Abstract
Purpose:
Osteonecrosis (ON) is a devastating complication of high dose corticosteroid therapy in cancer patients. Core decompression for prevention of bone collapse has been recently combined with the delivery of autologous concentrated bone marrow aspirates. The purpose of our study was to develop an imaging test for the detection of transplanted bone marrow cells in ON lesions.
Experimental Design:
In a prospective proof-of-concept clinical trial (NCT02893293), we performed serial MR imaging studies of nine hip joints of seven ON patients before and after core decompression. 24–48hours prior to the surgery, we injected ferumoxytol nanoparticles intravenously to label cells in normal bone marrow with iron oxides. During the surgery, iron labeled bone marrow cells were aspirated from the iliac crest, concentrated and then injected into the decompression track. Following surgery, patients received follow-up MRI up to 6 months after bone marrow cell transplantation.
Results:
Iron labeled cells could be detected in the access canal by a dark (negative) signal on T2*-weighted MR images. T2* relaxation times of iron labeled cell transplants were significantly lower compared to unlabeled cell transplants of control patients who were not injected with ferumoxytol (P = 0.02). Clinical outcomes of patients who received ferumoxytol-labeled or unlabeled cell transplants were not significantly different (P = 1), suggesting that the added ferumoxytol administration did not negatively affect bone repair.
Conclusions:
This immediately clinically applicable imaging test could become a powerful new tool to monitor the effect of therapeutic cells on bone repair outcomes after corticosteroid-induced osteonecrosis.
Keywords: High-dose corticosteroid therapy, avascular necrosis of the femoral head, ferumoxytol, core decompression
Introduction
Osteonecrosis (ON) is a debilitating and devastating complication of high-dose corticosteroid therapy: 15–47% of patients with leukemia and 3–44% of patients with systemic lupus erythematosus (SLE) develop an ON as a result of high-dose corticosteroid therapy (1–4). Seventeen to twenty-two percent of these patients progress to hip joint collapse (5,6) which leads to major long-term morbidities, such as severe joint pain requiring narcotic analgesia, impaired joint function and severely limited ambulation, ultimately requiring total joint replacement.
The pathogenesis of ON is multifactorial and involves corticosteroid-induced ischemia, necrosis of cells of the mesenchymal and hematopoietic lineages, as well as hypertrophy of fat cells which further compress microvessels and thereby, perpetuate ischemia (7). Progressive death of cells that provide structural support for the underlying bone ultimately leads to bone collapse. Interventions to save the affected bone are effective only if performed in a preventive manner (8). Various non-invasive and surgical procedures have been used to prevent progression to bone collapse (9). Core decompression has been established in many academic centers and involves drilling a track to an ON segment in order to release the presumed increased pressure in ON and facilitate revascularization. Recently, this core decompression procedure has been combined with the delivery of concentrated bone marrow aspirates containing mesenchymal stromal cells (MSCs) and enriched osteoprogenitor cells (10–12). These cell transplants are expected to facilitate the regeneration of normal bone marrow in ON through direct or indirect mechanisms. However, success or failure of this new treatment can only be diagnosed after several months to years (11,12). Our group has previously shown in a rat model that intravenously injected iron oxide nanoparticles are taken up by MSCs in the bone marrow and could be tracked with MRI after transplantation into osteochondral defects (13,14). Others have also noted in vivo labeling capacity of immune cells in the bone marrow (15,16).
An imaging test, which could directly track transplanted bone marrow cells in vivo could help us better understand the contribution of these cells to bone repair processes, diagnose complications earlier and facilitate the development of more successful cell therapies that can prevent bone collapse. The ability to track therapeutic cells non-invasively in vivo could have direct impact on patient management, e.g. by stratifying patients with unsuccessful or lost cell transplants to revision surgeries or alternative treatment options. To address this unmet clinical need, we developed an imaging test for the detection of bone marrow cell transplants in ON in a “first in patient” proof-of-concept clinical trial.
Materials and Methods
Study design
This prospective, non-randomized, HIPAA-compliant proof-of-concept clinical trial was approved by our institutional review board and performed under an investigator-initiated IND (111 154). The study was conducted in accordance with the Belmont Report. We invited pediatric and young adult patients from May 2015 until December 2017 to participate if they met the following inclusion criteria: (1) age 8–40 years, (2) avascular necrosis of the proximal femur (3) planned core decompression with transplantation of autologous bone marrow aspirates (4) willingness to give written informed consent. Patients were excluded if they had: (1) active leukemia, (2) contraindications to MRI, (3) hemosiderosis or hemochromatosis, or (4) if they were pregnant.
We recruited seven patients (mean age 30 ± 8.4 years; range: 17–38 years) with history of high dose corticosteroid treatment for leukemia (n=3), Hodgkin’s lymphoma (n=1), asthma (n=1), systemic lupus erythematodes (SLE) (n=1) or inflammation of unknown origin (n=1). The patients included four female (mean age 30 ± 9.5 years; range: 17–38 years) and three male patients (mean age 31 +/− 8.7 years; range: 21–36 years). The patients had nine early stage (ARCO stage 2) epiphyseal ON in their femoral heads: Three patients had ON of the right femoral head, two patients of the left femoral head and two patients had bilateral ON.
All patients received core decompression with transplantation of either iron labeled or unlabeled bone marrow aspirates. In order to achieve iron labeling of bone marrow cells in vivo, we injected ferumoxytol (Feraheme) intravenously as published previously (13). Four patients with 6 core decompression procedures received an intravenous injection of ferumoxytol at a dose of 5 mg Fe/kg at 24–48h before surgery. Three patients with 3 core decompression procedures did not receive ferumoxytol. Ferumoxytol labeled or unlabeled bone marrow cells were harvested by an iliac crest aspiration using an autologous cell aspiration and concentration system (Zimmer-Biomet, Warsaw, IN, USA) and mixed with demineralized bone matrix from DePuySynthes. Following core decompression, the graft matrix enriched with labeled or unlabeled bone marrow cells was implanted into the ON defect by an experienced orthopedic surgeon (SG). Follow-up imaging was performed by MRI.
To compare the clinical outcome of core decompression with and without labeled cells, we enrolled five additional control patients with seven femoral ON, which were treated with core decompression and unlabeled bone marrow cell transplants. These comprised one female and four male patients (mean age 30 ± 6.8 years; range: 21–38) with history of status post high dose corticosteroid treatment.
MR imaging
Patients included in our study received a pre-operative MRI and a follow-up MR scan at 1 week, 4–7 weeks and 6 months after bone marrow cell transplantation. MR imaging has been established as a highly sensitive and specific test for diagnosing early epiphyseal ON, before joint damage is apparent on bone scans or radiographs (17). MRI scans were obtained with a 3 Tesla MRI scanner (Discovery 750 MR, GE Healthcare), using a 32-channel torso phased array coil and the following pulse sequences: T1-weighted fast spin echo (FSE) sequence (TR=600 ms (517–721), TE=15 ms (5.8–19.7), flip angle (FA) = 90° (90°−160°), slice thickness (SL) = 3 mm (3–4.5)), T2-weighted fat saturated FSE sequence (TR=4450 ms (2399–4450), TE=61 ms (57–69), FA=125°, SL=4 mm (3–4)), short TI inversion recovery sequences (TR=5200 ms (5131–5282), TE=50 ms (47–54), inversion time=170 ms, FA=111°, SL=3 mm) and a flow-compensated 2D fast spoiled gradient recalled (FSPGR) sequence (TR=21.2 ms, TE=2.2 ms, inter-echo interval 2.2 ms, FA=25°, SL = 3 mm).
MR imaging analyses
Images were analyzed using Osirix (Pixmeo SARL). The decompression track was equally divided on coronal T2-weighted MR images in three parts: the proximal, mid and distal track. Each of these areas was manually outlined by one observer, who measured the signal-to-noise ratio (SNR) as the mean signal intensity of the outlined area, divided by the standard deviation of the background noise, which was measured in phase encoding direction within the field of view and outside of the patient. In addition, the iron signal in the same areas was quantified by measuring T2* relaxation times on corresponding T2* maps, which were generated from FSPGR sequences using the T2 fit map plugin of Osirix. Each of the areas were considered as an independent observation for MR imaging analyses.
Standard of reference
The extent of osseous necrosis on imaging studies was graded according to the Association Research Circulation Osseous (ARCO) classification (18,19). All patients had an ARCO stage 2 at baseline. Stable disease at 6 months after surgery was defined as equal or improved ARCO stage. Progressive disease was defined as progression to stage III or IV.
Statistical analyses
All experiments were analyzed using R version 3.4.4. SNR and T2* relaxation times were pairwise compared between labeled and unlabeled cell transplants, and between decompression track areas with and without visible iron labeled cells, using a mixed effects model including a random effect term accounting for correlation among the measures within a same patient. A Fisher’s exact test was applied for comparison of clinical outcomes of labeled and unlabeled cell transplants. In addition, differences in time to progression of ON from surgery between labeled and unlabeled cell transplants were assessed by log rank tests. Due to the small sample size and exploring purpose of this study, a P value of < 0.05 without adjustment for multiple comparisons was considered to indicate significant differences between experimental groups.
Results
Iron labeled bone marrow cells can be detected with MRI after their transplantation into ON
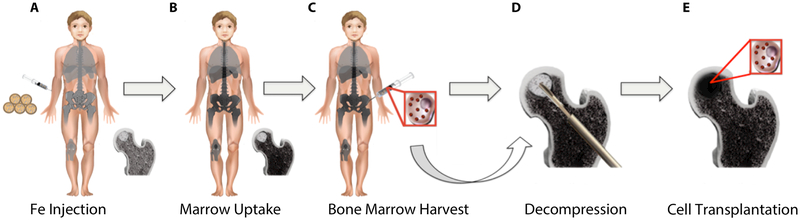
The overall concept of our study is shown in Fig. 1. Patients with ON received an intravenous injection of the iron supplement ferumoxytol prior to a scheduled core decompression in order to label bone marrow cells with iron, which can be detected by a dark signal on magnetic resonance images (MRI). 1–2 days later, the patients underwent a core decompression, bone marrow aspiration from the iliac crest and transplantation of concentrated iron labeled bone marrow cells through the decompression track into the ON in the femoral head. MRIs were performed before and within 1 week after the surgery, as well as at 4–7 weeks and 6 months in order to track transplanted iron labeled bone marrow cells in ON.
Fig. 1: Study Concept.
(A) 24–48 hours prior to a planned core decompression for ON treatment, patients received an intravenous injection of the FDA-approved iron supplement ferumoxytol. (B) Ferumoxytol is taken up by cells in normal bone marrow, leading to hypointense (dark) signal on MRI. (C) 24–48 hours after iron supplement administration, iron labeled bone marrow cells were harvested from the iliac crest during core decompression. (D) The osteonecrotic bone was decompressed by drilling a track to the ON through a minimally invasive procedure and (E) iron labeled bone marrow cells were injected through the decompression track.
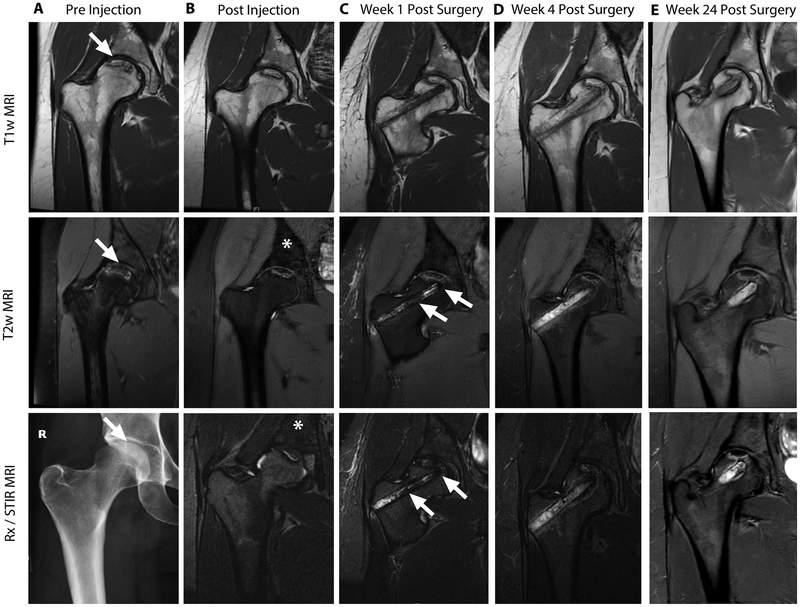
MR images before ferumoxytol administration showed a focal ON lesion in the proximal femoral epiphysis with a typical serpiginous border on T1- and T2-weighted MR images (Fig. 2A). All ON were consistent with stage 2 lesions according to the Association Research Circulation Osseous (ARCO) classification: The joint surfaces were intact and there was no evidence for subchondral fractures. This is important, because only joints without signs of bone collapse can be rescued by a core decompression. Next, patients received an intravenous injection of ferumoxytol. Post-contrast MR images showed a significant hypointense (dark) enhancement of the normal bone marrow on T2-weighted MR images (Fig. 2B).
Fig. 2: Serial MR images of osteonecrosis before and after core decompression and transplantation of iron labeled bone marrow cells.
(A) T1-weighted, T2-weighted and x-ray images of the right femur shows an osteonecrosis (arrows) in the femoral epiphysis with typical fat-equivalent center and serpiginous borders. (B) 24 hours after intravenous injection of ferumoxytol, the normal bone marrow in the iliac crest shows hypointense (dark) enhancement (asterisks) on T2-weighted MR images. (C) One week after core decompression and injection of iron labeled bone marrow cells, a hypointense (dark) signal is noted in the decompression track (arrows), consistent with delivery of iron labeled cells. MRI follow-up at (D) 4 weeks and (E) 24 weeks after core decompression and transplantation of labeled cells shows decline in iron signal over time. The femoral epiphysis did not collapse during this 6 month follow up period.
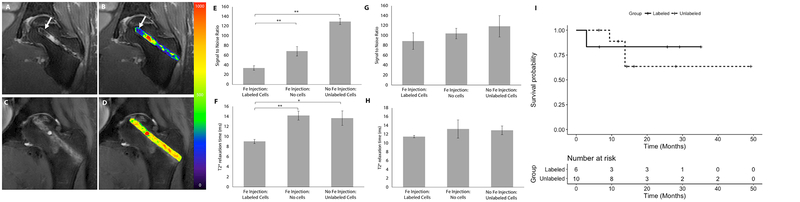
The patients underwent core decompression, harvest and concentration of iron labeled bone marrow cells from the iliac crest and transplantation of iron labeled bone marrow cells into the decompression track. T2-weighted MR images after injection of iron labeled marrow cells demonstrated hypointense (dark) signal in the decompression track and ON (Fig. 2C, Fig. 3A, B). By comparison, control patients who had received unlabeled bone marrow cell transplants did not show hypointense signal changes in the access canal (Fig. 3C, D). Signal-to-noise ratios (SNR) for ferumoxytol-labeled cell transplants were significantly lower compared to unlabeled cell transplants (33.82 ± 12.43 vs 129.56 ± 10.93; P = 0.002; Fig. 3E). Likewise, T2* relaxation times, which represent more robust measures of tissue iron concentrations, were significantly lower for ferumoxytol-labeled cell transplants than for unlabeled cell transplants (9.04 ± 0.7 vs. 13.7 ± 2.50; P = 0.02; Fig. 3F)
Fig. 3: Ferumoxytol labeled cell transplants can be detected in the decompression track after core decompression.
(A) Coronal T2-weighted MR image of the left femur of a patient, who was treated with core decompression and injection of iron labeled cells into the decompression track. Areas of hypointense (dark) signal (arrow) are noted in the decompression track, consistent with delivery of iron labeled cells (B) Superimposed color-coded signal intensities show areas of iron labeled cells (arrow) as displayed by blue color. (C) Coronal T2-weighted MR of the left femur of a patient, who was treated with core decompression and injection of unlabeled cells into the decompression track. Unlabeled cells are noted by an intermediate signal in the decompression track. (D) Superimposed color-coded signal intensities show medium ranged signal intensities (green/yellow) (E) Signal to Noise Ratios (SNR) and (F) T2* relaxation times during the first week after surgery for areas that showed iron signal compared to areas where iron labeled cell were not delivered and unlabeled controls show significantly lower SNR (P = 0.002, n = 18; P = 0.002, n = 10; respectively) and T2* relaxation times (P = 0.007, n = 9; P = 0.02, n = 6; respectively). (G) SNR and (H) T2* relaxation times at 4–7 weeks reveal no significant differences between the groups, suggesting interval iron metabolization. Data are means ± standard error of the mean. P values were determined by mixed effects model including a random effect term accounting for correlation among the measures within a same patient. (I) Time to progression of ON between labeled and unlabeled cell transplants are not significantly different (P = 0.8, n = 16). P value was determined by log rank test.
Within the decompression track of patients who had received iron labeled cell transplants, we noted areas that showed strong iron signal, presumably representing areas where iron labeled cells were delivered and areas that showed no iron signal, presumably representing areas where iron labeled cells were not delivered. We divided each decompression canal in three areas (proximal, mid and distal decompression canal) and compared SNR and T2* relaxation times of areas where cell transplants could be visually detected or not detected. SNR and T2* relaxation times were significantly lower for areas where cell transplants could be visually detected or not detected (68.45 ± 32.41, P = 0.002; 14.2 ± 2.18, P = 0.007; respectively) (Fig. 3E, F).
Iron supplement administration before decompression does not affect bone repair outcomes
To evaluate the long-term implications of ferumoxytol administrations on a decompression surgery, we investigated the MRI signal of transplanted cells over time and found a slow decline of the iron signal (Fig. 2D, E): Compared to unlabeled controls, SNR and T2* relaxation times of labeled cell transplants were not significantly different at 4–7 weeks (P > 0.05) (Fig. 3G, H). This implies either metabolization of the iron label or disappearance of the cell transplant or a combination of both.
To evaluate if the ferumoxytol administration prior to the core decompression had any effect on bone repair, we compared clinical outcomes of patients who did or did not receive ferumoxytol. Of six femoral heads treated with labeled cell transplants, one (17%) progressed to collapse. Of ten femoral heads treated with unlabeled cells, three (30%) progressed to collapse (Table 1). This difference was not significant (P = 1, Fisher’s exact test), suggesting that ferumoxytol administration before a core decompression did not adversely affect clinical outcomes. In addition, time to progression of ON from surgery between labeled and unlabeled cell transplants were also not significantly different (Fig. 3I; P = 0.8)
Table 1:
Absolute and relative (%) number of ON with labeled and unlabeled cells, which progressed to bone collapse.
| Labeled | Unlabeled | Total | |
|---|---|---|---|
| Progression | 1 (17%) | 3 (30%) | 4 (25%) |
| No progression | 5 (83%) | 7 (70%) | 12 (75%) |
| Total | 6 (100%) | 10 (100%) | 16 (100%) |
We noticed that one joint that progressed to collapse after administration of iron labeled cells had apparently received less cells compared to all other joints that did not collapse, as indicated by less iron signal in the treated decompression track (Supplementary Fig. S1). We quantified the hypointense (dark) area in the access canal through operator defined regions of interests (ROIs). In the femur that progressed to collapse, 3.1% of the area of the decompression track contained labeled cells. In the femur that did not progress to collapse, 16.6% ± 3.5% of the decompression track were covered by cells.
Overall, the collective successful outcome of ON treated with core decompression plus cell transplants was better than previously reported for core decompression alone: In our study, 12 of 16 femurs (75%) showed no collapse within one year or more after the intervention, compared to success rates of 53–71% for core decompression only, reported previously (20).
Discussion
Our data showed that a simple intravenous injection of the iron supplement ferumoxytol before a scheduled core decompression led to iron labeling of the bone marrow in patients. After harvest from bone marrow and transplantation into ON lesions, the iron labeled bone marrow cells could then be tracked with MRI in the early postoperative period. We previously proved in animal models that intravenously injected ferumoxytol nanoparticles are phagocytosed by bone marrow cells (13,14) and are slowly metabolized over time (21). We found that ferumoxytol nanoparticles accumulate in different cell populations in the bone marrow, that are capable of phagocytosis, including MSCs, macrophages, dendritic cells, and osteoprogenitor cells. In our previous work, we could show that ferumoxytol was taken up by MSCs, stored in lysosomes and had no effect on the viability and differentiation potential of the iron containing cells. Furthermore, MSCs could be tracked in rodents with MRI (13).
Other investigators reported the ability to track iron oxide nanoparticle labeled neural stem cells (22,23), autologous mesenchymal stem cells (24) and dendritic cells (25) with MRI in patients. In these previous studies, autologous cells were first harvested, then iron labeled in cell cultures, and then transplanted. Our approach is different in that we labeled bone marrow cells in vivo by intravenous injection of an FDA-approved iron supplement. The iron labeled cells could then be detected with MR imaging. This in vivo labeling approach is more practical in a clinical setting because it does not require any manipulation of the therapeutic cells and thereby, enables bone marrow cell harvest and transplantation in one surgery.
Corticosteroid induced ON leads to bone collapse in about 26% of patients (6). Core decompression can prevent or delay bone collapse in at least 50% of these patients (20). The addition of bone marrow cell transplants to classical decompression surgeries has improved outcomes compared to core decompression alone (11,26,27). It is discussed controversially, whether bone marrow cell transplants support ON repair directly or indirectly: Wang et al. and Lee et al. suggest that mesenchymal stromal cells and osteoprogenitor cells can directly regenerate bone injuries (28,29), Murphy et al. and Linero et al. suggest that MSCs support tissue repair through indirect paracrine mechanisms (30,31), Lim et al. and Pepke et al. question any therapeutic effect of bone marrow cells (32,33). However, Lim et al. and Pepke et al. also mention that numerous factors such as number of cells, area of transplantation, disease stage and follow-up period play an important role in clinical outcome. Our imaging test could be used to correlate the number and distribution of transplanted bone marrow cell transplants with outcomes. This information could be used to understand and optimize the effect of bone marrow cell transplants on bone repair outcomes. In addition, our imaging test could discriminate between successfully engrafting or lost cell transplants in the early postoperative period. This information is important for the treating physician, who could stratify patients with failed cell transplants to alternative operative and non-operative treatment options.
Our data showed that iron labeled and unlabeled cell transplants showed similar success rates in preventing bone collapse, confirming that our in vivo labeling approach did not affect the efficacy of the therapeutic cells. Previous studies showed that high intracellular iron concentrations and iron overload can impair chondrogenic differentiation (34,35) as well as osteogenic differentiation of MSCs and inhibit osteoblast activity, while facilitating osteoclast function (36). Our group has shown previously that this effect is dose-dependent and that the proliferation and function of MSCs is not impaired when the cells are loaded with less than 10 pg Fe per cell (37). Our investigations in preclinical models showed that intravenous injection of ferumoxytol (28 mg Fe/kg) leads to uptake of approximately 4.3 pg Fe per cell, which is below this threshold (13).
It has been reported previously, that MSC and osteoprogenitor cells bear the potential to migrate to the site of osteonecrotic bone lesions (38,39). In principle, our imaging test should be also suitable to investigate this process. Due to the avascular center, a newly developed osteonecrotic lesion takes up less ferumoxytol than surrounding healthy bone marrow. In case of an intrinsic migration of ferumoxytol labeled bone marrow cells from normal marrow to the site of injury, osteonecrotic lesions could become hypointense (dark) over time in MRI. However, tracking intrinsic in vivo migrations of ferumoxytol labeled cells to ON might involve fewer cells and therefore, require more sensitive pulse sequences.
We found that the quantity of bone marrow cells transplanted into ON correlates with clinical outcomes, which is in accordance with previously published findings (10,12,40,41). Hernigou et al. analyzed the number of transplanted progenitor cells in patients with osteonecrosis and found that femoral heads that received a low number of transplanted cells had higher risks of bone collapse than femoral heads that received a high number of cells (10). In another study, Hernigou et al. evaluated the amount of cells needed for treatment of nonunion of the tibial shaft in 60 patients and found a positive correlation between the volume of mineralized callus and the number of cells and concentration (40).
Bone marrow cells from ON patients may have less bone regeneration potential compared to healthy patients (12). Future studies will compare the in vitro bone-forming capacity of MSC and osteoprogenitor cells in ON patients and healthy controls. In patients who underwent bone marrow transplantation, MSC from the donor might be more effective in regenerating bone. Our imaging test can track the location, estimate the quantity and provide information about the phagocytic activity of bone marrow cells. For example, we previously noted that normal bone marrow cells of young patients take up more iron compared to bone marrow cells of older patients (42,43). Our imaging test cannot determine the efficacy of the transplanted cells to regenerate bone. However, in case our imaging studies suggest low quantities of iron labeled cells in the delivered bone marrow aspirates, we could increase the number of transplanted cells and/or add “off the shelf” MSC products or banked donor MSCs. We previously established ferumoxytol labeling procedures for ex vivo labeling of donor MSCs (21,44).
In summary, we provide proof-of-concept for a new imaging test, which allows to track autologous bone marrow derived cell transplants in patients with MRI, after a simple intravenous injection of an FDA approved iron supplement. The ability to directly detect and track iron labeled therapeutic cells in vivo, in patients, could help us to recognize inter-individual differences in the delivered quantity and location of transplanted cells and correlate results with tissue repair outcomes. This could ultimately improve our ability to develop more successful cell therapies. This new imaging test could become a powerful new tool to monitor the delivery and engraftment of bone marrow-derived therapeutic cells non-invasively in cancer patients with the ability to directly impact patient management. Future studies under this clinical trial will correlate MRI signal characteristics of iron labeled cells with clinical outcomes.
Supplementary Material
Translational Relevance.
Osteonecrosis (ON) due to corticosteroid therapy is a devastating complication in cancer patients. Core decompression with transplantation of bone marrow cells has shown promising results to prevent joint collapse. We developed an imaging test to track transplanted bone marrow cells in a “first in patient” clinical trial. We labeled bone marrow cells with a simple intravenous injection of an iron supplement. Iron labeled bone marrow cells were transplanted into ON and could be tracked with MRI. Tracking therapeutic cells in ON can improve our understanding of the role of cell transplants in bone regeneration processes, and our ability to develop successful cell therapies for joint repair. Based on our imaging results, patients could be stratified to revision surgeries, alternative treatment options or close follow-up examinations. By exploiting cell tracking techniques for monitoring engraftment outcomes, we anticipate alleviating long-term disabilities of cancer patients and related costs to our society.
Acknowledgments
We thank members of the Daldrup-Link lab for their helpful reviews and discussions of our study design and research results. We thank Jin Long from the Quantitative Sciences Unit at Stanford University and Ketan Yerneni for their excellent statistical consulting. Part of this work was performed at the Richard Lucas Center for MR Imaging and at the Stanford Nano Shared Facilities (SNSF) at Stanford University.
Financial Support: This work was supported by research grant 2R01AR054458 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Conflict of Interest Statement: The authors declare no potential conflicts of interest.
REFERENCES:
- 1.Ehmke TA, Cherian JJ, Wu ES, Jauregui JJ, Banerjee S, Mont MA. Treatment of osteonecrosis in systemic lupus erythematosus: a review. Curr Rheumatol Rep 2014;16(9):441 doi 10.1007/s11926-014-0441-8. [DOI] [PubMed] [Google Scholar]
- 2.Mattano LA Jr., Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2000;18(18):3262–72. [DOI] [PubMed] [Google Scholar]
- 3.Ojala AE, Paakko E, Lanning FP, Lanning M. Osteonecrosis during the treatment of childhood acute lymphoblastic leukemia: a prospective MRI study. Medical and pediatric oncology 1999;32(1):11–7. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro RC, Fletcher BD, Kennedy W, Harrison PL, Neel MD, Kaste SC, et al. Magnetic resonance imaging detection of avascular necrosis of the bone in children receiving intensive prednisone therapy for acute lymphoblastic leukemia or non-Hodgkin lymphoma. Leukemia 2001;15(6):891–7. [DOI] [PubMed] [Google Scholar]
- 5.Karimova EJ, Wozniak A, Wu J, Neel MD, Kaste SC. How does osteonecrosis about the knee progress in young patients with leukemia?: a 2- to 7-year study. Clinical orthopaedics and related research 2010;468(9):2454–9 doi 10.1007/s11999-010-1358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. The Journal of bone and joint surgery American volume 2010;92(12):2165–70 doi 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 7.Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World Journal of Orthopedics 2015;6(8):590–601 doi 10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce TP, Jauregui JJ, Elmallah RK, Lavernia CJ, Mont MA, Nace J. A current review of core decompression in the treatment of osteonecrosis of the femoral head. Curr Rev Musculoskelet Med 2015;8(3):228–32 doi 10.1007/s12178-015-9280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J 2017;99-B(10):1267–79 doi 10.1302/0301-620X.99B10.BJJ-2017-0233.R2. [DOI] [PubMed] [Google Scholar]
- 10.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clinical orthopaedics and related research 2002(405):14–23. [DOI] [PubMed] [Google Scholar]
- 11.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 2012;50(1):325–30 doi 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Hernigou P, Trousselier M, Roubineau F, Bouthors C, Chevallier N, Rouard H, et al. Stem Cell Therapy for the Treatment of Hip Osteonecrosis: A 30-Year Review of Progress. Clinics in Orthopedic Surgery 2016;8(1):1–8 doi 10.4055/cios.2016.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khurana A, Chapelin F, Beck G, Lenkov OD, Donig J, Nejadnik H, et al. Iron administration before stem cell harvest enables MR imaging tracking after transplantation. Radiology 2013;269(1):186–97 doi 10.1148/radiol.13130858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulte JW. Science to practice: can stem cells be labeled inside the body instead of outside? Radiology 2013;269(1):1–3 doi 10.1148/radiol.13131753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegers GM, Krishnamoorthy S, Gonzalez-Lara LE, McFadden C, Chen Y, Foster PJ. Pre-labeling of Immune Cells in Normal Bone Marrow and Spleen for Subsequent Cell Tracking by MRI. Tomography 2016;2(1):26–34 doi 10.18383/j.tom.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henning EC, Ruetzler CA, Gaudinski MR, Hu TC, Latour LL, Hallenbeck JM, et al. Feridex preloading permits tracking of CNS-resident macrophages after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 2009;29(7):1229–39 doi 10.1038/jcbfm.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miettunen PM, Lafay-Cousin L, Guilcher GM, Nettel-Aguirre A, Moorjani V. Widespread osteonecrosis in children with leukemia revealed by whole-body MRI. Clinical orthopaedics and related research 2012;470(12):3587–95 doi 10.1007/s11999-012-2579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardeniers J A new international classification of osteonecrosis of the ARCO Committee on terminology and classification. J Jpn Orthop Assoc 1992;66:18–20. [Google Scholar]
- 19.Meier R, Kraus TM, Schaeffeler C, Torka S, Schlitter AM, Specht K, et al. Bone marrow oedema on MR imaging indicates ARCO stage 3 disease in patients with AVN of the femoral head. European radiology 2014. doi 10.1007/s00330-014-3216-8. [DOI] [PubMed] [Google Scholar]
- 20.Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clinical orthopaedics and related research 1996(324):169–78. [DOI] [PubMed] [Google Scholar]
- 21.Khurana A, Nejadnik H, Chapelin F, Lenkov O, Gawande R, Lee S, et al. Ferumoxytol: a new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine (Lond) 2013;8(12):1969–83 doi 10.2217/nnm.12.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutova M, Frank JA, D’Apuzzo M, Khankaldyyan V, Gilchrist MM, Annala AJ, et al. Magnetic resonance imaging tracking of ferumoxytol-labeled human neural stem cells: studies leading to clinical use. Stem Cells Transl Med 2013;2(10):766–75 doi 10.5966/sctm.2013-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med 2006;355(22):2376–8 doi 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]
- 24.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010;67(10):1187–94 doi 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 2005;23(11):1407–13 doi 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 26.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone 2011;49(5):1005–9 doi 10.1016/j.bone.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 27.Hernigou P, Dubory A, Homma Y, Guissou I, Flouzat Lachaniette CH, Chevallier N, et al. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int Orthop 2018. doi 10.1007/s00264-018-3941-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang BL, Sun W, Shi ZC, Zhang NF, Yue DB, Guo WS, et al. Treatment of nontraumatic osteonecrosis of the femoral head with the implantation of core decompression and concentrated autologous bone marrow containing mononuclear cells. Arch Orthop Trauma Surg 2010;130(7):859–65 doi 10.1007/s00402-009-0939-0. [DOI] [PubMed] [Google Scholar]
- 29.Lee HS, Huang GT, Chiang H, Chiou LL, Chen MH, Hsieh CH, et al. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells 2003;21(2):190–9 doi 10.1634/stemcells.21-2-190. [DOI] [PubMed] [Google Scholar]
- 30.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013;45:e54 doi 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One 2014;9(9):e107001 doi 10.1371/journal.pone.0107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim YW, Kim YS, Lee JW, Kwon SY. Stem cell implantation for osteonecrosis of the femoral head. Exp Mol Med 2013;45:e61 doi 10.1038/emm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepke W, Kasten P, Beckmann NA, Janicki P, Egermann M. Core Decompression and Autologous Bone Marrow Concentrate for Treatment of Femoral Head Osteonecrosis: A Randomized Prospective Study. Orthop Rev (Pavia) 2016;8(1):6162 doi 10.4081/or.2016.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed 2004;17(7):513–7 doi 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 35.Roeder E, Henrionnet C, Goebel JC, Gambier N, Beuf O, Grenier D, et al. Dose-response of superparamagnetic iron oxide labeling on mesenchymal stem cells chondrogenic differentiation: a multi-scale in vitro study. PLoS One 2014;9(5):e98451 doi 10.1371/journal.pone.0098451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balogh E, Tolnai E, Nagy B Jr., Nagy B, Balla G, Balla J, et al. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Biophys Acta 2016;1862(9):1640–9 doi 10.1016/j.bbadis.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Daldrup-Link HE, Nejadnik H. MR Imaging of Stem Cell Transplants in Arthritic Joints. J Stem Cell Res Ther 2014;4(2):165 doi 10.4172/2157-7633.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fong LSE, Chan CK, Goodman SB. STEM CELL HOMING IN MUSCULOSKELETAL INJURY. Biomaterials 2011;32(2):395–409 doi 10.1016/j.biomaterials.2010.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibon E, Batke B, Jawad MU, Fritton K, Rao A, Yao Z, et al. MC3T3-E1 Osteoprogenitor Cells Systemically Migrate to a Bone Defect and Enhance Bone Healing. Tissue Engineering Part A 2012;18(9–10):968–73 doi 10.1089/ten.tea.2011.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. The Journal of bone and joint surgery American volume 2005;87(7):1430–7 doi 10.2106/jbjs.d.02215. [DOI] [PubMed] [Google Scholar]
- 41.Hernigou P, Guerin G, Homma Y, Dubory A, Chevallier N, Rouard H, et al. History of concentrated or expanded mesenchymal stem cells for hip osteonecrosis: is there a target number for osteonecrosis repair? Int Orthop 2018;42(7):1739–45 doi 10.1007/s00264-018-4000-1. [DOI] [PubMed] [Google Scholar]
- 42.Daldrup-Link HE, Link TM, Moller HE, Wiedermann D, Bonnemain B, Corot C, et al. Carboxymethyldextran-A2-Gd-DOTA enhancement patterns in the abdomen and pelvis in an animal model. European radiology 2001;11(7):1276–84 doi 10.1007/s003300000699. [DOI] [PubMed] [Google Scholar]
- 43.Klenk C, Gawande R, Uslu L, Khurana A, Qiu D, Quon A, et al. Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. Lancet Oncol 2014;15(3):275–85 doi 10.1016/S1470-2045(14)70021-X. [DOI] [PubMed] [Google Scholar]
- 44.Nejadnik H, Taghavi-Garmestani SM, Madsen SJ, Li K, Zanganeh S, Yang P, et al. The Protein Corona around Nanoparticles Facilitates Stem Cell Labeling for Clinical MR Imaging. Radiology 2018;286(3):938–47 doi 10.1148/radiol.2017170130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.