Abstract
Purpose:
The purpose of this study was to assess the effect of repetitive head impacts experienced by football players compared to non-contact athletes on dynamic postural control during both Single Task (ST) and Dual Task (DT) conditions.
Methods:
Thirty four football players wearing accelerometer instrumented helmets and 13 cheerleaders performed a dynamic postural control battery, consisting of ST and DT gait initiation, gait, and gait termination, both prior to and following the football season. A 2 (group) x 2 (time) repeated measures ANOVA compared performance across 32 dynamic postural outcomes. A linear regression was performed on postural control change scores with common head impact kinematics serving as the independent variables.
Results:
The football players experienced a mean of 538.1 ± 409.1 head impacts in the season with a mean linear acceleration of 27.8 ± 3.2 g’s. There were no significant interactions for any of the ST or DT dynamic postural control tasks. There was a significant relationship between head impact kinematics and the lateral center of pressure displacement during the anticipatory postural adjustment phase (r2 = 0.26, p=0.010) and transitional phase (r2 = 0.511, p=0.042) during ST gait initiation. For both measures, the number of impacts exceeding 98 g’s was the only significant predictor of decreased center of pressure displacement.
Conclusions:
A single competitive football season did not adversely affect dynamic postural control when comparing football players to cheerleaders who do not experience repetitive head impacts. Furthermore, there were limited relationships with head impact kinematics suggesting that a single season of football does not adversely affect most outcome measures of instrumented dynamic postural control. These findings are consistent with most studies which fail to identify clinical differences related to repetitive head impacts.
Keywords: Concussion, Subconcussive Impacts, balance, biomechanics
INTRODUCTION
While concussion remains a major public health issue and societal problem, recent years have seen an increased concern over repetitive head trauma, independent of concussion, which occurs through routine contact sports participation such as football and soccer.(1) These repeated head impacts (RHI) are the result of blows to the head which neither result in any overt concussion symptomology nor receive medical treatment. Animal studies suggest that RHI, not independently associated with cellular damage, result in neurological dysfunction when repeated in close temporal proximity and are associated with early development of cis-p-tauopathy, a potential precursor to chronic traumatic encephalopathy (CTE).(2–4) In humans, it is speculated that RHI may be associated with later life neuropathologies resulting from cumulative lifetime exposure and is likely mediated by age of first exposure, magnitude and timing of exposures, and other individual characteristics.(5) Most recently, an association between RHI and the development of CTE has been proposed based on animal modeling and the findings of CTE in athletes without prior diagnosed concussion.(4)
While the long term later life association with neuropathology remains an ongoing challenge given the obvious difficulties of longitudinal studies (e.g., tracking athletes for decades post-career),(6) the effects of RHI over the course of an athletic season are being utilized to model the short term effects. In collegiate student-athletes, findings to date are mixed and most clinical and behavioral assessments finding no differences over the course of a collision sports season. (7–11) Conversely, many neuroimaging studies do identify altered brain structure and/or function over the course of a similar collision sports season.(12–15) It is currently unknown if the changes observed in these neuroimaging studies represent compensatory adaptations or long lasting brain impairments.(13) Furthermore, inconsistency in neuroimaging changes (e.g., both increased and decreased fractional anisotrophy during diffusion tensor imaging) and lack of correlation with behavioral responses suggest caution must be exercised when interpreting these findings.(16) Whereas standard clinical and behavioral neurological examinations may lack sensitivity to identify neurological changes and neuroimaging findings may lack specificity, instrumented measures of postural control are well established markers of neurological health in diverse populations and may be well suited to elucidate changes.(17, 18)
Gait is a stable motor task over time in healthy young adults and routinely identifies postural control impairments across neurologically challenged populations including following concussion.(19) However, transitional dynamic tasks such as gait initiation (GI), the transition from quiet stance to locomotion, and planned gait termination (GT), the transition from locomotion to quiet stance, provide even greater challenges to the postural control system.(19–23) An additional advantage of gait related tasks is the known association between task performance and supraspinal structures and pathways based on animal, neuroimaging, and stroke related research.(24–26) During gait, the rhythmic activity is generated by the central pattern generators in the spinal cord; however, overriding supraspinal control remains present.(26) During transitional movements, the anticipatory postural adjustment (APA) phase of GI is likely controlled by the supplementary motor area whereas GT control largely resides in the dorsal lateral prefrontal cortex. (24–26) The addition of a cognitive challenge to these tasks, termed dual task (DT), requires the individual to share attentional capacity between the motor and cognitive challenges and increases the tasks discriminatory capabilities.(18) Not surprisingly, DT gait has routinely identified both acute and lingering postural control deficits post-concussion.(27, 28)
Neuroimaging studies have identified alterations associated with RHI whereas clinical and behavioral screening assessments have not; this suggests more sophisticated and sensitive clinical assessments may be required to identify subtle nervous system changes.(7, 9, 12) Furthermore, motor performance has received limited evaluation compared to cognitive function despite the general independence of the systems. Therefore, the purpose of this study was to assess the effect of RHI experienced by football players compared to non-contact athletes on dynamic postural control during both single (ST) and DT conditions at two institutions. We hypothesized that RHI would adversely affect postural control in the football group as compared to the non-contact athletes. Secondly, we hypothesized a dose response relationship would be present and those football participants with greater number and magnitudes of impact would experience greater deficits.
METHODS
Participants
There were two groups of participants from two institutions enrolled in this study, 1) 34 NCAA Division I football players (Football) wearing helmets instrumented with the Helmet Impact Telemetry System (HITS) from two institutions (one Football Championship Subdivision and one Football Bowl Subdivision) and 2) a University cheerleading (N=13; 11 Female, 2 Male) team (Cheer) which served as a non-repetitive head impact control group. (Table 1) The HITS system is only available in specific Riddell football helmets (Riddell, Inc., Chicago, IL.) and therefore the participants in the football group were delimited to those wearing Speed and Speed classic helmets and was roughly divided between skill (N=16) and lineman (N=18) positions. Potential participants were excluded if they self-reported neurological, visual, vestibular, or balance related pathologies which would adversely influence gait and balance. To maintain an ecologically valid sample, history of prior concussion was not an exclusion criteria; however, no participant had a currently clinically unresolved concussion at either testing session or suffered a concussion during the course of the season and all participants were medically cleared for full unrestricted participation during both the pre and post-season testing. All participants provided written informed consent as approved by the University of Delaware and Georgia Southern University institutional review boards.
Table 1.
Participant Demographics.
| Group | Age (years) | Height (cm)* | Weight (kg)* | Concussion History |
|---|---|---|---|---|
| Football | 20.2 ± 1.2 | 185.7 ± 7.1 | 103.4 ± 18.5 | 35.3% Prior Concussion 0.5 ± 0.8 concussions (R: 0 – 3) |
| Cheerleading | 19.8 ± 1.2 | 161.2 ± 9.3 | 58.3 ± 10.5 | 53.8% Prior Concussion 0.8 ± 1.0 concussions (R: 0 – 3) |
|
Player Position Distribution |
Offensive Line: 10 Wide Receiver: 5 Defensive Back: 5 Linebacker: 3 Defensive Line: 3 Running Back: 3 Special Teams: 2 Tight End: 2 Quarterback: 1 |
|||
There were significant differences between groups for height (p<0.001) and weight (p<0.001). There was no difference between groups for Concussion History (p=0.311) or Age (p=0.403). The player position is the primary position the individual participated during the course of the season.
Instrumentation
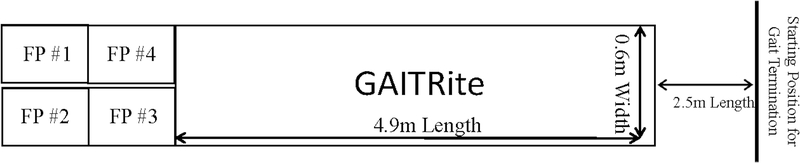
This project included two collaborating institutions and assessments were performed at both sites with the same testing equipment while one site also performed additional forceplate based assessments (N=28: 15 FB and 13 Control participants). (Figure 1) Gait performance was assessed using a 4.9 m GAITRite instrumented walkways (CIR Systems, Sparta, NJ, USA). The GAITRite is reliable (ICC: 0.8 – 0.98) and valid (ICC: 0.92–0.99) portable walkway gait analysis system with 18,432 sensors over a 0.6m (w) x 4.9m (l) grid that detect footfalls as the participant transverses the walkway.(29, 30) Kinetic data was collected at 1,000 Hz from four adjacent force plates (AMTI, Model OR-6, Watertown, MA.) embedded level with the laboratory floor.
Figure 1. Laboratory Set-up.
The participants begin each GI trial with one foot on force plate #1 and one foot on force plate #2. The participant initiated gait and their first heel strike impacted either force plate #3 or #4 and the participant continued down the GAITRite mat and stopped approximately 2.5 meters thereafter. The GT trials occurred in reverse direction with the individuals traversing the GAITRite mat before terminating gait with the penultimate step on either force plate #3 or #4 and the terminating step occurring with one foot on force plate #1 and one foot on force plate #2.
The football participant’s helmets were instrumented with HITS which has been extensively described previously.(31, 32) Briefly, HITS is comprised of 6 single axis accelerometers embedded inside a Riddell football helmet which communicates wirelessly with the Sideline Response System (SRS) computer.(31, 32) If the HITS accelerometer cannot immediately communicate with the SRS due to proximity or other reasons, the kinematic data is stored onboard the encoder and transmitted to the SRS once the system communication is restored. The impact data is recorded at 1,000 Hz in 40 ms time periods including 8 ms prior to the trigger and 32 ms thereafter.(31, 32) The accelerometers utilize established algorithms to calculate the resultant linear accelerations from the x-axis and y-aixs.(31, 32) The HITS system has been validated for recording the number of impacts, impact location (±0.41cm), and peak translational acceleration (4% error).(31–33) However, the lack of a z-axis results in an estimation of the angular or rotational acceleration and therefore the rotational accelerations are not utilized in current study.(31–35) The HITS data was cleaned to remove any impacts which occurred outside of game/practice times or any observed non-head impact measures (e.g., player observed throwing their helmet). The threshold for an impact was 10g’s which is commonly utilized with the HITS system.(7, 36, 37)
Procedures
All participants were tested on two occasions, 1) in July or August prior to the start of the athletic season, and 2) within 72 hours of the conclusion of the football season. The cheerleaders were tested at the same time points as the football players despite their season continuing to progress until April. There were two sites in this study and the same procedures were followed at each test site, but only one site had embedded force plates; thus 28 participants completed the GI and GT components and all 47 completed the gait trails. All participants were currently medically cleared for full unrestricted athletic participation at the time of testing and participants completed the following assessments;
Single Task GI, Gait, and GT:
All tasks were completed utilizing standard biomechanics laboratory protocols for gait and balance data collection. Participants began each GI trial standing with one foot on adjacent force plates, initiated movement with their self-selected limb in response to a verbal cue, traversed the 4.9 m walkway at a self-selected pace, and continued walking to a mark on the ground 2.5 m beyond the walkway.(38, 39) (Figure 1) Following a brief break, participants were then positioned at the floor mark ~2.5 m prior to the walkway and, in response to a verbal cue, traversed back down the walkway and terminated gait on the four force plates such that the penultimate step impacted force plate 3 or 4 and termination occurred on force plate 1 and 2.(21, 22) (Figure 1) Participants completed 5 trials each of GI and GT and thus 10 trials of gait.
Dual Task GI and Gait:
Participants began each trial in the same position as the ST trial; however, the initiation cue was a previously utilized cognitive challenge (months of the year backwards, spelling a word backwards, and serial 7’s).(27, 28) Participants were instructed to begin walking (GI) when the cognitive challenge was presented and continue answering the questions as they progressed down the walkway. Each participants completed 5 trials of DT GI and gait only, no DT GT trials were performed since the participant typically completed the cognitive challenge prior to terminating gait.
Data Analysis
Gait Initiation (GI):
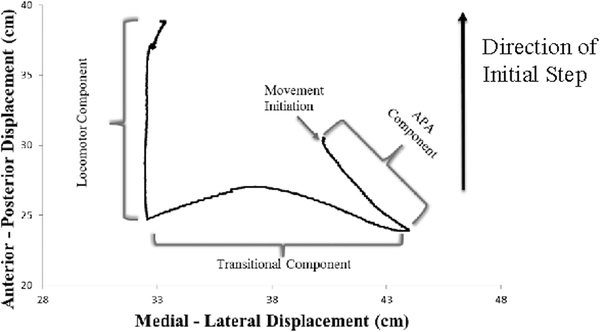
The displacement of the center of pressure (COP) was divided into three segments based on two landmarks as frequently described in the literature.(23, 39) (Figure 2A) Briefly, the COP initially is displaced posteriorly and towards the initial swing limb and this represents the anticipatory postural adjustment (APA) component of GI. Thereafter, the transitional phase occurs as the COP shifted from the initial swing limb to the stance limb as the swing limb unloads. Finally, the locomotor phase encompasses the COP anterior displacement under the initial stance limb as it progresses to toe-off. The anterior/posterior (AP) and medial-lateral (ML) displacement was calculated for each of the three phases.(23, 39)
Figure 2A.
Exemplar of Gait Initiation (GI) Center of Pressure (COP) displacement for Right Foot initial step. Movement initiation is operationally defined as the first change (± 2 SD from the mean of the first 500 ms of quiet stance) in posterior displacement of the COP. The APA component ends at the most posterior and medial position towards the initial swing limb. The transitional component ends at the maximum translation towards the initial stance limb. The locomotor component ends at toe-off of the initial stance limb.
Gait:
The GAITRite instrumented walkway provides common stepping characteristics.(29, 30) The outcomes measures in this study were mean step velocity, mean step length, mean heel to heel base of support (HHBOS), and the percentage of the gait cycle in stance, and mean of the left and right was utilized as the outcome measure for all dependent variables.(27) During the gait trials, the cognitive component was also assessed which included; 1) “correct answers” which was calculated as the total number of correct answers from the five DT trials, and 2) the “accuracy” of the answers which was defined as the number of correct answers divided by the total number of answers attempted.(27)
Gait Termination (GT):
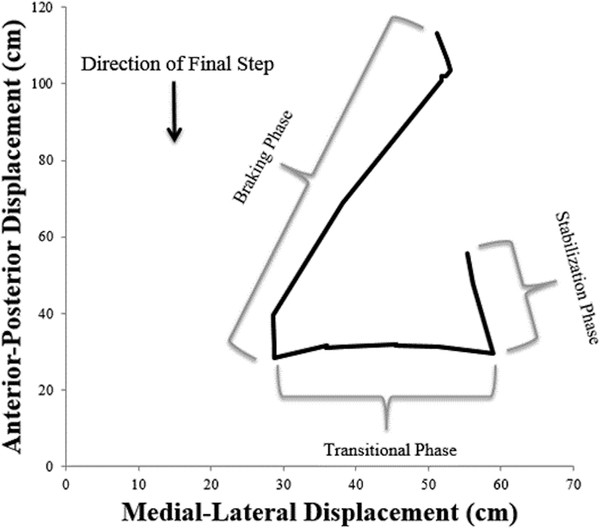
The COP displacements during GT are similar, but reversed, from GI.(21) (Figure 2B) The braking phase of GT occurs during a shift from the initial heel contact of the lead limb, during the penultimate step, to the ball of the terminating step foot during ground contact. During the transitional phase, COP is transferred underneath the lead limb, until the trailing limb concludes swing phase and a bipedal stance is resumed on the force plate. The stabilization phase is a final shift of the COP back to the center of the foot once both feet are planted.(21) Similar to GI, the AP and ML displacement was calculated for each of the phases.
Figure 2B.
Exemplar of Gait Termination (GT) Center of Pressure displacement. The Braking Phase represents the transition from penultimate step (left foot) to the termination step (right foot) with the left foot then ending forward locomotion during the Transitional and Stabilization phases.
Statistical Analysis
Descriptive statistics (mean and standard deviations) were calculated for all dependent variables to provide measures of central tendency and variability. To assess for changes over the course of the athletic season, separate 2 (group) x 2 (time) mixed design ANOVAs were performed for each dependent variable of interest. Significant interactions were followed-up with a pairwise comparison using Tukey procedure. If there was a difference at pre-season between groups, then an ANCOVA was performed with the pre-season serving as the covariate and the post-season serving as the dependent variable. Concussion history was not utilized as a covariate due to established challenges in accurately ascertaining lifetime concussion history.(40) Due to the lack of independence amongst variables, a more conservative alpha value of 0.01 was applied.
To assess for the role of head impacts on change in performance in the football players, separate linear regression analysis (enter method) was performed on the change score (post-season minus pre-season) for each dependent variable of interest. The four independent variables were; 1) number of head impacts, 2) mean linear acceleration, 3) cumulative linear acceleration, and 4) number of impacts exceeding 98 g’s which serves as a measure of a high impact threshold.(7) Following review of the variance inflation factors (VIF), the cumulative linear acceleration independent variable was removed from the model (VIF ≥20) and the remaining three independent variables served as predictors (VIF ≤5).
RESULTS
Head Impacts
There were a total of 18,295 head impacts (mean: 538.1 ± 409.1; Range: 80 – 1907; Median: 436) measured among all football participants with a mean of 5.3 ± 5.7 (Range: 0 – 16; Median: 5) impacts exceeding 98 g’s. The mean linear acceleration was 27.8 ± 3.2g’s (Range: 21.8 – 36.5; Median: 28.1). The distribution of helmet impacts were 1) Front of helmet (44.2 ± 15.4%), 2) Back of helmet (26.9 ± 13.1%), 3) Top of helmet (15.9 ± 10.9%), 4) Right side of helmet (6.7 ± 3.7%), 5) Left side of helmet (6.2 ± 3.1%).
There was no difference between groups for the number of days between the two testing sessions (Football: 131.3 ± 2.2 days and Cheerleading: 129.1 ± 1.9 days; p=0.796).
Effects of the Season
Gait Initiation
There were significant group differences at pre-season for both ST and DT APA M/L Displacement, transitional phase M/L Displacement, locomotor phase A/P Displacement with the football participants having larger displacements than cheer. (Table 2) Therefore, for these dependent variables, ANCOVAs were performed with the pres-sesaon value serving as the covariate.
Table 2.
Gait Initiation and Termination Displacement Results. Single Task (ST) and Dual Task (DT).
| Contact Athletes (N=15) |
Non-Contact Athletes (N=13) |
Interaction Effect p-value |
Regression Model | |||||
|---|---|---|---|---|---|---|---|---|
| Gait Initiation (ST) | Pre Mean ± SD |
Post Mean ± SD |
Pre Mean ± SD |
Post Mean ± SD |
R2 | F | P | |
| APA Posterior (cm) | 4.8 ± 1.9 | 4.7 ± 1.9 | 4.3 ± 1.4 | 4.0 ± 1.4 | 0.605 | 0.055 | 0.144 | 0.961 |
| APA Lateral (cm)* | 5.5 ± 1.8 | 5.5 ± 2.0 | 3.5 ± 1.2 | 3.7 ± 1.5 | 0.564 | 0.526 | 6.171 | 0.026‡ |
| Transition Posterior (cm) | −0.1 ± 1.7 | 0.2 ± 1.8 | 0.1 ± 2.7 | 0.4 ± 3.0 | 0.937 | 0.084 | 0.229 | 0.916 |
| Transition Lateral (cm)* | 19.5 ± 4.7 | 19.6 ± 4.2 | 13.6 ± 3.1 | 14.2 ± 3.2 | 0.285 | 0.378 | 3.832 | 0.042‡ |
| Locomotor Anterior (cm)* | 18.5 ± 2.0 | 18.1 ± 3.3 | 15.8 ± 2.5 | 15.1 ± 2.5 | 0.970 | 0.283 | 2.380 | 0.121 |
| Locomotor Lateral (cm) | 1.8 ± 0.9 | 2.6 ± 3.6 | 2.1 ± 1.2 | 1.8 ± 0.9 | 0.267 | 0.399 | 3.326 | 0.056 |
| Initial Step Length (cm) | 56.6 ± 3.1 | 58.7 ± 6.2 | 56.1 ± 3.9 | 58.7 ± 3.7 | 0.785 | 0.230 | 2.048 | 0.163 |
| Initial Step Velocity (cm/s) | 48.4 ± 4.6 | 51.8 ± 8.3 | 50.1 ± 4.9 | 53.8 ± 3.7 | 0.528 | 0.377 | 3.122 | 0.066 |
| Gait Termination | Pre Mean ± SD |
Post Mean ± SD |
Pre Mean ± SD |
Post Mean ± SD |
Interaction | R2 | F | P |
| Braking A/P | 60.5 ± 5.7 | 64.8 ± 8.0 | 62.8 ± 5.6 | 65.3 ± 5.7 | 0.595 | 0.156 | 0.461 | 0.763 |
| Braking M/L* | 21.6 ± 3.8 | 23.2 ± 4.7 | 16.4 ± 3.6 | 19.1 ± 2.5 | 0.444 | 0.166 | 1.696 | 0.227 |
| Transitional A/P | 5.6 ± 4.4 | 5.6 ± 4.3 | 5.8 ± 5.6 | 4.2 ± 2.8 | 0.433 | 0.362 | 2.989 | 0.073 |
| Transitional M/L* | 17.6 ± 4.6 | 18.7 ± 5.1 | 12.7 ± 2.6 | 12.8 ± 2.4 | 0.066 | 0.134 | 0.588 | 0.679 |
| Stabilization A/P | 1.9 ± 0.7 | 1.8 ± 0.6 | 2.8 ± 3.0 | 2.2 ± 0.9 | 0.565 | 0.120 | 0.625 | 0.655 |
| Stabilization M/L | 4.6 ± 3.4 | 5.1 ± 4.0 | 3.3 ± 2.2 | 2.6 ± 1.9 | 0.324 | 0.100 | 1.391 | 0.305 |
| Gait Initiation (DT) | Pre Mean ± SD |
Post Mean ± SD |
Pre Mean ± SD |
Post Mean ± SD |
Interaction | R2 | F | P |
| APA Posterior (cm) | 3.0 ± 1.5 | 3.4 ± 1.6 | 2.3 ± 0.8 | 3.0 ± 1.3 | 0.540 | 0.039 | 1.141 | 0.392 |
| APA Lateral (cm)* | 4.7 ± 2.4 | 4.1 ± 1.9 | 2.3 ± 1.1 | 3.3 ± 1.5 | 0.046 | 0.223 | 2.007 | 0.169 |
| Transition Posterior (cm) | 0.9 ± 2.1 | 0.4 ± 2.1 | 1.1 ± 2.9 | 1.0 ± 2.6 | 0.543 | 0.063 | 1.235 | 0.356 |
| Transition Lateral (cm)* | 18.7 ± 5.3 | 18.2 ± 4.1 | 14.7 ± 2.9 | 13.5 ± 3.0 | 0.798 | 0.077 | 0.749 | 0.581 |
| Locomotor Anterior (cm)* | 17.1 ± 2.6 | 16.6 ± 2.8 | 13.5 ± 2.6 | 15.1 ± 5.6 | 0.275 | 0.408 | 3.417 | 0.052 |
| Locomotor Lateral (cm) | 3.1 ± 3.1 | 1.6 ± 0.9 | 1.8 ± 1.2 | 2.3 ±2.2 | 0.073 | 0.010 | 1.034 | 0.436 |
| Initial Step Length (cm) | 55.1 ± 7.6 | 57.9 ± 7.0 | 56.8 ± 3.7 | 59.1 ± 3.5 | 0.719 | 0.076 | 0.754 | 0.578 |
| Initial Step Velocity (cm/s) | 41.7 ± 7.5 | 44.3 ± 9.2 | 42.1 ± 4.5 | 46.0 ± 6.9 | 0.427 | 0.168 | 1.708 | 0.224 |
Indicates a group difference at baseline and therefore an ANCOVA was performed with the baseline test serving as the covariate.
Indicates a significant regression.
There were no significant group by time interactions during ST GI. There were no significant group by time interactions during DT GI. (Table 2)
Gait
There were significant group differences at pre-season for HHBOS and Stance with the football participants having a wider base of support and higher percentage of their gait cycle in stance; therefore these were subsequently compared with an ANCOVA with the pre-season performance serving as the covariate. (Table 3) There were no significant pre-season differences for the cognitive component of the DT trials.
Table 3.
Gait Results. Single Task (ST) and Dual Task (DT).
| Contact Athletes (N=36) |
Non-Contact Athletes (N=13) |
Interaction Effect p-value |
Regression Model | |||||
|---|---|---|---|---|---|---|---|---|
| Gait: Single Task | Pre Mean ± SD |
Post Mean ± SD |
Pre Mean ± SD |
Post Mean ± SD |
R2 | F | P | |
| Step Velocity (m/s) | 1.26 ± 0.12 | 1.27 ± 0.12 | 1.29 ± 0.10 | 1.32 ± 0.13 | 0.305 | 0.068 | 0.508 | 0.730 |
| Step Length (m) | 0.69 ± 0.05 | 0.59 ± 0.06 | 0.65 ± 0.05 | 0.66 ± 0.05 | 0.914 | 0.004 | 0.097 | 0.440 |
| Heel-to-Heel BOS (m)* | 0.14 ± 0.03 | 0.13 ± 0.03 | 0.09 ± 0.03 | 0.10 ± 0.03 | 0.761 | 0.064 | 0.535 | 0.711 |
| Stance (% of gait cycle)* | 61.8 ± 1.2 | 61.6 ± 1.0 | 60.3 ± 0.09 | 60.2 ± 0.08 | 0.195 | 0.085 | 1.722 | 0.174 |
| Gait: Dual Task Motor | Pre Mean ± SD |
Post Mean ± SD |
Pre Mean ± SD |
Post Mean ± SD |
Interaction | R2 | F | P |
| Step Velocity (m/s) | 1.10 ± 0.16 | 1.15 ± 0.15 | 1.10 ± 0.12 | 1.16 ± 0.12 | 0.753 | 0.123 | 0.151 | 0.961 |
| Step Length (m) | 0.64 ± 0.06 | 0.65 ± 0.07 | 0.61 ± 0.05 | 0.62 ± 0.06 | 0.329 | 0.066 | 0.519 | 0.723 |
| Heel-to-Heel BOS (m)* | 0.15 ± 0.03 | 0.15 ± 0.04 | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.771 | 0.006 | 0.952 | 0.449 |
| Stance (% of gait cycle)* | 62.6 ± 1.4 | 62.2 ± 1.2 | 61.3 ± 0.7 | 61.0 ± 0.7 | 0.162 | 0.029 | 0.784 | 0.545 |
| Gait: Dual Task Cognitive | Pre Mean ± SD |
Post Mean ± SD |
Pre Mean ± SD |
Post Mean ± SD |
Interaction | R2 | F | P |
| Questions Answered (N) | 21.0 ± 5.5 | 22.6 ± 6.4 | 21.4 ± 4.1 | 21.8 ± 3.5 | 0.287 | 0.037 | 0.730 | 0.579 |
| Cognitive Accuracy (%) | 90.0 ± 9.2 | 91.1 ± 10.0 | 93.4 ± 8.4 | 94.6 ± 8.3 | 0.943 | 0.056 | 1.448 | 0.247 |
Indicates a group difference at baseline and therefore an ANCOVA was performed with the baseline test serving as the covariate.
There were no significant group by time interactions for the ST or DT stepping characteristics. There were no significant group by time interactions for the cognitive components of the DT trials. (Table 3)
Gait Termination
There were significant group differences at pres-season for braking phase and transitional phase COP medial lateral displacements with the Football participants having a larger displacement in both phases; therefore these were subsequently compared with an ANCOVA with the pres-season performance serving as the covariate.
There were no significant group by time interactions for COP displacement during Gait Termination. (Table 2)
Effects of Head Impact Kinematics
There were significant regression outcomes for APA M/L phase (R2 = 0.526, F=6.171, P=0.010; Β= −1.629, 95% CI: −11.426 – 8.169) and Transitional M/L phase (R2 = 0.511, F=3.832, P=0.042; Β= 4.576, 95% CI: −13.79 – 22.941) during ST GI. There were no relationships between head impact kinematics and any gait, GT, DT GI, or other ST GI variables. (Table 2)
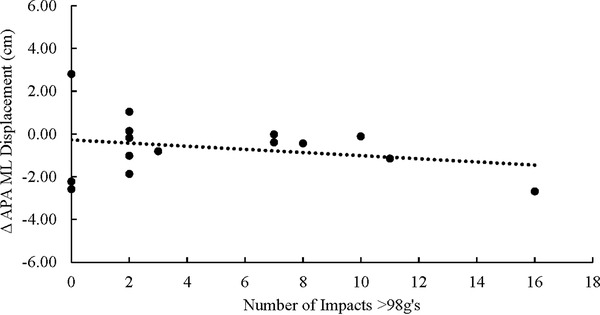
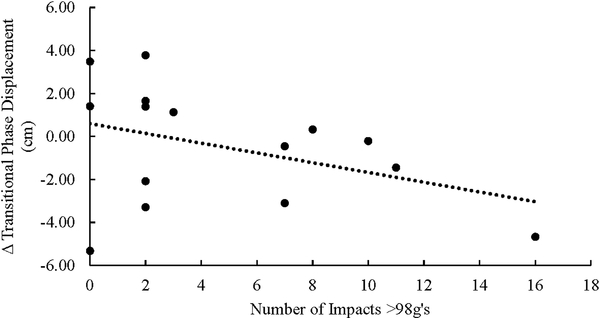
The number of impacts exceeding 98 g’s was the only independent predictor which was significant for APA M/L phase (Β=0.431, t=3.904, p=0.002). (Figure 3A) The number of impacts exceeding 98 g’s was the only independent predictor which was significant for changes in the Transitional M/L phase (Β=0.571, t=2.563, p=0.026). (Figure 3B) In both instances, an increased number of impacts >98G’s was associated with a reduction in the COP displacement.
Figure 3A.
There was significant relationship between the number of impacts exceeding 98g’s and the change in COP displacement during the APA lateral displacement phase of GI (P=0.002). The APA ML displacement is calculated as Post minus Pre, thus a negative number reflects decreased displacement and a positive number reflects increased displacement.
Figure 3B.
There was significant relationship between the number of impacts exceeding 98g’s and the change in COP displacement during the Transitional component of GI (P=0.026). The transitional phase displacement is calculated as Post minus Pre, thus a negative number reflects decreased displacement and a positive number reflects increased displacement.
DISCUSSION
Repetitive head impacts have been associated with neuroimaging changes over the course of a football season, but relatively few changes have been found in clinical or behavioral assessments.(7–15) Herein, instrumented dynamic postural control was assessed pre- and post-season in both football players wearing instrumented helmets and cheerleaders who served as non-RHI control participants. The primary finding of this study was no significant interactions in either ST or DT performance between groups over the course of the season. Furthermore, there were limited adverse relationships between head impact kinematics and dynamic postural control with only ST GI APA Lateral and Transitional displacement phases being associated with the number of ≥98g impact sustained. Taken together, these results suggest that dynamic postural control is not impaired by participation in a collegiate football season and head impact kinematics generally do not adversely affect postural control.
The neurological components essential to healthy dynamic postural control include the somatosensory, motor, and cognitive systems which allows the successful integration of the individual, task, and environment.(18) The attentional resources available from the cognitive systems vary depending on the task as well as the individual’s age and balance capabilities which makes DT an effective strategy to challenge the neurological systems of otherwise healthy young adults and has routinely identified post-concussion deficits.(18, 27, 28) Transitional motor tasks such as GI and GT further challenge the postural control systems and have also identified deficits post-concussion and in other neurological populations.(20–24) Thus, this test battery should be ideally situated to identify motor impairments associated with RHI over the course of a football season; however, no interactions were identified across 32 dependent variables including both ST and DT as well as transitional and steady state tasks.(19) While neuroimaging studies have routinely identified differences over the course of a season, it has been speculated the changes may originate from impaired neurovascular coupling which may not adversely affect motor planning or their associated descending pathways.(13) Furthermore, while RHI changes are commonly reported in high school athletes, fewer studies have identified changes in collegiate student-athletes.(12–15) It is unknown if these neuroimaging changes represent permanent changes in brain health or reflect compensatory changes which may allow for appropriate motor outcomes despite central neurological impairments.(13) Finally, it is possible that these findings accurately represent the effect of a football season’s related RHI and there was no adverse effects to the motor system.(11) Future work needs to continue to bridge the gap between neuroimaging and clinical or behavioral health outcomes to address the short term safety of collision sports which involve RHI.
There were two significant relationships, out of 32 assessed variables, between head impact kinematics, specifically the number of impacts exceeding 98 g’s, and ST GI performance. (Figures 3A and 3B) In both instances, increased number of high impacts predicted poorer performance as represented by decreased lateral COP displacement during both the APA phase and transitional phase of ST GI. The lateral COP displacement during the APA phase of GI is regulated by gluteus medius muscle activation and, in conjunction with the posterior COP displacement, is responsible for the decoupling of the COP from the Center of Mass during movement initiation and impairments have been noted in a multiple aging and neurological diseased populations.(20, 23, 24, 39) The COP lateral displacement during the transitional phase of GI accompanies the weight shift to the stance limb during the swing limb unloading and is rarely affected by neurological disorders.(20, 23, 24) Repeated high magnitude impacts have been associated with microhemorrhages, based on susceptibility weighted imaging, which could be related to impaired anticipatory postural adjustments (e.g., initial lateral displacement), but are unlikely to be affect the more mechanical transitional phase of GI.(13) Post-priori analysis failed to identify any balance or stepping characteristics (e.g., initial stance or step width, pre-movement limb loading) likely to predict COP transitional phase displacement, but the lack of 3D kinematics and electromyography limit the ability to explain this finding and these approaches should be incorporated in future studies. This finding is consistent with recent work by Slobounov who reported that neuroimaging changes, specifically changes in functional connectivity and cerebral blood flow, in collegiate football players who had more high impacts (>80 g’s) in practices during the season.(13) Similarly, McAllister reported an association between frequency and magnitude of RHI in college football players with both white matter changes and impaired cognition and suggested a subgroup of respondents may have elevated susceptibility to RHI.(12) Conversely, Gysland did not identify a relationship between high impact (>90 g’s) and performance on a clinical assessment battery.(7) It is important to note that the remaining 30 of 32 (93.8%) of dynamic postural control and cognitive variables showed no relationship to head impact kinematics. (Tables 2 and 3) Furthermore, the number of head impacts was not related to any of the 32 variables in this study which suggests that RHI alone, independent of magnitude, does not affect ST or DT dynamic postural control following a football season. This is important as RHI alone has been speculated to be strongly associated with later life neuropathological outcomes.(4) Taken together, these results suggest that neuroimaging, but not clinical or behavioral, changes are associated with high magnitude RHI and the results herein are generally consistent with this pattern.
The results of this study were consistent with previous reports of both head impact kinematics and dynamic postural control.(9, 12, 36, 37) Specifically, the football participants in this study experienced 538 ± 409 head impacts (range: 80 – 1907) over the course of the season with a mean linear acceleration of 27.8 ± 3.2 g’s. These values are consistent with earlier studies which frequently report ranges of 75 – 1400 impacts with means in the 400 – 600 range and mean linear accelerations in the 20 – 30 g range.(9, 12, 36, 37, 41) Over the course of a four year collegiate football career, these results suggest the average player would exceed 2,100 impacts which surpasses the proposed thresholds for elevated risk of impaired executive function and increased risk of depression in later life.(42)
While no differences in cognitive performance were noted between pre- and post-season nor was any relationship identified with head impact kinematics, it must be noted this study was limited by only utilizing a single cognitive modality, working memory, over a single football/ cheerleading season and future investigations should diversify cognitive assessments (e.g., stroop, N-back) preferably over multiple collegiate seasons. Furthermore, psychosocial outcomes were not assessed and therefore no conclusions relative to depression, anxiety, or other mental health condition can be implied. This investigation utilized a comprehensive dynamic postural control battery and noted few differences; however, future investigations could address this limitation by incorporating other postural control challenges (e.g., perturbations) as well as clinically feasible assessments (e.g., ST and DT Tandem Gait).(43) Cheerleaders experience fairly high rates of concussions, but are not generally considered a RHI sport and no cheerleader in this study was diagnosed with a concussion. Furthermore, there were clear differences in participant sex between groups and future studies should consider sex as a biological variable in their investigations; however, it is important to note there were few differences in baseline performances between the male football and mostly female (11/13) cheerleading groups. Finally, there is ongoing debate regarding the utilization of helmet mounted accelerometer systems,(33) but the HITS system is the most commonly utilized approach; however, these results are limited by the efficacy of the HITS system and rotational acceleration was not utilized as an independent variable.(31–35)
The results of this study failed to identify any widespread motor deficits across the course of a football season and limited relationships between head impacts kinematics and a comprehensive instrumented ST and DT postural control battery. While neuroimaging studies routinely identify differences, these results herein, consistent with prior outcomes, suggest a single season of football does not adversely affect performance across a variety of clinical or behavioral assessments. As the long term risk of collision sport participant remains an ongoing debate and can only be truly addressed with prospective longitudinal studies,(6) continued investigation into the short and mid-term effects of collision remains warranted.
Acknowledgements
This study was funded, in part, by a grant from the National Institute of Health/Neurological Disorders and Stroke (1R15NS070744). The funding agency had no role in the development of this manuscript or the decision to submit to Medicine and Science in Sports and Exercise.
Footnotes
The authors have no relationships with companies or manufacturers who will benefit from the results of the present study. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Publisher's Disclaimer: This study was funded, in part, by a grant from the National Institute of Health/Neurological Disorders and Stroke (1R15NS070744). The funding agency had no role in the development of this manuscript or the decision to submit to Medicine and Science in Sports and Exercise. The authors have no relationships with companies or manufacturers who will benefit from the results of the present study. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.McAllister T, McCrea M. Long-Term Cognitive and Neuropsychiatric Consequences of Repetitive Concussion and Head-Impact Exposure. J Athl Train. 2017;52(3):309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slemmer JE, Weber JT. The extent of damage following repeated injury to cultured hippocampal cells is dependent on the severity of insult and inter-injury interval. Neurobiol Dis. 2005;18(3):421–31. [DOI] [PubMed] [Google Scholar]
- 3.Shultz SR, MacFabe DF, Foley KA, Taylor R, Cain DP. Sub-concussive brain injury in the Long-Evans rat induces acute neuroinflammation in the absence of behavioral impairments. Behav Brain Res. 2012;229(1):145–52. [DOI] [PubMed] [Google Scholar]
- 4.Tagge CA, Fisher AM, Minaeva OV et al. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018;141(2):422–58. doi: 10.1093/brain/awx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailes JE, Petraglia AL, Omalu BI, et al. Role of subconcussion in repetitive mild traumatic brain injury A review. J Neurosurg. 2013;119(5):1235–45. [DOI] [PubMed] [Google Scholar]
- 6.Broglio SP, McCrea M, McAllister T et al. A National Study on the Effects of Concussion in Collegiate Athletes and US Military Service Academy Members: The NCAA-DoD Concussion Assessment, Research and Education (CARE) Consortium Structure and Methods. Sports Med. 2017;47(7):1437–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gysland SM, Mihalik JP, Register-Mihalik JK, et al. The Relationship Between Subconcussive Impacts and Concussion History on Clinical Measures of Neurologic Function in Collegiate Football Players. Ann Biomed Eng. 2012;40(1):14–22. [DOI] [PubMed] [Google Scholar]
- 8.Broglio SP, Eckner JT, Surma T, Kutcher JS. Post-concussion cognitive declines and symptomatology are not related to concussion biomechanics in high school football players. J Neurotrauma. 2011;28(10):2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAllister TW, Flashman LA, Maerlender A et al. Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology. 2012;78(22):1777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JR, Adamson GJ, Pink MM, Sweet JC. Comparison of preseason, midseason, and postseason neurocognitive scores in uninjured collegiate football players. Am J Sports Med. 2007;35(8):1284–8. [DOI] [PubMed] [Google Scholar]
- 11.Murray NG, Grimes KE, Shiflett ED et al. Repetitive head impacts do not affect postural control following a competitive athletic season. Int J Psychophysiol. 2017;3(17):30576–7. [DOI] [PubMed] [Google Scholar]
- 12.McAllister TW, Ford JC, Flashman LA et al. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82(1):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slobounov SM, Walter A, Breiter HC et al. The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: A multi-modal neuroimaging study. Neuroimage Clin. 2017;14:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazarian JJ, Zhu T, Zhong J et al. Persistent, Long-term Cerebral White Matter Changes after Sports-Related Repetitive Head Impacts. Plos One. 2014;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh R, Meier TB, Kuplicki R et al. Relationship of Collegiate Football Experience and Concussion With Hippocampal Volume and Cognitive Outcomes. JAMA. 2014;311(18):1883–8. [DOI] [PubMed] [Google Scholar]
- 16.Belanger HG, Vanderploeg RD, McAllister T. Subconcussive Blows to the Head: A Formative Review of Short-term Clinical Outcomes. J Head Trauma Rehabil. 2016;31(3):159–66. [DOI] [PubMed] [Google Scholar]
- 17.Moon Y, Sung J, An RP, Hernandez ME, Sosnoff JJ. Gait variability in people with neurological disorders: A systematic review and meta-analysis. Hum Mov Sci. 2016;47:197–208. [DOI] [PubMed] [Google Scholar]
- 18.Shumway-Cook A, Woollacott MH. Motor Control: Translating Research into Clinical Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 19.Buckley TA, Oldham JR, Caccese JB. Postural control deficits identify lingering post-concussion neurological deficits. J Sport Health Sci. 2016;5(1):61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang HA, Krebs DE. Dynamic balance control in elders: Gait initiation assessment as a screening tool. Arch Phys Med Rehabil. 1999;80(5):490–4. [DOI] [PubMed] [Google Scholar]
- 21.Oldham JR, Munkasy BA, Evans KM, et al. Altered dynamic postural control during gait termination following concussion. Gait Posture. 2016;49:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckley TA, Munkasy BA, Tapia-Lovler TG, Wikstrom EA. Altered gait termination strategies following a concussion. Gait Posture. 2013;38(3):549–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckley T, Oldham J, Munkasy B, Evans K. Decreased Anticipatory Postural Adjustments During Gait Initiation Acutely Post-Concussion. Arch Phys Med Rehabil. 2017;98(10):1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang W-H, Tang P-F, Wang Y-H, et al. Role of the premotor cortex in leg selection and anticipatory postural adjustments associated with a rapid stepping task in patients with stroke. Gait Posture. 2010;32(4):487–93. [DOI] [PubMed] [Google Scholar]
- 25.Wang JJ, Wai YY, Weng YH et al. Functional MRI in the assessment of cortical activation during gait-related imaginary tasks. J Neural Transm. 2009;116(9):1087–92. [DOI] [PubMed] [Google Scholar]
- 26.Movement Massion J., Posture and Equilibrium - Interaction and Coordination. Prog Neurobiol. 1992;38(1):35–56. [DOI] [PubMed] [Google Scholar]
- 27.Howell DR, Lynall RC, Buckley TA, Herman DC. Neuromuscular Control Deficits and the Risk of Subsequent Injury after a Concussion: A Scoping Review. Sports Med. 2018;17(10):018–0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howell DR, Stillman A, Buckley TA, et al. The utility of instrumented dual-task gait and tablet-based neurocognitive measurements after concussion. J Sci Med Sport. 2017;16(17):358–362. [DOI] [PubMed] [Google Scholar]
- 29.Menz HB, Latt MD, Tiedemann A, et al. Reliability of the GAITRite (R) walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20(1):20–5. [DOI] [PubMed] [Google Scholar]
- 30.Webster KE, Wittwer JE, Feller JA. Validity of the GAITRite (R) walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture. 2005;22(4):317–21. [DOI] [PubMed] [Google Scholar]
- 31.Duma SM, Manoogian SJ, Bussone WR et al. Analysis of real-time head accelerations in collegiate football players. Clin J Sport Med. 2005;15(1):3–8. [DOI] [PubMed] [Google Scholar]
- 32.Crisco JJ, Chu JJ, Greenwald RM. An algorithm for estimating acceleration magnitude and impact location using multiple nonorthogonal single-axis accelerometers. J Biomech Eng. 2004;126(6):849–54. [DOI] [PubMed] [Google Scholar]
- 33.Cummiskey B, Schiffmiller D, Talavage TM et al. Reliability and accuracy of helmet-mounted and head-mounted devices used to measure head accelerations. Proc Institut Mech Eng Part P-J Sports Eng Tech. 2017;231(2):144–53. [Google Scholar]
- 34.Breedlove KM, Breedlove EL, Robinson M et al. Detecting Neurocognitive and Neurophysiological Changes as a Result of Subconcussive Blows in High School Football Athletes. Athl Train Sports Health Care. 2014;6(3):119–27. [Google Scholar]
- 35.Rowson S, Brolinson G, Goforth M, et al. Linear and angular head acceleration measurements in collegiate football. J Biomech Eng. 2009;131(6):061016. [DOI] [PubMed] [Google Scholar]
- 36.Mihalik JP, Bell DR, Marshall SW, Guskiewicz KM. Measurement of head impacts in collegiate football players: an investigation of positional and event-type differences. Neurosurgery. 2007;61(6):1229–35. [DOI] [PubMed] [Google Scholar]
- 37.Crisco JJ, Wilcox BJ, Beckwith JG et al. Head impact exposure in collegiate football players. J Biomech. 2011;44(15):2673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckley TA, Vallabhajosula S, Oldham JD et al. Evidence of a Conservative Gait Strategy in Athletes with a History of Concussions. J Sport Health Sci. 2016;5(4):417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallabhajosula S, Buckley TA, Tillman MD, Hass CJ. Age and Parkinson’s disease related kinematic alterations during multi-directional gait initiation. Gait Posture. 2013;37(2):280–6. [DOI] [PubMed] [Google Scholar]
- 40.Llewellyn T, Burdette GT, Joyner AB, Buckley TA. Concussion Reporting Rates at the Conclusion of an Intercollegiate Athletic Career. Clin J Sport Medicine. 2014;24(1):76–9. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt JD, Phan TT, Courson RW, et al. The Influence of Heavier Football Helmet Faceguards on Head Impact Location and Severity. Clin J Sport Med. 2018;28(2):106–10. [DOI] [PubMed] [Google Scholar]
- 42.Montenigro PH, Alosco ML, Martin BM et al. Cumulative Head Impact Exposure Predicts Later-Life Depression, Apathy, Executive Dysfunction, and Cognitive Impairment in Former High School and College Football Players. Journal of Neurotrauma. 2017;34(2):328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oldham JR, DiFabio MS, Kaminski TW, et al. Efficacy of Tandem Gait to Identify Impaired Postural Control following Concussion. Med Sci Sports Exerc. 2018;50(6):1162–1168. [DOI] [PubMed] [Google Scholar]