Abstract
Purpose:
PD-1 blockade induces durable responses in patients with metastatic melanoma and prolongs relapse-free survival in patients with resected melanoma; however, current biomarkers do not consistently associate with patient responses. In this study we investigated the impact of nivolumab therapy on peripheral blood Tregs and its relation to patient outcomes.
Experimental Design:
Peripheral blood Tregs and conventional CD4+ T-cells from patients with resected high-risk melanoma treated with adjuvant nivolumab were assessed for gene expression changes by RNA-seq. Percentages of circulating Tregs and phosphorylated-STAT3 (pSTAT3) expression levels were assessed by flow cytometry and validated in an independent cohort of active disease patients. Suppressive function of Tregs was assessed in allogeneic mixed lymphocyte reactions.
Results:
Tregs from non-relapse patients had increased expression of proliferation associated genes. An increase in the proportion of circulating Tregs and pSTAT3 expression and a reduction in Treg suppressive capacity were observed in non-relapsing, but not relapsing patient samples 13 weeks after starting treatment. In vitro blockade of PD-1 increased Treg percentages and pSTAT3 expression, and reduced Treg suppressive function. PD-1 blockade also led to IL-10 production by T-cells, resulting in higher Treg proliferation. The addition of a STAT3 inhibitor ameliorated the increase in Tregs, enhanced suppressive function, and decreased T-cell IL-10 production in vitro.
Conclusions:
These results demonstrate that induction of pSTAT3, reduced suppressive function, and a paradoxical increase in Treg proliferation are novel correlates of patient benefit from PD-1 blockade.
Keywords: STAT3, Nivolumab, Tregs, Melanoma, Immunotherapy, Proliferation, PD-1, IL-10
Introduction
PD-1 blocking antibodies (e.g. nivolumab) have been effective in the treatment of a variety of malignancies. Nivolumab treatment of unresectable metastatic melanoma has demonstrated response rates up to ~40% (1) and efficacy as adjuvant therapy for surgically resected metastatic melanoma (2). However, not all patients benefit from PD-1 blockade and biomarkers associated with patient outcome are unclear.
Ligation of PD-1 expressing T-cells to PDL1 or PDL2 results in phosphorylation of SHP1/2 phosphatases, leading to ZAP70 dephosphorylation, RAS and PI3K signaling inhibition, BAFT activation, disruption of TCR and CD28 signaling, and, consequently, inhibition of T-cell activation, proliferation and effector functions (3–6). PDL1 expression by melanoma cells is associated with poor prognosis (7). Tumor PDL1 expression has been shown to be a biomarker of response to PD-1 blockade (8), but studies have shown that patient benefit may still occur for PDL1 negative tumors (9).
Regulatory T-cells (Tregs) are a heterogeneous population of T-cells responsible for maintaining self-tolerance (10), and have been shown to inhibit anti-tumor immune responses. Tregs function through a variety of mechanisms including production of immunosuppressive cytokines, downregulation of co-stimulation by antigen presenting cells, and direct killing of effector T-cells (11,12). Naturally occurring Tregs (nTregs) develop in the thymus and are characterized as CD4+CD127low/−CD25+/FOXP3+ (13). Conventional CD4+ T-cells (Tcons) can also acquire induced regulatory function which are termed iTregs or adaptive Tregs (13). TGFβ and IL-10 produced in the tumor microenvironment promote the expansion of nTregs and conversion of Tcons into iTregs (14,15). Tregs are overrepresented in the peripheral blood of metastatic melanoma patients (16), and increased ratios of Tregs to CD8+ T-cell tumor infiltrate correlate with reduced survival (17).
In ipilimumab treated patients, higher baseline levels of circulating Tregs are associated with improved survival (18). However, the importance of Tregs in patient response to PD-1 blockade remains unclear. Previous work from our group showed that PD-1 blockade increased resistance of effector T-cells to Treg suppression and directly reduced the suppressive function of Tregs in vitro (19). Herein, we sought to address the role of Tregs in resected metastatic melanoma patients after adjuvant treatment with the PD-1 blocking antibody nivolumab.
Materials and Methods
Patient Samples
PBMC were harvested from leukapheresis of metastatic or resected melanoma patients enrolled in clinical trials evaluating nivolumab therapy (clinicaltrials.gov NCT01176474(2), NCT01176461(9), NCT01783938(43)), purified through Ficoll Histopaque density gradient (GE Healthcare, Pittsburg, PA), and cryopreserved. All patients provided written informed consent, and studies were conducted in accordance with good clinical practice and the Declaration of Helsinki. All protocols were approved by the Institutional Review Board at Moffitt Cancer Center and New York University Langone Medical Center. Samples were coded with an anonymized number and ex vivo analyses of patient samples were performed blinded. For all in vitro assays, baseline metastatic melanoma patient samples were utilized, except in nTreg suppression assays.
Reagents
Recombinant human IL-2 (Aldesleukin, Prometheus Laboratories, San Diego, CA) was commercially obtained. Recombinant human IL-10 and TGFβ were purchased from R&D Systems (Minneapolis, MN). Stattic and rapamycin were obtained from Selleckchem (Houston, TX). Reagents were prepared per the manufacture, stored at −80°C and thawed immediately prior to use. αPD-1, αCD3, αCD28 and IgG antibodies were obtained from Biolegend (San Diego, CA). αPDL1 antibody was purchased from BioXCell (West Lebanon, NH). Nivolumab was provided by Bristol-Myers Squibb (New York, NY). Antibodies were stored at 4°C.
Treg Suppression Assays
In experiments assessing Treg suppression, Tregs (CD3+CD4+CD127−/lowCD25+) from baseline and week 13 post-nivolumab therapy were flow sorted to >98% purity from patient PBMC and co-cultured with allogeneic negatively-isolated CD8+ T-cells (EasySep kit, StemCell Technologies; Vancouver, Canada) and irradiated PBMC. After five days, cells were pulsed with 1μCi/well of tritiated thymidine for 18 hours and assessed for radioactive incorporation. Suppression was normalized against control cultures containing no Tregs (100% proliferation). Technical control wells contained only PBMC and Treg.
To assess PD-1/PDL1 blockade and STAT3 inhibition in vitro, nTregs were purified from healthy human donor PBMC by magnetic bead separation based on CD4 and CD25 expression to ~90% purity (Miltenyi, Auburn, CA). Isolated nTregs were expanded with Dynabeads Human T-Activator CD3/CD28 for T-Cell Expansion and Activation (ThermoFisher, Waltham, MA) at a nTreg to bead ratio of 1:1. RPMI culture medium was supplemented with 10% heat inactivated pooled human serum and recombinant human IL-2. Stattic (200nM), αPD-1 antibody (5ug/ml), αPDL1 (10ug/mL), DMSO (<0.01%), or IgG were added once on day 0 of expansion. After 3 days, nTregs were washed and plated with autologous CFSE labeled T-cells at 1:30 ratio (Treg:T-cell) plus CD3/CD28 beads at 1:30 ratio (beads:T-cell). T-cell proliferation was assessed by CFSE dilution after 3 days of culture using a FACSCANTO II flow cytometer (BD Biosciences, Franklin Lakes, NJ).
iTregs were generated by isolating naïve CD4+ T-cells from baseline patient PBMC using an EasySep kit, and culturing in a 10ug/mL OKT3 αCD3 antibody coated-plate plus 100nM rapamycin, 10ng/mL TGFβ, 300IU/mL IL-2, and 2ug/mL CD28.2 αCD28 antibodies. IgG, αPD-1, αPDL1 antibodies or stattic treatments were added as indicated. After seven days, cells were washed, counted and co-cultured at indicated ratios with autologous CD8+ T-cells (EasySep negative isolation) and 1:10 CD3/CD28 Dynabeads (bead:CD8+). After three days, Ki67 expression was assessed by flow cytometry. Values relative to Ki67 expression in CD8+ only groups (i.e. no Tregs) were graphed. In some experiments, supernatants were frozen for further cytokine evaluation by Luminex (Austin, TX).
RNA-Sequencing & Gene Set Enrichment Analysis
RNA-Seq was performed using the NuGen Ovation Encore Complete kit. Briefly, RNA was extracted from sorted T-cells using the Qiagen RNEasy Mini Kit, and 100 ng of RNA was used to generate cDNA and a strand-specific library following the manufacturer’s protocol (NuGEN Technologies, Inc., San Carlos, CA). The libraries were then sequenced on the Illumina NextSeq 500 v2 sequencer with a 75-base paired-end run in order to generate 30–40 million read pairs per sample. Sequence reads were aligned to the human genome in a splice-aware fashion using Tophat2(44). Aligned reads were then condensed into transcripts for calculation of differential expression at both the gene and transcript levels with the Cufflinks package(45). Transcript mapped to a single unique known gene, q<0.05 and a known log2 fold change was considered significant when comparing two groups.
Gene set enrichment was done using the MSigDB(46) web interface querying the HALLMARK(22) gene sets. Top ten gene sets with an FDR q-value <0.05 were used.
Flow Cytometry
Surface staining was performed in FACS buffer (PBS, 2mM EDTA, 2% fetal bovine serum), for 30 minutes at 4°C. Antibodies were obtained from BD Biosciences, Biolegend, eBioscience (San Diego, CA), or Cell Signaling (Danvers, MA). Cell viability was assessed by DAPI (50ng/mL). The FOXP3 transcription factor staining kit (eBioscience) was used for intracellular staining, and viability determined by Live Dead amine reactive dyes (ThermoFisher; Waltham, MA). LSR II (BD Biosciences) or Attune NxT (ThermoFisher) flow cytometers, and FlowJo v10 software (Ashland, Oregon) were used for data acquisition and analyses. Flow sorting of Tregs was performed in a FACSAria flow sorter (BD Biosciences). Gating strategy was based on viable CD4+CD25+CD127- cells (see Supplemental Figure 1) with a dump channel excluding CD8+, CD19+ and CD56+ cells.
For survival correlation data, percent change in pSTAT3 expression was calculated as: (week 13 gMFI - baseline gMFI)/baseline gMFI * 100%.
In Vitro Assays
CD3+ T-cells were negatively isolated using an EasySep kit, and cultured in X-VIVO media, supplemented with 10% AB human serum, L-glutamine, beta-mercaptoethanol, non-essential amino acids, HEPES, penicillin, streptomycin, gentamicin and 100IU/mL IL-2.
Supernatant cytokine levels were assessed by Luminex assays from R&D Systems (Minneapolis, MN).
Statistics
Statistical analyses were performed using GraphPad Prism 7 software. Type-two, two tailed t-tests were used for comparisons of two groups. One-way ANOVA were used for comparisons of three or more groups. Paired, two-tailed, t-tests were used to determine statistical significance of pre- versus post-treatment. Correlation significance and R2 was assessed using linear regression. P-values of ≤0.05 were considered significant.
Results
Changes in Gene Expression After Nivolumab Therapy Differ Based on Patient Outcome and T-cell Subset
To gain insight into the effects of nivolumab treatment on Tregs and conventional T-cells, CD14-CD56-CD19-CD3+CD4+CD127low/−CD25+ (Tregs (20,21)) and the remainder of the CD4+ population (Tcons) were flow sorted from pre- and post- adjuvant nivolumab treatment samples. Paired samples from peripheral blood mononuclear cells (PBMC) (baseline and week 13 of treatment) of seven patients free of disease (no evidence of disease [NED] at four years post-resection) and seven relapsing patients were assessed by RNA-Seq (Supplemental Figure 1A). Patient demographics and experimental values are reported in Supplemental Table 1. Changes in gene expression (post-treatment compared to baseline) were assessed for each patient and significantly changed genes (FDR corrected q≤0.05) determined for Tregs and Tcons. As staining for the intracellular marker FOXP3 is not compatible with retaining viable cells for downstream assessment, potential differences in expression of FOXP3 in NED and relapsing patient Tregs were evaluated in a separate experiment. No significant differences in FOXP3 expression were noted in baseline (Supplemental Figure 1B, p=0.385) or post-treatment (Supplemental Figure 1C, p=0.289) between NED and relapsing patients.
In Tcons, 189 genes were significantly changed in NED samples and 227 in relapsing patient samples (Supplemental Figure 1D). Seven genes overlapped between NED and relapse Tcons with an additional nine genes changed in both groups, but in opposing directions (Supplemental Table 2). In Tregs, 382 genes were significantly changed in NED samples and 256 in relapsing patients. Twenty-two genes overlapped between NED and relapse Tregs with an additional five genes changing in both groups in opposing directions (Supplemental Figure 1D, Supplemental Table 3). We next compared the overlap in significantly changed genes between Tcons and Tregs. In NED patient samples, four genes overlapped between Tcons and Tregs with an additional 18 overlapping but in opposing directions (Supplemental Figure 1D, Supplemental Table 4). In relapsing patient samples, 46 genes overlapped with an additional two overlapping but in opposing directions (Supplemental Figure 1D, Supplemental Table 5).
Non-relapsing patient Tregs have increased expression of proliferation-associated genes post-nivolumab.
The biological processes associated with these genes were assessed using gene-set enrichment analysis (GSEA) referenced to hallmark gene sets (22) (Supplemental Figure 1E). Analysis of Tcons showed upregulation of IFNγ response genes in both NED (q=1.74e-02) and relapsed (q=1.51e-07) patients (Supplemental Figure 1E). In Tregs, upregulation of IFNγ response hallmark was observed in relapsed (q=3.42e-09), but not NED patients (Supplemental Figure 1F). Oxidative phosphorylation (q=6.51e-05) was uniquely downregulated in NED Tregs, in contrast to the upregulation seen in NED Tcons (q=2.60e-02). Heme metabolism associated genes were downregulated in relapse Tregs (q=1.78e-03) and Tcons (q= 3.93e-02), in contrast to being upregulated in NED Tcons (q=2.60e-02) but not in NED Tregs. Proliferation associated hallmarks including G2M checkpoint (q=2.62e-59), E2F targets (q=1.34e-43) and mitotic spindle (q=7.21e-19) were only upregulated in NED Tregs. GSEA hallmark analysis of NED Tcon downregulated genes returned no results. These results suggest that the effects of nivolumab therapy on Tcons and Tregs gene expression are distinct.
Patients benefiting from adjuvant nivolumab therapy display an increase in circulating Tregs post-treatment.
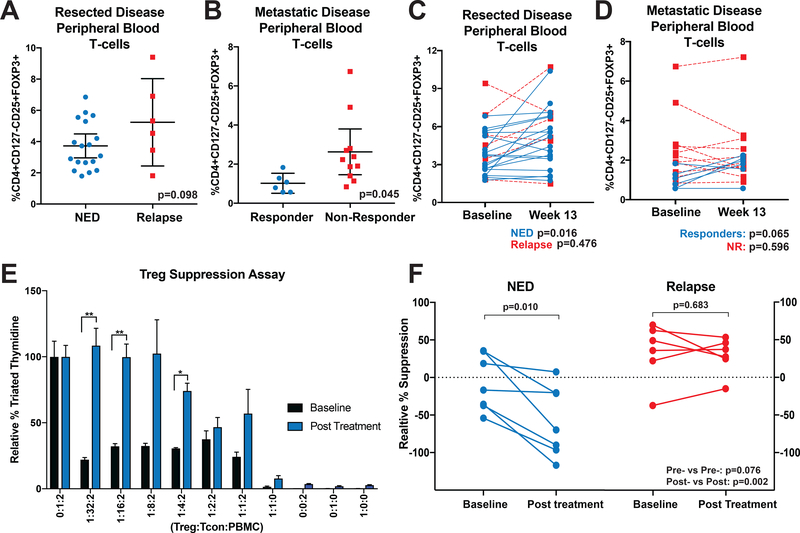
Given the observation that proliferation associated genes were upregulated in NED Tregs and that increased levels of circulating Tregs are associated with response to ipilimumab (18), we next assessed percentages of circulating Tregs by flow cytometry. The proportion of Tregs (CD4+CD25+CD127low/−FOXP3+) in relapsed compared to NED patients at baseline was not significantly different (p=0.098; Figure 1A) (NED n=18, relapsed n=6).
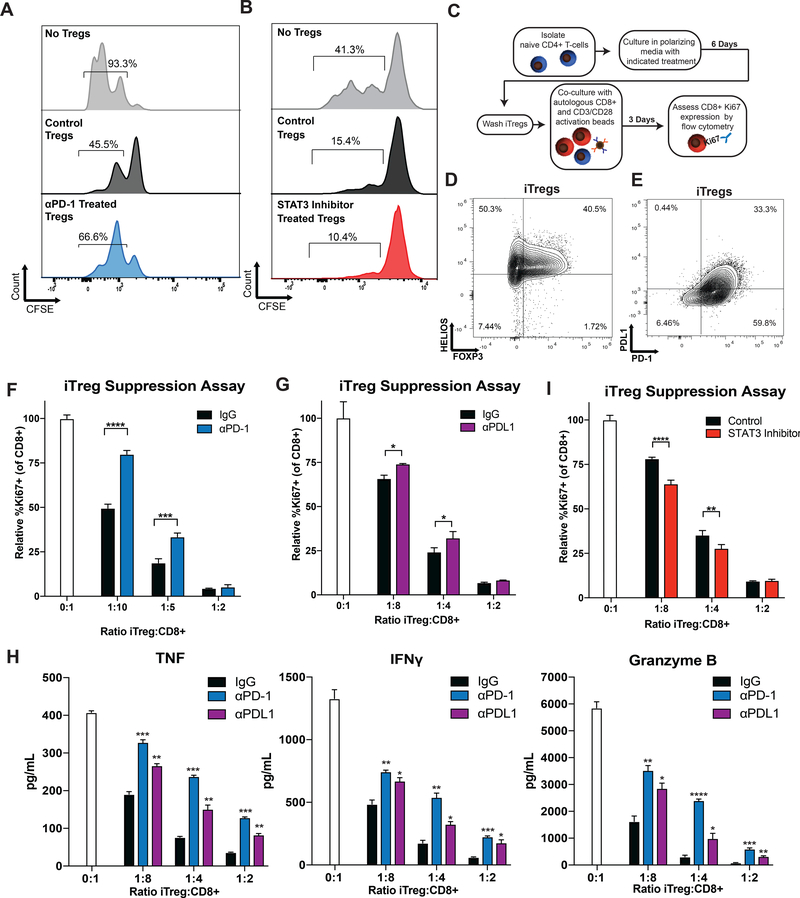
Figure 1. Nivolumab Therapy Reduces CD4+CD127low/−CD25+ T-cell Suppressive Function in Non-Relapsing Patients.
(A) Patient baseline PBMC samples were assessed by flow cytometry for Tregs (CD4+CD127-/lowCD25+FOXP3+) as a percentage of total CD3+ T-cells. (B) PBMC samples from patients with active disease treated with nivolumab were assessed as in (A). Error bars show mean with 95% confidence intervals. (C-D) Post-treatment samples from (C) resected disease patients and (D) active disease patients were likewise assessed. Paired patient samples are plotted with connecting lines. NED or responding patients are represented with blue lines and relapsing or non-responding patients with red, dotted lines. (E) Baseline (black bars) and after 13 weeks of nivolumab therapy (blue bars) patient PBMC CD4+CD127low/−CD25+ T-cells were isolated and co-cultured with allogeneic CD8+ T-cells and irradiated PBMC at indicated ratios. After 5 days, tritiated thymidine was added to cultures for a further 18 hours and cultures assessed for radioactive incorporation. Suppression was normalized against control cultures containing no CD4+CD127low/−CD25+ T-cells (100% proliferation). *p<0.05, **p<0.01. Error bars show standard error of the mean (SEM). (F) Additional patient CD4+CD127low/−CD25+ T-cells were likewise assessed for suppression at a 1:2:2 ratio. Patients are grouped based on outcome with intra-patient pre-treatment and post-treatment samples connected by solid lines. P-values are reported in graphs for paired analyses (above group plots) and for comparisons based on response (on bottom right of graph).
PBMC samples from patients with unresectable metastatic melanoma treated with nivolumab were likewise assessed for Tregs as a percentage of live cells. Unresectable disease patient demographics and experimental values are reported in Supplemental Table 6. As shown in Figure 1B, a significantly increased representation of Tregs was found in non-responding relative to responding patients (p=0.045).
The proportion of Tregs at week 13 of treatment with nivolumab was next assessed. No significant differences were found in the post-treatment levels of Tregs at week 13 for adjuvant treated (p=0.396), or active disease patients (p=0.324). However, in paired analyses NED patients displayed increased percentage of Tregs post-nivolumab (ratio paired t-test, n=18, p=0.016, ∆x̅=1.24%), but relapsing patients did not show a significant change (n=6, p=0.476) (Figure 1C). PBMC samples from active disease patients were likewise assessed for changes in the percentage of circulating Tregs. As shown in Figure 1D, responding patients had a borderline-significant increase in Treg percentages at week 13 after initiation of nivolumab treatment (n=6, p=0.065), while non-responding patients did not (n=11, p=0.596).
Tregs from non-relapsing patients have reduced suppressive function.
We next evaluated if Treg suppression was associated with patient outcomes. Tregs (CD4+CD127low/−CD25+) from resected disease patient samples were assessed in an allogeneic mixed lymphocyte reaction. Tregs were flow sorted (gating strategy shown in Supplemental Figure 2) from pre- and post-treatment (week 13) patient PBMC, then cultured with allogeneic antigen presenting cells (APCs) and CD8+ T-cells. Tritiated thymidine incorporation as an indicator of T-cell proliferation was assessed at different ratios of Tregs:CD8+:APCs. Tritiated thymidine counts were normalized to controls containing no Tregs (i.e. 0:1:2 ratio) for baseline and week 13 treatment independently. Suppressive function was assessed in seven adjuvant NED patients and six patients who had relapsed (representative assay shown in Figure 1E). Percent suppression, relative to CD8+ only cultures, at a ratio of 1:2:2 is shown in Figure 1F. While not significant (p=0.076), NED patients trended towards decreased levels of suppression at baseline relative to relapsed patients. Significantly reduced suppression in NED relative to relapsed was observed in week 13 samples (p=0.002). Paired analyses also showed a significant decrease in suppression from post-nivolumab Tregs of NED patients (p=0.010), but no changes in relapsed patient samples (p=0.683).
Tregs from patients benefiting from nivolumab have increased pSTAT3 expression post-treatment.
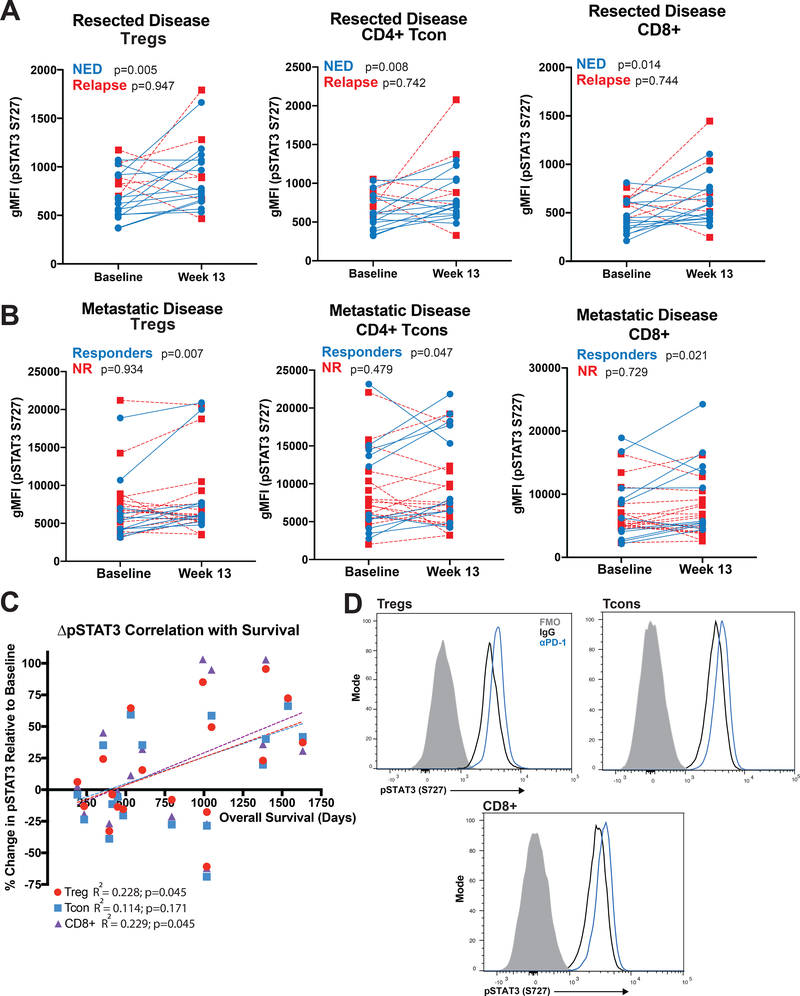
Previous studies have shown that Treg proliferation and function are regulated in part by STAT3 signaling (23,24). Therefore, we evaluated expression of pSTAT3 in PBMC samples from surgically resected, stage III/IV melanoma patients receiving adjuvant nivolumab. Circulating Tregs (CD4+CD25+CD127low/−FOXP3+) from NED patients displayed an increased percentage of pSTAT3 expressing cells at week 13 relative to baseline (Supplemental Figure 3C, 12/14 patients; p=0.004). This was not seen in relapsing patient samples (n=6, p=0.736). To determine if this effect was general to T-cells, we likewise evaluated Tcons and CD8+ T-cells from NED patients. Tcons had significant upregulation of pSTAT3 after nivolumab treatment (Supplemental Figure 3B, p=0.015) as did CD8+ T-cells (p=0.029). Of these populations, Tregs displaying the highest average change from baseline (∆x̅=11.7%). pSTAT3 geometric mean fluorescence intensity was similarly elevated post-treatment in Tregs (p=0.005), CD4+ Tcon (p=0.008) and CD8+ T-cells (p=0.014) from NED patients (Figure 2A). No significant changes were seen in any of these populations in relapsed patients.
Figure 2. PD-1 Blockade Increases Expression of Phosphorylated STAT3 in T-cells.
(A) Paired PBMC samples (baseline and week 13 post-nivolumab treatment initiation) from patients treated with adjuvant nivolumab were assessed by flow cytometry and the geometric mean fluorescent intensity (gMFI) of pSTAT3 S727 determined in Tregs (CD4+CD127-/lowCD25+FOXP3+), CD4+ Tcons and CD8+ T-cells. Blue lines connect paired NED patient samples and red, dotted lines connect relapsed patient samples. (B) PBMC samples from active metastatic disease patients treated with nivolumab were likewise assessed. (C) Changes in pSTAT3 intensity of PBMC Tregs (red circles), CD4+ Tcons (blue squares) and CD8+ T-cells (purple triangles) in metastatic disease patients are plotted in relation to overall patient survival. (D) CD3+ T-cells were isolated from baseline patient PBMC and cultured for 48 hours in the presence of IgG (black line histogram) or PD-1 blocking antibodies (blue line histogram), then assessed by flow cytometry for expression of pSTAT3 S727 in CD4+CD127-/lowCD25+ Tregs, CD4+ Tcons and CD8+ T-cells. Fluorescence minus one (FMO) histograms are shown in solid gray.
pSTAT3 expression was also assessed in circulating Tregs from unresectable, stage III/IV metastatic melanoma patients treated with nivolumab. Responding patients had an increase in Treg pSTAT3 levels (9/11 patients increased, p=0.007), while Tregs from patients with progressive disease did not display changes in pSTAT3 expression (n=17, p=0.934) (Figure 2B). Similar increases in pSTAT3 were seen in responding patients’ Tcons (p=0.047) and CD8+ T-cells (p=0.021), but was not observed in non-responders. We next evaluated the association between changes in Treg pSTAT3 expression and overall survival in metastatic patients receiving nivolumab therapy. Increased survival in patients with metastatic disease was positively correlated with increases in Treg (R2=0.228, p=0.045) and CD8+ T-cell (R2=0.229, p=0.045) pSTAT3 expression (Figure 2C).
PD-1 blockade enhances pSTAT3 expression in T-cells in vitro.
To determine whether the observed increases in T-cell pSTAT3 expression were a direct effect of PD-1 blockade, T-cells isolated from baseline PBMC samples of resected disease patients were cultured for 48 hours with the addition of PD-1 blocking antibody or IgG control. Tregs, Tcons and CD8+ T-cells from PD-1 blockade cultures had enhanced pSTAT3 expression relative to IgG (Figure 2D, Supplemental Figure 6A). These results suggest that blocking PD-1 increases T-cell pSTAT3 expression independent of interactions with an intermediate cell population (e.g. APC or tumor).
Patient T-cells express PD-L1.
Given the ability of αPD-1 to upregulate T-cell pSTAT3 in purified T-cell cultures, we hypothesized that T-cells may express PDL1. Baseline patient T-cells were cultured in vitro for 72 hours with or without the addition of CD3/CD28 activation. PDL1 expression was detected in activated T-cells with 28% of Tregs, 13% of Tcons and 10% of CD8+ expressing PDL1 in the representative flow plots shown in Supplemental Figure 4A.
We next evaluated Tregs (CD4+CD127-CD25+) from a small number of resected patient pre- and post-treatment PBMC samples to investigate potential differences in inhibitory molecule expression. A decrease in PD-1 expression was seen in all patient samples assessed (NED: n=5, p=0.008; Relapse: n=3, p=0.130) (Supplementary Figure 4B). Supplementary Figure 4C shows that Tregs expressed appreciable levels of PDL1 (15–41% positive). NED patient Tregs had a reduction in PDL1 expression (p=0.034), while no significant changes were seen in relapsing patients. No significant changes were seen in CTLA4 expression (Supplementary Figure 4D).
PD-1 blockade increases T-cell IL-10 production in vitro.
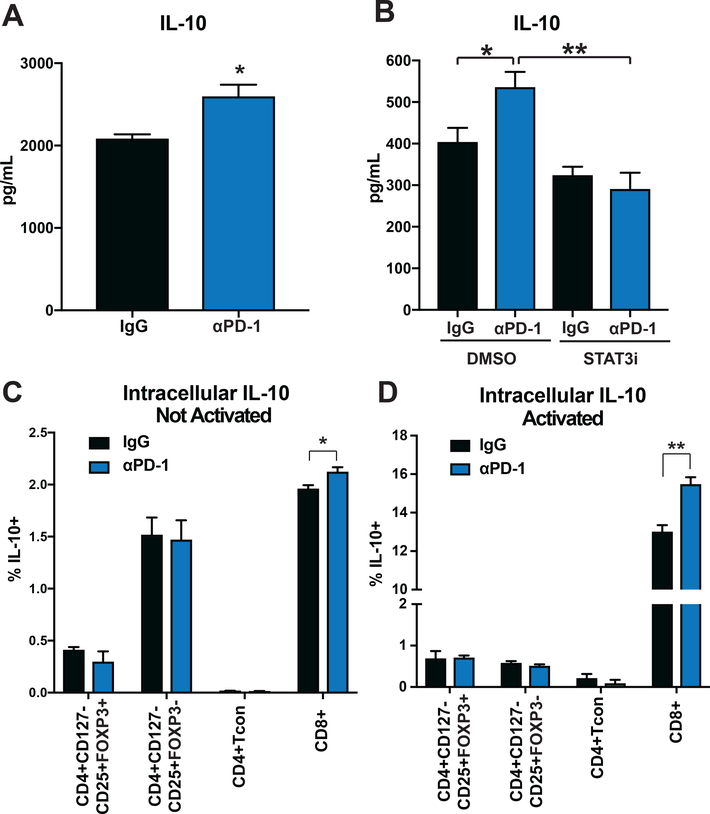
STAT3 positively regulates IL-10 expression, a cytokine with known roles in Treg expansion, differentiation and function (25,26). Therefore, we investigated the effects of PD-1 blockade on IL-10 production. Total T-cells isolated from baseline patient PBMC samples were cultured for two hours with PD-1 blocking antibody or IgG, CD3/CD28 stimulated for 72 hours, and cytokine production assessed. PD-1 blockade augmented IL-10 levels relative to IgG (p=0.023) (Figure 3A). Paired analysis showed an increase in IL-10 production (Supplemental Figure 6B, p=0.0289). To confirm the increase in IL-10 expression, T-cells were cultured overnight with IgG or PD-1 blocking antibodies and subsequently activated for six hours via CD3/CD28. Assessment of IL-10 mRNA demonstrated upregulation of IL-10 expression (Supplemental Figure 5).
Figure 3. PD-1 Blockade Increases Expression of IL-10.
(A) CD3+ T-cells were isolated by negative magnetic separation from baseline patient PBMC, cultured for two hours with IgG or αPD-1 (5ug/mL) and then activated with CD3/CD28 Dynabeads for 72 hours. Supernatant IL-10 levels were assessed in a Luminex assay. (B) T-cells were treated with IgG or αPD-1 (5ug/mL), and stattic (200nM) or DMSO control. IL-10 production was assessed by Luminex. (C-D) Isolated CD3+ T-cells were evaluated by intracellular flow cytometry for IL-10 expression after treatment with IgG or αPD-1 (5ug/mL) (C) without or (D) with the addition of CD3/CD28 Dynabeads. Indicated cell populations were gated on and percentage of IL-10 positive cells determined. All bar graphs illustrate means +/− SEM for triplicates/group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
We next investigated whether IL-10 production was dependent on pSTAT3. Addition of the STAT3 inhibitor static (27) to cultures reversed the PD-1 blocking antibody mediated increase in IL-10 (p=0.0094) (Figure 3B). STAT3 inhibition did not significantly alter IL-10 production in IgG treated samples (p=0.2993) (Supplemental Figure 6C). IL-10 levels were reduced in all samples when STAT3 inhibitor was added to PD-1 blocking antibody (p=0.0023) (Supplemental Figure 6D).
To determine the cellular source of the increased IL-10, total T-cells isolated from baseline patient PBMC samples were cultured for 72 hours with PD-1 blocking antibody or IgG and assessed for IL-10 expression by intracellular flow staining. Indicated T-cell subsets (e.g. CD8+, Treg) were gated on and the percentage of IL-10 expressing cells assessed for each subset. Figure 3C shows an increase in CD8+ T-cell IL-10 production without (p=0.0429), and with CD3/CD28 activation (Figure 3D, p=0.0087) in PD-1 blocking antibody treated cultures relative to IgG. Paired analysis is shown in Supplemental Figure 6E (p=0.0446) and 6F (p=0.0192). CD4+ T-cell populations did not show differences in IL-10 production.
IL-10 production enhanced by PD-1 blockade is associated with increased Treg percentages in vitro.
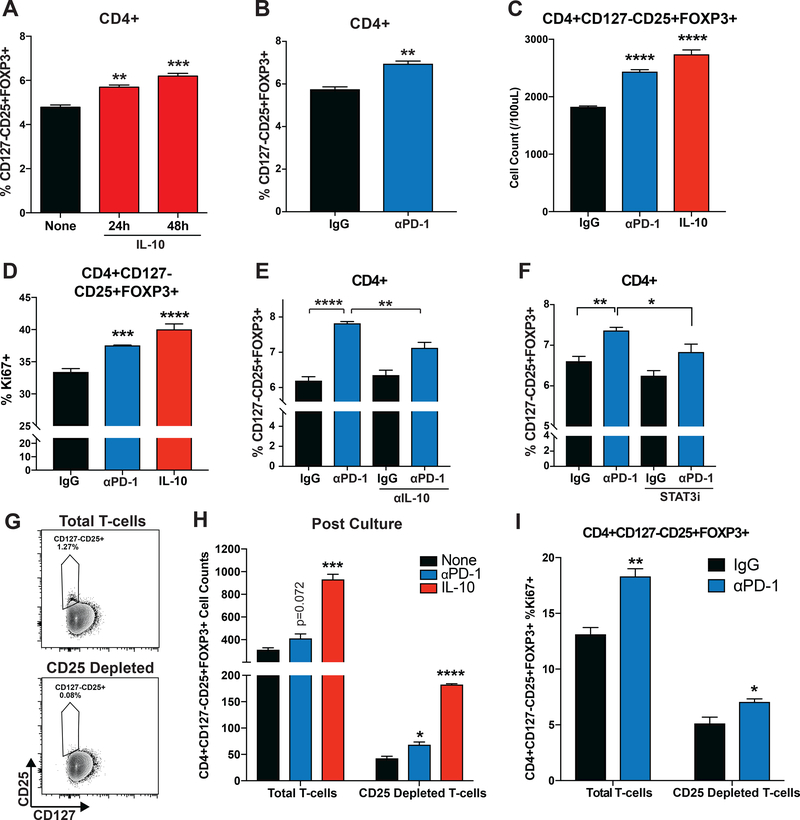
As IL-10 has been demonstrated to induce differentiation and proliferation of Tregs(26), we investigated if the observed increase in pSTAT3 and IL-10 production mediated by PD-1 blockade influenced Treg percentages in vitro. T-cells isolated from baseline patient PBMC were cultured with 50ng/mL recombinant IL-10 for 24 or 48 hours, and Tregs evaluated as a percentage of the CD4+ population. Addition of IL-10 increased the proportion of CD4+CD25+ CD127low/−FOXP3+ Tregs as a percentage of the CD4+ population at 24 (p=0.0005) and 48 hours (p<0.0001) (Figure 4A). Paired analysis displayed upregulation of Treg percentages at 48 hours (p=0.0006) (Supplemental Figure 6I). In a similar experiment, T-cells were cultured with PD-1 blocking antibody or IgG and evaluated for proportion of Tregs as a percentage of the CD4+ population at 48 hours. PD-1 blockade increased Treg percentages relative to IgG (p=0.0011, Figure 4B). Paired analysis of samples showed upregulation (p=0.0007) (Supplemental Figure 6H). Figure 4C demonstrates increases in Tregs evaluating CD4+CD25+ CD127low/−FOXP3+ cell counts after culturing with αPD-1 (p=0.0001) or IL-10 (p=0.0001). PD-1 blocking antibody also increased expression of Ki67 in Tregs (p=0.0002) (Figure 4D). Paired analysis showed upregulation in all samples assessed (p=0.0059) (Supplemental Figure 6G).
Figure 4. PD-1 Blockade Increases Treg Numbers.
(A) CD3+ T-cells were isolated from baseline patient PBMC and cltured with the addition of IL-10 (50ng/mL) at 24 or 48 hours prior to analysis by flow cytometry. CD4+CD127-/lowCD25+ Tregs as a percentage of the CD4+ population are graphed. (B) T-cells isolated from baseline patient PBMC were cultured with IgG or αPD-1 (5ug/mL) for 48 hours and assessed by flow cytometry for Tregs as a percentage of CD4+ T-cells. (C) T-cells isolated from baseline patient PBMC were cultured with IgG, αPD-1 (5ug/mL) or IL-10 (50ng/mL) for 48 hours and assessed for CD4+CD127-/lowCD25+FOXP3+ (Treg) cell counts by flow cytometry. (D) T-cells isolated from baseline patient PBMC were cultured with IgG or αPD-1 (5ug/mL) with CD3/CD28 Dynabeads for 48 hours and assessed by flow cytometry for expression of Ki67 by Tregs. (E) T-cells were cultured with IgG or αPD-1 (5ug/mL) with or without the addition of αIL-10 neutralization (10ug/mL) for 48 hours. Tregs as a percentage of CD4+ T-cells were assessed by flow cytometry. (F) T-cells were cultured with IgG or αPD-1 (5ug/mL) with or without the addition of the STAT3 inhibitor (stattic) for 48 hours. Tregs as a percentage of CD4+ T-cells were assessed by flow cytometry. (G) Isolated T-cells were split and half depleted of CD25+ cells. Cells were then assessed by flow cytometry. Contour plots of CD127 vs. CD25 expression in live CD4+ is shown for baseline (top) and CD25 depleted (bottom) samples. Results shown are representative of three samples assessed in two independent experiments. (H) Total T-cell and CD25 depleted T-cell samples were cultured with αPD-1 (5ug/mL) or IL-10 (50ng/mL) and assessed for CD4+CD127-/lowCD25+FOXP3+ Treg cell counts by flow cytometry. (I) Total T-cell and CD25 depleted T-cell samples were cultured with IgG or αPD-1 (5ug/mL) and assessed for Ki67 expression in CD4+CD127-/lowCD25+FOXP3+ Tregs by intracellular flow cytometry. Results shown are representative of four samples assessed over two independent experiments. All bar graphs illustrate means +/− SEM for triplicates/group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
To determine if the higher Treg percentages observed in cultures with PD-1 blocking antibodies were mediated by increased IL-10 production, IL-10 neutralizing antibodies were added to cultures of T-cells treated with PD-1 antibody or IgG. Neutralization reduced Treg percentages relative to PD-1 blockade alone (p=0.0009) but had no impact on IgG cultures (p=0.3218) (Figure 4E). Paired analysis showed that neutralizing IL-10 did not change Treg percentages relative to IgG (Supplemental Figure 6J), but reduced Treg percentages when added to PD-1 blockade (p=0.0149; Supplemental Figure 6K).
To determine whether the observed increases in Treg proportions were dependent on pSTAT3 induction, T-cells were cultured with IgG or PD-1 blocking antibodies with or without STAT3 inhibitor (stattic). Addition of stattic to PD-1 blocking antibody-treated cultures reduced Treg percentages (p=0.0205) but not when added to IgG-treated cultures (p=0.0887) (Figure 4F). Paired analyses showed that stattic decreased Treg percentages when added to PD-1 antibody-treated cultures (p=0.0068; Supplemental Figure 6M), but not when added to IgG-treated controls (p=0.3290; Supplemental Figure 6L).
To address if the observed increases in Tregs resulted from inducible Tregs (iTregs), baseline patient samples were isolated for CD3+ T-cells (Figure 4G top panel) and a portion of those cells depleted for CD25 (Figure 4G bottom panel). Cells were then cultured in the presence of PD-1 blocking antibody or recombinant IL-10 for 72 hours and assessed for CD4+CD25+CD127low/−FOXP3+ Treg counts. Exogenous IL-10 increased Treg numbers in both total T-cell (p=0.0002) and CD25-depleted cultures (p<0.0001) (Figure 4H). An increase over control was seen in the PD-1 blocking antibody-treated CD25-depleted culture (p=0.0129). PD-1 blockade also increased Ki67 expression in CD25-depleted (p<0.05) and total (p<0.01) T-cells (Figure 4I).
Treg suppression is reduced in vitro by PD-1/PDL1 blockade and enhanced by STAT3 inhibition.
To determine the effects of PD-1 blockade on nTreg suppressive function in vitro, isolated Tregs were pre-treated with PD-1 blocking antibodies or IgG, and equivalent numbers of Tregs subsequently co-cultured with CFSE-labeled autologous T-cells plus CD3/CD28 activation. After three days, cultures were assessed by flow cytometry for proliferation of target T-cells. PD-1 blockade decreased Treg suppression, as evidenced by an increased percentage of proliferating Tcons (no Treg control: 93.3%, IgG: 45.5%, PD-1 antibody treated: 66.6% proliferating Tcons) (Figure 5A). To address the role of STAT3 in the suppressive function of Tregs, a similar in vitro nTreg assay was performed using DMSO or STAT3 inhibitor (stattic) pre-treated Tregs. Tregs treated with STAT3 inhibitor had increased suppressive function relative to DMSO (no Treg control: 41.3%, DMSO: 15.4%, STAT3 inhibitor: 10.4% proliferating Tcons) (Figure 5B).
Figure 5. PD-1 and PDL1 Blockade Reduces While STAT3 Inhibition Enhances iTreg Suppressive Function.
(A-B) Human nTregs were treated with αPD-1 (5ug/ml), stattic (200nM), DMSO, or IgG and expanded with CD3/CD28 Dynabeads (1:1) for 3 days, then washed. nTreg suppression was evaluated in cultures with CFSE-loaded autologous T cells (1:30, nTreg:T-cell), stimulated by CD3/CD28 Dynabeads (1:30). (A) Histograms showing proliferation peaks (by CFSE dilution) for cultures with no Tregs (grey histogram), IgG pre-treated Tregs (black histograms) and αPD-1 pre-treated Tregs (blue histograms) are shown. (B) Histograms showing proliferation peaks for cultures with no Tregs (grey histogram), DMSO pre-treated Tregs (black histograms) and stattic pre-treated Tregs (red histograms) are illustrated. The percentages of cells with ≥1 division are given above each corresponding histogram. One representative of two independent experiments is shown for each. (C) Naïve CD4+ T-cells were cultured in iTreg polarizing conditions with the addition of specified treatments. T-cells were washed and cultured with autologous CD8+ T-cells at indicated ratios with the addition of CD3/CD28 Dynabeads. (D) Polarized CD4+ T-cells were assessed by flow cytometry for expression of FOXP3 (x-axis) and HELIOS (y-axis). (E) Polarized cells were also assessed for PD-1 (x-axis) and PDL1 (y-axis) expression. (F) Ki67 expression by CD8+ T-cells cultured with iTregs was assessed by flow cytometry and graphed relative to cultures containing only CD8+ T-cells (white bars). IgG controls are illustrated in black and αPD-1 pre-treated iTregs illustrated in blue. Results shown are representative of six samples assessed in six independent experiments. (G) The suppressive function of IgG (black bars) and PDL1 blocking antibody (purple bars) pre-treated iTregs were likewise assessed. Results shown are representative of three samples assessed over two independent experiments. (H) TNF (left panel), IFNγ (middle panel), and Granzyme B (right panel) cytokine production from iTreg suppression assays was assessed. Results shown are representative of three samples from three independent experiments. (I) An iTreg polarization experiment was performed with stattic (red bars) or control (black bars) pre-treatment, and CD8+ Ki67 expression assessed. Results shown are representative of five samples assessed over three independent experiments. Error bars are +SEM for triplicate samples assessed. **p<0.01, ***p<0.001, ****p<0.0001, ****p<0.00001.
As the data suggest that blocking PD-1 increases iTregs, we also evaluated the effects of PD-1 blockade and STAT3 inhibition on the suppressive function of iTregs. Naïve CD4+ T-cells from baseline patient PBMC samples were polarized with or without the addition of PD-1 blocking antibodies or other indicated treatments. Polarized cells were washed before addition to autologous CD8+ T-cells as outlined in Figure 5C. To determine efficacy of polarization, polarized CD4+ T-cells were evaluated by flow cytometry for expression of the transcription factors FOXP3 and HELIOS (Figure 5D) as well as PD-1 and PDL1 (Figure 5E). Polarized CD4+ T-cells expressed high levels of HELIOS (90.8% positive) and FOXP3 (42.2% positive). These cells also expressed high levels of PD-1 (93% positive) and PDL1 (33.7% positive). No differences were observed between IgG and PD-1 cultured iTregs in expression of these markers or TGFβ production (data not shown).
Culture of naïve CD4+ T-cells with PD-1 blocking antibodies decreased suppressive function at 1:10 (Treg:CD8+) (p<0.0001) and 1:5 (p=0.0004) (Figure 5F). Given the observation that patient T-cells and polarized iTregs express PDL1, we hypothesized that similar reduction in suppressive function could be achieved with PDL1 blockade. As seen in Figure 5G, the addition of PDL1 blocking antibodies reduced suppression at 1:8 (p=0.0179) and 1:4 (p=0.0263) iTreg:CD8+ ratios. Production of inflammatory cytokines was also evaluated in iTreg suppression assays. Cultures containing iTregs displayed reduction in TNF, IFNγ, and Granzyme B relative to CD8+ only cultures (0:1 ratio) (Figure 5H). However, iTregs polarized in the presence of PD-1 (p<0.01) or PDL1 blocking antibodies (p<0.05) had an increase in all three cytokines, at all ratios relative to IgG control iTregs.
To determine the effects of STAT3 inhibition in iTreg function, naïve CD4+ T-cells were polarized as before, but with the addition stattic. STAT3 inhibition augmented iTreg suppression at 1:8 (p=0.0004) and 1:4 ratios (p=0.0397) (Figure 5I).
Discussion
Our data showed that after nivolumab treatment, circulating Tregs from patients with clinical benefit had reduced suppressive function, increased proliferation, and increased pSTAT3 expression. These changes were absent in patients without benefit. Increased pSTAT3 expression was also observed in CD4+ Tcons and CD8+ T-cells in benefiting patients. PD-1 blockade in vitro of purified T-cells recapitulated these effects, supporting a direct effect on T-cells rather than mediated through tumor or APC interactions. In vitro assays demonstrated that PD-1 blockade increased T-cell IL-10 production in a STAT3 dependent manner, which mediated the increase in Treg percentages. However, no differences were noted in the in vitro effects of PD-1 blockade between NED and relapsing patient samples (data not shown), limiting the use of these assays as predictive of patient outcomes to PD-1 blockade. These data support a model in which PD-1 blockade induces STAT3 signaling in Tregs, reducing suppressive function while increasing the production of IL-10 by CD8+ T-cells, resulting in Treg proliferation. Increases in Treg proliferation were seen in NED patient RNA-Seq gene signatures, circulating Treg proportions in NED patients, and in vitro assays. While an accompanying increase in nTreg proliferation cannot be ruled out, these data suggest that induction of iTregs by enhanced IL-10 production mediates the increase in Tregs.
How the impact on peripheral Tregs by PD-1 blockade correlates to changes in tumor-infiltrating Tregs in patients with unresectable disease remains to be investigated. Studies have demonstrated that the phenotype and function of intra-tumor Tregs is similar but enhanced relative to that of circulating Tregs (e.g. increased suppressive function in the tumor and enhanced inhibitory molecule expression)(28). The major patient population in this study was surgically resected and free of tumor, limiting the ability to address this hypothesis. Given the scarcity of tumor biopsies from patients, particularly paired (pre- and post-treatment), future work should determine if the effects observed here are also observed in murine pre-clinical models.
PD-1 blockade exerted effects on T-cell isolated cultures in the absence of tumor or APCs. PDL1 was robustly expressed in patient T-cells, suggesting that interaction of PD-1 with PDL1 expressed on T-cells may play an important role in T-cell function and efficacy of immunotherapies. In support of this hypothesis, a recent study has demonstrated that high levels of PDL1 expression by CD8+ T-cells are associated with progression of disease after ipilimumab treatment (29). Our data show that peripheral blood Tregs from resected patients express high levels of PD-1, CTLA4 and PDL1. Interpretations are limited by the low sample numbers available for assessment, but a significant reduction in PDL1 expression was observed in NED patients. In contrast, 2/3 relapsed patient samples assessed had an increase in PDL1 expression. These results highlight a potential important role of Treg PDL1 expression in patient outcome, which should be investigated in future studies.
Post-treatment peripheral blood nTregs had reduced suppressive function in NED patients, and in vitro assays showed similar decreases in the suppressive function of PD-1 exposed iTregs. While not significant, pre-treatment Treg suppression was notably lower in NED patients. Future validation of alterations in suppressive function, including in active disease patients, may offer larger sample sizes and statistical power to determine whether baseline differences influence patient outcomes.
In some patients, Tregs assessed for suppressive activity resulted in enhanced CD8+ T-cell proliferation over no Treg controls (i.e. negative suppressive values). The Tregs used in these assays were isolated using the marker set CD4+CD127low/−CD25+, as intracellular staining of FOXP3 is incompatible with downstream use in functional assays. In assessing FOXP3 expression in circulating Tregs, no significant differences were found between NED and relapsing patients, suggesting that differences in FOXP3 expression could not explain differences in suppression. These limitations illustrate the need for further research identifying surface markers that uniquely distinguish suppressive T-cells.
STAT3 has important roles in Treg biology including acting as a co-transcription factor with FOXP3 and regulating IL-10 gene expression amongst other STAT3 targets (30). In agreement with our data, STAT3 expression in Tregs was previously shown to be associated with decreased suppressive function (31). Increased amounts of pSTAT3+ Tcons are associated with immune activation in systemic lupus erythematosus (25) and graft-versus-host disease after allogeneic hematopoietic cell transplantation (32), suggesting enhanced reactivity. However, the role of Tcon pSTAT3 expression in the context of malignancy and patient response to therapies still remains to be fully addressed. Our results show that pSTAT3 induction in all populations of T-cells is associated with patient outcome, and future work will specifically address the consequences of pSTAT3 induction in effector T-cells.
The duality of STAT3 and IL-10 in the tumor-immune context is becoming increasingly clear. STAT3 signaling and IL-10 production by melanomas are associated with worse prognosis (33,34), and our data, along with that of other groups (15), show that IL-10 production results in the formation of iTregs. However, STAT3 and IL-10 are also necessary for CTL effector function and memory formation, requisites for a productive anti-tumor immune response (35–37). Likewise, Tregs are associated with poor patient outcome in a variety of malignancies (38), but are also critical in inhibiting carcinogenesis through suppression of inflammation (39). Our results suggest more complex roles of IL-10 in the context of checkpoint blockade of melanoma. Studies have shown increased IL-10 expression in immune cells after PD-1 blockade(40), association of IL-10 levels with patient response (41), and a potential anti-tumor efficacy of pegylated IL-10 therapy (42), supporting the need for evaluating the contextual effects and clinical impact of IL-10.
Our data demonstrates an important role of Tregs in the outcome of metastatic melanoma patients after nivolumab therapy and reveal three potential pharmacodynamic biomarkers of melanoma patient response to nivolumab: increased T-cell pSTAT3 expression, decreased Treg suppressive function, and increased Treg proliferation. As pharmacodynamic biomarkers, these are not useful in stratifying patient treatments, but instead give further insight into potential novel mechanism of patient response and generate rationale for combination therapies. Data from in vitro experiments link these three biomarkers in a model where nivolumab directly induces Treg pSTAT3, reducing suppression and resulting in IL-10 production leading to proliferation.
Supplementary Material
Statement of Significance.
PD-1 blockade induces durable responses in patients with metastatic melanoma and prolongs relapse-free survival in patients with resected melanoma; however, biomarkers of response and clinical benefit remain unknown. The data presented herein support a role of Tregs in melanoma patient outcome after nivolumab therapy, demonstrating that induction of pSTAT3 in T-cells and an accompanying reduction in Treg suppressive function with a paradoxical increase in Treg percentages are novel correlates of patient benefit with PD-1 blockade.
Acknowledgements:
This work was supported by grant funding from the National Cancer Institute (NIH NCI R01 CA175732–01). We extend our appreciation to Christine Horak, Don Jackson and Bristol-Myers Squibb for their insight and assistance as well as for providing nivolumab. We also extended our appreciation to the Genomics Core Facility, Sean Yoder, the Flow Cytometry Core Facility, and Jodie Kroger at Moffitt Cancer Center for their assistance.
Financial Support: NIH NCI R01 CA175732–01
Abbreviations:
- Treg
Regulatory T-cell
- nTreg
Natural Treg
- iTreg
Inducible Treg
- Tcon
Conventional T-cell
- pSTAT3
Phosphorylated STAT3
- NED
No evidence of disease
- APCs
Antigen Presenting Cells
- PBMC
Peripheral blood mononuclear cells
- SEM
Standard Error of the Mean
Footnotes
Disclosures
David M. Woods: Stock ownership in Bristol Myers Squibb.
Rupal Ramakrishnan: None.
Andressa S. Laino: None.
Anders Berglund: None.
Brian Betts: None.
Jeffrey S. Weber: Receives compensation from Bristol-Myers Squibb for consulting and serving on ad boards. Named on a PD-1 biomarker patent by Biodesix.
References
- 1.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30 [DOI] [PubMed] [Google Scholar]
- 2.Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res 2015;21:712–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nature medicine 2010;16:1147–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 2004;574:37–41 [DOI] [PubMed] [Google Scholar]
- 5.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal 2012;5:ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017;355:1428–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massi D, Brusa D, Merelli B, Ciano M, Audrito V, Serra S, et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2014;25:2433–42 [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 2013;31:4311–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008;133:775–87 [DOI] [PubMed] [Google Scholar]
- 11.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008;8:523–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol 2009;21:1105–11 [DOI] [PubMed] [Google Scholar]
- 13.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 2009;30:626–35 [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine 2003;198:1875–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. The Journal of experimental medicine 2002;196:247–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. Journal of immunology 2004;173:1444–53 [DOI] [PubMed] [Google Scholar]
- 17.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. British journal of cancer 2011;105:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin Cancer Res 2016;22:2908–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol 2009;21:1065–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. The Journal of experimental medicine 2006;203:1701–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. The Journal of experimental medicine 2006;203:1693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 2015;1:417–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 2010;32:605–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betts BC, Veerapathran A, Pidala J, Yu XZ, Anasetti C. STAT5 polarization promotes iTregs and suppresses human T-cell alloresponses while preserving CTL capacity. J Leukoc Biol 2014;95:205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedrich CM, Rauen T, Apostolidis SA, Grammatikos AP, Rodriguez Rodriguez N, Ioannidis C, et al. Stat3 promotes IL-10 expression in lupus T cells through trans-activation and chromatin remodeling. Proc Natl Acad Sci U S A 2014;111:13457–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu P, Santner-Nanan B, Hu M, Skarratt K, Lee CH, Stormon M, et al. IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. Journal of immunology 2015;195:3665–74 [DOI] [PubMed] [Google Scholar]
- 27.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chemistry & biology 2006;13:1235–42 [DOI] [PubMed] [Google Scholar]
- 28.Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. British journal of cancer 2013;109:2629–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacquelot N, Roberti MP, Enot DP, Rusakiewicz S, Ternes N, Jegou S, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nature communications 2017;8:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hossain DM, Panda AK, Manna A, Mohanty S, Bhattacharjee P, Bhattacharyya S, et al. FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity 2013;39:1057–69 [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Li B, Dang E, Jin L, Fan X, Wang G. Impaired function of regulatory T cells in patients with psoriasis is mediated by phosphorylation of STAT3. J Dermatol Sci 2016;81:85–92 [DOI] [PubMed] [Google Scholar]
- 32.Betts BC, Sagatys EM, Veerapathran A, Lloyd MC, Beato F, Lawrence HR, et al. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response. J Leukoc Biol 2015;97:807–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee I, Fox PS, Ferguson SD, Bassett R, Kong LY, Schacherer CW, et al. The expression of p-STAT3 in stage IV melanoma: risk of CNS metastasis and survival. Oncotarget 2012;3:336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torisu-Itakura H, Lee JH, Huynh Y, Ye X, Essner R, Morton DL. Monocyte-derived IL-10 expression predicts prognosis of stage IV melanoma patients. Journal of immunotherapy 2007;30:831–8 [DOI] [PubMed] [Google Scholar]
- 35.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. Journal of immunology 2006;177:2565–74 [DOI] [PubMed] [Google Scholar]
- 36.Fujii S, Shimizu K, Shimizu T, Lotze MT. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood 2001;98:2143–51 [DOI] [PubMed] [Google Scholar]
- 37.Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity 2011;35:806–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Scientific reports 2015;5:15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res 2005;65:3998–4004 [DOI] [PubMed] [Google Scholar]
- 40.Lamichhane P, Karyampudi L, Shreeder B, Krempski J, Bahr D, Daum J, et al. IL10 Release upon PD-1 Blockade Sustains Immunosuppression in Ovarian Cancer. Cancer Res 2017;77:6667–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nature medicine 2018;24:144–53 [DOI] [PubMed] [Google Scholar]
- 42.Naing A, Papadopoulos KP, Autio KA, Ott PA, Patel MR, Wong DJ, et al. Safety, Antitumor Activity, and Immune Activation of Pegylated Recombinant Human Interleukin-10 (AM0010) in Patients With Advanced Solid Tumors. J Clin Oncol 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL, Jr., Lawrence DP, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 2016;17:943–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009;25:1105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature biotechnology 2010;28:511–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.