Abstract
Aims:
Type 2 diabetes in lean individuals has recently come to attention. We assessed type 2 diabetes prevalence and the associated risk factors in underweight and normal weight individuals in two ethnic populations.
Methods:
We conducted cross-sectional analyses, using representative samples of 4,930 Asian Indians from the CARRS-Chennai Study and 2,868 Whites from the NHANES Survey. Diabetes was defined as use of glucose lowering medication, fasting glucose ≥ 126 mg/dl, or 2 hour glucose ≥ 200 mg/dl. Body mass index (BMI) was classified using WHO standard criteria.
Results:
Prevalence of type 2 diabetes by BMI varied by ethnicity and sex. In men, type 2 diabetes prevalence was 5.4% and 23.5% in underweight and normal weight Asian Indians and 0.0% and 6.1% in underweight and normal weight Whites. In women, the prevalence was 5.6% and 13.6% in underweight and normal weight Asian Indians and 2.3% and 2.8% in underweight and normal weight Whites. Adjustment for waist circumference, insulin resistance, and insulin secretion did not explain the increased prevalence in Asian Indians.
Conclusions:
These findings suggest significant ethnic differences in type 2 diabetes prevalence without overweight or obesity. Future studies should examine the pathophysiology of type 2 diabetes development in lean individuals.
Keywords: Type 2 diabetes, Asian Indian, ethnicity, underweight, normal weight, body mass index
1. Introduction
Overweight and obesity are well known risk factors for type 2 diabetes [1–5]. However, some populations (particularly those in or from Asia and Africa) are at risk of type 2 diabetes at much lower levels of body mass indices (BMI) than other ethnic groups [6–8]. Furthermore, type 2 diabetes is increasingly being reported in normal weight and underweight individuals [9–13]. For example, in a nationally representative sample from China, type 2 diabetes prevalence was 4.5% in individuals with BMI < 18.5 kg/m2 and 7.6% in individuals with BMI 18.5–24.9 kg/m2 [10]. Similar results were reported from a more recent nationally representative survey from mainland China, in which the prevalence of type 2 diabetes as 7.8% in individuals with BMI < 25 kg/m2[11]. Furthermore, a study examining the prevalence of type 2 diabetes in Zambia and the Western Cape of South Africa found that while the prevalence of type 2 diabetes was 2.9% in Zambia, two-thirds of these cases were in those who were underweight or normal weight [13]. Similarly, while the overall prevalence of type 2 diabetes was 9.4% in the Western Cape, nearly two-thirds of these cases were in those with BMI < 25 kg/m2 [13].
Investigating type 2 diabetes in non-obese subpopulations may expand knowledge above and beyond the connection between insulin resistance and type 2 diabetes risk, and may reveal novel aspects in disease etiology, and pathophysiology, and potentially direct to new approaches to preventing and managing the disease. Such studies are particularly important in populations living in or with origin from low- and middle-income countries, such as India, other parts of Asia, Africa, which are currently experiencing an extremely high burden of type 2 diabetes in parallel with dual burdens of under and over-nutrition [14–16].
We therefore investigated the prevalence of type 2 diabetes by BMI in a population-based sample of Asian Indians living in Chennai, India, from the Center for Cardio-Metabolic Risk Reduction in South Asia (CARRS study) and compared it to Whites from the National Health and Nutrition Examination Survey (NHANES) in the United States. We also examined factors associated with the prevalence of type 2 diabetes in underweight/normal weight compared to overweight/obese individuals.
2. Materials and Methods
2.1. Study population
In brief, CARRS is a multi-site cohort study consisting of two urban cities in India (New Delhi, and Chennai) and one in Pakistan (Karachi). Recruitment and baseline cross-sectional data collection was done in 2010–2011 [17]. For the purposes of this study, data were analyzed from Chennai, India only, as this was the only site to collect both fasting and two hour plasma glucose samples. Chennai is a large metropolitan city with a population of approximately 8 million people [18] and is located in the South Indian state of Tamil Nadu. In order to be representative of Chennai, households were selected for participation using multi-stage random sampling technique [17]. A total of 6,921 individuals aged ≥ 20 were screened for participation. For this study we limited our population to the 4,950 (72%) participants who were either previously diagnosed with type 2 diabetes, or who provided fasting and two hour post-challenge glucose measurements. While the NHANES classifies individuals with type 1 diabetes as those who started using insulin within one year of diabetes diagnosis, were currently using insulin, and were diagnosed prior to the age of 30 [19], CARRS did not collect information on insulin use specifically. Therefore, we also excluded 39 participants who were diagnosed with diabetes prior to the age of 30 as a best method to exclude individuals with type 1 diabetes as well as 5 participants with negative HOMA-β values for a total sample of 4,906 individuals. All participants in CARRS-Chennai were considered Asian Indian.
NHANES is a cross-sectional survey conducted by the US Centers for Disease Control and Prevention’s National Center for Health Statistics. The survey is designed to be representative of the US civilian, non-institutionalized population on the basis of a complex multi-stage, biennial probability sample [20]. After completing an in home questionnaire, participants attended a mobile examination clinic where they received a questionnaire in addition to physical and laboratory examinations. In order to be in accordance with the time frame of CARRS, cycles 2007–2008, 2009–2010, and 2011–2012 were combined for analysis. We limited the analysis to adults aged 20 years and older. Including all ethnicity/ethnic groups, a total of 24,731 were screened for participation. Of those, 17,713 (72%) provided questionnaire data, and 17,085 (69%) participated in the mobile examination. Participants who self-reported as “other ethnicity,” Mexican American (Hispanic), Other Hispanic (Hispanic), or Non-Hispanic Black (Black), (9,935 (56%)) or who were currently pregnant (34 (0.4%)) were excluded from analysis. We also excluded 1,266 (16%) participants who were over the age of 75 to remain in concordance with the upper age group included in CARRS, as well as 26 participants who started insulin therapy within one year of diabetes diagnosis, were currently using insulin, and were diagnosed with diabetes prior to the age of 30 to ensure that we excluded individuals with type 1 diabetes [19] In addition, we excluded 6 individuals who had negative values of HOMA-β. Of the remaining 6,452 participants, 580 participants (9%) were previously diagnosed with diabetes. We excluded 3,579 individuals who had missing values for two hour post challenge glucose or for previously diagnosed diabetes. We thus limited our population to the 2,873 individuals who met inclusion criteria and had either a previous diabetes diagnosis or either fasting or two hour post challenge glucose measurements, and self-identified as White. Details regarding the eligibility criteria, questionnaire, and examination components in NHANES and CARRS are listed in Table 1. Additional details of each study have been previously published [17,20].
Table 1.
Characteristics of Participants with Type 2 Diabetes by BMI and Ethnicity
| Asian Indian-CARRS | White-NHANES | |||
|---|---|---|---|---|
| Underweight/Normal Weight | Overweight/Obese | Underweight/Normal Weight | Overweight/Obese | |
| N (%) | 338 (17.1) | 640 (26.7)* | 76 (4.0) | 634 (14.6) * |
| Age (years) | 53.1 ± 11.7 | 48.5 ± 10.6* | 55.5 ± 12.7 | 58.5 ± 11.8 |
| Height (cm) | 158.2 ± 9.1 | 154.7 ± 8.5* | 170.7 ± 8.8 | 169.2 ± 9.6 |
| Waist Circumference (cm) | 82.4 ± 10.3 | 93.4 ± 9.7* | 90.0 ± 7.4 | 114.9 ± 14.0* |
| Fasting Glucose (mmol/L) | 9.5 ± 4.1 | 8.7 ± 3.6* | 7.3 ± 2.6 | 7.9 ± 3.1 |
| 2-hr Glucose (mmol/L) | 15.2 ± 5.6 | 14.6 ± 4.9 | 11.2 ± 4.0 | 12.3.3 ± 3.9 |
| †Fasting Insulin (pmol/L) | 74.5 ± 1.5 | 80.5 ± 1.5* | 59.0 ± 3.9 | 116.9 ± 4.7* |
| †HOMA-P (μIU/ml/mmol/l) | 47.3 ± 27.7 | 51.5 ± 22.1* | 50.8 ± 1.1 | 90.0 ± 1.0* |
| †HOMA-IR (μIU/ml*mmol/l) | 4.0 ± 7.1 | 4.3 ± 6.3 | 2.7 ± 1.4 | 5.6 ± 1.0* |
| †Triglycerides (mmol/L) | 1.9 ± 0.4 | 1.9 ± 0.3 | 1.2 ± 0.1 | 1.6 ± 0.3* |
| HDL (mmol/L) | 1.1 ± 0.3 | 1.0 ± 0.2 | 1.4 ± 21.1 | 1.2 ± 13.2* |
| Systolic Blood Pressure (mmHg) | 132.5 ± 21.8 | 131.6 ± 19.9 | 124.4 ± 23.2 | 127.9 ± 17.6 |
| Diastolic Blood Pressure (mmHg) | 85.2 ± 12.9 | 85.9 ± 11.4 | 69.6 ± 12.4 | 69.7 ± 13.3 |
| Known Diabetes (previously diagnosed) | 77.1% | 68.2%* | 65.8% | 78.1%* |
Data are presented as means ± standard deviation
Denotes statistical significance compared to underweight/normal weight, P < 0.05
Data are presented as geometric means ± standard deviation
2.2. Definitions and Measurements
In both the CARRS and NHANES studies, type 2 diabetes was defined by previous physician diagnosis, the use of glucose lowering medication, or fasting plasma glucose ≥ 126 mg/dl and/or two hour post-challenge glucose ≥ 200 mg/dl, and by exclusion of possible type 1 diabetes from clinical presentation [21]. In CARRS individuals with a diabetes diagnosis prior to the age of 30 were excluded, while in NHANES, participants who started insulin therapy within one year of diabetes diagnosis, were currently using insulin, and were diagnosed with diabetes prior to the age of 30 were excluded to ensure only individuals with type 2 diabetes were included in the study [19]. Plasma glucose was analyzed using the hexokinase method in both studies. Estimates of inherent insulin resistance and insulin secretion in participants were generated using HOMA modeling [15]. HOMA-β was used to measure insulin secretion and was calculated as [20*I0(μIU/ml) / G0 (mmol/l)- 3.5]. HOMA-IR was used to measure insulin resistance and was calculated as [I0(μIU/ml) * G0 (mmol/l)/22.5] [22].
For both Asian Indian and White participants, BMI was classified according to World Health Organization (WHO) standard cut-points for underweight (BMI < 18.5 kg/m2) normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), and obesity (BMI ≥30 kg/m2) [23]. When comparing characteristics of those with type 2 diabetes by BMI status and ethnicity/ethnicity, the underweight and normal weight categories were combined as were the overweight and obese categories due to the small number of underweight individuals in the NHANES sample. We also conducted a sensitivity analysis using WHO-Asian cut-points for underweight (BMI < 18.5 kg/m2) normal weight (BMI 18.5–22.9 kg/m2), overweight (BMI 23.0–27.4 kg/m2), and obesity (BMI ≥27.5 kg/m2) [24] in Asian Indian participants.
2.3. Statistical Analysis
All analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC) or SAS callable SUDAAN (version 9, Research Triangle Institute) software. Data from CARRS and NHANES were set together into a single dataset for analysis. Sampling weights for each survey were created independently in order to maximize the representativeness of each sample and were maintained upon combined analysis. Participant characteristics were stratified by ethnicity and BMI and were compared using conditional marginal distributions and Wald chi-squared tests. Weighted crude type 2 diabetes prevalence values and 95% confidence intervals were estimated by ethnicity and sex. Prevalence ratios of type 2 diabetes were estimated using log binomial regression models for underweight/normal weight individuals and for overweight/obese individuals separately. Multivariate regression models were adjusted for age, sex, waist circumference, standardized HOMA-IR, and standardized HOMA-β.
3. Results
The total sample of 7,774 participants was comprised of 4,906 Asian Indian and 2,868 White participants. The weighted BMI composition of the sample by ethnicity was 5.8% underweight, 38.5% normal weight, 38.2% overweight and 17.6% obese in Asian Indians; and 1.2% underweight, 30.4% normal weight, 34.0% overweight, and 34.3% obese in Whites. In sensitivity analyses using the WHO-Asian cut-points for BMI, 5.8% of Asian Indians were classified as underweight, 22.6% were normal weight, 37.5% were classified as overweight, and 34.1% were obese.
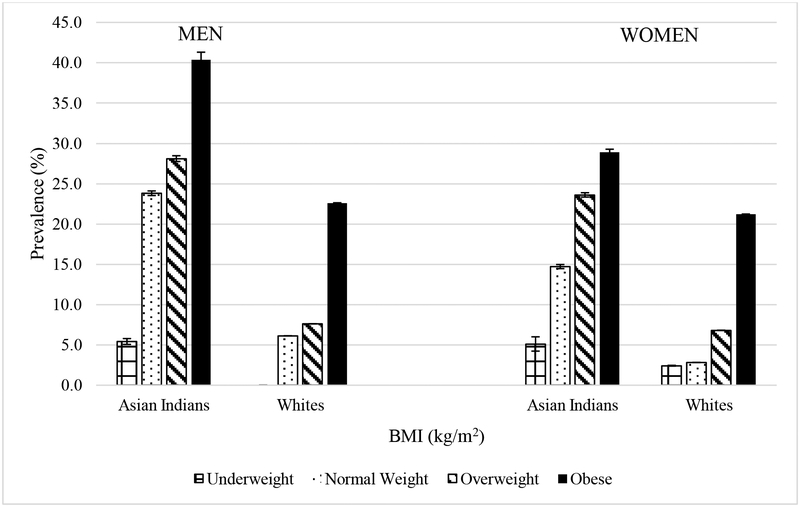
The prevalence of type 2 diabetes by BMI category varied by ethnicity and sex (Figure 1). In underweight men, the prevalence of type 2 diabetes was 5.4% in Asian Indians. However, no White men who were underweight had type 2 diabetes. In normal weight men, the prevalence of type 2 diabetes was 23.5% and 6.1% in Asian Indians and Whites respectively. In underweight women, the prevalence of type 2 diabetes was 5.6% in Asian Indians and 2.3% in Whites. In normal weight women, the prevalence of type 2 diabetes was 13.6% and 2.8% in Asian Indians and Whites respectively. In both sexes, Asian Indians also had a greater prevalence of type 2 diabetes in the overweight and obese categories compared to White individuals.
Figure 1.
Prevalence of Type 2 Diabetes by BMI and Ethnicity in CARRS and MASALA
Regarding the prevalence ratios of type 2 diabetes, among men, Asian Indians who were underweight had 5.4 times greater prevalence of type 2 diabetes than their White counterparts. Asian Indian men who were normal weight, overweight, or obese had a 3.9 times, 3.7 times, and 1.8 times significantly greater prevalence of type 2 diabetes than White men who were normal weight overweight, or obese. Among women, Asian Indians who were underweight normal weight, overweight or obese had 2.2 times, 5.2 times, 3.5 times, and 1.4 times greater prevalence of type 2 diabetes respectively compared to White women who were underweight, normal weight, overweight, and obese respectively. Similar trends were found in sensitivity analyses using the WHO Asian BMI cut-points for the Asian Indian population (Figure 1b).
Table 1 details the characteristics of individuals with type 2 diabetes by ethnicity and BMI category. The categories of underweight and normal weight were combined as were the categories of overweight and obese due to the small number of underweight participants in the NHANES sample. In Asian Indians, those with type 2 diabetes who were underweight/normal weight were significantly older, taller, and had smaller waist circumference measures than those who were overweight/obese. Asian Indians with type 2 diabetes who were underweight/normal weight also had significantly higher fasting glucose measures, lower fasting insulin, poorer β-cell function, and a greater prevalence of previously diagnosed diabetes compared to those who were overweight/obese. In Whites, those with type 2 diabetes who were underweight/normal weight had significantly smaller mean waist circumference measures, lower fasting insulin, poorer β-cell function, less insulin resistance, and greater prevalence of previously diagnosed type 2 diabetes compared to those who were overweight/obese. Similar results were found using the WHO-Asian cut-points for BMI (Supplemental Table 1).
In multivariable log binomial regression models (Table 2), in underweight/normal weight individuals, after adjusting for age, sex, waist circumference, HOMA-IR and HOMA-β, there Asian Indians were 1.8 times more likely to have type 2 diabetes compared to Whites. When using ages 20–29 years as the referent, those who were 30–39 years old had a greater prevalence of type 2 diabetes compared to those who were 40–59 years or those who were aged 60 and above. Male sex was associated with increased prevalence of type 2 diabetes, as was increasing waist circumference and quartile of HOMA-IR. Decreased HOMA-β was also associated with increased prevalence of type 2 diabetes in those who were underweight or normal weight. Amongst those who were overweight or obese, after adjusting for age group, sex, quartile of waist circumference, quartile of HOMA-IR and quartile of HOMA-β, Asian Indians had a decreased prevalence of type 2 diabetes compared to Whites. The prevalence of type 2 diabetes increased sequentially with each age category, as well as with each quartile of waist circumference, and HOMA-IR. Decreased HOMA-β was also associated with greater prevalence of type 2 diabetes.
Table 2.
Multivariate Adjusted Prevalence Ratios of Type 2 Diabetes Among Underweight/Normal Weight and Overweight/Obese Individuals
| Underweight/Normal Weight | Overweight/Obese | |
|---|---|---|
| Prevalence Ratio (95% CI) | Prevalence Ratio (95% CI) | |
| Demographic, Behavioral, Or Body Fat Covariate | Multivariate Adjusted* | Multivariate Adjusted* |
| Race/Ethnicity | ||
| White | 1.0 (Reference) | 1.0 (Reference) |
| Asian Indian | 1.8 (1.7, 1.8) | 0.9 (0.9, 0.9) |
| Age (years) | ||
| 20–29 | 1.0 (Reference) | 1.0 (Reference) |
| 30–39 | 3.2 (3.1, 3.2) | 2.2 (2.2, 2.2) |
| 40–59 | 2.6 (2.6, 2.6) | 2.9 (2.9, 2.9) |
| 60+ | 2.9 (2.8, 2.9) | 6.1 (6.1, 6.1) |
| Sex | ||
| Men | 1.0 (Reference) | 1.0 (Reference) |
| Women | 1.1 (1.1, 1.1) | 1.2 (1.2, 1.2) |
| Waist Circumference (cm) | ||
| 40–73 | 1.0 (Reference) | 1.0 (Reference) |
| 73−80 | 1.2 (1.2, 1.2) | 1.3 (1.1, 1.4) |
| 80–87.0 | 1.9 (1.9, 1.9) | 1.4 (1.3, 1.5) |
| 87+ | 3.0 (3.0, 3.0) | 1.8 (1.6, 1.9) |
| HOMA-IR (Standardized) | ||
| 0.08–0.53 | 1.0 (Reference) | 1.0 (Reference) |
| 0.53–0.75 | 1.3 (1.3, 1.3) | 0.8 (0.8, 0.8) |
| 0.75–1.09 | 2.8 (2.8, 2.8) | 1.6 (1.6, 1.6) |
| 1.09+ | 9.5 (9.5, 9.5) | 8.2 (8.1, 8.2) |
| HOMA-β (Standardized) | ||
| 0.06–1.07 | 1.0 (Reference) | 1.0 (Reference) |
| 1.07–1.5 | 0.4 (0.4, 0.4) | 0.4 (0.4, 0.4) |
| 1.5–2.18 | 0.2 (0.2, 0.2) | 0.2 (0.2, 0.2) |
| 2.18+ | 0.1 (0.1, 0.1) | 0.2 (0.2, 0.2) |
Each factor is adjusted for every other factor in the table
Compared to those who were underweight/overweight, those who were overweight/obese had less prevalence of type 2 diabetes in the 30–39 age group, but increased prevalence in those who were aged 60 and older. Increasing waist circumference had a greater associated with type 2 diabetes prevalence in those who were underweight/normal weight compared to those who were overweight or obese in the third and fourth quartiles, as did all quartiles of HOMA-IR. Similar results were also found when using the WHO-Asian cut-points for BMI, in multivariable log-binomial regression models (Supplemental Table 2).
4. Discussion
In this cross-sectional study of two large population-based cohorts, we found that Asian Indians had a greater prevalence of type 2 diabetes in all BMI categories compared to White individuals. We also noted that while type 2 diabetes was present in individuals who were underweight and normal weight, in both Asian Indian and White populations, Asian Indians had a 2.8 times greater prevalence of type 2 diabetes in the underweight category of BMI, and a 4.6 time greater prevalence in the normal weight category than did White individuals, and both Asian Indian men and women exhibited a higher prevalence of type 2 diabetes at lower levels of BMI than their White counterparts.
Overweight and obesity are well known risk factors for type 2 diabetes. However, our study findings indicate that there is a large proportion of individuals with type 2 diabetes even in the absence of elevated body mass index. Furthermore, the prevalence of type 2 diabetes in normal weight Asian Indians was even higher than that of overweight Whites. The results of our study are in accordance with a study estimating the prevalence of diabetes in White, African-American, Native Hawaiian, Japanese and Latino Americans by BMI category. Results of this study noted that while there was a proportion of individuals from all ethnic groups who were underweight and had diabetes, the prevalence of type 2 diabetes in underweight individuals was higher in all ethnic groups compared to Whites [25]. Similarly, a study analyzing data on US immigrant adults from the National Health Interview Survey to determine the prevalence of diabetes across 9 geographical regions of birth, found that diabetes prevalence among normal weight immigrants from Africa and the Indian subcontinent was higher than that of obese immigrants from Europe and South America [26]. Our results add additional evidence to the notion that type 2 diabetes exists in a substantial proportion of individuals who are without the traditional risk factor of increased body weight. It is possible that individuals who are underweight/normal weight may develop type 2 diabetes through a differing pathophysiological pathway than those who are overweight/obese. In our study, both Asian Indian and White participants with type 2 diabetes who were underweight had lower fasting insulin, and poorer insulin secretion as measured by HOMA-β compared to those with type two diabetes who were overweight or obese. These results were similar to earlier studies from India that found significantly higher fasting plasma glucose and lower fasting insulin in lean individuals with type 2 diabetes compared to those who were overweight or obese [27,28]. In our study, there were no differences in insulin resistance as measured by HOMA-IR in Asian Indians with type 2 diabetes who were underweight/normal weight compared to those who were overweight/obese. However, in White individuals, those with type 2 diabetes who were overweight/obese were also more insulin resistant than those who were underweight or normal weight. We also found that after adjusting for additional risk factors, Asian Indians no longer had an increased prevalence of type 2 diabetes compared to Whites in those who were overweight or obese. However, adjustment for age, sex, waist circumference, HOMA-IR and HOMA-β did not completely explain the increased prevalence of type 2 diabetes in Asian Indians compared to Whites, thereby suggesting that additional factors may play an important role. Furthermore, being in the second or third quartile of waist circumference or having increased measures of HOMA-IR was more strongly associated with diabetes risk in those who were underweight or normal weight compared to those who were overweight or obese, thereby suggesting that individuals with type 2 diabetes who are lean may have a phenotype that is more susceptible to metabolic disturbances. However, given that our study was cross-sectional in nature, it is not clear as to the relative contributions of insulin secretion and insulin resistance on diabetes development much earlier in the natural history, and whether this varies by BMI status. Therefore, further studies are needed to explore these differences.
Our study directly compared the prevalence of type 2 diabetes by BMI and ethnicity/ethnicity using two large, population based surveys using both self-report and laboratory measures. However, the results of our study should be interpreted in the context of several limitations. While glucose and insulin were analyzed in different laboratories, both used the same assays for analysis, thereby reducing intra-laboratory bias. Additionally, assays from the laboratory in Chennai have been run against a reference laboratory in the US and show a high concordance of r=0.945. Furthermore, while there were differences in the sampling frames, both studies are large, population-based samples that are representative either of the US, or an urban city in India. While the results of this study cannot be generalized to the entire Indian population, results from a recent nationally representative study from India reported an overall type 2 diabetes prevalence of 7.3%. However, mean BMI in the population was 22.1 kg/m2 thereby indicating a high disease burden in a country with relatively low mean BMI [29]. Furthermore, many rural areas of India are now starting to urbanize and experience dual burdens of over and underweight, as well as increases in diabetes prevalence [29–33]. Lastly, the cross sectional nature of our study does not allow us to assess the relative contributions of insulin resistance and insulin secretion throughout the natural history of diabetes progression in individuals who are normal weight/underweight compared to those who are overweight/obese. While elevated insulin resistance appears to be strongly associated with diabetes prevalence in both underweight/normal weight and overweight/obese populations, it is not clear as to whether the level of insulin secretion was sufficient to compensate for increased insulin resistance early in the natural history, and whether this differed by BMI status or ethnicity/ethnicity.
In conclusion, we found that type 2 diabetes is present in underweight and normal weight individuals in both White and Asian Indian populations. However, the prevalence of type 2 diabetes in underweight and normal weight groups was significantly higher in Asian Indians compared to Whites. Elevated measures of waist circumference and insulin resistance and decreased insulin secretion were associated with increased diabetes prevalence in both the underweight/normal weight, as well as the overweight/obese groups. However, adjustment for these factors did not explain the increased diabetes prevalence in Asian Indians compared to Whites in the underweight/normal weight category. Therefore further research is needed to determine the etiology behind the increased risk of type 2 diabetes underweight and normal weight populations, particularly with regards to pathophysiological pathways of development.
Supplementary Material
Highlights.
It is possible that a substantial proportion of individuals develop type 2 diabetes in the absence of overweight or obesity.
Compared to Whites, a large proportion of Asian Indians have a high type 2 diabetes prevalence even in the underweight and normal weight categories of body mass index.
The differences in type 2 diabetes prevalence in lean individuals between groups were not explained by differences in waist circumference, insulin resistance, or insulin secretion.
Additional studies should examine the pathophysiological mechanisms leading to type 2 diabetes development in lean individuals.
Acknowledgements
The authors have no conflicts of interest. The CARRS Study was funded in part by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under Contract No. HHSN268200900026C, and the United Health Group, Minneapolis, MN, USA., KMV Narayan was funded in part by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number P30DK111024.
Role of the Funding Source: The funding sources had no involvement in the collection, analysis, and interpretation of the data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–94. doi:10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tulloch-Reid MK, Williams DE, Looker HC, Hanson RL, Knowler WC. Do Measures of Body Fat Distribution Provide Information on the Risk of Type 2 Diabetes in Addition to Measures of General Obesity? Diabetes Care 2003;26:2556–61. doi:10.2337/diacare.26.9.2556. [DOI] [PubMed] [Google Scholar]
- [3].Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005;81:555–63. [DOI] [PubMed] [Google Scholar]
- [4].Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, et al. WEIGHT AS A RISK FACTOR FOR CLINICAL DIABETES IN WOMEN. Am J Epidemiol 1990;132:501–13. doi:10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- [5].Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The Disease Burden Associated With Overweight and Obesity. JAMA 1999;282:1523–9. doi:10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- [6].Oza-Frank R, Ali MK, Vaccarino V, Narayan KV. Asian Americans: diabetes prevalence across US and World Health Organization weight classifications. Diabetes Care 2009;32:1644–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care 2011;34:1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gupta LS, Wu CC, Young S, Perlman SE. Prevalence of Diabetes in New York City, 2002–2008 Comparing foreign-born South Asians and other Asians with US-born whites, blacks, and Hispanics. Diabetes Care 2011;34:1791–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Little M, Humphries S, Patel K, Dodd W, Dewey C. Factors associated with glucose tolerance, pre-diabetes, and type 2 diabetes in a rural community of south India: a cross-sectional study. Diabetol Metab Syndr 2016;8:21. doi:10.1186/s13098-016-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of Diabetes among Men and Women in China. N Engl J Med 2010;362:1090–101. doi:10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- [11].Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA 2017;317:2515–23. doi:10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miyakawa M, Shimizu T, Van Dat N, Thanh P, Thuy PTP, Anh NTH, et al. Prevalence, perception and factors associated with diabetes mellitus among the adult population in central Vietnam: a population-based, cross-sectional seroepidemiological survey. BMC Public Health 2017;17:298. doi:10.1186/s12889-017-4208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bailey SL, Ayles H, Beyers N, Godfrey-Faussett P, Muyoyeta M, du Toit E, et al. Diabetes mellitus in Zambia and the Western Cape province of South Africa: Prevalence, risk factors, diagnosis and management. Diabetes Res Clin Pract 2016;118:1–11. doi:10.1016/j.diabres.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Griffiths PL, Bentley ME. The nutrition transition is underway in India. J Nutr 2001;131:2692–2700. [DOI] [PubMed] [Google Scholar]
- [15].Shetty PS. Nutrition transition in India. Public Health Nutr 2002;5:175–182. [DOI] [PubMed] [Google Scholar]
- [16].Popkin BM, Horton S, Kim S, Mahal A, Shuigao J. Trends in Diet, Nutritional Status, and Diet-related Noncommunicable Diseases in China and India: the Economic Costs of the Nutrition Transition. Nutr Rev 2001;59:379–90. doi:10.1111/j.1753-4887.2001.tb06967.x. [DOI] [PubMed] [Google Scholar]
- [17].Nair M, Ali MK, Ajay VS, Shivashankar R, Mohan V, Pradeepa R, et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health 2012;12:701. doi:10.1186/1471-2458-12-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Census of India Website : Office of the Registrar General & Census Commissioner, India n.d. http://www.censusindia.gov.in/2011census/PCA/A4.html (accessed April 23, 2018).
- [19].Menke A, Orchard TJ, Imperatore G, Bullard KM, Mayer-Davis E, Cowie CC. The Prevalence of Type 1 Diabetes in the United States. Epidemiol Camb Mass 2013;24:773–4. doi:10.1097/EDE.0b013e31829ef01a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].NHANES - Survey Methods and Analytic Guidelines n.d. http://www.cdc.gov/nchs/nhanes/survey_methods.htm (accessed February 18, 2016).
- [21].American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010;33:S62–9. doi:10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- [23].Organization WH, others. Obesity: preventing and managing the global epidemic: report of a WHO consultation on obesity, Geneva, 3–5 June 1997 1998. [PubMed] [Google Scholar]
- [24].WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet Lond Engl 2004;363:157–63. doi:10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- [25].Maskarinec G, Grandinetti A, Matsuura G, Sharma S, Mau M, Henderson BE, et al. Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis 2009;19:49–55. [PMC free article] [PubMed] [Google Scholar]
- [26].Oza-Frank R, Narayan KM. Overweight and diabetes prevalence among US immigrants. Am J Public Health 2010;100:661–8. doi:10.2105/AJPH.2008.149492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mohan V, Vijayaprabha R, Rema M, Premalatha G, Poongothai S, Deepa R, et al. Clinical profile of lean NIDDM in South India. Diabetes Res Clin Pract 1997;38:101–8. doi:10.1016/S0168-8227(97)00088-0. [DOI] [PubMed] [Google Scholar]
- [28].Das S, Samal KC, Baliarsinha AK, Tripathy BB. Lean (underweight) NIDDM-peculiarities and differences in metabolic and hormonal status-a pilot study. J Assoc Physicians India 1995;43:339–342. [PubMed] [Google Scholar]
- [29].Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol 2017;5:585–96. doi:10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- [30].Hwang CK, Han PV, Zabetian A, Ali MK, Narayan KM. Rural diabetes prevalence quintuples over twenty-five years in low- and middle-income countries: A systematic review and meta-analysis. Diabetes Res Clin Pract 2012;96:271–85. doi:10.1016/j.diabres.2011.12.001. [DOI] [PubMed] [Google Scholar]
- [31].Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297. [DOI] [PubMed] [Google Scholar]
- [32].Misra P, Upadhyay RP, Misra A, Anand K. A review of the epidemiology of diabetes in rural India. Diabetes Res Clin Pract 2011;92:303–11. doi:10.1016/j.diabres.2011.02.032. [DOI] [PubMed] [Google Scholar]
- [33].Little M, Humphries S, Patel K, Dewey C. Factors associated with BMI, underweight, overweight, and obesity among adults in a population of rural south India: a cross-sectional study. BMC Obes 2016;3:12. doi:10.1186/s40608-016-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.