Abstract
Public health plays an important role in ensuring access to interventions that can prevent disease, including implementation of evidence-based genomic recommendations. We used the Centers for Disease Control and Prevention (CDC) Science Impact Framework to trace the impact of public health activities and partnerships on implementation of the 2009 Evaluation of Genomic Applications in Practice and Prevention (EGAPP™) Lynch Syndrome (LS) screening recommendation and the 2005 and 2013 United States Preventive Services Task Force (USPSTF) BRCA1 and BRCA2 testing recommendations.
The EGAPP and USPSTF recommendations have each been cited by >300 peer-reviewed publications. CDC funds selected states to build capacity to integrate these recommendations into public health programs, through education, policy, surveillance, and partnerships. Most state cancer control plans include genomics-related goals, objectives, or strategies. Since the EGAPP recommendation, major public and private payers now provide coverage for LS screening for all newly diagnosed colorectal cancers. National guidelines and initiatives, including Healthy People 2020, included similar recommendations and cited the EGAPP and USPSTF recommendations. However, disparities in implementation based on race, ethnicity, and rural residence remain challenges. Public health achievements in promoting evidence-based use of genomics for prevention of hereditary cancers can inform future applications of genomics in public health.
Keywords: Lynch Syndrome, hereditary breast and ovarian cancer, public health genomics, Science Impact Framework, evaluation
Public health approaches to promoting health and preventing disease are population-based but frequently target population subgroups, defined by characteristics such as race or ethnicity and rural or urban residential status. These subgroups might not represent the majority of those at risk, but often have a substantially greater risk than the general population. An emerging role for public health is to help find people at highest risk for hereditary cancer syndromes, a subgroup of the population at increased risk for cancer, especially at a younger age. Women with breast cancer 1 (BRCA1) and breast cancer 2 (BRCA2) pathogenic variants have a 69-72% risk of breast cancer and a 17-44% risk of ovarian cancer by age 80, compared with lifetime breast and ovarian cancer risks of 12% and 1%, respectively, for women in the general population.1,2 Women with Lynch syndrome (LS) have a 35% risk of colorectal cancer by age 70 and men have a 45% risk, compared with 4.5% lifetime risk for the general population.3,4 Women with Lynch syndrome have a 15-60% risk of endometrial cancer, compared with 2.7% lifetime risk in the general population.4
BRCA pathogenic variants cause about 3% of breast cancers and 10% of ovarian cancers,5 while LS accounts for about 3% of colorectal cancers.3 Although most people diagnosed with breast, ovarian, or colorectal cancer do not have a BRCA or LS-related pathogenic variant, those carrying these pathogenic variants can find out about their cancer risk prior to any signs of disease, and take preventive measures early, when they are most likely to be effective. For women with BRCA pathogenic variants, prophylactic mastectomy can reduce breast cancer risk 85-100% and prophylactic oophorectomy can reduce ovarian cancer risk 69-100% and breast cancer risk 37-100%.5 Women with BRCA pathogenic variants can start screening with mammography earlier, as recommended by the United States Preventive Services Task Force (USPSTF),6 and with Magnetic Resonance Imaging (MRI), as recommended by the American Cancer Society.7 For individuals with LS, colorectal cancer screening reduces their lifetime colorectal cancer risk by about 62%.3
Evidence-based recommendations are an important first step in identifying and providing interventions to those at risk for disease, and translating these guidelines into public health practice is crucial for their implementation. Use of genomics in public health, especially in chronic disease prevention, is still emerging, and evaluations of ongoing efforts are important to provide the evidence base to show the impact of incorporating genomics into chronic disease prevention, to encourage and inform future efforts. Here, we use the Centers for Disease Control and Prevention (CDC) Science Impact Framework (SIF) 8 to trace the influence of public health activities on the prevention of hereditary cancers. We focus on LS and BRCA-associated hereditary breast and ovarian cancer (HBOC), starting with the USPSTF 2005 and 2013 recommendation, “BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing”2,9 and the 2009 Evaluation of Genomic Applications in Practice and Prevention (EGAPP™) recommendation, “Genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives.”3 We selected the CDC SIF because it takes a broader approach to evaluation, measuring the impact of science beyond journal citations, and considers short term indicators that support long term impact, with an emphasis on contribution rather than attribution. The CDC SIF8 considers five spheres of influence: Disseminating Science, Creating Awareness, Catalyzing Action, Effecting Change, and Shaping the Future (Figure 1).
Figure 1.
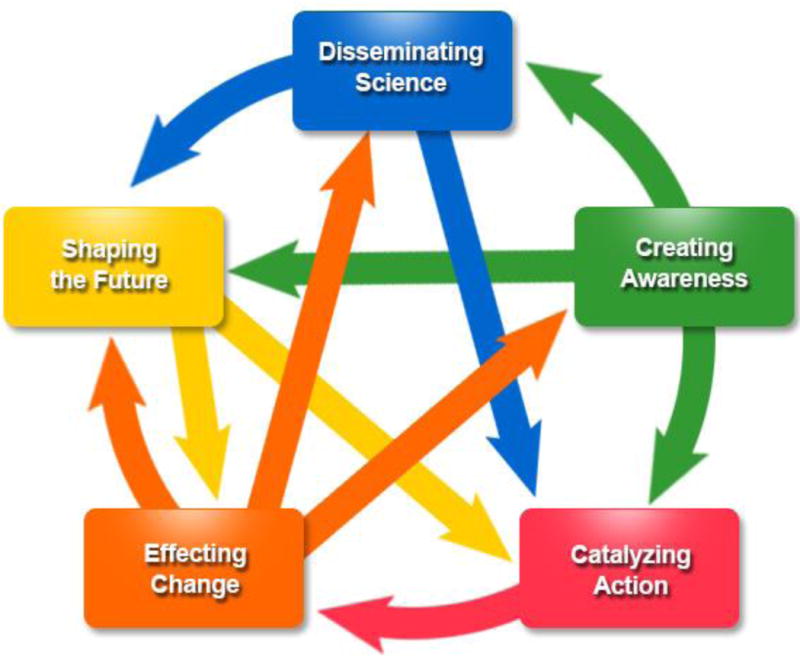
CDC’s Science Impact Framework8 illustrates the “Historical Tracing Method” with 5 domains of CDC scientific influence that define degrees of impact that may not be chronological. The degree of impact is not necessarily a progression; therefore, events captured may not be reflected at every domain. In addition, there may be loop-back at any point. Health outcomes are the ultimate goal, driven by the 5 domains of influence. Disseminating science can include publication of findings in peer-reviewed journals, presentation at conferences, or through other media channels. Creating awareness involves receiving recognition and may include awards, general awareness, or acceptance of a concept or findings by scientific community or policy makers, generating new discussion. Catalyzing action may include partnerships and collaborations, technology creation, congressional hearings or bills, or introduction in practice. Effecting change may include building public health capacity, legal or policy change, cultural, social, or behavioral change, or economic change. Shaping the future may include new hypotheses or strategies, implementation of new programs/initiatives, or quality improvement.
DISSEMINATING SCIENCE
In 2009, the CDC-sponsored, independent EGAPP Working Group published a landmark evidence-based recommendation that genetic testing for LS should be offered to all individuals newly diagnosed with colorectal cancer,3 unlike previous guidelines, which recommended targeted screening based on age, family history, and other factors. Finding those with LS would allow their relatives to be tested for the same pathogenic variant and, if positive, to take steps to prevent cancer or find it early, including colonoscopies at a younger age. If everyone in the U.S. with newly diagnosed colorectal cancer were tested, about 4,000 people each year would be identified with LS. Testing the blood relatives of these 4,000 people could potentially identify multiple relatives in each family with LS who would benefit from evidence-based interventions.
To assess the evidence base prior to issuing the recommendation, the EGAPP working group conducted a systematic review on LS testing for all individuals with newly diagnosed colorectal cancer to reduce morbidity and mortality, updating an Evidence Report from the Agency for Healthcare Research and Quality.10 The supplementary review10 focused on clarifying the LS case definition, removing family history assessment as a requirement for screening, determining the clinical validity of testing, identifying benefits and harms of testing for patients and their relatives, and providing a cost analysis.
The USPSTF published evidence-based recommendations in 2005, revised in 2013, on use of family health history to identify women at risk for BRCA pathogenic variants (B rating).2,9 The recommendations focus on women with a family health history of cancer consistent with a BRCA pathogenic variant who have not had breast, ovarian, tubal, or peritoneal cancer themselves and provide guidance targeted to primary care providers on referral for BRCA genetic counseling and testing. To identify women who should be referred for genetic counseling and, if indicated after counseling, BRCA genetic testing, the 2005 recommendation listed specific family history criteria, while the 2013 revision stated that primary care providers should screen women with one of several screening tools. An estimated 5% of women in the U.S.—about 6 million women aged ≥18 years—meet the 2005 USPSTF referral criteria (2014 U.S. census estimates).11
CREATING AWARENESS
Over 340 peer-reviewed publications have cited the EGAPP LS screening recommendation, including cost-effectiveness studies supporting its universal screening approach in the U.S.12 and studies describing implementation of universal LS screening of newly diagnosed colorectal cancers13 (Supplementary Table 1). In a 2009 survey, 29% of National Cancer Institute (NCI)-designated Comprehensive Cancer Centers, 16% of American College of Surgeons-accredited Community Hospital Comprehensive Cancer Programs, and 0% of Community Hospital Cancer Programs reported performing universal LS screening for all patients newly diagnosed with colorectal cancer.14 In a 2013 survey15 of pathology laboratories, about half reported screening all or nearly all colorectal cancers for LS, suggesting that LS screening rates had increased since 2009 and the publication of the EGAPP recommendation. 15
Over 400 peer-reviewed publications have cited the 2005 and/or 2013 USPSTF recommendations. These include studies on implementing breast cancer risk assessment for women without a personal history of breast cancer, either in primary care settings16 or among women receiving screening mammograms17 (Supplemental Table 1). Also included are studies evaluating hereditary cancer risk assessment tools and protocols,18 assessing primary care clinicians’ ability to determine hereditary cancer risk,19 identifying ways to improve cancer risk assessment and access to genetic services for those at-risk,20 and describing prevalence of and characteristics associated with referrals, genetic counseling, and testing for HBOC21 (Supplemental Table 1).
To educate clinicians about the recommendations, CDC collaborated with Medscape on expert commentaries22 on LS and the EGAPP recommendation in 2011 and BRCA pathogenic variants and the USPSTF recommendation in 2014. CDC partnered with the Georgia, Michigan, and Oregon Departments of Health and the National Coalition for Health Professional Education in Genomics (now the Jackson Laboratory Clinical and Continuing Education Program) to create an online continuing medical education (CME) course on HBOC,23 with >7,000 sessions since its launch in February 2014 (D. Duquette, personal communication). The American Medical Association and the College of American Pathologists developed CME courses on Lynch syndrome. The 2016 CDC Grand Rounds, “Cancer and Family History: Using Genomics for Prevention” and summary publication discussed public health approaches to hereditary cancers and focused on LS and HBOC, including the EGAPP and USPSTF recommendations.24 The Grand Rounds reached >790 participants, and the resulting publication has an Altmetric score of 58 as of September, 2017, ranking in the top 5% of all research outputs scored by Altmetric.
CDC developed the Know:BRCA risk assessment tool,25 launched in 2014, to help women and their health care providers assess their risk for BRCA pathogenic variants. CDC launched the Bring Your Brave campaign26 in 2015 to increase young women’s knowledge about breast health and risk factors for early onset breast cancer, including BRCA pathogenic variants.
CATALYZING ACTION
CDC funding helped establish selected state health departments’ capacity to integrate HBOC into public health programs, starting in 2008 and continuing in 2011. The most recent funding in 2014 included LS and focused on education, policy, and surveillance. Trivers et al.27 used CDC’s SIF to evaluate funded states’ HBOC activities.
Educational activities
Educational activities of CDC-funded state health departments include small media targeting providers and the public, online and in-person presentations and training, websites, publications, promotion of educational programs through provider incentives, creation of screening tools, development of genomics competencies and curriculum, technical assistance, national and state health observances, outreach events, and health education campaigns. For example, the Connecticut Department of Public Health (DPH) developed an educational booklet for providers, Cancer Genomics Best Practices for Connecticut Healthcare Providers, and the Michigan Department of Health and Human Services (MDHHS) developed a handheld provider tool, the Cancer Family History Guide© and a form and patient education booklet for providers to use to obtain written informed consent prior to pre-symptomatic or predictive genetic testing as mandated by Michigan law. Several states issued proclamations for LS Awareness Day on March 22, 2017, indicating that increasing LS awareness is a state priority. Michigan issued proclamations for HBOC Awareness Week September 25-October 1, 2016.
Policy and systems change activities
Policy and systems change activities conducted by CDC-funded states include developing cancer genomics program infrastructure, forming advisory committees, including genomics in state cancer plans, developing policy guidance documents for institutions and policy makers, working with state cancer registries to include data elements on cancer family history and other genetic data, working with payers to promote coverage according to EGAPP and USPSTF recommendations, working with community clinics serving low income populations to include family history risk assessment, implementing a process in which laboratory reports on new colorectal cancer diagnoses are immediately forwarded to the local hospital cancer registrar and board‐certified genetic counselor, encouraging compliance with American College of Surgeons Commission on Cancer Standards on Genetic Counseling and Risk Assessment, and educating stakeholders about state licensure for genetic counselors.
We assessed state cancer plans currently available online for genomics terms similar to those used in Laufman et al.:28 gene, genetic, genomics, heredity, hereditary, heritability, family history, DNA, high risk, risk assessment, and first-degree relative (Table 1). The majority of states (71%, 36/51, including Washington, D.C., Table 1) include genomics-related goals, objectives, or strategies in their state cancer control plans, and the number has continued to increase, even as cancer control plans have become more streamlined. (Laufman et al.’s 2012 study found that 32/50 (64%) state cancer plans included genomics-related goals, strategies, or objectives.28) Most cancer control plans that are up-to-date (72%, 21/29) include genomics-related goals, objectives, or strategies. Five state cancer plans include goals, objectives, or strategies on LS screening of all newly diagnosed colorectal cancers. Nineteen state cancer control plans include goals, objectives, or strategies that address LS or family history of colorectal cancer, 23 plans include goals, objectives, or strategies that address HBOC or BRCA testing, and an additional 10-11 include goals, objectives, or strategies that are relevant to HBOC and LS but use more general terms, such as hereditary cancer or family history of cancer.
Table 1.
Genomics, hereditary breast and ovarian cancer, and Lynch syndrome in state cancer plans.
| State | Period covered | Genetics-related term | Genomics-related goal/objective/strategy | Hereditary breast and ovarian cancer-specific goal/objective/strategy | Lynch-specific goal/objective/strategy | Screening all newly diagnosed colorectal cancers for Lynch syndrome goal/objective/strategy |
|---|---|---|---|---|---|---|
| Alabama | 2011-2015 | Yes | Yes | Yes | Yes* | No |
| Alaska | 2016-2020 | Yes | Yes | Yes | Yes | No |
| Arizona | 2014-2018 | Yes | Yes | Yes | Yes | No |
| Arkansas | NS | Yes | Yes | Yes | Yes | No |
| California | 2011-2015 | Yes | Yes | Yes | No | No |
| Colorado | 2016-2020 | Yes | Yes | Yes | Yes | Yes |
| Connecticut | 2014-2017 | Yes | Yes | Yes* | Yes* | No |
| Delaware | 2012-2016 | No | No | No | No | No |
| Florida | 2015 | Yes | Yes | Yes | Yes* | No |
| Georgia | 2014-2019 | Yes | Yes | Yes | Yes | No |
| Hawaii | 2016-2020 | Yes | Yes | Yes | Yes | No |
| Idaho | 2016-2020 | Yes | Yes | No | No | No |
| Illinois | 2012-2015 | Yes | No | No | No | No |
| Indiana | 2010-2014 | Yes | No | No | No | No |
| Iowa | 2018-2022 | Yes | Yes | Yes* | Yes* | No |
| Kansas | 2017-2021 | Yes | Yes | Yes | Yes | No |
| Kentucky | 2016 | Yes | No | No | No | No |
| Louisiana | 2017-2021 | Yes | Yes | Yes | Yes | Yes |
| Maine | 2016-2020 | Yes | No | No | No | No |
| Maryland | 2016-2020 | Yes | Yes | Yes | Yes | No |
| Massachusetts | 2012-2016 | Yes | No | No | No | No |
| Michigan | 2016-2020 | Yes | Yes | Yes | Yes | Yes |
| Minnesota | 2025 | Yes | Yes | Yes | Yes | Yes |
| Mississippi | 2006-2011 | Yes | Yes | Yes* | Yes | No |
| Missouri | 2016-2020 | Yes | No | No | No | No |
| Montana | 2016-2021 | Yes | Yes | Yes | Yes* | No |
| Nebraska | 2017-2022 | Yes | No | No | No | No |
| Nevada | 2016-2020 | Yes | Yes | Yes | No | No |
| New Hampshire | 2015-2020 | No | No | No | No | No |
| New Jersey | 2008-2012 | Yes | Yes | Yes | Yes | No |
| New Mexico | 2012-2017 | Yes | Yes | Yes* | Yes* | No |
| New York | 2012-2017 | Yes | Yes | Yes | Yes* | No |
| North Carolina | 2014-2020 | Yes | Yes | No | No | No |
| North Dakota | 2011-2016 | Yes | Yes | Yes | Yes | No |
| Ohio | 2015-2020 | Yes | Yes | Yes* | Yes* | No |
| Oklahoma | 2006-2010 | No | No | No | No | No |
| Oregon** | 2005-2010 | Yes | Yes | Yes* | Yes | No |
| Pennsylvania | 2013-2018 | No | No | No | No | No |
| Rhode Island | 2013-2018 | No | No | No | No | No |
| South Carolina | 2011-2015 | Yes | Yes | Yes* | Yes* | No |
| South Dakota | 2015-2020 | Yes | Yes | Yes | Yes | No |
| Tennessee | 2013-2017 | Yes | Yes | Yes* | Yes | No |
| Texas | 2012 | Yes | No | No | No | No |
| Utah | 2016-2020 | Yes | Yes | Yes | Yes | No |
| Vermont | 2016-2020 | Yes | No | No | No | No |
| Virginia | 2013-2017 | Yes | Yes | Yes* | Yes* | No |
| Washington** | 2009-2013 | Yes | Yes | No | No | No |
| Washington, D.C. | 2013-2018 | Yes | Yes | Yes | No | No |
| West Virginia | 2016-2020 | Yes | Yes | Yes | Yes | Yes |
| Wisconsin | 2016-2020 | Yes | Yes | Yes* | Yes* | No |
| Wyoming | 2016-2020 | Yes | No | No | No | No |
Goals, objectives, or strategies are relevant to hereditary breast and ovarian cancer or Lynch syndrome but use more general terms, such as hereditary cancer or family history of cancer.
State plans, including cancer plan, combined into one comprehensive plan for all chronic conditions. We analyzed the earlier cancer plan.
Surveillance activities
Surveillance activities include surveys of providers, patients, and payers to assess knowledge, interest, and current practices regarding family history, hereditary cancers, and genetic testing; addition of questions on family history of cancer and hereditary cancers to state surveys such as the Behavioral Risk Factor Surveillance System (BRFSS); hospital chart reviews to track the number of newly diagnosed colorectal cancers screened for LS; and data collection through state-specific surveillance systems.
Using data from surveys, like BRFSS, states have been able to estimate the state prevalence of personal and family history of breast, colorectal, and other cancers, assess awareness of and interest in genetic testing for hereditary cancer syndromes, and track state progress toward national goals and objectives and state cancer plan goals, objectives, or strategies. A study using data from the 2006-2009 Oregon BRFSS29 found that health care providers were more likely to discuss cancer risk, screening for breast cancer, and health behaviors changes with patients with a family health history of breast cancer, compared with those without a family history of breast cancer. Women who discussed breast cancer screening with their providers were more likely to have mammograms than those who did not discuss it. Analyses using the Michigan BRFSS30 showed a two-fold increase in the percentage of Michigan women with a significant family health history of breast and/or ovarian cancer (based on the 2005 USPSTF criteria) who received genetic counseling (8.5-8.8% in 2012 to 16.0% in 2015), with about 10% of adult Michigan women meeting the 2005 USPSTF criteria for genetic counseling referral. Data from the 2010 Michigan BRFSS31 showed that a higher percentage of adults with a personal or family history of colorectal cancer reported having a colon cancer screening than those who did not report a personal or family history of colorectal cancer (80.4% vs. 65.3%). A 2008 Oregon BRFSS study found that respondents with a family history of colorectal cancer were more likely to report that their health care provider discussed colorectal cancer screening (OR=4.2 [95% CI 2.4-7.4]), they had colorectal screening within the recommended time period (OR=2.2 [95% CI 1.3-3.9], and they made lifestyle changes to prevent colorectal cancer (OR=2.6 [95% CI 1.7-4.0).32
Partnerships and other activities
State cancer genetics programs have partnered with cancer registries, clinical facilities, healthcare providers, health systems, public and private payers, policy makers, other state, regional, and federal programs, academic institutions, community organizations, advocacy groups, and industry. The LS Screening Network (LSSN),33 created in 2011, fosters collaboration and data sharing among institutions routinely screening newly diagnosed colorectal or endometrial cancers for LS. Thus far, 122 leading cancer institutions in 30 states have applied for LSSN membership, and 95 institutions and partners in 30 states are active members. Ninety-five percent of LSSN member institutions report that they used the EGAPP recommendation to justify or support their universal or routine LS screening of colorectal cancer cases (D. Duquette, personal communication). LSSN evolved from a 2010 CDC stakeholder meeting on universal colorectal cancer tumor screening for LS.34
One of the first approaches that some states have used to implement hereditary cancer activities has been bidirectional reporting using cancer registries to identify individuals at increased risk for LS (those with colorectal cancer and endometrial cancer <age 50) and HBOC (those with breast cancer < age 50 or ovarian cancer). These programs reported aggregate numbers of patients at increased risk to the reporting institution or provider, and in some cases, contacted the patient to inform them of their risk. While bidirectional reporting might not change clinical outcomes, in part due to the time elapsed between cancer diagnosis and reporting back of risk, state health departments used it as an educational tool to promote compliance with the EGAPP, USPSTF, and other recommendations.35 The MDHHS cancer genomics program reported back 10,340 colorectal cancer cases, 3,025 breast cancers in women <age 50, 1,985 people with multiple -related or LS-related primary cancers, 459 endometrial cancer cases <age 50, 127 ovarian cancers, and 147 male breast cancers to 145 reporting institutions (D. Duquette, personal communication). The Connecticut DPH reported back >3,700 cancer cases for possible HBOC evaluation and received requests from 70% of participating hospitals for Grand Rounds presentations on prevention, early detection, and genetic counseling and testing for hereditary cancers.35 A 2009 project in Colorado36 reported back hereditary colorectal cancer information on 575 cases to 412 health care providers and 181 patients. HBOC bidirectional reporting programs are described further in Trivers et al.27
Using CDC-funded state activities as models, CDC developed the Public Health Genomics Toolkit37 to assist other state health departments in implementing the EGAPP and USPSTF recommendations. The Toolkit has been visited over 20,000 times since its launch and includes resources such as patient and provider fact sheets on LS and HBOC, summaries of the EGAPP and USPSTF recommendations, pamphlets and sample letters to help those with LS or BRCA pathogenic variants share information about their diagnoses with family members, and a slide set for states to use for educating providers and institutions about LS and HBOC, all of which can be customized to suit states’ needs. To provide further access to public health genomics activities at the state level, CDC created the State Implementation Activities Clickable Map,38 which provides state-by-state information on HBOC and LS activities and has been visited >65,000 times.
EFFECTING CHANGE
For implementation of the USPSTF and EGAPP recommendations in the clinical setting, health insurance coverage for services related to BRCA testing and universal LS screening may be necessary. The Patient Protection and Affordable Care Act (ACA) requires many health plans to provide in-network coverage without cost-sharing for preventive services with a USPSTF rating of “A” or “B,” which includes the BRCA testing recommendation.39 A clarification in May 201540 stipulated that ACA coverage included women with a personal history of cancer, and the Centers for Medicare and Medicaid Services (CMS) Local Coverage Determinations on BRCA1 and BRCA2 Genetic Testing41 allow for regional coverage of BRCA genetic counseling and testing for Medicare beneficiaries with personal histories of breast, ovarian, and other cancers that fit specific criteria for increased risk for a BRCA pathogenic variant. Thus, coverage, depending on the source, can potentially be provided for individuals both with and without personal histories of BRCA-related cancers who meet certain criteria and have not previously undergone BRCA genetic testing. An MDHHS study,42 using data prior to the ACA (2008-2012), found that insurance or out-of-pocket cost concerns were a substantial barrier for BRCA testing in women (with and without personal histories of breast or ovarian cancer) who had received BRCA genetic counseling which indicated that they were candidates for testing. A recent paper found a correlation between ACA coverage and increased BRCA testing in women with a family history of breast and/or ovarian cancer.43 Major private payers44 and CMS45 now provide coverage for LS screening, with some, including CMS, covering screening for all individuals diagnosed with colorectal cancer and citing the EGAPP recommendation.
The reach of the EGAPP and USPSTF recommendations and public health efforts to implement these recommendations have been magnified by the inclusion of similar recommendations in other guidelines and initiatives. Following the EGAPP recommendation, ten national and international recommendations have included universal LS screening.4,46–54 Recommendations from at least seven national and international organizations include strategies for identification of women at risk for BRCA pathogenic variants and cite the USPSTF recommendation.55–59
BRCA testing and LS screening were included in national initiatives aimed at improving health. The Healthy People 2020 genomics objectives,60 which cite the USPSTF and EGAPP recommendations, are “Increase the proportion of women with a family history of breast and/or ovarian cancer who receive genetic counseling” and “Increase the proportion of persons with newly diagnosed colorectal cancer who receive genetic testing to identify LS (or familial colorectal cancer syndromes).” The 2016 NCI Cancer MoonshotSM Blue Ribbon Panel Report61 recommended a LS Demonstration Project which includes LS screening of all new colorectal cancers in the U.S. and cited the EGAPP recommendation. The Report included an HBOC Demonstration Project focused on genetic testing of men with breast cancer, women with breast cancer <age 50, and women with ovarian cancer. While the USPSTF recommendation starts with unaffected women whose risk is identified through their family history, it acknowledges that testing should ideally first be done in a family member who has had a BRCA-related cancer. Also, the Demonstration Project would extend testing to relatives of individuals who test positive for a pathogenic variant.
Recent studies indicate that LS screening of colorectal cancer patients is not yet universal. A population-based study on those diagnosed in 2011 in Louisiana,62 found that only 23% of the 274 colorectal cancer patients aged ≤50 years were screened for LS. However, studies on institutions implementing universal screening have seen higher rates.13 As one measure of efficacy of the work to increase LS screening of all colorectal cancers, LSSN member institutions have screened >31,000 colorectal cancer cases since 2008, and LSSN provides a forum for providers to discuss questions on cases and screening (D. Duquette, personal communication). LSSN recently received funding for its database, which will provide an opportunity to track LS screening nationally across its member institutions.
Recent studies have shown increases in BRCA testing rates, and cancer family history has surpassed personal history of breast or ovarian cancer as the indication for testing.63 This is consistent with more women being identified and tested in accordance with the USPSTF guidelines.
Studies have shown disparities by race and ethnicity and rural and urban residential status in identification and treatment of those with hereditary cancer syndromes, highlighting opportunities for public health approaches which address these disparities. Black breast cancer survivors are less likely than breast cancer survivors of other races to have HBOC genetic counseling or testing.64–66 The most commonly reported reason was that their health care provider had not recommended genetic services,64,65 and health care providers primarily serving minority populations are less likely to refer or order genetic testing for their patients.67 Blacks with BRCA pathogenic variants are less likely to tell their relatives about their pathogenic variant, and relatives are less likely to be tested for the pathogenic variant.68 Furthermore, black women with BRCA pathogenic variants have lower rates of risk-reducing salpingo-oophorectomy than BRCA carriers of other races.65 Similarly for colorectal cancer, colonoscopy screening at ages 40-49 for first degree relatives of those with colorectal cancer was lower among blacks than whites.69 Hispanic women with early-onset breast cancer are also less likely to undergo BRCA testing, compared with non-Hispanic white women.66
Disparities have also been observed for those living in rural areas. A recent study70 looking at women with employee-sponsored insurance found that BRCA testing rates were lower in non-metropolitan areas compared with metropolitan areas, although the differences decreased over the study period, especially in younger women. Women living in non-metropolitan areas also were less likely to receive certain preventive interventions. Universal LS screening of colorectal cancers is less common in community hospitals, which tend to serve rural populations, compared with institutions with comprehensive cancer centers or programs,14 which tend to be located in more urban areas.
Efforts to increase risk assessment and genetic testing in populations with lower rates have shown success. For example, a study offering genetic counseling and testing for HBOC to women at a safety-net hospital in which 78% of patients were from racial and ethnic minority groups had high uptake rates for these services.71 Future public health efforts can impact disparities in the implementation of the EGAPP and USPSTF recommendations.
SHAPING THE FUTURE
Work by the public health community to promote HBOC risk assessment and universal LS screening has helped lead to new initiatives and programs. In 2015, the National Academies of Sciences, Engineering, and Medicine’s Roundtable on Genomics and Precision Health formed the Genomics and Population Health Action Collaborative (GPHAC)72 to identify opportunities for genomics to improve population health, prevent disease, and reduce health disparities; inform and engage stakeholders about implementation of genomics; and explore ways to integrate evidence-based genomic applications into population health programs at the health care-public health interface. GPHAC focused on LS and HBOC and included evaluation and implementation working groups in the first year. The GPHAC evaluation working group finalized its work with the completion of a white paper72 on “Building the Evidence Base for Genomics in Public Health: Implications for Decision Making, Public Policy, and Population Health Planning.” To address issues specific to health disparities in implementation of hereditary cancer risk assessment and prevention, the implementation working group created a subgroup focused on health disparities. In 2017, working groups were formed on cascade screening and population-based screening to address how to broaden identification of individuals with LS and BRCA pathogenic variants beyond screening of those who already have cancer, using either a cascade screening approach, in which family members of individuals identified with LS or BRCA pathogenic variants are offered genetic counseling and testing for the pathogenic variant that their relative has, or a population-based screening approach to identify those at-risk independent of their family history.
Some academic medical centers and health systems have already launched cascade screening programs for LS and BRCA pathogenic variants, and some state health departments have worked on defining the role of public health in cascade screening. However, further work is needed, including clinician and patient education about the importance and process of cascade screening. Using data reported by Myriad and data from the state BRCA Clinical Network surveillance system, the Michigan Department of Community Health showed that single site testing, used in cascade screening, increased by only 12.1% from 2008 to 2011, compared with increases of 72.2% in sequencing and 370.9% in rearrangement testing (D. Duquette, personal communication). MDHHS survey data showed that individuals identified with a BRCA pathogenic variant and those who tested negative in a family with a known BRCA pathogenic variant shared their BRCA testing results with an average of 11.7 and 9.2 relatives, respectively. However, only 2.2 and 1.1 of their relatives had subsequent BRCA testing (D. Duquette, personal communication). Health disparities in cascade screening have been observed. Among those receiving HBOC genetic counseling, black patients present with a known familial pathogenic variant at lower rates than whites (3.3% vs. 13.4%), thus showing lower rates of cascade screening (D. Duquette, personal communication).
NCI convened a 2017 workshop73 on “Approaches to Blue Ribbon Panel Recommendations: The Case of LS” to review the Blue Ribbon Panel recommendations and discuss health care delivery, knowledge gaps, and resources needed for implementation. The workshop helped lead to 2018 NCI funding for implementation research on hereditary cancers, including LS and HBOC.74
SUMMARY
We used CDC’s SIF to document the trajectory and influence of public health activities to implement genomics, starting with the EGAPP and USPSTF recommendations. The SIF is beneficial for examining the effects of public health activities to translate recommendations, which are broad and often difficult to delineate and measure on a causal pathway. A single product rarely produces impact in isolation but rather produces effects in combination with other contributions. Effects may not follow chronologically. The SIF accounts for all of this. One challenge in using the SIF is establishing links between events and outcomes without a set protocol or database to search, especially retrospectively when opportunities to collect further data may not be available. Also, the SIF lacks measures of magnitude or quantitation of effect size.
Our evaluation shows how cancer prevention programs have translated clinical evidence-based recommendations for identification of individuals at risk for hereditary cancer syndromes into activities for public health practice, with the long term goal of improving health. For example, MDHHS worked to raise awareness and establish systems to improve HBOC screening and showed a two-fold increase in genetic counseling for women with a family history consistent with HBOC, using BRFSS data. Inclusion of LS- and HBOC-related goals, objectives, and strategies in state cancer plans and national initiatives, such as Healthy People 2020 and the Cancer Moonshot, can provide the impetus for state health departments to implement hereditary cancer activities. CDC funds the central state-based cancer registries, which could provide further opportunities for collaborating at the state level to move these agenda forward. Evaluating activities of CDC-funded states is important to increase the evidence base for future efforts by state health departments and others.
The continued role of public health is crucial to ensure that health disparities in identification and treatment of hereditary cancer syndromes are addressed. These disparities have been recognized in some cases but work is needed to effect change. The GPHAC health disparities working group focuses on identifying and addressing these disparities, and the Cancer Moonshot funding includes the development of “optimum strategies for reaching diverse communities such as rural, racial/ethnic minorities and low-socioeconomic groups.” Evaluations of this work will be needed to see if they result in changes in health disparities in hereditary cancer risk assessment and prevention.
Comparing implementation of the USPSTF and EGAPP recommendations is informative. HBOC-related recommendations and federal funding were available earlier than LS-related ones. Clinicians responsible for carrying out the recommendations differ: primary care providers for USPSTF and pathologists for EGAPP, requiring different target audiences for provider education. Patients participate directly in HBOC screening through sharing family history information, unlike with LS tumor tissue testing. Tracking LS screening using available databases is challenging: the biochemical test used for most LS screening has multiple applications, so its use does not necessarily indicate LS screening. However, the LSSN database will address this gap. The success of the family history-based USPSTF recommendation might impede progress of the EGAPP recommendation, as providers performing LS screening might assume that family history information is required, highlighting the importance of clear public health messaging that all colorectal cancers should be screened.
Public health efforts to implement hereditary cancer prevention activities can serve as a model for future integration of genomics into public health approaches to chronic disease prevention, such as integration of familial hypercholesterolemia into heart disease prevention activities. To help those in the public health and clinical arenas determine which genomic applications are ready for implementation, CDC created an evidence-based classification of genomic tests and family history, the Tier Table Database.75 The Tier Table ranks genomic applications in three tiers according to level of evidence supporting their use, with Tier 1 applications having a synthesized evidence base supporting implementation in practice. LS screening of all newly diagnosed colorectal cancers, based on the EGAPP recommendation, and use of HBOC family history for risk prediction for BRCA genetic counseling referral, based on the USPSTF recommendation, are two of >40 Tier 1 tests. CDC works to promote implementation of Tier 1 genomic applications, and public health efforts to translate the EGAPP and USPSTF recommendations into public health activities can inform this work.
Supplementary Material
Footnotes
None of the authors have a conflict of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA, United States Preventive Services Task Force Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281. doi: 10.7326/M13-2747. [DOI] [PubMed] [Google Scholar]
- 3.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Guidelines Version 1.2017 Genetics/Familial High-Risk Assessment: Colon. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Accessed February 5, 2018.
- 5.Nelson HD, Pappas M, Zakher B, Mitchell JP, Okinaka-Hu L, Hu L, Fu R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: A systematic review to update the U.S. preventive services task force recommendation. Annals of Internal Medicine. 2014;160(4):255–266. doi: 10.7326/M13-1684. [DOI] [PubMed] [Google Scholar]
- 6.Siu AL, United States Preventive Services Task Force Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 7.Saslow D, Boetes C, Burke W, et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA: A Cancer Journal for Clinicians. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Science Making a Difference - Five Domains of Influence. Available at: https://www.cdc.gov/od/science/impact/index.htm. Accessed February 5, 2018.
- 9.United States Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med. 2005;143(5):355–361. doi: 10.7326/0003-4819-143-5-200509060-00011. [DOI] [PubMed] [Google Scholar]
- 10.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11(1):42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healthy People 2020 Progress Review: Cancer and Genomics. Available at: https://www.healthypeople.gov/sites/default/files/HP2020_Cancer_and_Genomics_Progress_Review_Slides.pdf. Accessed February 5, 2018.
- 12.For example; Grosse SD, Palomaki GE, Mvundura M, Hampel H. The cost-effectiveness of routine testing for Lynch syndrome in newly diagnosed patients with colorectal cancer in the United States: corrected estimates. Genet Med. 2015;17(6):510–511. doi: 10.1038/gim.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For example; Cohen SA, Laurino M, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122(3):393–401. doi: 10.1002/cncr.29758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beamer LC, Grant ML, Espenschied CR, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for lynch syndrome among US Cancer Programs and follow-up of abnormal results. Journal of Clinical Oncology. 2012;30(10):1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volmar KE, Idowu MO, Souers RJ, Nakhleh RE. Molecular testing in anatomic pathology and adherence to guidelines: A college of American pathologists Q-probes study of 2230 testing events reported by 26 institutions. Archives of Pathology and Laboratory Medicine. 2015;139(9):1115–1124. doi: 10.5858/arpa.2014-0513-CP. [DOI] [PubMed] [Google Scholar]
- 16.For example; Brannon Traxler L, Martin ML, et al. Implementing a Screening Tool for Identifying Patients at Risk for Hereditary Breast and Ovarian Cancer: A Statewide Initiative. Annals of Surgical Oncology. 2014;21(10):3342–3347. doi: 10.1245/s10434-014-3921-1. [DOI] [PubMed] [Google Scholar]
- 17.For example; Destounis S, Arieno A, Morgan R. Implementation of a risk assessment program in a breast-imaging community practice. Breast Cancer. 2016;23(2):273–278. doi: 10.1007/s12282-014-0569-4. [DOI] [PubMed] [Google Scholar]
- 18.For example; Acheson LS, Zyzanski SJ, Stange KC, Deptowicz A, Wiesner GL. Validation of a self-administered, computerized tool for collecting and displaying the family history of cancer. Journal of Clinical Oncology. 2006;24(34):5395–5402. doi: 10.1200/JCO.2006.07.2462. [DOI] [PubMed] [Google Scholar]
- 19.For example; Burke W, Culver J, et al. Genetic assessment of breast cancer risk in primary care practice. American Journal of Medical Genetics, Part A. 2009;149(3):349–356. doi: 10.1002/ajmg.a.32643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.For example; Cohen SA, Nixon DM. A collaborative approach to cancer risk assessment services using genetic counselor extenders in a multi-system community hospital. Breast Cancer Research and Treatment. 2016;159(3):527–534. doi: 10.1007/s10549-016-3964-z. [DOI] [PubMed] [Google Scholar]
- 21.For example; Bellcross CA, Leadbetter S, Alford SH, Peipins LA. Prevalence and healthcare actions of women in a large health system with a family history meeting the 2005 USPSTF recommendation for BRCA genetic counseling referral. Cancer Epidemiology Biomarkers and Prevention. 2013;22(4):728–735. doi: 10.1158/1055-9965.EPI-12-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medscape CDC Expert Commentary. Genetic Testing for Lynch Syndrome in Colorectal Cancer. Available at: http://www.medscape.com/viewarticle/735522. Genetics and BRCA in Primary Care. Available at: http://www.medscape.com/viewarticle/832522. Accessed February 5, 2018.
- 23.Hereditary Breast and Ovarian Cancer (HBOC) Available at: https://learn.education.jax.org/browse/hpe/cme/courses/hboc. Accessed February 5, 2018.
- 24.Rodriguez JL, Thomas CC, Massetti GM, et al. CDC Grand Rounds: Family History and Genomics as Tools for Cancer Prevention and Control. MMWR Morb Mortal Wkly Rep. 2016;65(46):1291–1294. doi: 10.15585/mmwr.mm6546a3. [DOI] [PubMed] [Google Scholar]
- 25.Know:BRCA Tool. Available at: https://www.cdc.gov/cancer/breast/young_women/knowbrca.htm. Accessed February 5, 2018.
- 26.Bring Your Brave Campaign. Available at: https://www.cdc.gov/cancer/breast/young_women/bringyourbrave/index.htm. Accessed February 5, 2018.
- 27.Trivers KF, Rodriguez JL, Cox SL, Crane BE, Duquette D. The Activities and Impact of State Programs to Address Hereditary Breast and Ovarian Cancer, 2011–2014. Healthcare (Basel) 2015;3(4):948–963. doi: 10.3390/healthcare3040948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laufman JD, Duquette D, Trepanier A. Evaluation of state comprehensive cancer control plans for genomics content. Preventing Chronic Disease. 2012;9(12) doi: 10.5888/pcd9.120190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zlot AI, Cox SL, Silvey K, Leman R. The effect of chronic disease family history on healthcare provider practice and patient behavior among Oregonians. Public Health Genomics. 2012;1(3–4):189–200. doi: 10.1159/000335555. [DOI] [PubMed] [Google Scholar]
- 30.Fussman CSJ, Duquette D. Michigan BRFSS Surveillance Brief. 3. Vol. 10. Lansing, MI: Michigan Department of Health and Human Services, Lifecourse Epidemiology and Genomics Division; 2016. Breast and Ovarian Cancer Personal/Family History and Genetic Counseling Utilization Among Michigan Women. [Google Scholar]
- 31.McLosky J, Anderson B, Duquette D, Fussman C. Colorectal Cancer and Genetic Testing Among Michigan Adults t. Michigan BRFSS Surveillance Brief. 2012;3 [Google Scholar]
- 32.Zlot AI, Silvey K, Newell N, Coates RJ, Leman R. Family history of colorectal cancer: Clinicians’ preventive recommendations and patient behavior. Prev Chronic Dis. 2012;9:E21. [PMC free article] [PubMed] [Google Scholar]
- 33.Mange S, Bellcross C, Cragun D, et al. Creation of a network to promote universal screening for Lynch syndrome: the LynchSyndrome Screening Network. J Genet Couns. 2015;24(3):421–427. doi: 10.1007/s10897-014-9770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellcross CA, Bedrosian SR, Daniels E, et al. Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: summary of a public health/clinical collaborative meeting. Genet Med. 2012;14(1):152–162. doi: 10.1038/gim.0b013e31823375ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senier L, Lee R, Nicoll L. The strategic defense of physician autonomy: State public health agencies as countervailing powers. Soc Sci Med. 2017;186:113–121. doi: 10.1016/j.socscimed.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowery JT, Axell L, Vu K, Rycroft R. A novel approach to increase awareness about hereditary colon cancer using a state cancer registry. Genet Med. 2010;12(11):721–725. doi: 10.1097/GIM.0b013e3181f1366a. [DOI] [PubMed] [Google Scholar]
- 37.Tier 1 Genomic Applications Toolkit for Public Health Departments. Available at: https://www.cdc.gov/genomics/implementation/toolkit/index.htm. Accessed February 5, 2018.
- 38.State Genomics Implementation Map. Available at: https://phgkb.cdc.gov/PHGKB/stateMapStartPage.action. Accessed February 5, 2018.
- 39.H.R.3590 - Patient Protection and Affordable Care Act. Available at: https://www.congress.gov/bill/111th-congress/house-bill/3590. Accessed February 5, 2018.
- 40.FAQS ABOUT AFFORDABLE CARE ACT IMPLEMENTATION (PART XXVI) Available at: https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/Downloads/aca_implementation_faqs26.pdf. Accessed February 5, 2018.
- 41.For example, Local Coverage Determination (LCD): MolDX: BRCA1 and BRCA2 Genetic Testing (L36082). Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=36082&ver=26&CoverageSelection=Both&ArticleType=All&PolicyType=Final&s=All&KeyWord=brca&KeyWordLookUp=Title&KeyWordSearchType=And&bc=gAAAACAAAAAAAA%3d%3d&. Accessed February 5, 2018 and Local Coverage Determination (LCD): MolDX: BRCA1 AND BRCA2 Genetic Testing (L36163). Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=36163&ver=3&SearchType=Advanced&CoverageSelection=Local&PolicyType=Both&s=&AdvSearchName=5%7c2&KeyWord=BRCA1+and+BRCA2&KeyWordLookUp=Title&KeyWordSearchType=Exact&kq=true&bc=IAAAACAAAAAAAA%3d%3d&, Accessed February 5, 2018.
- 42.Hayden S, Mange S, Duquette D, Petrucelli N, Raymond VM, Partners BCN. Large, Prospective Analysis of the Reasons Patients Do Not Pursue BRCA Genetic Testing Following Genetic Counseling. J Genet Couns. 2017 doi: 10.1007/s10897-016-0064-5. [DOI] [PubMed] [Google Scholar]
- 43.Han X, Jemal A. Recent Patterns in Genetic Testing for Breast and Ovarian Cancer Risk in the US. Am J Prev Med. 2017 doi: 10.1016/j.amepre.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 44.For example, BlueCross BlueShield of North Carolina Corporate Medical Policy: Genetic Testing for Colon Cancer. Available at: http://www.bcbsnc.com/assets/services/public/pdfs/medicalpolicy/genetic_testing_for_colon_cancer.pdf. BlueCross BlueShield of Rhode Island Medical Coverage Policy: Genetic Testing for Lynch Syndrome and Other Inherited Intestinal Polyposis Syndromes-PREAUTH. Available at: https://www.bcbsri.com/sites/default/files/polices/GeneticTestingLynchSyndrome.pdf. HMSA Genetic Testing for Lynch Syndrome And Other Inherited Colon Cancer Syndromes. Available at: https://hmsa.com/portal/provider/MM.02.007_Genetic_Testing_for_Lynch_Syndrome_and_Other_Inherited_Colon_Cancer_Syndromes_042216.pdf. Aetna: Genetic Testing. Available at: http://www.aetna.com/cpb/medical/data/100_199/0140.html. Cigna Medical Coverage Policy: Tumor Profiling, Gene Expression Assays and Molecular Diagnostic Testing for Hematology/Oncology Indications. Available at: https://cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/medical/mm_0520_coveragepositioncriteria_tumor_profiling.pdf. Cigna Medical Coverage Policy: Genetic Testing for Hereditary Cancer Susceptibility Syndromes. Available at: https://cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/medical/mm_0518_coveragepositioncriteria_genetic_cancer_syndromes.pdf. Humana Medical Coverage Policy: Genetic Testing for Colorectal Cancer Susceptibility. Available at: http://apps.humana.com/tad/tad_new/Search.aspx?criteria=Lynch&searchtype=freetext&policyType=both. Anthem Medical Policy: Genetic Testing for Colorectal Cancer Susceptibility. Available at: https://www.anthem.com/medicalpolicies/policies/mp_pw_c166601.htm. United Healthcare Medical Policy: Genetic Testing. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Genetic_Testing.pdf. Accessed February 5, 2018.
- 45.For example, Local Coverage Determination (LCD): MolDX: Genetic Testing for LYNCH SYNDROME (L35349) Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=35349&ver=14&CoverageSelection=Both&ArticleType=All&PolicyType=Final&s=All&KeyWord=lynch+syndrome&KeyWordLookUp=Title&KeyWordSearchType=And&bc=gAAAACAAAAAAAA%3d%3d&. Local Coverage Determination (LCD): Genetic Testing for LYNCH SYNDROME (L34912). Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=34912&ver=13&CoverageSelection=Both&ArticleType=All&PolicyType=Final&s=All&KeyWord=lynch+syndrome&KeyWordLookUp=Title&KeyWordSearchType=And&bc=gAAAACAAAAAAAA%3d%3d&. Local Coverage Determination (LCD): MolDX: Genetic Testing for LYNCH SYNDROME (L36370). Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=36370&ver=8&CoverageSelection=Both&ArticleType=All&PolicyType=Final&s=All&KeyWord=lynch+syndrome&KeyWordLookUp=Title&KeyWordSearchType=And&bc=gAAAACAAAAAAAA%3d%3d&. Local Coverage Determination (LCD): MolDX: Genetic Testing for LYNCH SYNDROME (L36374). Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=36374&ver=7&CoverageSelection=Both&ArticleType=All&PolicyType=Final&s=All&KeyWord=lynch+syndrome&KeyWordLookUp=Title&KeyWordSearchType=And&bc=gAAAACAAAAAAAA%3d%3d&. Local Coverage Determination (LCD): MolDX: Genetic Testing for LYNCH SYNDROME (L35024). Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=35024&ver=36&CoverageSelection=Both&ArticleType=All&PolicyType=Final&s=All&KeyWord=lynch+syndrome&KeyWordLookUp=Title&KeyWordSearchType=And&bc=gAAAACAAAAAAAA%3d%3d&. Local Coverage Determination (LCD): MolDX: Genetic Testing for LYNCH SYNDROME (L36793). Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=36793&ver=4&CoverageSelection=Both&ArticleType=All&PolicyType=Final&s=All&KeyWord=lynch+syndrome&KeyWordLookUp=Title&KeyWordSearchType=And&bc=gAAAACAAAAAAAA%3d%3d&. Local Coverage Determination (LCD): Pathology and Laboratory: Genetic Testing for LYNCH SYNDROME (L35553). Available at: https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=35553&ver=6&CoverageSelection=Both&ArticleType=All&PolicyType=Final&s=All&KeyWord=lynch+syndrome&KeyWordLookUp=Title&KeyWordSearchType=And&bc=gAAAACAAAAAAAA%3d%3d&. Accessed February 5, 2018.
- 46.Balmana J, Balaguer F, Cervantes A, Arnold D, Group EGW Familial risk-colorectal cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2013;24(Suppl 6):vi73–80. doi: 10.1093/annonc/mdt209. [DOI] [PubMed] [Google Scholar]
- 47.Duffy MJ, Lamerz R, Haglund C, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134(11):2513–2522. doi: 10.1002/ijc.28384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol. 2014;109(8):1159–1179. doi: 10.1038/ajg.2014.186. [DOI] [PubMed] [Google Scholar]
- 49.Rubenstein JH, Enns R, Heidelbaugh J, Barkun A, Clinical Guidelines Committee American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Lynch Syndrome. Gastroenterology. 2015;149(3):777–782. doi: 10.1053/j.gastro.2015.07.036. quiz e716–777. [DOI] [PubMed] [Google Scholar]
- 50.Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J Mol Diagn. 2017;19(2):187–225. doi: 10.1016/j.jmoldx.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015;33(2):209–217. doi: 10.1200/JCO.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223–262. doi: 10.1038/ajg.2014.435. quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weissman SM, Burt R, Church J, et al. Identification of individuals at risk for Lynch syndrome using targeted evaluations and genetic testing: National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Colorectal Cancer joint practice guideline. J Genet Couns. 2012;21(4):484–493. doi: 10.1007/s10897-011-9465-7. [DOI] [PubMed] [Google Scholar]
- 54.National Institute for Health and Care Excellence. Molecular testing strategies for Lynch syndrome in people with colorectal cancer. Available at: https://www.nice.org.uk/guidance/dg27/chapter/1-Recommendations. Accessed February 5, 2018.
- 55.Singapore Cancer Network Cancer Genetics W. Singapore Cancer Network (SCAN) Guidelines for Referral for Genetic Evaluation of Common Hereditary Cancer Syndromes. Ann Acad Med Singapore. 2015;44(10):492–510. [PubMed] [Google Scholar]
- 56.Berliner JL, Fay AM, Cummings SA, Burnett B, Tillmanns T. NSGC practice guideline: risk assessment and genetic counseling for hereditary breast and ovarian cancer. J Genet Couns. 2013;22(2):155–163. doi: 10.1007/s10897-012-9547-1. [DOI] [PubMed] [Google Scholar]
- 57.Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17(1):70–87. doi: 10.1038/gim.2014.147. [DOI] [PubMed] [Google Scholar]
- 58.Wilson RD, Langlois S, SOGC Genetics Committee Genetic considerations for a woman’s annual gynaecological examination. J Obstet Gynaecol Can. 2012;34(3):276–284. doi: 10.1016/S1701-2163(16)35189-1. [DOI] [PubMed] [Google Scholar]
- 59.Breast and Ovarian. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed February 5, 2018.
- 60.Healthy People 2020 Genomics Objective. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/genomics. Accessed February 5, 2018.
- 61.Cancer MoonshotSM Blue Ribbon Panel Report. Available at: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel. Accessed February 5, 2018.
- 62.Karlitz JJ, Hsieh MC, Liu Y, et al. Population-Based Lynch Syndrome Screening by Microsatellite Instability in Patients </=50: Prevalence, Testing Determinants, and Result Availability Prior to Colon Surgery. Am J Gastroenterol. 2015;110(7):948–955. doi: 10.1038/ajg.2014.417. [DOI] [PubMed] [Google Scholar]
- 63.Chen Z, Kolor K, Grosse SD, et al. Trends in utilization and costs of BRCA testing among women aged 18–64 years in the United States, 2003–2014. Genet Med. 2017 doi: 10.1038/gim.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCarthy AM, Bristol M, Domchek SM, et al. Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. Journal of Clinical Oncology. 2016;34(22):2610–2618. doi: 10.1200/JCO.2015.66.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cragun D, Weidner A, Lewis C, et al. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017 doi: 10.1002/cncr.30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levy DE, Byfield SD, Comstock CB, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genetics in Medicine. 2011;13(4):349–355. doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shields AE, Burke W. Results of a national survey. Genetics in Medicine. 2008;10(6):404–414. doi: 10.1097/GIM.0b013e3181770184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fehniger J, Lin F, Beattie MS, Joseph G, Kaplan C. Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. Journal of Genetic Counseling. 2013;22(5):603–612. doi: 10.1007/s10897-013-9592-4. [DOI] [PubMed] [Google Scholar]
- 69.Tsai MH, Xirasagar S, de Groen PC. Persisting Racial Disparities in Colonoscopy Screening of Persons with a Family History of Colorectal Cancer. J Racial Ethn Health Disparities. 2017 doi: 10.1007/s40615-017-0418-1. [DOI] [PubMed] [Google Scholar]
- 70.Kolor K, Chen Z, Grosse SD, et al. BRCA Genetic Testing and Receipt of Preventive Interventions Among Women Aged 18–64 Years with Employer-Sponsored Health Insurance in Nonmetropolitan and Metropolitan Areas - United States, 2009–2014. MMWR Surveill Summ. 2017;66(15):1–11. doi: 10.15585/mmwr.ss6615a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Komenaka IK, Nodora JN, Madlensky L, et al. Participation of low-income women in genetic cancer risk assessment and BRCA 1/2 testing: the experience of a safety-net institution. Journal of Community Genetics. 2016;7(3):177–183. doi: 10.1007/s12687-015-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Action Collaboratives. Genomics and Population Health—A Precision Health Activity. Available at: http://www.nationalacademies.org/hmd/Activities/Research/GenomicBasedResearch/Innovation-Collaboratives/Genomics-and-Population-Health.aspx. Accessed February 5, 2018.
- 73.Approaches to Blue Ribbon Panel Recommendations: The Case of Lynch Syndrome. Available at: https://cancercontrol.cancer.gov/lynch-syndrome-workshop/. Accessed February 5, 2018.
- 74.RFA-CA-17-041: Approaches to Identify and Care for Individuals with Inherited Cancer Syndromes (U01) Available at: https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-17-041.html. Accessed February 5, 2018.
- 75.Dotson WD, Douglas MP, Kolor K, et al. Prioritizing genomic applications for action by level of evidence: a horizon-scanning method. Clin Pharmacol Ther. 2014;95(4):394–402. doi: 10.1038/clpt.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


