Abstract
Background and aims:
Fat radiodensity, measured via CT Hounsfield units (HU), is a potential marker of fat quality. We sought to determine the cross-sectional associations of total heart fat (TAT) and aortic perivascular fat (PVAT) radiodensity with cardiovascular risk factors, coronary artery calcification (CAC), and aortic calcification (AC) in midlife women.
Methods:
Fat radiodensity, CAC, and AC were quantified using CT scans. A total of 528 women (mean age: 50.9 ± 2.9 years; 37% Black) were included in analyses.
Results:
Women in the lowest TAT radiodensity tertile were more likely to have adverse cardiovascular risk factors. Independent of cardiovascular risk factors, women in the middle and high TAT radiodensity tertiles were less likely to have CAC (OR (95% CI): 0.32 (0.18, 0.59); 0.43 (0.24, 0.78), respectively) compared with women in the lowest TAT radiodensity tertile. Although adjusting for BMI attenuated the overall association, women in the middle TAT radiodensity tertile remained at significantly lower odds of CAC when compared to the low radiodensity tertile, 0.47 (0.24, 0.93), p=0.03. No significant associations were found for PVAT radiodensity and calcification measures in multivariable analysis.
Conclusions:
Lower TAT radiodensity was associated with a less favorable cardiometabolic profile. Women with mid-range TAT radiodensity values had a lower odds of CAC presence, independent of CVD risk factors and BMI. More research is necessary to understand radiodensity as a surrogate marker of fat quality in midlife women.
Keywords: fat radiodensity, cardiovascular, vascular calcification, midlife women
Introduction
Adipose tissue is a complex, metabolically active organ with potential endocrine, paracrine, and vasocrine effects1-3 that conveys varying cardiovascular risk depending on the location in the body.4 Cardiovascular fat volumes, fat surrounding the heart and the vasculature, are predictors of vascular calcification (coronary (CAC) and aortic calcification (AC)) independent of other measures of adiposity.4–6 CAC strongly predicts future cardiovascular disease (CVD) events and mortality;7, 8 while AC predicts all-cause mortality.9 Interestingly, compared to healthy premenopausal women, healthy postmenopausal women have higher volumes of cardiovascular fat,10 with greater volumes of the fat located outside the pericardium, known as paracardial adipose tissue (PAT), being significantly associated with a greater risk of CAC.11 These findings suggest a role for cardiovascular fat volumes, particularly PAT, in the increased CVD risk reported after menopause.10, 12
Most recently, attention has been focused on the quality of adipose tissue surrounding the heart and vasculature as a novel marker of CVD risk.13–15 Adipose tissue quality characteristics including hypertrophy, hyperplasia,16 hypoxia,17, 18 macrophage infiltration,19 capillary density,18 and adipocyte type20 are potentially associated with cardiovascular risks. Research of these fat quality characteristics in humans is limited due to the invasiveness of the procedures.18, 19 Fat radiodensity measured by computed tomography (CT) Hounsfield Units (HU), is a surrogate marker of adipose tissue quality.13–15 Higher adipose tissue radiodensity (more positive) may indicate adipocytes that are densely packed with mitochondria, multiple lipid droplets, and higher levels of vascularization and innervation.21, 22 In contrast, lower fat radiodensity (more negative) may be indicative of hypertrophic adipocytes with higher levels of lipid content thus representing a lower quality adipose tissue.14, 16, 21 Several studies have evaluated fat radiodensity as a risk factor for CVD in different settings with varying levels of consistency.13–15, 23–26
To the best of our knowledge, no studies have evaluated the associations between total heart adipose tissue (TAT: inside / outside the pericardium) and aortic perivascular adipose tissue (PVAT: along the descending aorta) radiodensity values with CVD risk factors and vascular calcification in midlife women. The objectives of our study were to determine the cross-sectional associations of TAT and PVAT radiodensity values with CVD risk factors, CAC, and AC in women at midlife. We hypothesized that women with higher TAT and PVAT radiodensity values would have lower odds of both CAC and AC presence and a more favorable cardiovascular risk profile.
Materials and methods
Study population
The Study of Women’s Health Across the Nation (SWAN) is a multicenter, communitybased prospective study of the menopause transition with previously reported design and objectives. 27 The SWAN Heart ancillary study was designed to measure subclinical atherosclerosis among White and Black women at the Pittsburgh and Chicago study sites. The SWAN Cardiovascular Fat ancillary study was designed to quantify volumes of cardiovascular fat depots among SWAN Heart participants using previously attained CT images. A total of 562 out of 608 SWAN Heart participants who had readable CT scans were included. For the current analyses, participants were excluded if they were missing both AC and CAC readings, had a history of stroke, angina, or heart attack, or had undergone surgical menopause. A total of 528 women were included in the PVAT analyses. Due to either poor image quality or scans that did not encompass the designated anatomical boundaries for the TAT depot an additional 40 participants were excluded from TAT analyses, leaving a total of 488 women. The institutional review board at each site approved the study protocol and all participants signed informed consent prior to participation.
CAC and AC measurements and quantification
Calcification of the coronary arteries and descending and abdominal aorta was measured using electron-beam CT with an Imatron C-150 Ultrafast scanner (GE-Imatron, South San Francisco, CA). Three passes were performed: 1) to determine anatomical landmarks, 2) to provide 30 to 40 contiguous 3-mm-thick transverse images of the coronary arteries from the level of the aortic root to the apex of the heart, 3) to provide cross-sectional 6-mm images of the aorta from the aortic arch to the iliac bifurcation. Scans were scored according to the Agatston method using a DICOM workstation and AccuImage, Inc. software (South San Francisco, CA) at the University of Pittsburgh.28 Calcification was considered present if there were at least 3 contiguous pixels with a radiodensity ≥130 HU. CAC was quantified for each of the 4 major coronary arteries and then summed for a total Agatston score; while AC was quantified as a single total Agatston score. Excellent intra-class correlation coefficients of at least 0.98 have been reported for both CAC and AC.29
Cardiovascular fat depot measurements and quantification
As previously described, TAT was defined as the combination of the fat inside and outside the pericardium (Figure 1).10 TAT radiodensity and volume were quantified at the Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center, CA, USA, using the same set of images acquired during the CAC electron-beam CT scanning. TAT volumes were determined from 15 mm above to 30 mm below the superior extent of the left main coronary artery. Using volume analysis software (GE Healthcare, Waukesha, WI, USA), adipose tissue was distinguished from the remainder of the heart tissue by a threshold of −190 to −30 HU. The aorta and bronchus defined the posterior border, while the chest wall defined the anterior border. Posterior mediastinum and peri-aortic adipose tissues were not included. For exploratory analysis, epicardial fat (EAT) was defined as the fat inside the pericardium. 10
Figure 1:
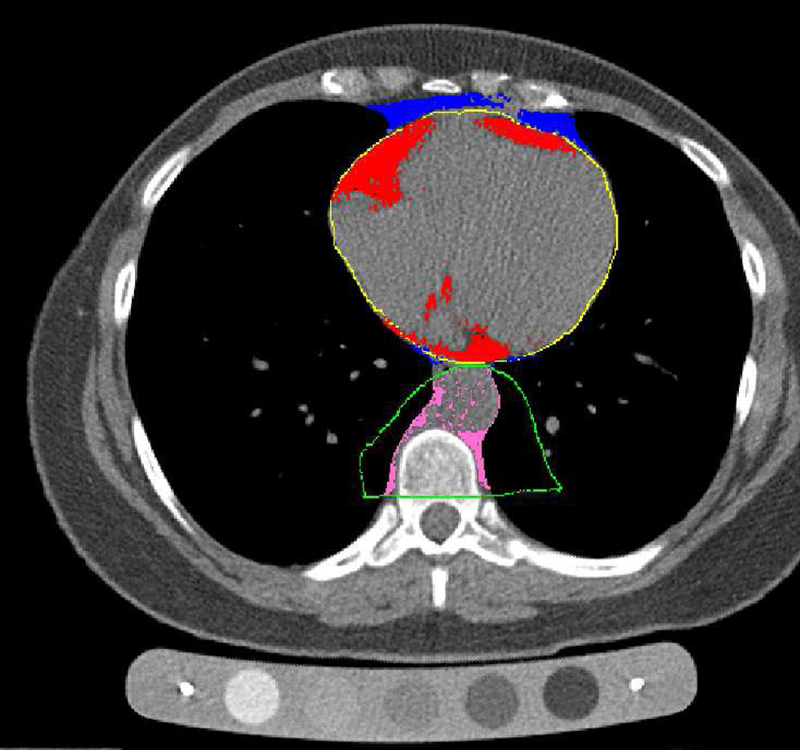
Cardiovascular fat depots: typical cross-sectional CT image including the heart (red=epicardial fat, blue=paracardial fat, yellow=pericardium) and the aorta (pink=aorta PVAT, green=anatomically oriented boundary for aorta PVAT inclusion)
Excellent within- and between-reader Spearman correlation coefficients of 0.97 have been reported for each cardiovascular fat volume.10 Pracon et al. used a similar methodology for assessing EAT radiodensity and found excellent within- and between-reader intra-class correlation coefficients of 0.99 and 0.97. 25
PVAT was quantified at the Ultrasound Research Lab at the University of Pittsburgh using the same set of images acquired during the AC electron-beam CT scanning. PVAT was defined as the adipose tissue immediately surrounding the descending aorta (Figure 1). Using a dedicated image analysis workstation equipped with Slice-O-Matic v4.3 (Tomovision, Magog, Quebec, Canada), adipose tissue was distinguished from other tissues by a threshold of −190 to 30 HU. The pulmonary bifurcation marked the proximal border and the first lumbar vertebrae served as the distal border. The anterior borders included the left bronchus, esophagus, and eventually the interior border of the crus of the diaphragm, while the anterior border of the vertebral foramen served as the posterior border. It is expected that the length of the evaluated part of the descending aorta within the anatomic landmarks described above (aorta length, mm) may vary across participants. Therefore, aorta length was estimated from table position number at first included CT slice and table position number at last included CT slice, and accounted for in analysis involved PVAT and calcification measures.
Excellent intra-reader and inter-reader intra-class coefficients of at least 0.998 have been reported for PVAT volume.6 For PVAT radiodensty, data from a random subset of our study population (n=18) showed excellent inter-reader reproducibility statistics; with a Spearman correlation of 0.99 and an inter-reader intra-class correlation coefficient of 0.97.
Study covariates
Body mass index (BMI) was calculated as measured weight in kilograms divided by measured height in square meters. Blood pressure was measured twice and averaged. As previously described, triglycerides, high-density lipoprotein cholesterol (HDL-C), and lowdensity lipoprotein cholesterol (LDL-C) were assayed at the Medical Research Laboratories (Lexington, KY, USA); and race, age, financial strain, and current smoking status were selfreported.30 The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated from insulin and glucose as (fasting insulin (mU/L) x fasting glucose (mmoles/L))/22.5.31 Diabetes was defined as a maximum glucose ≥ 126 or taking diabetes medication. Financial Strain was derived from the interview question, “How hard is it for you to pay for the very basics like food, housing, medical care, and heating?” The answers were dichotomized as “somewhat hard to very hard” and “not hard at all”. Cardiovascular medication was defined as taking blood pressure and/or lipid- lowering medication.
Menopausal status was determined using self-reported bleeding patterns and categorized as follows: 1) premenopausal: menses in the last 3 months with no change in regularity in the last 12 months; 2) early peri-menopausal: menses in the last 3 months with some change in regularity during the prior 12 months; 3) late peri-menopausal: no menses within the last 3 months, but some menstrual bleeding over the prior 12 months; and 4) postmenopausal: no menses within the last 12 months. For the current analyses, premenopausal and early peri-menopausal were combined into one group; late peri-menopausal and postmenopausal were combined into a second group; and women taking hormone therapy comprised a third group.10
Statistical analyses
Participant characteristics were summarized and presented as mean ± standard deviation for normally distributed variables; median (Q1, Q3) for skewed variables; and frequency (percentage) for categorical variables. Triglycerides, PVAT and TAT volumes, HOMA-IR, and LDL-C were log-transformed due to skewed distributions. The presence of CAC was defined as an Agatston score of greater than or equal to 10 and the presence of AC was defined as an Agatston score of greater than or equal to 100.32, 33 Cardiovascular fat radiodensity values were first analyzed as continuous variables. These preliminary analyses suggested a non-linear effect. Therefore, the main analyses reported in the current manuscript utilized tertiles of radiodensity for each fat depot. Chi-square and analysis of variance tests were used to assess differences across tertiles of TAT and PVAT radiodensity (Table 1).
Table 1:
Characteristics of the study population across tertiles of TAT and PVAT radiodensity
| TAT radiodensity tertiles (N=488) |
PVAT radiodensity tertiles (N=528) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Low radiodensity → High radiodensity | Low radiodensity → High radiodensity | |||||||
| Characteristics | -91 to −81a
(−83.1 ± 2.2)b Low (n=152) |
-80 to −78a
(−79.0 ± 0.8)b Middle (n=162) |
-77 to −67a
(−75.3 ± 1.8)b High (n=174) |
p | -95.2 to −84.7a
(−86.8 ± 1.8)b Low (n=179) |
-84.7 to −82.0a
(−83.4 ± 0.8)b Middle (n=172) |
-82.0 to −68.3a
(−79.4 ± 2.2)b High (n=177) |
p |
| Age (years) | 51.4 ± 3.0 | 50.7 ± 3.0 | 50.9 ± 2.7 | 0.13c | 51.1 ± 3.1 | 50.8 ± 2.8 | 50.9 ± 2.8 | 0.59c |
| Whites, n (%) | 126 (83) | 110 (68) | 70 (40) | <0.001 | 129 (72) | 103 (60) | 99 (56) | 0.01 |
| Menopausal status, n (%) | 0.06 | 0.82 | ||||||
| Pre-/early peri- | 71 (47) | 91 (56) | 107 (61) |
100 (56) |
100 (58) | 95 (54) | ||
| Late peri-/post | 66 (43) | 57 (35) | 49 (28) | 63 (35) | 57 (33) | 61 (34) | ||
| Hormone users | 17 (10) | 14 (9) | 18 (10) | 16 (11) | 15 (9) | 21 (12) | ||
| Smoking, n (%) | 31 (20) | 33 (20) | 24 (14) | 0.19 | 33 (18) | 36 (21) | 23 (13) | 0.14 |
| Financial strain, n (%) | 44 (31) | 43 (28) | 60 (36) | 0.32 | 53 (31) | 51 (31) | 58 (34) | 0.85 |
| SBP (mmHg) | 119.0 ± 15.7 | 118.5 ± 14.9 | 121.6 ± 17.7 | 0.14c | 119.4 ± 14.8 | 118.5 ± 17.5 | 121.1 ± 17.5 | 0.36c |
| DBP (mmHg) | 75.6 ± 9.5 | 75.8 ± 9.9 | 76.8 ± 10.4 | 0.28c | 75.8 ± 9.5 | 75.6 ± 9.8 | 76.8 ± 10.7 | 0.37c |
| LDL-C (mg/dL) | 120.0 | 117.0 | 110.0 | 0.02c | 113.0 | 114.0 | 117.0 | 0.47c |
| (100.0, 148.0) | (97.0, 141.0) | (95.0, 131.5) | (95.0, 140.0) | (98.0, 133.5) | (97.0, 141.0) | |||
| HDL-C (mg/dL) | 54.2 ± 13.4 | 59.3 ± 14.6 | 58.2 ± 15.0 | 0.02c | 54.2 ± 13.6 | 57.7 ± 15.0 | 59.7 ± 14.2 | 0.004c |
| Triglycerides (mg/dL) | 120.0 (87.0, 174.0) |
98.5 (76.0, 136.8) |
85.0 (68.0, 110.0) |
<0.001 c |
109.0 (79.0, 156.0) |
95.0 (73.0, 128.0) |
92.0 (71.0, 125.0) |
<0.001 c |
| BP/lipid-lowering medication, n (%) | 26 (17) | 33 (20) | 38 (22) | 0.555 | 44 (25) | 28 (16) | 32 (18) | 0.119 |
| Diabetes, n (%) | 10 (7) | 5 (3) | 9 (5) | 0.353 | 9 (5) | 8 (5) | 10 (6) | 0.912 |
| HOMA-IR | 2.3 (1.7, 3.9) | 1.9 (1.4, 2.8) | 1.9 (1.4, 3.1) | 0.04c | 2.1 (1.6, 3.9) | 1.9 (1.3, 3.2) | 2.0 (1.5, 2.8) | 0.16c |
| BMI (kg/m2) | 30.8 ± 6.3 | 28.0 ± 5.4 | 29.0 ± 6.7 | 0.02c | 30.1 ± 6.5 | 28.4 ± 6.4 | 29.1 ± 5.7 | 0.13c |
| TAT, cm3 | 66.3 (51.5, 93.5) | 44.3 (33.5, 59.7) | 36.7 (29.6, 47.8) |
<0.001 c |
51.7 (35.3, 71.5) | 45.3 (33.9, 59.8) | 46.5 (35.0, 65.9) | 0.06c |
| PVAT, cm3 | 37.3 (29.6, 45.1) | 29.1 (23.9, 36.5) | 26.9 (21.8, 32.8) |
<0.001 c |
34.2 (25.0, 44.5) | 28.8 (23.4, 37.2) | 29.2 (24.0, 35.6) |
<0.001 c |
| Aorta length, mm | N/A- | N/A- | N/A- | - | 160.7 ± 14.9 |
163.3 ± 15.1 |
168.5 ± 16.2 |
<0.001 |
| CAC score | 2.8 (0.0, 13.7) | 0.0 (0.0, 3.1) | 0.0 (0.0, 4.5) | 0.002c | 0.0 (0.0, 7.9) | 0.0 (0.0, 6.4) | 1.7 (0.0, 7.9) | 0.91c |
| CAC ≥10, n (%) | 46 (30) | 21 (13) | 33 (19) | <0.001 | 42 (23) | 38 (22) | 35 (20) | 0.70 |
| AC score | 31.5 (2.4, 168.0) | 6.1 (0.0, 66.0) | 8.3 (0.0, 45.0) |
<0.001 c |
21.0 (0.0, 138.0) | 6.7 (0.0, 69.0) | 16.0 (0.0, 63.0) | 0.21c |
| AC ≥100, n (%) | 50 (33) | 30 (19) | 28 (16) | <0.001 | 51 (29) | 37 (22) | 32 (18) | 0.05 |
Hounsfield Unit tertile range.
Average ± standard deviation radiodensity Hounsfield Units.
p value for trend. Mean ± standard deviation. Median (interquartile range). n (%).
TAT, total heart adipose tissue; PVAT, aortic perivascular adipose tissue; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance; BMI, body mass index; CAC, coronary artery calcium; AC, aortic calcification
Logistic regression was used to assess the relationships between cardiovascular fat radiodensity tertiles (TAT and PVAT, separate models) as the independent variable and the presence of CAC and AC, separately, as the dependent variable. Effect modifications by race and menopausal status were individually assessed in each model.
Given the strong effect of BMI on the main outcome (p<0.0001) and the strong correlations between BMI and cardiovascular fat depots (correlation coefficient range ~0.6–0.7) as well as between TAT density and TAT volume (correlation coefficient =0.5) it was challenging to include all these variables in one single model. Adjusting for cardiovascular fat volumes resulted in poor model fit statistics/quality (Hosmer–Lemeshow test or Akaike information criterion (AIC)). Therefore, we explored the associations between cardiovascular fat radiodensity and calcification stratified by tertiles of cardiovascular fat volume. Additional exploratory analyses evaluated epicardial adipose tissue (EAT) radiodensity separately.
Finally, we assessed financial strain, diabetes, and cardiovascular medication (BP and lipid lowering medications) as potential confounders in our multivariable analyses. Although they are well established risk factors for cardiovascular disease, they were not associated with both the exposure and outcome variables and did not improve the fit of our models. Therefore, our models did not include these variables.
Results
Cardiovascular risk factors and vascular calcification across cardiovascular fat radiodensity tertiles- unadjusted analysis
The characteristics of the participants by tertiles of TAT and PVAT radiodensity are presented in Table 1. The radiodensity values ranged from −91.0 HU to −67.0 HU for TAT and ranged from −95.2 HU to −68.3 HU for PVAT. On average, 21.1% of the study participants had CAC≥10 while 22.3% had AC≥100. Women in the lowest TAT radiodensity tertile were significantly more likely to be White, have higher LDL-C, triglycerides, HOMA-IR, BMI, TAT, and PVAT volumes, and lower HDL-C. They were also more likely to have CAC (≥10) and AC (≥100) present (30% versus 13% and 19%, and 33% versus 19% and 16%, respectively). Women in the lowest PVAT radiodensity tertile were significantly more likely to be White, have lower HDL-C, higher triglycerides, greater PVAT volume and shorter aorta length. Additionally, they tended to have more AC (≥100, p=0.054). No other differences, including the presence of CAC (≥10), were found across PVAT tertiles.
Adjusted analyses of TAT radiodensity with vascular calcification
When compared to the low TAT radiodensity tertile, the odds of having CAC were significantly lower for the middle and high radiodensity tertiles in the partially adjusted model (model 2), Table 2. After further adjustment for BMI (model 3), the overall association was attenuated; however, the odds for having CAC remained significantly lower for the middle radiodensity tertile compared to the lowest tertile. In the final model (model 4), the results remained consistent. The odds of having AC were significantly lower for the middle and high TAT radiodensity tertiles in model 3 when compared to the low radiodensity tertile. In the final model (model 4), the results were attenuated and no longer significant. There were no significant interactions between TAT radiodensity tertiles and race or menopausal status for the presence of CAC or AC.
Table 2:
Multivariable logistic regression for the associations between TAT radiodensity tertiles and the presence of CAC ≥10 and AC ≥100
| CAC ≥10 presence (n=488) |
AC ≥100 presence (n=486) |
||||
|---|---|---|---|---|---|
|
TAT
radiodensity tertiles |
OR (95% CI) | p |
TAT
radiodensity tertiles |
OR (95% CI) | p |
| Model 1 | <0.001 | Model 1 | <0.001 | ||
| Middle vs. Low | 0.34 (0.19, 0.61) | <0.001 | Middle vs. Low | 0.46 (0.27, 0.77) | 0.003 |
| High vs. Low | 0.54 (0.32, 0.90) | 0.02 | High vs. Low | 0.38 (0.23, 0.65) | <0.001 |
| Model 2 | <0.001 | Model 2 | <0.001 | ||
| Middle vs. Low | 0.32 (0.18, 0.59) | <0.001 | Middle vs. Low | 0.45 (0.26, 0.78) | 0.004 |
| High vs. Low | 0.43 (0.24, 0.78) | 0.01 | High vs. Low | 0.35 (0.20, 0.64) | <0.001 |
| Model 3 | 0.09 | Model 3 | 0.02 | ||
| Middle v.s Low | 0.47 (0.24, 0.93) | 0.03 | Middle vs. Low | 0.56 (0.32, 0.99) | 0.04 |
| High vs Low | 0.66 (0.34, 1.31) | 0.24 | High vs. Low | 0.44 (0.24, 0.82) | 0.01 |
| Model 4 | 0.08 | Model 4 | 0.12 | ||
| Middle vs. Low | 0.46 (0.23, 0.91) | 0.03 | Middle vs. Low | 0.59 (0.32, 1.10) | 0.09 |
| High vs. Low | 0.67 (0.33, 1.36) | 0.26 | High vs. Low | 0.52 (0.26, 1.03) | 0.06 |
TAT, total heart adipose tissue; CAC, coronary artery calcification; AC, aortic calcification; Model 1: unadjusted; Model 2: adjusted for age, race, site, menopausal status; Model 3: model 2 + body mass index; Model 4: model 3 + log-transformed triglycerides, current smoking status, and systolic blood pressure
PVAT radiodensity and vascular calcification
No associations between PVAT radiodensity tertiles and the presence of CAC were found in any of the models, Table 3. The odds of the presence of AC (≥100) were significantly lower for the high radiodensity tertile when compared to the low tertile in model 2. After additional adjustment for BMI (model 3), the results were attenuated to non-significance. Additional adjustment for log-transformed triglycerides, current smoking status, systolic blood pressure and aorta length (model 4) did not change our findings. There were no significant interactions etween PVAT radiodensity and race or menopausal status for the presence of CAC or AC.
Table 3:
Multivariable logistic regression for the associations between PVAT radiodensity tertiles and the presence of CAC ≥ 10 and AC ≥ 100
| CAC ≥ 10 presence (n=528) |
AC ≥ 100 presence (n=526) |
||||
|---|---|---|---|---|---|
|
PVAT
radiodensity tertiles |
OR (95% CI) | p |
PVAT
radiodensity tertiles |
OR (95% CI) | p |
| Model 1 | 0.70 | Model 1 | 0.05 | ||
| Middle vs.
Low |
0.92 (0.56, 1.52) | 0.76 | Middle vs.
Low |
0.69 (0.42, 1.12) | 0.13 |
| High vs. Low | 0.80 (0.48, 1.33) | 0.40 | High vs. Low | 0.55 (0.33, 0.91) | 0.02 |
| Model 2 | 0.54 | Model 2 | 0.04 | ||
| Middle vs.
Low |
0.91 (0.54, 1.54) | 0.73 | Middle vs.
Low |
0.68 (0.41, 1.12) | 0.13 |
| High vs. Low | 0.74 (0.44, 1.27) | 0.28 | High vs. Low | 0.52 (0.31, 0.87) | 0.01 |
| Model 3 | 0.55 | Model 3 | 0.17 | ||
| Middle vs.
Low |
1.35 (0.74, 2.48) | 0.33 | Middle vs.
Low |
0.81 (0.48, 1.38) | 0.44 |
| High vs. Low | 1.03 (0.56, 1.87) | 0.93 | High vs. Low | 0.60 (0.35, 1.02) | 0.06 |
| Model 4 | 0.47 | Model 4 | 0.22 | ||
| Middle vs.
Low |
1.32 (0.69, 2.53) | 0.40 | Middle vs.
Low |
0.69 (0.38, 1.27) | 0.24 |
| High vs. Low | 0.89 (0.46, 1.74) | 0.74 | High vs. Low | 0.59 (0.31, 1.10) | 0.09 |
PVAT, aortic perivascular adipose tissue; CAC, coronary artery calcification; AC, aortic calcification; Model 1: unadjusted; Model 2: adjusted for age, race, site, menopausal status; Model 3: model 2 + body mass index; Model 4: model 3 + log-transformed triglycerides, current smoking status, systolic blood pressure and aorta length
Exploratory analyses
In analyses stratified by TAT volume, significant associations between TAT radiodensity and the presence of CAC (≥10) and AC (≥100) were evident only in the highest TAT volume stratum (Supplemental Table 1). In the high TAT volume strata, the middle TAT radiodensity tertile was inversely associated with the presence of CAC (≥10) and AC (≥100) in the unadjusted and adjusted models. No significant associations between PVAT radiodensity and vascular calcification were found for the PVAT volume strata (Supplemental Table 2).
We found no associations between EAT radiodensity and the presence of CAC (≥10) (Supplemental Table 3). The highest EAT radiodensity was inversely associated with AC presence (≥100) in the partially adjusted model (model 2); however, the associations were not significant adjusting for BMI (model 3), Supplemental Table 3.
Discussion
Our cross-sectional study of midlife women has three main findings: 1) TAT radiodensity was associated with several CVD risk factors, such that women in the lowest radiodensity tertile had a less favorable cardiovascular risk profile, 2) women with mid-range TAT radiodensity values had a lower odds of CAC presence (≥10), independent of CVD risk factors and BMI, and 3) we did not find any significant associations between PVAT radiodensity and CAC or AC, and the associations of PVAT radiodensity with CVD risk factors were less pronounced.
Our study provides a look at the radiodensity of cardiovascular fat depots that have not been previously assessed in midlife women. We found a marked difference in several CVD risk factors across tertiles of TAT radiodensity with the higher TAT radiodensity values being more favorable. Consistent with our study, Rosenquist et al. and Lee et al. found that, within Framingham Heart Study MDCT substudy participants, more positive radiodensity values for VAT and SAT volumes were associated with more favorable cardiometabolic and adipokine profiles.14, 34 In a recent study by Lu et al., lower (more negative) epicardial and paracardial fat radiodensity values were associated with high-risk plaque among men and women with suspected acute coronary syndrome in univariate analyses.35 These associations were attenuated and no longer significant in multivariable analyses.35
However, results from studies assessing associations between fat radiodensity and vascular calcification are not in agreement with our findings on TAT radiodensity and CAC. Pracon et al. found that higher EAT radiodensity values were positively correlated with CAC severity among participants suspected of coronary artery disease in minimally adjusted analyses.25 Among Framingham Heart Study MDCT substudy participants, Alvey et al. found that higher VAT radiodensity was positively associated with both CAC and abdominal aortic calcification, higher SAT radiodensity was positively associated with CAC; and EAT radiodensity was not associated with either measure of calcification.13 Shields et al. found PVAT radiodensity was positively associated with AC, independent of CVD risk factors, including BMI, among women with SLE.15 Our EAT radiodensity exploratory findings where we found no association with CAC were consistent with Alvey et al. and Lu et al.13, 35 In a study among men and women with confirmed or suspected coronary artery disease, Mahabadi et al. found that EAT radiodensity was only mildly correlated with EAT volume and positively associated with type-I myocardial infarction.36 In a study among primarily male patients suspected of CVD, Marwan et al. found that pericoronary fat radiodensity was positively associated with coronary atherosclerotic plaque in unadjusted analyses.37
There are several potential factors that may have contributed to the inconsistency between our current findings and those previously reported. First, not all studies assessed the same fat depots, which may differ in adipocyte-specific characteristics, such as metabolic activity and embryonic origin.38 Two recent studies evaluated pericoronary fat radiodensity among men and women with suspected CVD and found that the radiodensity varied with distance from the coronary lumen and between distal and proximal arteries, suggesting that fat characteristics, and possibly quality, differ even within the EAT depot.37, 39 Second, our vascular calcification measures differed in categorization (e.g., CAC ≥ 10 vs CAC ≥ 100 vs. continuous CAC) and arterial bed (e.g., calcification within the abdominal aorta).13, 25 Third, our study population included generally healthy Black and White women with no history of cardiovascular disease, while the other studies included both men and women in a White population, participants with suspect cardiovascular disease, or unhealthy women with an autoimmune disease associated with chronic inflammation.15 Lastly, we chose to categorize fat radiodensity into tertiles to provide a unique look at the non-linear relationship between fat radiodensity and vascular calcification.
We hypothesized that lower cardiovascular fat radiodensity would be associated with a less favorable cardiovascular profile for both fat depots. This hypothesis was based on current literature suggesting that lower CT radiodensity may represent lipid-laden hypertrophic adipocytes thought to secrete higher amounts of pro-inflammatory substances and increased levels of free fatty acids.22, 40, 41 In addition, as adipose tissue expands beyond a certain threshold, a lack of angiogenesis may cause hypoxic conditions within the adipose tissue resulting in hypoxia.42 Some studies have shown that capillary density and blood flow are reduced in obesity18 and that hypertrophic adipocytes may increase to a size too large for oxygen to diffuse the distance to the adipocyte mitochondria.17, 42, 43 A hypoxic state exacerbates an inflammatory response, including macrophage accumulation, insulin resistance, and other adverse cardiometabolic characteristics.17, 43, 44 To further complicate our understanding of fat radiodensity, adipose tissue with higher lipid content, hypertrophic cells, and lower capillary density, characteristics of low radiodensity, may, in fact, promote adipose tissue fibrosis.18, 21, 43 Fibrosis may cause adipose tissue dysfunction and insulin resistance43 and is represented by higher CT radiodensity values, which could convolute the associations between fat radiodensity and calcification.13, 15 Given the contradicting radiodensity findings across studies, it may be that this area of investigation is now uncovering a U-shaped association between fat radiodensity and subclinical cardiovascular disease manifestations such as vascular calcification. Perhaps the extremes of low and high radiodense cardiovascular adipose tissue both represent low quality adipose tissue: 1) high lipid white fat (for the lower end) and 2) dense fibrotic fat (for the upper end), which may also be dependent on sex, age, and disease state. Future studies with larger sample sizes should assess this possibility.
Strengths and limitations
Our study has some limitations including the cross-sectional observational study design preventing assessment of temporality, introducing possible unmeasured or residual confounders, and providing a level of uncertainty regarding casual effect reversal. Our study was limited to healthy midlife women of Black or White race and may not be generalizable to the extended population. AC was quantified along the descending thoracic and abdominal aorta, while PVAT was assessed along the descending thoracic aorta, which may have contributed to our null findings between PVAT radiodensity and AC. Although the current analyses of PVAT were not adjusted for aorta length, our previous work comparing aorta length between a lupus population and their age-, race-matched controls did not show any differences.6 Previously, we found greater EAT volumes were significantly associated with CAC, and PAT volumes were associated with CAC dependent on menopausal status and estradiol levels.13 In this work, we found no association between EAT radiodensity and CAC; however, TAT is the sum of EAT and PAT and we suspect that the PAT portion may be driving the TAT association with CAC. Since our PAT volume was derived from TAT and EAT volumes, PAT radiodensity could not be directly assessed. This study has several strengths, including being the first to evaluate surrogate markers of fat quality for TAT and PVAT in midlife women along with providing additional information for understanding the use of radiodensity as a surrogate marker of fat quality. We also utilized the well-established, community-based SWAN study to assess the CVD risk factors, menopausal status, and subclinical atherosclerosis.
Conclusions
We found that midlife women with lower TAT and PVAT radiodensity values tended to have a less favorable cardiovascular profile. TAT was more strongly associated with CVD risk factors than PVAT and women with mid-range TAT radiodensity values had a lower odds of CAC presence. Future studies with a larger sample size should evaluate these associations across fat volume strata. Longitudinal studies evaluating the change in radiodensity may help determine whether low radiodensity fat becomes fibrotic over time.
Supplementary Material
Highlights.
-
•
Lower TAT and PVAT radiodensities are associated with a less favorable CVD risk profile in midlife women.
-
•
Women with mid-range TAT radiodensity have lower odds of CAC presence independent of risk factors and BMI.
-
•
Our study showed PVAT radiodensity is not related to CAC or AC in women at midlife.
Acknowledgements
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Financial support
This work was supported by financial support: The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). SWAN Heart was supported by the National Heart, Lung, and Blood Institute (NHLBI) (Grants HL065581, HL065591).
The SWAN Cardiovascular Fat Ancillary Study was supported by an award from the American Heart Association (AHA) Great River Affiliation Clinical Research Program: 12CRP11900031. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
Conflicts of Interest
Dr. Hanley has nothing to disclose; Dr. Shields has nothing to disclose, Dr. El Khoudary reports grants from AHA, during the conduct of the study; Dr. Matthews has nothing to disclose; Dr. Brooks reports grants from Gilead Science Inc, outside the submitted work; Dr. Janssen reports grants from NIH, during the conduct of the study; Dr. Budoff reports grants from NIH, during the conduct of the study; grants from GE, outside the submitted work; Dr. Sekikawa has nothing to disclose; Dr. Mulukutla has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kershaw EE and Flier JS, Adipose tissue as an endocrine organ, The Journal of clinical endocrinology and metabolism, 2004;89:2548–2556. [DOI] [PubMed] [Google Scholar]
- [2].Yudkin JS, Eringa E and Stehouwer CD, “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease, Lancet, 2005;365:1817–1820. [DOI] [PubMed] [Google Scholar]
- [3].Iacobellis G and Barbaro G, The double role of epicardial adipose tissue as pro- and anti-inflammatory organ, Horm Metab Res, 2008;40:442–445. [DOI] [PubMed] [Google Scholar]
- [4].Ahmadi N, Nabavi V, Yang E, Hajsadeghi F, Lakis M, et al. , Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium, Acad Radiol, 2010;17:1518–1524. [DOI] [PubMed] [Google Scholar]
- [5].Bettencourt N, Toschke AM, Leite D, Rocha J, Carvalho M, et al. , Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden, Int J Cardiol, 2012;158:26–32. [DOI] [PubMed] [Google Scholar]
- [6].Shields KJ, Barinas-Mitchell E, Gingo MR, Tepper P, Goodpaster BH, et al. , Perivascular adipose tissue of the descending thoracic aorta is associated with systemic lupus erythematosus and vascular calcification in women, Atherosclerosis, 2013;231:129135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jain A, McClelland RL, Polak JF, Shea S, Burke GL, et al. , Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: the multi-ethnic study of atherosclerosis (MESA), Circulation. Cardiovascular imaging, 2011;4:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, et al. , Absence of coronary artery calcification and all-cause mortality, JACC. Cardiovascular imaging, 2009;2:692700. [DOI] [PubMed] [Google Scholar]
- [9].Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, et al. , Calcified atherosclerosis in different vascular beds and the risk of mortality, Arteriosclerosis, thrombosis, and vascular biology, 2012;32:140–146. [DOI] [PubMed] [Google Scholar]
- [10].El Khoudary SR, Shields KJ, Janssen I, Hanley C, Budoff MJ, et al. , Cardiovascular Fat, Menopause, and Sex Hormones in Women: The SWAN Cardiovascular Fat Ancillary Study, The Journal of clinical endocrinology and metabolism, 2015;100:33043312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].El Khoudary SR, Shields KJ, Janssen I, Budoff MJ, Everson-Rose SA, et al. , Postmenopausal Women With Greater Paracardial Fat Have More Coronary Artery Calcification Than Premenopausal Women: The Study of Women’s Health Across the Nation (SWAN) Cardiovascular Fat Ancillary Study, Journal of the American Heart Association, 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gorodeski GI, Impact of the menopause on the epidemiology and risk factors of coronary artery heart disease in women, Experimental gerontology, 1994;29:357–375. [DOI] [PubMed] [Google Scholar]
- [13].Alvey NJ, Pedley A, Rosenquist KJ, Massaro JM, O’Donnell CJ, et al. , Association of fat density with subclinical atherosclerosis, Journal of the American Heart Association, 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, et al. , Visceral and subcutaneous fat quality and cardiometabolic risk, JACC. Cardiovascular imaging, 2013;6:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shields KJ, El Khoudary SR, Ahearn JM and Manzi S, Association of aortic perivascular adipose tissue density with aortic calcification in women with systemic lupus erythematosus, Atherosclerosis, 2017;262:55–61. [DOI] [PubMed] [Google Scholar]
- [16].Veilleux A, Caron-Jobin M, Noel S, Laberge PY and Tchernof A, Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women, Diabetes, 2011;60:1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, et al. , Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation, Diabetes, 2007;56:901911. [DOI] [PubMed] [Google Scholar]
- [18].Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, et al. , Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response, Diabetes, 2009;58:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, et al. , Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects, Arteriosclerosis, thrombosis, and vascular biology, 2008;28:1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahmadi N, Hajsadeghi F, Conneely M, Mingos M, Arora R, et al. , Accurate detection of metabolically active “brown” and “white” adipose tissues with computed tomography, Acad Radiol, 2013;20:1443–1447. [DOI] [PubMed] [Google Scholar]
- [21].Hu HH, Chung SA, Nayak KS, Jackson HA and Gilsanz V, Differential computed tomographic attenuation of metabolically active and inactive adipose tissues: preliminary findings, Journal of computer assisted tomography, 2011;35:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Baba S, Jacene HA, Engles JM, Honda H and Wahl RL, CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies, Journal of nuclear medicine : official publication, Society of Nuclear Medicine, 2010;51:246–250. [DOI] [PubMed] [Google Scholar]
- [23].Rosenquist KJ, Massaro JM, Pedley A, Long MT, Kreger BE, et al. , Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality, The Journal of clinical endocrinology and metabolism, 2015;100:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Therkelsen KE, Pedley A, Rosenquist KJ, Hoffmann U, Massaro JM, et al. , Adipose tissue attenuation as a marker of adipose tissue quality: Associations with six-year changes in body weight, Obesity, 2016;24:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pracon R, Kruk M, Kepka C, Pregowski J, Opolski MP, et al. , Epicardial adipose tissue radiodensity is independently related to coronary atherosclerosis. A multidetector computed tomography study, Circulation journal : official journal of the Japanese Circulation Society, 2011;75:391–397. [DOI] [PubMed] [Google Scholar]
- [26].Franssens BT, Nathoe HM, Leiner T, van der Graaf Y, Visseren FL, et al. , Relation between cardiovascular disease risk factors and epicardial adipose tissue density on cardiac computed tomography in patients at high risk of cardiovascular events, Eur J Prev Cardiol, 2017;24:660–670. [DOI] [PubMed] [Google Scholar]
- [27].Sower M CS, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J, SWAN: a multicenter, multiethnic, community-based cohort study of owmen and the menopausal transition , In: Lobo RA KJ, Marcus R (ed), Menopause: Biology and Pathobiology, New York, NY, Academic Press, 2000:175–188. [Google Scholar]
- [28].Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., et al. , Quantification of coronary artery calcium using ultrafast computed tomography, Journal of the American College of Cardiology, 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- [29].Sutton-Tyrrell K, Kuller LH, Edmundowicz D, Feldman A, Holubkov R, et al. , Usefulness of electron beam tomography to detect progression of coronary and aortic calcium in middle-aged women, The American journal of cardiology, 2001;87:560–564. [DOI] [PubMed] [Google Scholar]
- [30].Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, et al. , Sexhormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN), Circulation, 2005;111:1242–1249. [DOI] [PubMed] [Google Scholar]
- [31].Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, et al. , Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes, Diabetes Care, 2001;24:362–365. [DOI] [PubMed] [Google Scholar]
- [32].Kaczmarska E, Kepka C, Dzielinska Z, Pracon R, Kryczka K, et al. , What is the optimal cut-off point for low coronary artery calcium score assessed by computed tomography? Multi-Detector Computed Tomography ANIN Registry, Postepy w kardiologii interwencyjnej = Advances in interventional cardiology, 2013;9:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].El-Saed A, Curb JD, Kadowaki T, Okamura T, Sutton-Tyrrell K, et al. , The prevalence of aortic calcification in Japanese compared to white and Japanese-American middle-aged men is confounded by the amount of cigarette smoking, Int J Cardiol, 2013;167:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee JJ, Pedley A, Hoffmann U, Massaro JM, Keaney JF Jr., et al. , Cross-Sectional Associations of Computed Tomography (CT)-Derived Adipose Tissue Density and Adipokines: The Framingham Heart Study, Journal of the American Heart Association, 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lu MT, Park J, Ghemigian K, Mayrhofer T, Puchner SB, et al. , Epicardial and paracardial adipose tissue volume and attenuation - Association with high-risk coronary plaque on computed tomographic angiography in the ROMICAT II trial, Atherosclerosis, 2016;251:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mahabadi AA, Balcer B, Dykun I, Forsting M, Schlosser T, et al. , Cardiac computed tomography-derived epicardial fat volume and attenuation independently distinguish patients with and without myocardial infarction, PLoS One, 2017;12:e0183514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Marwan M, Hell M, Schuhback A, Gauss S, Bittner D, et al. , CT Attenuation of Pericoronary Adipose Tissue in Normal Versus Atherosclerotic Coronary Segments as Defined by Intravascular Ultrasound, Journal of computer assisted tomography, 2017;41:762–767. [DOI] [PubMed] [Google Scholar]
- [38].Iacobellis G and Bianco AC, Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features, Trends Endocrinol Metab, 2011;22:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hell MM, Achenbach S, Schuhbaeck A, Klinghammer L, May MS, et al. , CT-based analysis of pericoronary adipose tissue density: Relation to cardiovascular risk factors and epicardial adipose tissue volume, J Cardiovasc Comput Tomogr, 2016;10:52–60. [DOI] [PubMed] [Google Scholar]
- [40].Koutsari C and Jensen MD, Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity, Journal of lipid research, 2006;47:1643–1650. [DOI] [PubMed] [Google Scholar]
- [41].Skurk T, Alberti-Huber C, Herder C and Hauner H, Relationship between adipocyte size and adipokine expression and secretion, The Journal of clinical endocrinology and metabolism, 2007;92:1023–1033. [DOI] [PubMed] [Google Scholar]
- [42].Trayhurn P, Hypoxia and adipose tissue function and dysfunction in obesity, Physiological reviews, 2013;93:1–21. [DOI] [PubMed] [Google Scholar]
- [43].Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, et al. , Hypoxiainducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue, Molecular and cellular biology, 2009;29:4467–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE Jr., et al. , Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance, The Journal of clinical endocrinology and metabolism, 2011;96:E1990–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


