster of mutations. The management of advanced cancers with these mutations remains elusive, without an approved inhibitor.
Experimental Design:
Preclinical models of a representative set of EGFR exon 20 insertion mutations to evaluate the efficacy of different inhibitors and description of the clinical outcome of an advanced lung cancer.
Results:
We show that select first, second and third generation EGFR inhibitors are unable to deter common EGFR exon 20 insertion mutants in concentrations that spare the wild-type kinase. Nonetheless, EGFR exon 20 insertion mutants associate with the heat shock protein 90 (Hsp90) chaperone system. We exploit this vulnerability to show that the non-geldanamycin Hsp90 inhibitor luminespib (formerly AUY922) degrades EGFR exon 20 mutations, downstream targets and induces apoptosis. In addition, a patient whose EGFR inhibitor-insensitive lung adenocarcinoma harbored an EGFR exon 20 insertion mutation had a confirmed radiographic response to luminespib.
Conclusions:
The report confirms that EGFR exon 20 mutations are dependent on Hsp90 and are readily inhibited by the Hsp90 inhibitor luminespib; a treatment strategy that has been pursued in a confirmatory clinical trial (NCT01854034) for this group of lung adenocarcinomas that currently represent an unmet clinical need in precision oncology.
Keywords: mutation, lung cancer, adenocarcinoma, EGFR exon 20 insertion, mutation, kinase inhibitor, Hsp90, luminespib, AUY922
INTRODUCTION
Precision medicine is an important palliative modality in the management of advanced lung adenocarcinomas [1], with oral kinase inhibitors approved for advanced tumors with epidermal growth factor receptor (EGFR) mutations (gefitinib, erlotinib, icotinib, afatinib, olmutinib and osimertinib), anaplastic lymphoma kinase (ALK) rearrangements (alectinib, crizotinib, ceritinib and brigatinib), ROS proto-oncogene 1 (ROS1) rearrangements (crizotinib) or B-Raf proto-oncogene, serine/threonine kinase (BRAF) V600E mutation (dabrafenib plus trametinib). EGFR mutations, first identified in 2004, mostly cluster around the kinase domain of the receptor [2]. The most frequent EGFR mutations - more than 75% of mutations found in non-small-cell lung cancers (NSCLCs) - are indels around exon 19 and the exon 21 L858R, which are oncogenic and hypersensitive to reversible (1st generation), irreversible (2nd generation) and covalent mutation-specific (3rd generation) EGFR tyrosine kinase inhibitors (TKIs); hence the approval labels based on these biomarkers [2]. Other less prevalent but clinically-relevant EGFR mutations that predict for response to gefitinib, erlotinib, osimertinib or afatinib include: exon 18 indels/E709X, exon 18 G719X, exon 19 insertions, exon 20 A763_Y764insFQEA, exon 20 S768I, exon 21 L861Q, exon 18–25 kinase domain duplication and EGFR rearrangements [2]. EGFR TKI monotherapy is not curative in the advanced setting, and heterogeneous acquired resistance to gefitinib, erlotinib, icotinib and afatinib occurs due to EGFR mutations, oncogene bypass tracks or histological transformation [3, 4]. The most prevalent resistance is mediated by EGFR-T790M, a gatekeeper mutation that can be inhibited by covalent mutation-specific EGFR TKIs; with osimertinib worldwide and olmutinib in South Korea being the approved clinical drugs [5].
However, not all EGFR mutants share the same sensitivity pattern to approved EGFR inhibitor classes. The third most prevalent kinase domain EGFR mutation class encompasses in-frame insertions and indels within or following the regulatory C-helix amino-acids of exon 20 [6, 7], which account for approximately 10% of all EGFR mutations in NSCLC. These structural oncogene variants still engage phosphatidylinositol-3-kinases (PI3K)/protein kinase B (AKT) and extracellular-signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) downstream pathways with EGFR dependency [7]. Our group has previously shown that the segment of the kinase domain pocket - in which 1st and 2nd generation drug binding occurs - of exon 20 insertion mutants is structurally similar to wild-type (WT) EGFR, thereby negating a therapeutic window for use of gefitinib, erlotinib and afatinib [7] with clinically-achievable WT EGFR sparing doses [7, 8]. The lack of significant clinical responses to approved doses of 1st, 2nd and 3rd generation EGFR TKIs corroborates the preclinical data [5–11], and reiterates that EGFR exon 20 insertion mutated tumors have an unmet clinical need within the realm of precision oncology. Although new EGFR exon 20 mutation selective inhibitors are undergoing preclinical and clinical development (Supp. Table 1), none is expected to receive regulatory approval within the immediate future. To circumvent this issue, we have started to identify non-EGFR specific therapies that may be effective against EGFR exon 20 insertion mutated NSCLCs. The heat shock protein 90 (Hsp90) chaperone complex protects cellular proteins from degradation by the ubiquitin-proteasome system and this system is coopted by driver oncogenes (such as EGFR mutants) plus cell cycle proteins [12]. In this report, we provide preclinical rationale and early clinical data to support the use of the non-geldanamycin resorcinol-containing triazolone Hsp90 inhibitor in clinical development luminespib [13–15] for this unique class of lung cancers.
METHODS
Patient and case report:
The clinical and radiographic data used for this study was obtained from ongoing Institutional Review Board (IRB) approved protocols at our institutions. The case was identified as a participant from the aforementioned clinical trial: NCT01124864 [15]. Written informed consent was obtained from the patient when entering NCT01124864 and the review of data was conducted in accordance with recognized ethical guidelines.
Reagents:
Erlotinib, afatinib and rociletinib were purchased from LC Laboratories. Luminespib was purchased from Selleckchem. All reagents were dissolved in dimethyl sulfoxide and stored at −80°C.
Cell culture:
Ba/F3 cell lines, BID007, NCI-H1975 (H1975), NCI-H3255 (H3255) and PC9 cells were obtained, authenticated and maintained in RPMI 1640 medium (Mediatech) supplemented with 10% fetal bovine serum, as previously described [7]. The Ba/F3 EGFR-WT cell system (Supp. Fig. 1) was derived in an identical fashion as other EGFR mutants [7] and, as an overexpression system for WT EGFR, it is used to compare the differential effects of inhibitors with EGFR mutant proteins [8]. All cells were grown at 37°C in a humidified atmosphere with 5% CO2. Cells were thawed and tested for lack of mycoplasma contamination (MycoAlert Mycoplasma Detection Kit, Lonza) prior to experiments that were initiated within the initial one to five passages.
Cell line proliferation assays:
Cells were plated in each well of 96-well plates and then were treated in the appropriate medium with or without EGFR or Hsp90 inhibitors for up to 3 days. Cell viability was determined by CellTiter 96 Aqueous One solution proliferation kit (Promega), as previously described [7]. Inhibitory proliferation curves and the 50% inhibitory concentration (IC50) were generated using GraphPad Prism 6 (GraphPad Software).
Western blotting and antibodies:
Western blot lysates and preparation were performed as previously described. Immunoprecipitation was achieved using protein G agarose to sepharose beads for preclearing lysates and capturing the immunocomplex after incubation with the immunoprecipitating antibody. In cell line experiments, treatment with inhibitors for the specified number of hours (figure legends) was performed to determine the response of Hsp90, EGFR and its downstream signals to inhibitors. Immunoglobulin G (IgG) and anti-HA tag antibodies were obtained from Abcam. Total EGFR and β-actin antibodies were obtained from Santa Cruz Biotechnology. Total ERK 1/2 antibody was obtained from BD Transduction Laboratories. Phospho-EGFR (pT1068) antibody was purchased from Invitrogen. Hsp90, Hsp70, phospho-AKT (pS473), total AKT, phospho-ERK (pT202/pY204), PARP, and cleaved PARP antibodies were obtained from Cell Signaling Technology. Primary antibodies were diluted 1:1,000, while secondary antibodies were diluted 1:10,000.
Statistical analysis:
Proliferation experiments were performed using three replicates (when not otherwise detailed). Median IC50s and standard deviations were generated using GraphPad Prism 6 (GraphPad Software).
RESULTS
Preclinical data to support lack of therapeutic window of common EGFR exon 20 mutants to 1st, 2nd and 3rd generation EGFR TKIs:
A panel of representative EGFR exon 20 mutations (A763_Y764insFQEA, Y764_V765insHH, M766_A767insAI, D767_V769dupASV, D770_N771insSVD, D770_N771insNPG, H773_V774insH), EGFR TKI hypersensitive mutations (exon 19 deletion delL747_P753insS and L858R) and 1st/2nd generation EGFR TKI resistant mutations (delL747_P753insS+T790M and L858R+T790M) driving an isogenic Ba/F3 cell line system was utilized to probe inhibitory concentrations in comparison to WT EGFR (Fig. 1). When we analyzed the representative 1st generation EGFR TKI erlotinib, only the EGFR-exon 19 deletion, L858R and A763_Y764insFQEA dependent Ba/F3 cells had inhibitory concentrations that were exceedingly lower than those of the WT EGFR driven cell (Fig. 1A). The identical pattern was observed with the 2nd generation irreversible EGFR TKI afatinib (Fig. 1B). In all other cells, the driver EGFR mutant led to 50% inhibitory concentrations (IC50s) in 72 hour proliferation assays that exceed WT EGFR for erlotinib (Fig. 1A) and afatinib (Fig. 1B). In the case of the 3rd generation mutation-selective covalent EGFR TKI rociletinib (an inhibitor with superb in vitro profile but limited pharmacokinetic translation [Supp. Table 1]), the cells with the lowest IC50s harbored delL747_P753insS+T790M and L858R+T790M followed by delL747_P753insS and L858R (Fig. 1C). All EGFR exon 20 insertions had IC50s that exceed that of the WT EGFR-driven system (Fig. 1C). The phosphorylation of EGFR was only repressed in cells that were inhibited by the respective EGFR TKI (Fig. 1). These results showed that EGFR exon 20 insertion mutants do not have a therapeutic window in relation WT EGFR when treated with common 1st, 2nd and 3rd generation EGFR TKIs.
Figure 1. Lack of therapeutic window of EGFR exon 20 mutants to representative EGFR inhibitors in Ba/F3 cells dependent on EGFR.
A. Dose-inhibition curves and standard deviations of 3 experiments for erlotinib using Ba/F3 cells driven by wild type (WT) EGFR or other EGFR mutants after 72 hours, at nanomolar (nM) concentrations indicated. Mean IC50 value is indicated in ascending order. Western blot results showing the intracellular signaling effects of increasing concentrations of erlotinib after 24 hours of exposure in select Ba/F3 cells, as indicated. B. Dose-inhibition curves and standard deviations of 3 experiments for afatinib using Ba/F3 cells after 72 hours, at concentrations indicated. Mean IC50 value is indicated in ascending order. Western blot results showing the intracellular signaling effects of increasing concentrations of afatinib after 24 hours of exposure in select Ba/F3 cells as indicated. C. Dose-inhibition curves and standard deviations of 3 experiments for rociletinib using Ba/F3 cells after 72 hours, at concentrations indicated. Mean IC50 value is indicated in ascending order. Western blot results showing the intracellular signaling effects of increasing concentrations of afatinib after 24 hours of exposure in select Ba/F3 cells as indicated. Immunoblotting was performed against indicated proteins: EGFR (phospho [p] and total) and β-actin.
However, we and others have described that in-development doses of approved (osimertinib) or novel exon 20 mutation-specific (poziotinib and TAK-788) EGFR TKIs have shown some degree of preclinical activity [8, 16–18] and are ongoing further clinical development (Supp. Table 1).
EGFR exon 20 mutants can be found in complex with Hsp90 and can be degraded by Hsp90 inhibitors:
To evaluate whether the Hsp90 complex is responsible for degradation of EGFR exon 20, as do other EGFR mutant proteins [12], we performed a co-immunoprecipitation reaction using the human influenza hemagglutinin (HA) tag that is part of our EGFR mutant proteins [7] with Hsp90 (Fig. 2A). Both the typical EGFR exon 20 insertion mutant D770_N771insSVD and the atypical mutant A763_Y764insFQEA disclosed physical association with the Hsp90 complex (Fig. 2A). The Hsp90 inhibitor luminespib, in the same experiments, led to dissociation and degradation of EGFR mutant proteins (Fig. 2A). In a time-dependent manner using preclinical models driven by EGFR-D770_N771insSVD and A763_Y764insFQEA mutated proteins, luminespib led to augmentation of Hsp70 (a protein signature of Hsp90 inhibition in view of the difficulty in measuring Hsp90 functionally), degradation of EGFR, downregulation of the activity of AKT and ERK, and induction of apoptosis as measured by cleavage of poly ADP-ribose polymerase (PARP) at the cellular level (Fig. 2B).
Figure 2. EGFR exon 20 mutants associate with the Hsp90 chaperone system and are inhibited by the Hsp90 inhibitor luminespib.
A. Immunoprecipitation (IP) of human influenza hemagglutinin (HA) tag that is part of our EGFR mutant proteins and Hsp90 plus immunoglobulin G (IgG) control in Ba/F3 cells dependent on EGFR-L858R (LR), L858R+T790M (LRTM), D770_N771insSVD (SVD) and A763_Y764insFQEA (FQEA). IP results in the absence or presence of 16 hours of treatment with luminespib. Immunoblotting was performed against indicated proteins: HA-tag, EGFR and Hsp90. B. Time course of alterations in Hsp90 and EGFR plus their targets after inhibition with up to 24 hours of luminespib (as indicated) in Ba/F3 cells driven by the EGFR exon 20 mutants D770_N771insSVD and A763_Y764insFQEA. Immunoblotting was performed against indicated proteins: Hsp90, Hsp70, PARP (full length [Fl] and cleaved [Cl]), EGFR (phospho [p] and total), AKT (p and total), ERK (p and total) and β-actin. C. Dose-inhibition curves and standard deviations of 3 experiments for luminespib using Ba/F3 cells driven by wild type (WT) EGFR or other EGFR mutants after 72 hours, at nanomolar (nM) concentrations indicated. Mean IC50 value is indicated in ascending order. Western blot results showing the intracellular signaling effects of increasing concentrations of luminespib after 24 hours of exposure in select Ba/F3 cells, as indicated. Immunoblotting was performed against indicated proteins: EGFR (phospho [p] and total) and β-actin.
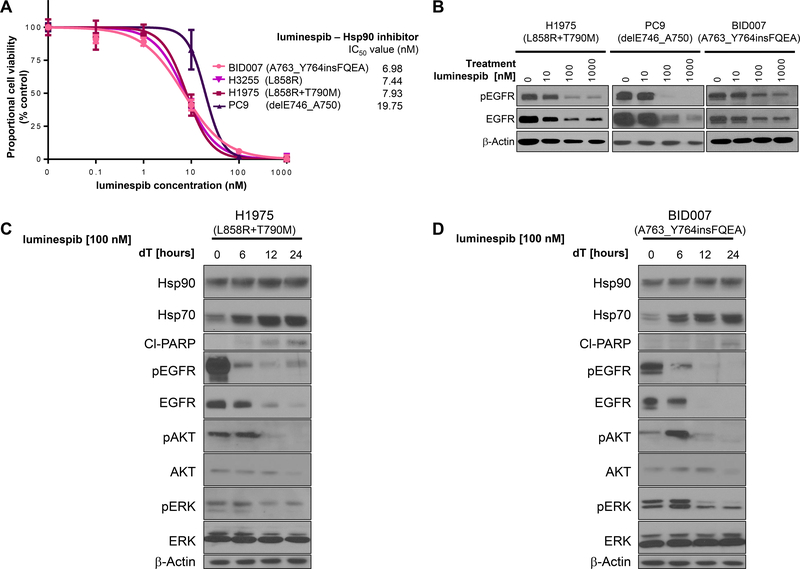
Turning back to our extensive panel of representative Ba/F3 cells driven by EGFR exon 20 mutations, we showed that the Hsp90 inhibitor luminespib disclosed a similar pattern of IC50s in a dose-dependent manner when comparing these mutant cells with EGFR-exon 19 deletions, L858R or T790M dependent cells (Fig. 2C); and even with cells driven by overexpression of WT EGFR (Fig. 2C and Supp. Fig. 1). The range of median IC50s in all EGFR mutants tested did not veer from the 1.8 to 8.2nM range, in significant contrast to the many fold difference in EGFR TKI experiments (Fig. 1). The pattern of short-term EGFR degradation induced by increasing concentrations of luminespib was also similar between EGFR-D770_N771insSVD, A763_Y764insFQEA, L858R or L858R+T790M (Fig. 2C).
As the aforementioned data was obtained using the Ba/F3 system, we confirmed the role of Hsp90 inhibition using NSCLC cell lines. The EGFR exon 20 insertion mutated cell line BID007 (harboring as the driver oncogene EGFR-A763_Y764insFQEA) had inhibitory curves to increasing concentrations of luminespib that were near identical to other EGFR mutated cell lines (Fig. 3A). The levels of EGFR degradation induced by luminespib were also similar between BID007, PC9 (EGFR-delE746_A750) and H1975 (EGFR-L858R+T790M) in a dose-dependent manner (Fig. 3B). Luminespib also induced Hsp70, degraded EGFR, downregulated the activity of AKT and ERK, and induced apoptosis over the latter time points during the course of a day in BID007 (Fig. 3D). These data confirmed in distinct preclinical models that Hsp90 inhibition should undergo further clinical testing in EGFR exon 20 insertion mutated NSCLCs.
Figure 3. EGFR exon 20 mutated NSCLC cell lines are inhibited by the Hsp90 inhibitor luminespib.
A. Dose-inhibition curves and standard deviations of 3 experiments for luminespib using NSCLCs cell lines harboring different EGFR mutations (as indicated) as driver oncogenes after 72 hours, using nanomolar (nM) concentrations indicated. Mean IC50 value is indicated in ascending order. B. Western blot results showing the intracellular signaling effects of increasing concentrations of luminespib after 24 hours of exposure in H1975, PC9 and BID007 as indicated. Immunoblotting was performed against indicated proteins, EGFR (phospho [p] and total) and β-actin. C. and D. Time course of alterations in Hsp90 and EGFR plus their targets after inhibition with up to 24 hours of luminespib (as indicated) in H1975 cells (C) and BID007 cells (D). Immunoblotting was performed against indicated proteins: Hsp90, Hsp70, PARP (cleaved [Cl]), EGFR (phospho [p] and total), AKT (p and total), ERK (p and total) and β-actin.
Early evidence of clinical activity of luminespib in EGFR exon 20 insertion mutated lung cancer:
Our investigators cared for a patient with advanced NSCLC that was enrolled in the previously published phase 2 trial of luminespib in molecularly defined patients with advanced lung cancer (ClinicalTrials.gov identifier NCT01124864) [15]. The patient was a never smoker woman who presented at age 79 years-old with recurrent lung adenocarcinoma with intra and extra-thoracic disease burden. Initial therapy with oral erlotinib at 150 mg/day was ineffective and resulted in symptomatic tumor progression within 1 month of use.
The patient then received, on the aforementioned clinical trial, intravenous luminespib at 70 mg/m2 weekly and achieved a partial response (Fig. 4A) that was confirmed in subsequent imaging studies (Fig. 4B). The patient continued on luminespib for 18 weeks prior to radiographic progressive disease (Fig. 4B), and died 6 months later without intervening systemic therapy. This case spawned the design of a dedicated phase 2 trial of luminespib for EGFR exon 20 insertion mutated NSCLC, NCT01854034, as discussed below.
Figure 4. Response to luminespib in a patient with lung adenocarcinoma harboring an EGFR exon 20 insertion mutation.
A. Positron emission tomography-computed tomography (PET-CT) scans disclosing fluorine-18-fluorodeoxyglucose (FDG) avid lesions (as indicated by white arrows [PET image] and white circles [cross sectional CT image]) in a woman with advanced EGFR-D770_N771insGL mutated lung adenocarcinoma before (baseline) and after luminespib therapy. Decrease in size of lesions is noted. B. Sum of largest target lesions in millimeter (mm) as defined by response evaluation criteria in solid tumors (RECIST) version 1.1 over the course of therapy with luminespib (repeat imaging studies performed at baseline, 6, 12 and 18 weeks prior to progression). Luminespib was started at 70 mg/m2 weekly and then dose-reduced due to toxicity to 54 mg/m2 at week 4 of therapy. The patient achieved a confirmed partial response by RECIST prior to RECIST-based progression at week 18, as indicated.
DISCUSSION
The clinical management of advanced lung cancers with EGFR exon 20 insertion mutations remains elusive, without an approved or commercially active oral inhibitor obtainable. As detailed here and in prior reports [5–11], the currently available EGFR TKIs gefitinib, erlotinib, icotinib, afatinib, olmutinib and osimertinib at their approved doses are unable to induce significant radiographic regressions. The development of novel EGFR TKIs that are selective for EGFR exon 20 insertions is of utmost importance for thoracic oncologists and a few novel inhibitors have shown initial preclinical and clinical activity (Supp. Table 1). Poziotinib (formerly HM781–36B) is a pan-ErbB TKI that had limited activity in the setting of tumors with EGFR-L858R and EGFR-exon 19 deletions with enhancement for EGFR-T790M [19, 20], and therefore had initial clinical development halted. More recently, a moderate size EGFR inhibitor screen against EGFR exon 20 insertion mutations in preclinical models disclosed selective activity of poziotinib and a single institution clinical trial has reported unconfirmed partial responses in the majority of cases [17]. A confirmatory phase 2 trial of poziotinib (NCT03066206) for this population commenced on 2017 and is expected to end by 2021. TAK-788 (formerly AP32788) is a novel EGFR inhibitor designed to match the unique crystal structure model of the most common EGFR exon 20 insertion mutant proteins [7] and it has shown consistently in initial preclinical models a favorable therapeutic window (lower inhibitory concentrations than EGFR WT) in EGFR exon 20 mutant models [18]. The first-in-human clinical trial of TAK-788 (NCT02716116) was initialed in 2016 and due to promising initial results has been expanded from dose escalation to expansion cohorts [18], with an estimated completion date by 2020. Due to the close structural similarity of EGFR (also known as ErbB1) and Erb-B2 Receptor Tyrosine Kinase 2 (ErbB2, also known as HER2) and the promiscuity of TKIs [21], the EGFR exon 20 mutation selective inhibitors poziotinib and TAK-788 are also being developed for ERBB2 exon 20 mutated lung adenocarcinomas [17, 18]. We hope one of these drugs has a favorable toxicity profile and is found to be effective in the majority of EGFR and ERBB2 exon 20 insertion mutated NSCLCs.
Notwithstanding, it seems that the clinical path for approval of poziotinib and/or TAK-788 will take time even in the absence of major toxicities and - as for all EGFR/ErbB2 TKIs - biologic resistance will hinder long-term outcomes of monotherapy with any approved TKI. To circumvent these fallacies, the preclinical and clinical development of therapies with distinct mechanisms of action is valid. We attempted to highlight the Hsp90 system as a potential target. The non-mutant EGFR is a potential client of the Hsp90 chaperone complex but kinase mutant EGFRs (including exon 19 deletions, L858R and T790M) are intimately dependent on the chaperone properties of Hsp90 [22, 23]. The data presented here establish that EGFR exon 20 insertion mutants are bona fide clients of Hsp90, in a manner similar to more prevalent EGFR mutant proteins, and that exploiting this dependency with Hsp90 inhibitors has merit.
Various compounds have been developed to target the Hsp90 chaperone system with different levels of clinical development. Some are geldanamycin Hsp90 inhibitors (tanespimycin/17-AAG, alvespimycin/17-DMAG and retaspimycin/IPI-504), purine-scaffold based inhibitors (BIIB021/CNF2024), non-geldanamycin resorcinol-containing triazolone inhibitors (luminespib and ganetespib/STA-9090) among others [23]. Although none has reached regulatory approval, there have been encouraging reports of activity in subgroups of NSCLC - most notably in oncogene driven cohorts [15]. Other groups have reported the preclinical activity of the geldanamycin inhibitor tanespimycin in EGFR and ERBB2 mutated cell lines [24] but this compound has significant clinical toxicities. In preclinical models of EGFR and ERBB2 mutated cells [22, 24], Hsp90 inhibition is active to a degree that exceeds the clinical experience with this class of agents and novel models are necessary to better reflect the full spectrum of response/resistance in patients. The limitations of Hsp90 inhibitors that have undergone clinical development, including luminespib, need to be addressed. These include lack of optimized biomarkers for adequate pharmacodynamic/pharmacokinetic dosing schemes in patients, the inherited toxicities associated with Hsp90 inhibition that can limit maximum dosing, the induction/upregulation of pro-survival proteins (such as can be seen with Hsp70 as noted in Figs. 2 and 3) upon Hsp90 monotherapy that may limit the effects of client oncogene inhibition, the lack of selectivity of chaperone dependency for a particular driver oncogene (as seen in Fig. 2 in relation to different EGFR proteins), and the lack of complete understanding of downstream signaling cascades plus other chaperones that may not be inhibited after Hsp90 inhibition in humans [22–26].
We chose to focus on luminespib in view of its reported clinical activity in traditional EGFR mutated NSCLCs and the fact that a dedicated clinical trial of luminespib 70 mg/m2 weekly was underway for patients with EGFR exon 20 mutated NSCLC. In a phase 2 study, luminespib was associated with a confirmed radiographic response rate that exceeded 15% in patients with all types of EGFR mutated NSCLC with an acceptable adverse event profile notable for mild visual-related disorders in the majority of cases [15]. The only responder, in this initial trial, with EGFR exon 20 mutated NSCLC is reported in detail here (Fig. 4) and spawned our group to complete a dedicated clinical trial of luminespib in this patient population (NCT01854034). Initial results of this Simon two-stage design clinical trial were reported in 2015, with sufficient responses within the initial strata of cases to expand the trial to its conclusion [25]. The final results of the NCT01854034 trial, which completed accrual in 2016, will be reported in full elsewhere detailing the response rate, progression-free survival and toxicities of the complete cohort of 29 cases with EGFR exon 20 mutated NSCLC. If the final response rate exceeds 15%, as seen for other oncogene driven NSCLCs [15], this will provide the proof-of-concept clinical information to stimulate further study of Hsp90 inhibition for these NSCLCs - this level of response activity is generally superior to that seen with evidence-based second line strategies (either the cytotoxic agent docetaxel or immune checkpoint inhibitors) in unselected cases of advanced lung cancer.
It is possible to speculate that some type of Hsp90 inhibitor alone or in combination with other therapies could be clinically developed for the management of EGFR and/or ERBB2 mutated NSCLC in the TKI-naïve or TKI-resistant setting, including tumors harboring EGFR exon 20 insertion mutations. Additional preclinical and clinical studies are warranted.
Supplementary Material
TRANSLATIONAL RELEVANCE.
EGFR exon 20 insertions account for up to 10% of all EGFR mutations in lung adenocarcinomas and represent a group of lung adenocarcinomas that currently have an unmet clinical need in precision oncology without an approved inhibitor. These mutants are dependent on the Hsp90 system and are readily inhibited by the Hsp90 inhibitor luminespib; a treatment strategy that has been evaluated in a confirmatory clinical trial (NCT01854034) by our group.
ACKNOWLEDGEMENTS:
This work was funded in part through a Lung Cancer Foundation of America-International Association for the Study of Lung Cancer grant (to D. B. Costa), an American Cancer Society grant RSG 11–186 (to D. B. Costa), and National Institutes of Health (NIH)/National Cancer Institute (NCI) grants R37 CA218707 (to D. B. Costa), R01 CA169259 (to S. Kobayashi) and R21 CA178301 (to S. Kobayashi).
Acknowledgements/Funding: This work was funded in part through a Lung Cancer Foundation of America-International Association for the Study of Lung Cancer grant (to D. B. Costa), an American Cancer Society grant RSG 11–186 (to D. B. Costa), and National Institutes of Health (NIH)/National Cancer Institute (NCI) grants R37 CA218707 (to D. B. Costa), R01 CA169259 (to S. Kobayashi) and R21 CA178301 (to S. Kobayashi).
Footnotes
Disclosure of Potential Conflicts of interest: DBC has consulting fees and honoraria from Pfizer, Astra-Zeneca and ARIAD/Takeda. ZP has received consulting fees from Boehringer Ingelheim, AstraZeneca, Novartis, AbbVie, GuarantHealth and ARIAD/Takeda. GRO has received consulting fees from AstraZeneca, ARIAD/Takeda, Invivata, and honoraria from Guardant. LVS has received consulting fees from AstraZeneca, Genentech, Bristol-Myers Squibb and Pfizer, and honoraria from AstraZeneca. SSK has consulting fees from Pfizer and Ono Pharmaceutical. No other conflict of interest is stated.
REFERENCES
- [1].Gerber DE, Gandhi L, Costa DB. Management and future directions in non-small cell lung cancer with known activating mutations. Am Soc Clin Oncol Educ Book 2014;e353–e365. [DOI] [PubMed] [Google Scholar]
- [2].Costa DB. Kinase inhibitor-responsive genotypes in EGFR mutated lung adenocarcinomas: moving past common point mutations or indels into uncommon kinase domain duplications and rearrangements. Transl Lung Cancer Res 2016;5:331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786–92. [DOI] [PubMed] [Google Scholar]
- [4].Ohashi K, Maruvka YE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol 2013;31:1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689–99. [DOI] [PubMed] [Google Scholar]
- [6].Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23–e31. [DOI] [PubMed] [Google Scholar]
- [7].Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013;5:216ra177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hirano T, Yasuda H, Tani T, et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget 2015;6:38789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830–8. [DOI] [PubMed] [Google Scholar]
- [10].Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 2013;12:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013;8:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shimamura T, Shapiro GI. Heat shock protein 90 inhibition in lung cancer. J Thorac Oncol 2008;3:S152–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sessa C, Shapiro GI, Bhalla KN, et al. First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. Clin Cancer Res 2013;19:3671–80. [DOI] [PubMed] [Google Scholar]
- [14].Garon EB, Finn RS, Hamidi H, et al. The HSP90 inhibitor NVP-AUY922 potently inhibits non-small cell lung cancer growth. Mol Cancer Ther 2013;12:890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Felip E, Barlesi F, Besse B, et al. Phase 2 Study of the HSP-90 Inhibitor AUY922 in Previously Treated and Molecularly Defined Patients with Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2017. [DOI] [PubMed] [Google Scholar]
- [16].Floc’h N, Martin MJ, Riess JW, et al. Anti-tumor activity of osimertinib, an irreversible mutant-selective EGFR tyrosine kinase inhibitor, in NSCLC harboring EGFR Exon 20 Insertions. Mol Cancer Ther 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Elamin Y, Robichaux J, Lam V, et al. OA 12.01 The Preclinical and Clinical Activity of Poziotinib, a Potent, Selective Inhibitor of EGFR Exon 20 Mutant NSCLC. J Thorac Oncol 2017;12:S1776 (meeting abstract). [Google Scholar]
- [18].Doebele RC, Horn L, Spira A, et al. P2.06–007 A Phase 1/2 Trial of the Oral EGFR/HER2 Inhibitor AP32788 in Non–Small Cell Lung Cancer (NSCLC): Topic: Phase I/II Trials. J Thorac Oncol 2017;12:S1072–S1073 (abstract). [Google Scholar]
- [19].Kim TM, Lee KW, Oh DY, et al. Phase 1 Studies of Poziotinib, an Irreversible Pan-HER Tyrosine Kinase Inhibitor in Patients with Advanced Solid Tumors. Cancer Res Treat 2017;doi: 10.4143/crt.2017.303. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Han JY, Lee KH, Kim SW, et al. A Phase II Study of Poziotinib in Patients with Epidermal Growth Factor Receptor (EGFR)-Mutant Lung Adenocarcinoma Who Have Acquired Resistance to EGFR-Tyrosine Kinase Inhibitors. Cancer Res Treat 2017;49:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Costa DB, Jorge SE, Moran JP, et al. Pulse Afatinib for ERBB2 Exon 20 Insertion-Mutated Lung Adenocarcinomas. J Thorac Oncol 2016;11:918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shimamura T, Li D, Ji H, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Res 2008;68:5827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 2010;10:537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xu W, Soga S, Beebe K, et al. Sensitivity of epidermal growth factor receptor and ErbB2 exon 20 insertion mutants to Hsp90 inhibition. Br J Cancer 2007;97:741–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Piotrowska Z, Costa DB, Huberman M, et al. Activity of AUY922 in NSCLC patients with EGFR exon 20 insertions. J Clin Oncol 2015;33:8015 (abstract). [Google Scholar]
- [26].Butler LM, Ferraldeschi R, Armstrong HK, Centenera MM, Workman P. Maximizing the Therapeutic Potential of HSP90 Inhibitors. Mol Cancer Res 2015;13:1445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.