Abstract
In sickle cell disease (SCD), ‘disease severity’ associates with increased RBC adhesion to quiescent endothelium, but the impact on activated endothelium is not known. Increased concentrations of free heme result from intravascular hemolysis in SCD. Heme is essential for aerobic metabolism, and plays an important role in numerous biological processes. Excess free heme induces reactive oxygen species generation and endothelial activation, which are associated with cardiovascular disorders including atherosclerosis, hypertension, and thrombosis. Here, we utilized an endothelialized microfluidic platform (Endothelium-on-a-chip) to assess adhesion of sickle hemoglobin-containing red blood cells (HbS RBCs), from adults with homozygous SCD, to heme-activated human endothelial cells (EC) in vitro. Confluent EC monolayers in microchannels were treated with pathophysiologically relevant levels of heme in order to simulate the highly hemolytic intravascular milieu seen in SCD. RBC adhesion to heme-activated ECs varied from subject to subject, and was associated with plasma markers of hemolysis (LDH) and reticulocytosis, thereby linking those RBCs that are most likely to adhere with those that are most likely to hemolyze. These results re-emphasize the critical contribution made by heterogeneous adhesive HbS RBCs to the pathophysiology of SCD. We found that adhesion of HbS RBCs to heme-activated ECs varied amongst individuals in the study population, and associated with biomarkers of hemolysis and inflammation, age, and a recent history of transfusion. Importantly, the microfluidic approach described herein holds promise as a clinically feasible Endothelium-on-a-chip platform with which to study complex heterocellular adhesive interactions in SCD.
INTRODUCTION
Increased hemolysis, driven by inherited conditions, such as sickle cell disease, or infections, such as malaria, may lead to increased levels of heme and reactive oxygen species (ROS), which may result in cellular damage or apoptosis.1–3 Heme is a metal complex required for aerobic metabolism. Heme serves many critical homeostatic functions, such as oxygen transfer, respiration, drug detoxification, and regulation of protein synthesis and cell differentiation.4–6 However, the accumulation of free heme in plasma, due to an imbalance of heme biosynthesis and catabolism or from intravascular red blood cell (RBC) breakdown (hemolysis), can be toxic to surrounding tissues through the induction of heme-derived reactive oxygen species (ROS).7 Further, heme-enhanced oxidative stress leads to vascular inflammation and endothelial injury/activation.8,9 Heme-exposed endothelial cells (ECs) upregulate the expression of ICAM-1, VCAM-1, E-selectin, P-selectin, and vWF through multiple pathways, including TLR4.10,11 Recently, it was shown that heme activation of ECs played a critical role in initiating vaso-occlusion in a murine model of sickle cell disease (SCD).11 This activation process may be enhanced via transfer of cell-free heme within RBC-derived microparticles into the endothelium.12 Free heme may also induce initiation of coagulation by promoting the synthesis of both endothelial- and leukocyte-derived tissue factor and thrombin generation.13,14
Microchannels functionalized with ECs mimic the intravascular environment and thereby allow the study of various pathological conditions in vitro.15,16 Such endothelialized systems have recently been designed and implemented to simulate vaso-occlusion in SCD and measure protein expression in response to changes in wall shear stress.17,18 However, we are unaware of previous studies in which a microfluidic platform has been employed to systematically study the association of RBC adhesion to activated endothelium with clinical phenotype. This is in part because of the technical challenges that are associated with the manufacture of endothelialized microfluidic systems in a consistent and high-throughput manner, which can then be implemented in a large clinical population. Here, we introduce a new microfluidic approach to the fabrication of microfluidic channels seeded with human endothelial cells utilizing a lamination-based technique and laser micro-machined components (Endothelium-on-a-chip). This allowed us to assess HbS RBC adhesion to human endothelial cells that were stimulated with pathophysiologic levels of heme in a clinically diverse adult SCD population.
Adhesion of HbS RBCs to the endothelium and associated adhesion molecules is heterogeneous and linked to disease severity as was shown by us and others.19–23 This heterogeneity exists within an individual patient due to distinct RBC subpopulations with impaired biophysical properties as well as at a population level in which individuals with SCD present varying clinical phenotypes.24–27 Nonetheless, heterogeneous adhesion of RBCs in a SCD population has yet to be adequately addressed, perhaps because many important pathophysiologic studies have been conducted using inbred sickle cell mice with RBCs that are homogeneous within the population.11,28,29 In contrast, humans with SCD exhibit a more heterogeneous distribution of RBCs, as we have previously shown22,27,30,31, likely because of confounding co-inherited disease-modifying genes. We hypothesized that endothelial activation in SCD is associated with enhanced RBC adhesion in a range that reflects clinical heterogeneity and disease severity.
Our integrated clinical and Endothelium-on-a-chip microfluidic approach allowed us to determine the impact of individual pathophysiologic components in a model incorporating only human-derived cells, e.g., HbS RBCs and activated endothelial cells. At its simplest, these are the variables at play in the adhesion of an RBC to the endothelium. Here, we hold one of those two variables, activated endothelium, constant through treating (heme-activated) cultured endothelial cells in a consistent manner. We interrogate RBC adhesion to this activated endothelium using clinical blood samples acquired from adults with SCD who are followed in our clinic, thereby examining the contribution to abnormal adhesion at an activated endothelium that is made by the RBC. In this, we highlight for the first time the correlative relationship between RBC adhesion to heme-activated endothelium and clinical phenotypes associated with a hemolytic plasma milieu in vivo.
METHODS
Subjects and sample collection
Sickle blood samples were obtained from de-identified adult subjects (≥18 years) with homozygous (HbSS) SCD at University Hospitals CWRU (Cleveland, OH), under an Institutional Review Board (IRB) approved protocol. All samples were collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes, and subjects’ hemoglobin types were confirmed by high-performance liquid chromatography (HPLC). Only subjects infected with HIV or hepatitis C were ineligible for this study. The clinical phenotypes of the study population are summarized in Table S1.
Endothelium-on-a-chip fabrication and endothelialization of microfluidic channels
Microfluidic channels were fabricated using a lamination technique, as previously described.22 Briefly, the channel geometry was determined by laser micro-machined double-sided adhesive (DSA) films that were placed between a top polymethyl methacrylate (PMMA) cap and bottom microscope glass slide, forming a uniform flow domain (Figure 1A). The assembled microchannels were rinsed serially with PBS, 100% ethanol, and GMBS following a 20-minute incubation. Thereafter, another washing step was performed using 100% ethanol and PBS before loading the microchannels with a fibronectin solution at a concentration of 0.2 mg/mL. Fibronectin-loaded microchannels were incubated at 37 °C for 1 hour for complete protein immobilization on the GMBS-functionalized surface. To prepare the Endothelium-on-a-chip microchannels, human umbilical vein endothelial cells were seeded into fibronectin-coated microchannels at a density of 8x106 cells/mL and incubated for 4 hours at 37°C and 5% CO2 to allow cell attachment and spreading, while replacing the culture medium in the microchannels every hour (Figure S1A). An initial seeding density of 1x106 cell/mL did not yield a confluent monolayer over the microchannel surface after 4 hours (Figure S2), hence we consistently used a seeding density of 8x106 cells/mL throughout this study.
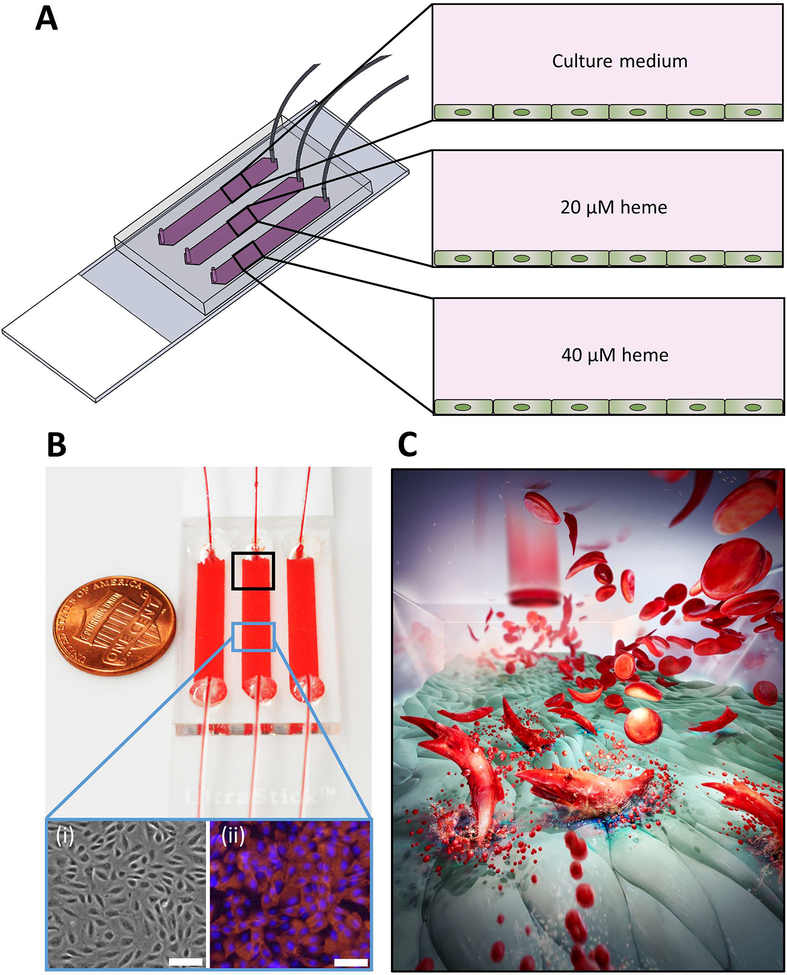
Figure 1. The microfluidic system and probing sickle RBC adhesion to activated endothelial cells in microfluidic channels.
(A) Schematic illustration of the endothelialized microchannels and treatment of ECs by culture medium alone (control) or supplemented with 20 or 40 μM heme. (B) SCD blood samples flown over non-activated and heme-activated EC. The phase-contrast (i) and fluorescent (ii) images of immobilized ECs demonstrate a confluent layer of cells. (C) The pathophysiological process is illustrated whereby hemolyzing sickle RBCs release hemoglobin that is converted to heme in plasma where it activates the endothelial monolayer, resulting in increased RBC adhesion. Scale bars = 20 μm. (Illustration credit: Grace Gongaware)
Heme activation of endothelial cells
Prior to blood perfusion, immobilized human endothelial cells (EC) were incubated with 20 μM or 40 μM heme-containing RPMI-1640 for 60 minutes in 37 °C (Figure 1B). The control microchannels were loaded with heme-free RPMI-1640 and incubated under the same conditions. Heme stock solution was prepared by dissolving bovine hemin in 0.1 M NaOH solution to obtain a final heme concentration of 40 mM. Next, the stock solution was diluted using RPMI-1640 (FBS and antibiotics-free) to the final working concentrations of 20 and 40 μM, and the pH of both solutions was adjusted by addition of 0.1 M HCl.
Sample preparation and blood perfusion over ECs in microchannels
SCD blood samples were centrifuged, at 500g for 5 minutes at room temperature, to isolate RBCs, free from EDTA and cytokine-containing plasma. EDTA can negatively affect endothelial cell adhesion, and patient cytokines may complicate interpretation of adhesion results. Isolated RBCs were then washed with PBS three times and re-suspended in fresh culture medium supplemented with 10 mM HEPES at a hematocrit of 25% (Figure S1B) A total sample volume of 15 μl was perfused into the devices at a shear stress of 1 dyne/cm2, corresponding to a typical value observed in human post-capillary venules. Non-adherent RBCs were rinsed away by injecting fresh culture medium into the microchannels at 1 dyne/cm2 (Figure S1C). By visual inspection of adhesion, contamination by platelets and WBCs was negligible. Figure 1C depicts RBCs flowing through an endothelialized microchannel and the interaction of cell-free hemoglobin and cell-free heme with endothelial cells.
Fluorescent labeling of endothelial proteins
Following the 4-hour static incubation, firmly attached and spread quiescent ECs were rinsed with fresh culture medium and fixed with 4% PFA for 20 minutes at room temperature. Fixed cells were then permeabilized with 0.1% Triton-X for 30 minutes in room temperature. Thereafter, the microchannels were injected with fluorescently labeled antibodies against cell nuclei, actin filaments, and vascular endothelium cadherin (VE-cadherin) followed by a 30-minute incubation in dark. Cell cytoskeleton (Figure S3A), VE-cadherin (Figure S3B), and combined fluorescent images with cell nuclei (Figure S3C) were recorded at 10X and 20X (Figure S3). Positive staining for VE-cadherin in Figures S3B&C demonstrates the formation of cell-cell junctions along the microchannel surface.
Fluorescent labeling of VCAM-1 and P-selectin on ECs
Following the incubation, both the quiescent and heme-activated immobilized ECs were washed with fresh culture medium and fixed with 4% PFA for 20 minutes at room temperature. Next, fixed EC monolayers were rinsed with PBS two times and loaded with fluorescently labeled antibodies against cell nucleus, VCAM-1, and P-selectin followed by a 30-minute incubation in room temperature. Fluorescent images of labeled proteins were then obtained at multiple locations throughout the microchannel surface for quiescent, 20 μM and 40 μM heme-activated ECs (Figure 2A). Calculations of fluorescence intensities were carried out using ImageJ software.32
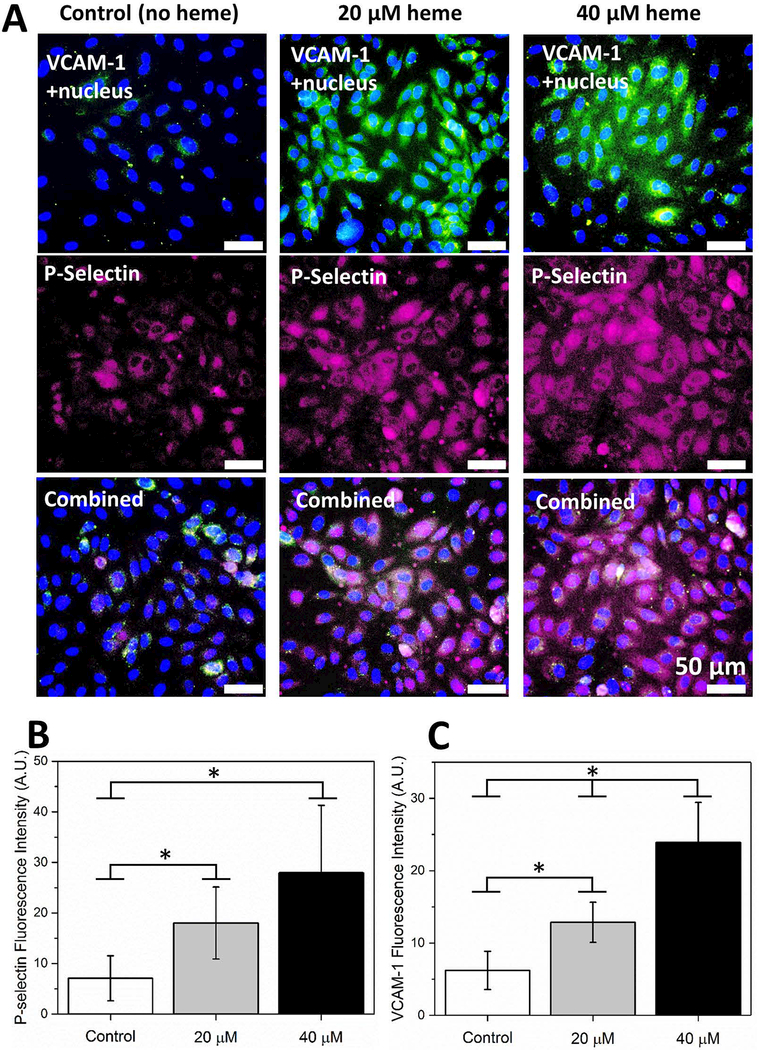
Figure 2. Activation of ECs with heme results in VCAM-1 and P-selectin expression in a concentration dependent manner.
(A) ECs were treated with RPMI containing 0 μM, 20 μM, and 40 μM heme for 60 minutes in 37 °C and incubated with fluorescently labeled antibodies against VCAM-1 and P-selectin following a fixing step with 4% PFA. Cell nuclei were stained with DAPI. (B) Expression of P-selectin was significantly elevated in 20 μM and 40 μM heme-treated ECs (p<0.05, one-way ANOVA) compared to quiescent ECs while a significant difference was absent between 20 μM and 40 μM heme-treated ECs. (C) Similarly, VCAM-1 expression was significantly greater in heme-activated ECs in comparison with quiescent ECs, 40 >20 μM heme (P<0.05). The horizontal brackets and stars between different groups indicate statistically significant difference based on a one-way ANOVA test (n=5 in each group, P<0.05). Error bars = 50 μm.
Statistical Methods
Data acquired in this study were reported as mean ± standard error of the mean (SEM). All statistical analyses were carried out using Minitab 18 Software (Minitab Inc., State College, PA). Data were analyzed for normality followed by either non parametric Mann-Whitney U test or parametric one-way ANOVA. Statistical significance was set at 95% confidence level for all tests (p < 0.05).
RESULTS
Heme activation mediates the expression of P-selectin and VCAM-1 on ECs
Endothelial expression of P-selectin (Figure 2B) and VCAM-1 (Figure 2C) significantly increased with heme activation (p<0.05, one-way ANOVA), for VCAM-1 in a concentration dependent manner.
HbS RBC adhesion to immobilized ECs
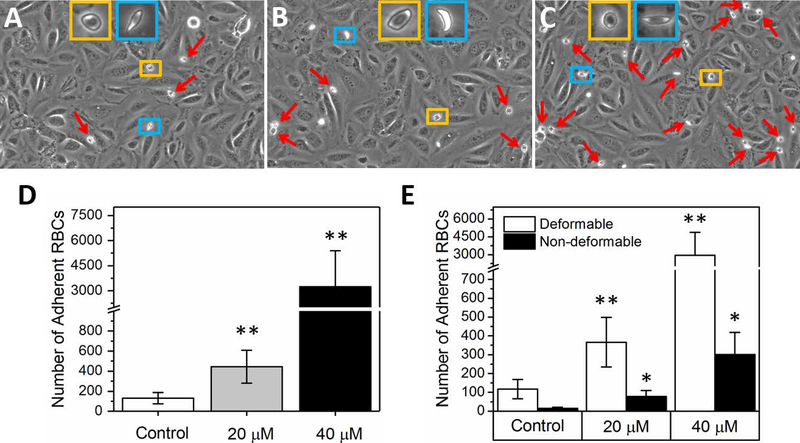
More HbS RBCs adhered to heme-activated than quiescent ECs, with significant differences observed at both 20 μM and 40 μM levels of heme activation (Figure 3, p<0.01, Mann-Whitney). Representative images of adherent RBCs acquired inside the microchannels with quiescent, 20 μM heme-activated, and 40 μM heme-activated ECs, respectively, are shown (Figures 3A-C). Of note, no visible morphologic damage to ECs was observed in heme-treated microchannels. We have previously reported distinct adhesive and biomechanical properties of deformable and non-deformable RBCs from subjects with SCD22 as also illustrated in Figure S4. Deformable HbS RBCs maintained a characteristic biconcave morphology and were capable of deforming to a certain extent under shear flow (Figure S4A), while non-deformable HbS RBCs lacked a biconcave morphology and retained a characteristic elliptical shape (Figure S4A), with impaired ability to deform even at high flow shear rates up to 1000 s−1 (Figure S4B). Both RBC subpopulations demonstrated significantly enhanced adhesion to heme-activated endothelial cells, but the majority of adherent RBCs were deformable (Figure 3E, p<0.01, Mann-Whitney). Our results further suggested that HbS RBC adhesion to activated ECs was subject dependent, as shown by the total number of adherent RBCs variability between quiescent (2–870 RBCs, mean 131), 20 μM (28–2104 RBCs, mean 444), and 40 μM (88–32900 RBCs, mean 3246) to heme activated ECs in 16 subjects with SCD.
Figure 3. Sickle RBC adhesion to heme-activated ECs in vitro.
Representative adherent RBC images to non-activated (A), 20 μM (B) and 40 μM heme-activated (C) ECs are illustrated. (D) Overall, sickle-RBC adhesion is low to quiescent ECs (Control group, N=15) and increases with heme activation depending on the concentration (N=16). (E) Both deformable and non-deformable HbS RBC sub-populations exhibit an adhesive response while adhesion of deformable RBCs was significantly greater in each condition. In (A), (B), and (C), deformable RBCs are shown in yellow rectangles while the non-deformable cells, which may be irreversibly sickled, are demonstrated in blue rectangles. Red arrows indicate adherent RBCs. P values in (D) and (E) computed based on the Mann-Whitney test and the stars at the top of the bars indicate statistically significant difference (one star: p<0.05 and two stars: p<0.01).
Clinical implications of heme-mediated RBC adhesion to ECs
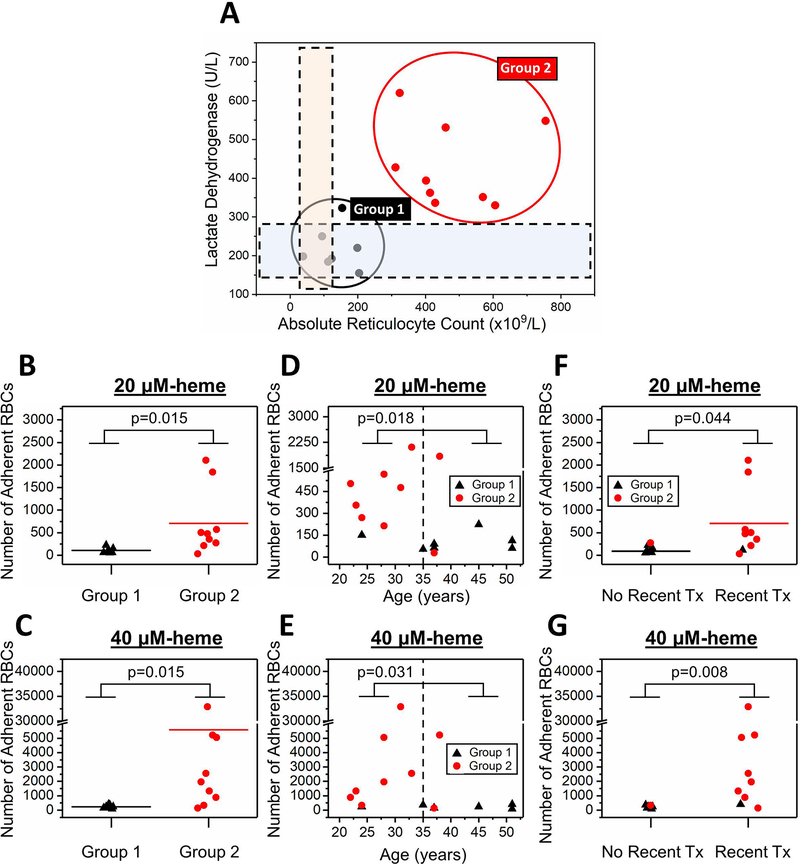
To determine whether subjects’ clinical phenotypes impacted adhesion profiles of their HbS RBCs, we analyzed univariate models via the k-means clustering method and identified 2 subgroups with distinct lactate dehydrogenase (LDH, signifying hemolysis) levels and absolute reticulocyte counts (ARCs) (Figure 4A). Subjects in Group 1 (N=7) had significantly lower LDH levels and ARCs compared to subjects in Group 2 (N=9, mean LDH: 217 vs. 433 IU, respectively, p<0.0001, one-way ANOVA; mean ARC: 132 vs. 475 x 109/L, respectively, p<0.0001, one-way ANOVA). Because we observed two very distinct subject subpopulations based on their LDH and ARCs, we asked whether subjects in Group 1 and Group 2 would also differ in terms of other clinical variables. Accordingly, we found that 1 out of 7 subjects in Group 1, compared with 7 out of 9 subjects in Group 2, had higher than normal WBC counts (Figure S5A, mean WBC: 7.4 vs. 13.6 x109/L, p=0.002, one-way ANOVA). Similarly, all subjects in Group 1, compared with only 3 of 9 subjects in Group 2, had absolute neutrophil counts (ANCs) above the normal range (Figure S5B, mean ANC: 3941 vs. 7643x109/L, p=0.05, one-way ANOVA).
Figure 4. RBC adhesion to heme-activated ECs correlates with subject clinical phenotype including hemolytic & inflammatory biomarkers, age, and transfusion history.
(A) Two distinct subject subpopulations are present based on plasma LDH levels and ARCs. The study population was segregated into two groups: Group 1 (N=7) and Group 2 (N=9) via k-means clustering analysis. The dashed rectangular regions represent normal ranges for the given clinical parameters. RBCs from subjects in Group 2 (N=9), with significantly increased levels of LDH and ARC, have greater adhesion to both 20 μM (B) and 40 μM (C) heme-activated ECs compared to the RBCs from subjects in Group 1 (N=7, p=0.015 & p=0.015, one-way ANOVA). (D, E) 6 subjects out of 7 in Group 1 (lower RBC adhesion profile) were ≥35 years of age, while only 2 subjects out of 9 in Group 2 (higher RBC adhesion profile) were ≥35 years of age. Adhesion of RBCs from subjects younger than 35 years of age (N=8) was significantly higher to both 20 μM (D) and 40 μM (E) heme-activated ECs (p=0.018 & p=0.031, Mann-Whitney). Subjects with a recent transfusion (Tx) therapy (<3 months) had significantly higher RBC adhesion to both 20 μM (F) and 40 μM (G) heme-activated ECs (p=0.044 & p=0.008, Mann-Whitney). 4 out of 7 subjects with no recent Tx record were on hydroxyurea (HU) while the other 3 subjects were on supportive care at the time of sample collection. 2 out of 9 subjects with a recent Tx record were also receiving HU therapy. Only 1 subject out of 7 who did not have recent Tx was in Group 2 whereas that ratio was 8 out of 9 among the subjects with recent Tx. N=7 for the no recent Tx subject group and N=9 for the recent Tx subject group. The horizontal brackets between Groups 1 and 2 indicate statistically significant difference. N represents the number of subjects.
We next compared the adhesion of RBCs from subjects in Group 1 and Group 2. Our results showed that RBCs from Group 1 subjects demonstrated significantly lower mean adhesion, compared with subjects in Group 2, to both 20 μM and 40 μM heme-activated ECs (Figure 4B, 107 vs. 2032, respectively for 20 μM heme, p=0.015, one-way ANOVA; and Figure 4C, 706 vs. 5590, p=0.015, respectively for 40 μM heme, one-way ANOVA). Adhesion profiles of HbS RBCs to heme-activated ECs also differed depending on whether the sample came from a young or older adult (<35 vs ≥35 years of age). We observed that only 1 of 8 young adults belonged to the low LDH/low ARC Group 1, while 6 of 8 older adults belonged to this group (Figures 4D&E). The mean age in Group 1 was greater than that seen in Group 2 (mean age: 40 vs 29, respectively, p=0.016, one-way ANOVA). Older adults displayed significantly lower RBC adhesion to both 20 and 40 μM heme-activated ECs in comparison with the younger subjects (Figure 4D, mean adhesion: 308 vs 579, respectively to 20 μM, p=0.018, Mann-Whitney; Figure 4E, mean adhesion: 841 vs 5651, respectively to 40 μM, p=0.031, Mann-Whitney).
Finally, subjects who had received blood transfusions within 3 months prior to the index experiment (N=9, 2 of whom were also on hydroxyurea) had higher RBC adhesion to heme-activated ECs compared to subjects without recent blood transfusions (N=7, 4 of whom were on hydroxyurea, Figure 4F, mean RBC adhesion: 129 vs. 688, respectively to 20 μM, p=0.044 Mann-Whitney; Figure 4G, mean RBC adhesion: 221 vs. 5598, respectively to 40 μM, p=0.008 Mann-Whitney). The clinical phenotypes of all subjects based on recent history of transfusion are summarized in Table S2.
DISCUSSION
In this study, we developed an Endothelium-on-a-chip microfluidic adhesion assay seeded with human ECs to study HbS RBC adhesion in a clinically relevant manner. Earlier studies employing endothelialized platforms to probe RBC adhesion in vitro largely depended on static incubation of RBCs within microfluidic chambers, where the effect of micro-physiological flow conditions on the adhesion dynamics were negated.19–21,33–36 Recently, a number of endothelialized microfluidic systems have been designed and implemented to study various pathophysiological conditions.15,17,18,37–40 The main advantage of these systems stems from their ability to provide a precisely controlled flow environment, with which to better mimic the physiological milieu and with which to impose pathological scenarios. Although recent advancements in micro-system development have significantly contributed to our understanding of various hematologic diseases, the focus on technical aspects of fabrication and endothelialization has hindered the use of these platforms in a more clinically-useful fashion. Therefore, in this work, we adopted a lamination-based fabrication technique using laser micro-machined parts in constructing the micro-devices that can be performed within 5 minutes. Additionally, we optimized the microchannel endothelialization process to allow confluent EC monolayer growth following a 4-hour incubation, which facilitated the production of endothelialized microchannels in large quantities and timely manner for a clinically relevant use.
Further, the large-scale design of this Endothelium-on-a-chip microfluidic platform affords a significantly greater surface area compared to currently available endothelialized microfluidic systems (i.e. 32 mm2 vs 0.1 mm2).17,18,37 Having a large interrogation surface area significantly improves our ability to capture rare adhesive events for clinical samples with low adhesion potential. Furthermore, the relatively higher volume of the microchannel is likely to prevent any blockage or clogging when clinical samples with higher adhesion or aggregation rates are tested. Moreover, the use of gas impermeable components in our microchannel fabrication process allows the clinically relevant experiments to be carried out in a standard laboratory setting without the need for a specialized culture chamber, as gas exchange between the blood/media and the outside environment is limited. In contrast, the conventional lithography based platforms made of PDMS permit rapid exchange of gases, which necessitates maintaining the microchannel inside a culture chamber, impairing the clinical applicability of the system. Finally, having a gas-impermeable closed-loop flow system allows imaging on a heated-microscope plate, eliminating the need for expensive and complex on-stage incubators during image acquisition.
In our model, we found consistent expression of VE-cadherin at cell-to-cell junctions after a static incubation of ECs for 4 hours, indicating the maintenance of endothelial cell functionality and integrity.41–46 We treated these monolayers with pathological levels of heme in order to mimic the highly hemolytic microvascular conditions often triggered in SCD patients due to the short lifespan of HbS RBCs.47,48 Plasma heme levels, and by extension serum LDH levels, are significantly greater in these patients compared to healthy controls.49 Earlier studies reported plasma heme concentrations up to 20 μM in a group of SCD subjects at steady state, but this can increase further during a VOC due to significantly shortened RBC lifespan and excessive hemolysis.50–53 Therefore, we used heme concentrations that simulate physiological and pathophysiological conditions in SCD, and assessed HbS-containing RBC adhesion to both quiescent and activated ECs.
In an early study, it has been shown that heme treatment of endothelial cells induced the expression of adhesion molecules VCAM-1, ICAM-1, and E-selectin due to increased oxidative stress.10 More recently, the role of toll like receptor 4 (TLR4) in heme-induced endothelial activation was identified, where the activation of TLR4 signaling resulted in increased expression of VCAM-1, ICAM-1, E-selectin, P-selectin, IL-1, IL-6, IL-8, and tissue factor via a pathway involving the activation of NF-κB phospho-p65.11 This mechanism explains that the activation of TLR4 signaling leads to the production of ROS, which triggers degranulation of Weibel-Palade bodies and rapid immobilization of P-selectin and vWF on the endothelial surface.11 However, the presence of these adhesion molecules on the endothelial surface may not necessarily reflect a homogeneous adhesive response by red blood cells from a clinically heterogeneous patient population. Indeed, the extensive heterogeneity in clinical severity among individuals with SCD has been correlated with a myriad of clinical parameters, such as serum hemolytic biomarkers and levels of different hemoglobin types (i.e. HbS, HbF etc.).54–58 Nevertheless, the general point of view in the literature is that an activated endothelium should yield similar changes in RBC adhesion profiles, which neglects the individual and heterogeneous contribution of RBCs in this process. Here, we show that HbS RBC adhesion to heme-activated endothelial cells significantly varies depending on subject clinical phenotypes, including hemolytic and inflammatory biomarkers, age, and transfusion history. One of the most critical findings in this study is that individuals with SCD may have remarkably distinct RBC adhesion profiles even at a similar degree of endothelial activation.
In concert with previously published work 10,11,59,60, we observed enhanced expression of VCAM-1 and P-selectin following endothelial activation with 20 μM and 40 μM heme. We also detected expression of these adhesion molecules, albeit at a much lower level, on quiescent ECs, which likely explains the observed low level adhesion of HbS RBC to these cells. We then demonstrated significantly increased HbS RBC adhesion to 20 and 40 μM heme-activated ECs compared to non-activated cells (Figure 3D), primarily by deformable RBCs (Figure 3E), in line with earlier reports by Mohandas and Evans.61 The total number of adherent RBCs to heme-activated ECs was in the range of 28 to 2104 (20 μM) and 88 to 32900 (40 μM), suggesting a significant contribution to adhesion levels by the RBCs themselves, independent of the degree of EC activation. Furthermore, this heterogeneity was in strong association with subject clinical phenotypes, as we show.
High levels of LDH, and by inference heme, as well as ARCs in SCD have been previously linked to disease severity.49,58,62–65 However, the dynamics of RBC adhesion and its relationship to ongoing hemolysis has not been well understood. Although the toxic byproducts, including cell-free heme, accumulate in plasma during intravascular hemolysis and lead to an inflamed and highly activated endothelial lining, whether this results in uniform elevation in RBC adhesion to the endothelium was not clear. In our earlier work22,23, we showed that RBCs from subjects with higher LDH and reticulocytes adhered to immobilized laminin more significantly, in both normoxic and hypoxic conditions, compared with the subjects who had lower hemolytic biomarker levels, revealing that RBCs that hemolyze may be more adhesive (or vice versa), independent from the deleterious impacts of hemolysis on the endothelium. Similarly, our results in this work suggest that RBCs from subjects with a more severe hemolytic phenotype have greater adhesion to heme-activated ECs, supporting the key contribution of adhesive RBCs to the “hyperhemolysis paradigm” in SCD.66–68 Our results collectively suggest that the degree of RBC adhesion differs significantly among individuals with SCD, and may be in strong association with hemolysis, despite the presence of a consistently activated endothelium with a pro-inflammatory phenotype.
Subjects with markedly increased levels of LDH and of ARC, associated with greater RBC adhesion, also had significantly higher WBC counts and ANCs, indicating a heightened inflammatory state in addition to hemolysis. The role of WBCs in altering RBC biomechanical properties, including adhesiveness and deformability, has yet to be fully elucidated. Earlier, we found a possible link between WBC counts and shear-dependent adhesion of HbS RBCs31, which is consistent with findings here that increased WBCs may be associated with HbS RBCs that are more adhesive to heme-activated ECs.
Increased mortality in young adults with SCD remains a significant challenge despite recent dramatic improvements in care.69–72 Here, most (7/8) subjects under the age of 35 were in the more clinically active group, Group 2. On the other hand, most (6/8) older subjects, ≥35 years of age, were in the lower LDH, lower ARC, lower RBC adhesion group, Group 1. We speculate that adults with SCD who have RBCs that adhere more to heme-activated ECs may die earlier, leaving individuals with a lower adhesion profile to comprise the older adult population. This study suggests a direct association between hemolysis-driven elevated RBC adhesion to ECs and early death in SCD.
Of note, higher adhesion to heme-activated ECs (20 or 40 μM heme) was seen in RBCs from subjects with a recent (<3 months) transfusion history, suggesting a worse adhesion phenotype in transfused subjects. This seemingly paradoxical observation is similar to our earlier finding23, in which RBCs from transfused subjects displayed significantly greater adhesion to laminin under hypoxic conditions. Whether transfusions impact RBC adhesion in individuals or conversely, whether individuals who require transfusions have an intrinsically worse disease phenotype is not yet clear.
Vaso-occlusive crises (VOC), vasculopathy, and hemolysis are the clinical hallmarks of SCD and can lead to systemic organ damage and early mortality. Although SCD is characterized by a point mutation that affects RBC biophysical properties, many different cell types contribute to the pathogenesis of the disease, and it has been shown that VOC episodes in SCD are caused by complex heterocellular adhesive amongst between RBCs, WBCs, platelets, and ECs.73–78 Moreover, the interplay between these cellular components is often magnified by complex plasma proteins that may act as molecular bridges between circulating blood cells and the activated endothelial layer.78 Therefore, having a thorough grasp of underlying VOC mechanisms in SCD requires the simultaneous examination of interactions between all these cellular components as well as the pertinent molecular pathways. However, this study is principally focused toward better understanding the isolated role of RBCs, which are highly heterogeneous within the SCD population, and adhesion to activated human ECs. Indeed, our findings highlight the importance of heterogeneous RBC adhesion to heme-activated human ECs, in particular the strong association of this adhesion with clinical phenotypes. In addition, removal of plasma from blood samples allowed us to precisely control the extent of heme-driven endothelial activation, without additional interference by cytokines or other endothelial-activating agents in the plasma that may have varied from subject to subject.
In conclusion, we have designed and implemented an endothelialized microfluidic approach with which to interrogate adhesion of HbS RBCs to both quiescent and heme-activated endothelial cells in vitro. Our technique in designing and manufacturing the microfluidic channels seeded with endothelial cells is a relatively low labor-intensive and high throughput methodology that facilitates the incorporation of such systems into a standard laboratory setting for clinically relevant applications. Accordingly, our findings obtained using this endothelialized micro-platform revealed enhanced adhesion of HbS RBCs to heme-activated endothelial cells in a subject specific fashion. Notably, the heterogeneity in the adhesion levels solely reflects on the heterogeneous adhesivity of RBCs from different subjects, without reference to the variable activation states of ECs that are likely found in vivo. The RBC-driven subject-specific adhesion profiles developed in this study displayed significant association with hemolytic and inflammatory biomarkers, including LDH, ARC, and WBC counts. In addition, we found that subjects with SCD who are undergoing RBC transfusion therapy may experience exaggerated HbS RBC to heme-activated EC interactions, which may also reflect a more severe clinical phenotype. Future investigations will examine the role of adhesion proteins that are upregulated on heme-activated endothelium, including ICAM-1, VCAM-1, E-selectin, P-selectin, and vWF, in this adhesion process. Understanding the important collective interplay between RBCs, WBCs, and platelets with heme-activated ECs will better characterize the multicellular adhesion paradigm for VOC in SCD, and may enable us develop more effective strategies for both the monitoring and treatment of this disease.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants #2013126 and #2015191 from the Doris Duke Charitable Foundation. U. A. G. and J. A. L. acknowdlege National Heart Lung and Blood Institute R01HL133574, which supported this study in part. U. A. G. acknowledge National Science Foundation CAREER Award 1552782, which supported this study in part. N.S.K and A.I. were supported in part by National Institutes of Health U01HL117659. The authors acknowledge with gratitude the contributions of patients and clinicians at Seidman Cancer Center (University Hospitals, Cleveland). U. A. G. would like to thank the Case Western Reserve University, University Center for Innovation in Teaching and Education (UCITE) for the Glennan Fellowship, which supports the scientific art program and the art student internship in CASE-BML. The authors would like to thank Greg Learn for his technical guidance in cell culture and Grace Gongaware from Cleveland Institute of Art for her scientific illustration used in this work.
Footnotes
AUTHOR CONTRIBUTIONS
E. K., N. K., U. A. G. developed the idea and designed the study. E. K. performed the experiments. E. K., U. A. G. analyzed the data. E. K., A. I., J. A. L., N. K., U. A. G. discussed and interpreted the data. E. K. wrote the manuscript, A. I., J. A. L., N. K., U. A. G. edited the mauscript. J. A. L. provided the patient samples.
CONFLICT-OF-INTEREST DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- 1.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653-1662. [DOI] [PubMed] [Google Scholar]
- 2.Phillips RE, Pasvol G. Anaemia of Plasmodium falciparum malaria. Baillieres Clin Haematol. 1992;5(2):315-330. [DOI] [PubMed] [Google Scholar]
- 3.Goyette RE, Key NS, Ely EW. Hematologic changes in sepsis and their therapeutic implications. Semin Respir Crit Care Med. 2004;25(6):645-659. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157(3):175-188. [DOI] [PubMed] [Google Scholar]
- 5.Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood. 2007;109(7):2693-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe-Matsui M, Muto A, Matsui T, et al. Heme regulates B-cell differentiation, antibody class switch, and heme oxygenase-1 expression in B cells as a ligand of Bach2. Blood. 2011;117(20):5438-5448. [DOI] [PubMed] [Google Scholar]
- 7.Hunt RC, Handy I, Smith A. Heme-mediated reactive oxygen species toxicity to retinal pigment epithelial cells is reduced by hemopexin. J Cell Physiol. 1996;168(1):81-86. [DOI] [PubMed] [Google Scholar]
- 8.Balla J, Vercellotti GM, Jeney V, et al. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol Nutr Food Res. 2005;49(11):1030-1043. [DOI] [PubMed] [Google Scholar]
- 9.Dutra FF, Bozza MT. Heme on innate immunity and inflammation. Front Pharmacol. 2014;5:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagener FA, Feldman E, de Witte T, Abraham NG. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997;216(3):456-463. [DOI] [PubMed] [Google Scholar]
- 11.Belcher JD, Chen C, Nguyen J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camus SM, De Moraes JA, Bonnin P, et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015;125(24):3805-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setty BN, Betal SG, Zhang J, Stuart MJ. Heme induces endothelial tissue factor expression: potential role in hemostatic activation in patients with hemolytic anemia. J Thromb Haemost. 2008;6(12):2202-2209. [DOI] [PubMed] [Google Scholar]
- 14.Sparkenbaugh EM, Chantrathammachart P, Wang S, et al. Excess of heme induces tissue factor-dependent activation of coagulation in mice. Haematologica. 2015;100(3):308-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, Chen J, Craven M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109(24):9342-9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song JW, Cavnar SP, Walker AC, et al. Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS One. 2009;4(6):e5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai M, Kita A, Leach J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122(1):408-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannino RG, Myers DR, Ahn B, et al. "Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions". Sci Rep-Uk. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980;302(18):992-995. [DOI] [PubMed] [Google Scholar]
- 20.Hebbel RP, Yamada O, Moldow CF, Jacob HS, White JG, Eaton JW. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980;65(1):154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebbel RP, Moldow CF, Steinberg MH. Modulation of erythrocyte-endothelial interactions and the vasocclusive severity of sickling disorders. Blood. 1981;58(5):947-952. [PubMed] [Google Scholar]
- 22.Alapan Y, Kim C, Adhikari A, et al. Sickle cell disease biochip: a functional red blood cell adhesion assay for monitoring sickle cell disease. Translational research : the journal of laboratory and clinical medicine. 2016;173:74-91 e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M, Alapan Y, Adhikari A, Little JA, Gurkan UA. Hypoxia-enhanced adhesion of red blood cells in microscale flow. Microcirculation. 2017;24(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaul DK, Fabry ME, Windisch P, Baez S, Nagel RL. Erythrocytes in sickle cell anemia are heterogeneous in their rheological and hemodynamic characteristics. J Clin Invest. 1983;72(1):22-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Dao M, Lykotrafitis G, Karniadakis GE. Biomechanics and biorheology of red blood cells in sickle cell anemia. Journal of biomechanics. 2017;50:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Du E, Dao M, Suresh S, Karniadakis GE. Patient-specific modeling of individual sickle cell behavior under transient hypoxia. PLoS computational biology. 2017;13(3):e1005426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alapan Y, Little JA, Gurkan UA. Heterogeneous red blood cell adhesion and deformability in sickle cell disease. Sci Rep. 2014;4:7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaul DK, Fabry ME, Costantini F, Rubin EM, Nagel RL. In vivo demonstration of red cell-endothelial interaction, sickling and altered microvascular response to oxygen in the sickle transgenic mouse. J Clin Invest. 1995;96(6):2845-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaul DK, Kollander R, Mahaseth H, et al. Robust vascular protective effect of hydroxamic acid derivatives in a sickle mouse model of inflammation. Microcirculation. 2006;13(6):489-497. [DOI] [PubMed] [Google Scholar]
- 30.Alapan Y, Matsuyama Y, Little JA, Gurkan UA. Dynamic deformability of sickle red blood cells in microphysiological flow. Technology. 2016;4(2):71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kucukal E, Little JA, Gurkan UA. Shear dependent red blood cell adhesion in microscale flow. Integrative biology : quantitative biosciences from nano to macro. 2018;10(4):194-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoover R, Rubin R, Wise G, Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979;54(4):872-876. [PubMed] [Google Scholar]
- 34.Hebbel RP, Eaton JW, Steinberg MH, White JG. Erythrocyte/endothelial interactions in the pathogenesis of sickle-cell disease: a "real logical" assessment. Blood Cells. 1982;8(1):163-173. [PubMed] [Google Scholar]
- 35.Setty BN, Stuart MJ. Vascular cell adhesion molecule-1 is involved in mediating hypoxia-induced sickle red blood cell adherence to endothelium: potential role in sickle cell disease. Blood. 1996;88(6):2311-2320. [PubMed] [Google Scholar]
- 36.Adragna NC, Fonseca P, Lauf PK. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood. 1994;83(2):553-560. [PubMed] [Google Scholar]
- 37.Tsvirkun D, Grichine A, Duperray A, Misbah C, Bureau L. Microvasculature on a chip: study of the Endothelial Surface Layer and the flow structure of Red Blood Cells. Sci Rep. 2017;7:45036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen MB, Whisler JA, Frose J, Yu C, Shin Y, Kamm RD. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat Protoc. 2017;12(5):865-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakurai Y, Hardy ET, Ahn B, et al. A microengineered vascularized bleeding model that integrates the principal components of hemostasis. Nat Commun. 2018;9(1):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan OF, Sefton MV. Endothelial cell behaviour within a microfluidic mimic of the flow channels of a modular tissue engineered construct. Biomed Microdevices. 2011;13(1):69-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26(5):441-454. [DOI] [PubMed] [Google Scholar]
- 42.Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci. 2013;126(Pt 12): 2545-2549. [DOI] [PubMed] [Google Scholar]
- 43.Dejana E, Giampietro C. Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol. 2012;19(3):218-223. [DOI] [PubMed] [Google Scholar]
- 44.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16(2):209-221. [DOI] [PubMed] [Google Scholar]
- 45.Lampugnani MG, Dejana E. The control of endothelial cell functions by adherens junctions. Novartis Found Symp. 2007;283:4-13; discussion 13–17, 238–241. [DOI] [PubMed] [Google Scholar]
- 46.Harris ES, Nelson WJ. VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol. 2010;22(5):651-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bensinger TA, Gillette PN. Hemolysis in sickle cell disease. Archives of internal medicine. 1974;133(4):624-631. [PubMed] [Google Scholar]
- 48.London IM, Shemin D, et al. Heme synthesis and red blood cell dynamics in normal humans and in subjects with polycythemia vera, sickle-cell anemia, and pernicious anemia. The Journal of biological chemistry. 1949;179(1):463-484. [PubMed] [Google Scholar]
- 49.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383-1389. [DOI] [PubMed] [Google Scholar]
- 51.Jison ML, Munson PJ, Barb JJ, et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104(1):270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968;32(5):811-815. [PubMed] [Google Scholar]
- 53.Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life. 2012;64(1):72-80. [DOI] [PubMed] [Google Scholar]
- 54.Quinn CT. Minireview: Clinical severity in sickle cell disease: the challenges of definition and prognostication. Experimental biology and medicine. 2016;241(7):679-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127(3):750-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor JGt, Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT, Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclusive pain. PLoS One. 2008;3(5):e2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens MC, Hayes RJ, Vaidya S, Serjeant GR. Fetal hemoglobin and clinical severity of homozygous sickle cell disease in early childhood. The Journal of pediatrics. 1981;98(1):37-41. [DOI] [PubMed] [Google Scholar]
- 58.Mikobi TM, Lukusa Tshilobo P, Aloni MN, et al. Correlation between the Lactate Dehydrogenase Levels with Laboratory Variables in the Clinical Severity of Sickle Cell Anemia in Congolese Patients. PLoS One. 2015;10(5):e0123568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4(12):1392-1396. [DOI] [PubMed] [Google Scholar]
- 60.Soares MP, Seldon MP, Gregoire IP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. Journal of immunology. 2004;172(6):3553-3563. [DOI] [PubMed] [Google Scholar]
- 61.Mohandas N, Evans E. Sickle erythrocyte adherence to vascular endothelium. Morphologic correlates and the requirement for divalent cations and collagen-binding plasma proteins. J Clin Invest. 1985;76(4):1605-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Driscoll S, Height SE, Dick MC, Rees DC. Serum lactate dehydrogenase activity as a biomarker in children with sickle cell disease. British journal of haematology. 2008;140(2):206-209. [DOI] [PubMed] [Google Scholar]
- 63.Damanhouri GA, Jarullah J, Marouf S, Hindawi SI, Mushtaq G, Kamal MA. Clinical biomarkers in sickle cell disease. Saudi journal of biological sciences. 2015;22(1):24-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darbari DS, Onyekwere O, Nouraie M, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. The Journal of pediatrics. 2012;160(2):286-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920-1923. [DOI] [PubMed] [Google Scholar]
- 66.Hebbel RP. Reconstructing sickle cell disease: a data-based analysis of the "hyperhemolysis paradigm" for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol. 2011;86(2):123-154. [DOI] [PubMed] [Google Scholar]
- 67.Gladwin MT. Revisiting the hyperhemolysis paradigm. Blood. 2015;126(6):695-696. [DOI] [PubMed] [Google Scholar]
- 68.Aragona E, Kelly MJ. Hyperhemolysis in sickle cell disease. J Pediatr Hematol Oncol. 2014;36(1):e54-56. [DOI] [PubMed] [Google Scholar]
- 69.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639-1644. [DOI] [PubMed] [Google Scholar]
- 70.Lanzkron S, Carroll CP, Haywood C Jr, Mortality rates and age at death from sickle cell disease: U.S., 1979–2005. Public health reports. 2013;128(2):110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prabhakar H, Haywood C, Jr, Molokie R. Sickle cell disease in the United States: looking back and forward at 100 years of progress in management and survival. Am J Hematol. 2010;85(5):346-353. [DOI] [PubMed] [Google Scholar]
- 72.Maitra P, Caughey M, Robinson L, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica. 2017;102(4):626-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15(4):384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J, Kim K, Hahm E, et al. Neutrophil AKT2 regulates heterotypic cell-cell interactions during vascular inflammation. J Clin Invest. 2014;124(4):1483-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jimenez MA, Novelli E, Shaw GD, Sundd P. Glycoprotein Ibalpha inhibitor (CCP-224) prevents neutrophil-platelet aggregation in Sickle Cell Disease. Blood Adv. 2017;1(20):1712-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Hematology Am Soc Hematol Educ Program. 2013;2013:362-369. [DOI] [PubMed] [Google Scholar]
- 78.Kaul DK, Finnegan E, Barabino GA. Sickle red cell-endothelium interactions. Microcirculation. 2009;16(1):97-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.