Abstract
Objective:
To assess geographic variation in cystoscopy rates among women versus men with suspected bladder cancer, lending insight into gender-specific differences in cystoscopic evaluation.
Methods:
We conducted a cross-sectional study of all fee-for-service Medicare beneficiaries within 306 Hospital Referral Regions (HRRs) who received care in 2014. For each HRR, we calculated the age- and race-adjusted cystoscopy rate for women and men as our primary outcome. The rate was the number of beneficiaries who underwent cystoscopy for bladder cancer symptoms (using procedure and ICD-9 diagnosis codes) divided by all beneficiaries in the HRR. We used the coefficient of variation to compare relative variability of cystoscopy rates.
Results:
Overall, 173,551 women (n=14.8 million) and 286,090 men (n=11.5 million) underwent cystoscopy in 2014. While women received less cystoscopies compared to men (mean 11.0 vs. 23.5 per 1,000, p<0.001), there was greater variation in cystoscopy rates among women (coefficient of variation 27.5 vs. 23.5, p=0.010). When restricting to ICD-9 codes for hematuria only, women continued to demonstrate greater variation in cystoscopy rates (coefficient of variation 27.8 vs. 24.2, p=0.022). Findings were robust across larger HRR sizes – thereby removing some random variation seen in smaller HRRs – as well as across years 2010, 2011, 2012, and 2013.
Conclusions:
Cystoscopy rates are lower in women than men, likely due to their lower bladder cancer incidence. However, there is greater variation in cystoscopy rates among women with symptoms of bladder cancer. This may reflect increased provider uncertainty whether to refer and work-up women with suspected bladder cancer.
Keywords: bladder cancer, geographic variation, cystoscopy
INTRODUCTION
Nearly 80,000 new cases of bladder cancer are diagnosed each year with over 15,000 deaths estimated annually.1 While only 23% of new bladder cancers are diagnosed among women, more women than men (31–43% vs. 26–28%) are diagnosed with advanced disease.2–5 Advanced stage at diagnosis generally predicts worse outcomes, and bladder cancer is no exception.6 Thus, survival among women with bladder cancer is worse than among men, with 77% of women surviving beyond 5 years compared to 82% of men.7 This difference in survival between women and men has been attributed in part to more advanced disease stage at presentation.4
Many believe the gender disparity in disease stage at presentation is related to delayed bladder cancer diagnosis among women,8–10 who may not always undergo diagnostic cystoscopy as readily as men. For example, painless hematuria in older men may raise more immediate concern for bladder cancer. In contrast, painless hematuria and irritative voiding symptoms in older women may also be associated with a urinary tract infection.9,11 Indeed, both patient- and physician-related factors contribute to the longer delay in diagnosis observed among women.12,13 Suspected factors include patient decision to first seek care based on symptoms,14,15 primary care provider assessment for appropriate urologic referral,11,16,17 and urologist discretion to potentially rule in (or out) bladder cancer with thorough work-up and cystoscopy.11 In both women and men, the interplay between these and additional factors likely contributes to variation in the number of diagnostic cystoscopies performed for suspected bladder cancer on a population level.
To lend some insight into differences in cystoscopic evaluation between women and men on a population level, we sought to understand the geographic variation in cystoscopy rates among women versus men with suspected bladder cancer. We hypothesized that there would be greater variation in diagnostic cystoscopy procedures among women than men, reflecting greater uncertainty in referral and work-up. If true, future work could then examine the extent to which intensity of cystoscopic evaluation is associated with stage at the time of bladder cancer diagnosis among women and men, taking a first step towards further understanding and ultimately reducing gender disparities in bladder cancer.
METHODS
Overview of Design
We conducted a cross-sectional study of all fee-for-service Medicare beneficiaries aged 65 to 99 enrolled in Parts A and B during the entire calendar year 2014. Our primary interest was in comparing and contrasting cystoscopy rates among women and men within the 306 Hospital Referral Regions (HRRs) in the United States. HRRs are regional health care markets for tertiary medical care and were the unit of analysis.18 Thus, we calculated adjusted cystoscopy rates performed for symptoms associated with suspected bladder cancer for each of the 306 HRRs, stratified by gender. We then assessed variation in rates across HRRs, separately for women and men, and compared the magnitude of variation between them. The Dartmouth College Committee for the Protection of Human Subjects exempted the study from review.
Outcome
The primary outcome was the HRR-specific rate of cystoscopy in 2014, stratified by gender. The denominator for this rate was the total number of female or male beneficiaries with at least one Medicare claim residing in each HRR. The numerator for this rate was the number of beneficiaries within each HRR who underwent at least one cystoscopy procedure for symptoms associated with bladder cancer. To estimate that number, we used Current Procedural Terminology (CPT) codes (Supplemental Table 1) to identify diagnostic cystoscopy procedures. We only included cystoscopy procedures from claim line-items with an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code that may indicate a possible bladder cancer diagnosis (e.g. hematuria, bladder cancer, urinary tract infection, dysuria, urinary urgency, Supplemental Table 1). We included bladder cancer diagnosis codes because urologic coding practices may vary.19 For instance, a diagnostic cystoscopy initially performed for hematuria that reveals a bladder cancer could be coded as hematuria or bladder cancer; we therefore analyzed an initially broad range of diagnosis codes to ensure we did not miss procedures that were done for bladder cancer diagnosis. Finally, only one cystoscopy procedure was counted per beneficiary. This avoided over-counting procedures among patients who may have been diagnosed with a non-muscle-invasive bladder cancer and were then followed with surveillance cystoscopy procedures, as per non-muscle-invasive bladder cancer guidelines.20
Statistical Analysis
All HRR-specific rates were age- and race-adjusted using indirect adjustment as customary of The Dartmouth Atlas of Health Care.18 We used the two-sample t-test to compare cystoscopy rates between women and men across HRRs. We used the coefficient of variation as a measure of relative variability for cystoscopy rates among women and men. The coefficient of variation is defined as the standard deviation divided by the mean and multiplied by 100 to give a percentage; therefore, the coefficient of variation provides a normalized measure of dispersion that adjusts for differing means. We used Feltz & Miller‟s asymptotic test as our test of equality for the coefficients of variation (R-package “cvequality” Version 0.1.3).21,22 We conducted additional analyses restricted to only HRRs with at least 20,000 and 50,000 beneficiaries. Similar to our prior work,23 this was done to remove some of the random variation that can be encountered in HRRs with a smaller number of total beneficiaries. All analyses were performed with Stata 15.0 and R.
Sensitivity Analysis
We conducted two sensitivity analyses. First, we restricted analyses to cystoscopy procedures associated with ICD-9 diagnosis codes for hematuria (Supplemental Table 1) – the most common initial symptom of bladder cancer regardless of gender.12 This was done to evaluate whether possible over-counting of cystoscopy procedures associated with the broader list of ICD-9 diagnosis codes used in the main analyses affected our results. Second, to ensure findings were consistent across years, we repeated our cross-sectional analyses for calendar years 2010, 2011, 2012, and 2013.
RESULTS
There were almost 15 million female and 12 million male Medicare beneficiaries in 2014 of whom 173,551 and 286,090 underwent at least one cystoscopy procedure, respectively (Supplemental Table 2). Among 306 HRRs in the United States, 286 HRRs had >20,000 beneficiaries and 174 had >50,000 beneficiaries in 2014; HRRs contained an average of 86,053 Medicare beneficiaries. The adjusted mean cystoscopy rate across 306 HRRs was less than half among women than men (mean 11.0 vs. 23.5 per 1,000 beneficiaries, p-value < 0.001), a consistent finding when restricting analysis by HRR size (Table 1). Figure 1 shows the adjusted rate of cystoscopy procedures across the 306 HRRs for women and men. Each dot in the Turnip Plot represents an HRR graphed to its corresponding adjusted cystoscopy rate along the Y-axis, and the figure illustrates the lower rates among women as well as a substantial amount of variation across HRRs.
Table 1:
Variation Measures for Adjusted Cystoscopy Rates per 1,000 Beneficiaries in 2014
| All HRRs [n = 306] |
HRRs >20,000 Beneficiaries [n = 286] |
HRRs >50,000 Beneficiaries [n = 174] |
||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| Mean (SD) | 11.0 (3.0) | 23.5 (5.5) | 11.1 (3.0) | 23.7(5.4) | 11.6 (2.8) | 24.7 (5.0) |
| p-value* | < 0.001 | < 0.001 | < 0.001 | |||
| Coefficient of Variation | 27.5 | 23.5 | 27.1 | 22.9 | 23.8 | 20.2 |
| p-value†, SD / Mean | 0.010 | 0.008 | 0.043 | |||
Abbreviations: SD, Standard Deviation; HRR, Hospital Referral Region
2-sample t-test
Feltz and Miller‟s Asymptotic Test
Figure 1:
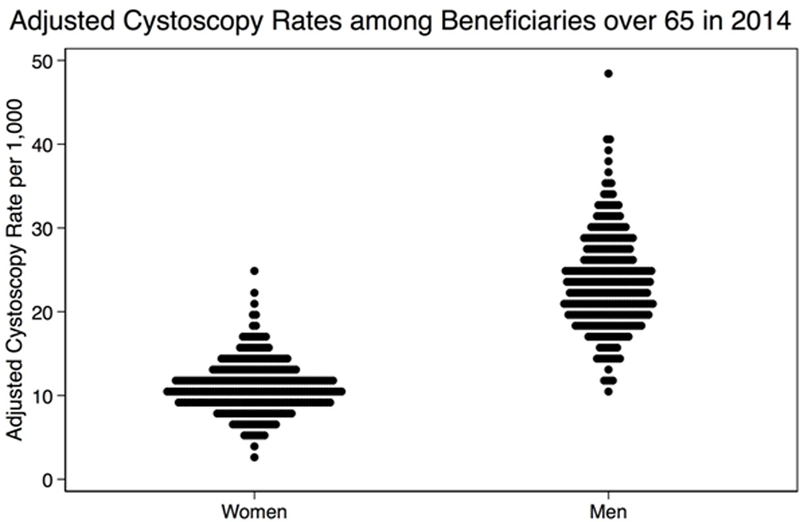
Turnip plot graphing the age- and race-adjusted cystoscopy rates among female and male Medicare beneficiaries in 2014. Each dot represents a Hospital Referral Region.
There was significantly greater variation in adjusted cystoscopy rates among women than men (coefficient of variation 27.5 vs. 23.5, p-value = 0.010). This finding persisted when removing some of the random variation present in smaller HRRs by restricting analyses to larger HRRs with >20,000 and >50,000 beneficiaries (Table 1). Figure 2 visually demonstrates this larger amount of variation among women than men when normalizing to the gender-specific mean cystoscopy rates across all 306 HRRs, those with >20,000 beneficiaries, and those with>50,000 beneficiaries. In the Turnip Plot, each dot represents an HRR, and the greater spread of HRRs among women compared to men along a Y-axis of gender-specific means portrays the greater geographic variation in adjusted cystoscopy rates.
Figure 2:
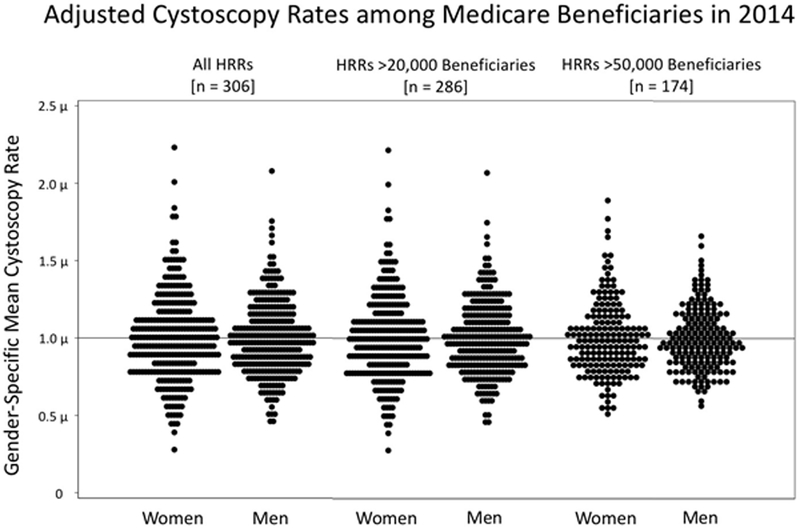
Turnip plots graphing the age- and race-adjusted cystoscopy rates among female and male Medicare beneficiaries in 2014 along a Y-axis of gender-specific means. Each dot represents a Hospital Referral Region (HRR). The distribution provides a visual representation of the normalized variation in cystoscopy procedures by gender. Results are shown for all HRRs (left), those with >20,000 beneficiaries (middle), and those with >50,000 beneficiaries (right).
We performed a sensitivity analysis restricted to claim line-items of cystoscopy procedures associated with ICD-9 diagnosis codes for hematuria only. Of the almost 15 million female and 12 million male Medicare beneficiaries included, 118,395 women and 240,812 men underwent at least one such procedure during calendar year 2014. Women again had a lower mean cystoscopy rate than men (mean 7.5 vs. 19.8 per 1,000 beneficiaries, p-value < 0.001). There remained greater normalized variation in cystoscopy rates (coefficient of variation 27.8 vs. 24.2, p-value = 0.022). Results from this sensitivity analysis were robust across HRR sizes (Table 2). In additional sensitivity analyses, findings were not substantially different in calendar years 2010, 2011, 2012, or 2013.
Table 2:
Sensitivity Analysis Restricted to Hematuria ICD-9 Codes for Adjusted Cystoscopy Rates per 1,000 Beneficiaries in 2014
| All HRRs [n = 306] |
HRRs >20,000 Beneficiaries [n = 286] |
HRRs >50,000 Beneficiaries [n = 174] |
||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| Mean (SD) | 7.5 (2.1) | 19.8 (4.8) | 7.5 (2.1) | 19.9 (4.7) | 7.9 (2.0) | 20.8 (4.4) |
| p-value* | < 0.001 | < 0.001 | < 0.001 | |||
| Coefficient of Variation | 27.8 | 24.2 | 27.5 | 23.5 | 24.8 | 21.2 |
| p-value†, SD / Mean | 0.022 | 0.012 | 0.049 | |||
Abbreviations: SD, Standard Deviation; HRR, Hospital Referral Region
2-sample t-test
Feltz and Miller‟s Asymptotic Test
DISCUSSION
In this population-based study of all fee-for-service Medicare enrollees in 2014, rates of diagnostic cystoscopy were significantly lower in women than in men – a finding one would expect given that fewer women have bladder cancer than men.1 Importantly, there was significantly greater geographic variation in cystoscopy procedures among women with suspected bladder cancer than among men. Our results persisted when restricting by HRR size to remove random noise from smaller regions, suggesting these gender-specific patterns in diagnostic cystoscopy are robust. The greater variation in cystoscopy rates among women may reflect the greater uncertainty among providers regarding who should be referred to urology and worked-up.
Appropriate and timely referrals to urology and work-up with imaging and cystoscopy have important implications for the quality of bladder cancer care, as delays in diagnosis are associated with worse outcomes.6,24 Accordingly, the American Urological Association recommends a cystoscopy procedure in any patient 35-years and older presenting with asymptomatic microhematuria and in all patients with gross hematuria after ruling out benign causes.25 As such, providers must weigh the possibility of more common and benign etiologies like urinary tract infection with the risk of more sinister disease.
Several studies have sought to understand the gender disparity in diagnostic stage between women and men with bladder cancer. On the patient level, in the absence of recommended screening tests for individuals at average risk, timely bladder cancer diagnosis depends on both the patient‟s ability to recognize early symptoms and his or her willingness to seek care.15,26 In a survey of newly diagnosed bladder cancer patients, 55% of women reported direct consultation with a urologist when initial symptoms arose compared to 78% of men.15 On the provider level, timely diagnosis depends on an appropriate level of suspicion for bladder cancer and on avoiding misdiagnoses. Women with bladder cancer had greater than twice the likelihood of initially being diagnosed with a urinary tract infection than men prior to diagnostic work-up.11 Additionally, differences in referral patterns exist between men and women. At a Midwest managed care organization, 28% of women with hematuria were referred for urologic evaluation compared to 47% of men.16 More recent investigations based on linked Surveillance, Epidemiology, and End Results (SEER)–Medicare data have corroborated that women are less promptly referred to a urologist and more likely to experience delays in hematuria evaluation.17
Our study complements prior findings and adds to the body of literature by providing a population-level assessment of geographic variation in cystoscopy for all fee-for-service Medicare beneficiaries with suspected bladder cancer. Greater geographic variation is commonly interpreted as a proxy for differing patient behavior and clinical uncertainty.27 Thus, the significantly greater geographic variation in cystoscopy among women than men with suspected bladder may in part be due to differences in patient behavior, specifically who will or will not seek direct urologic care for symptoms. In addition, it may reflect increased uncertainty among providers whether to refer and work-up women with symptoms that could be associated with urinary tract infections, but also with bladder cancer.
The present study is not without limitations. First, it excludes beneficiaries participating in Health Maintenance Organizations, persons residing outside of the United States, and patients below 65-years-old. However, as bladder cancer is primarily a disease of older adults (median age at diagnosis 79 years),1 Medicare data should reflect care among the majority of bladder cancer patients. Second, given the cross-sectional nature of our data, we were unable to ascertain whether cystoscopy preceded or followed a diagnosis of bladder cancer and thus was a diagnostic or surveillance cystoscopy. As a result, we likely overestimated the rate of diagnostic cystoscopy, capturing some surveillance cystoscopy procedures among patients with a history of bladder cancer. To address this as much as possible, we only counted one procedure per beneficiary and year. Third, our study – as any study utilizing Medicare claims data – may have been affected by varying coding practices. For example, a diagnostic cystoscopy procedure that was performed for hematuria and revealed a bladder tumor may have been coded with either a hematuria or bladder cancer code. Thus, we included bladder cancer diagnosis codes in our primary analyses but also conducted sensitivity analyses restricted to ICD-9 codes for hematuria only, which confirmed our main findings. Lastly, we acknowledge that HRRs are defined based on referral and receipt of tertiary neurologic and cardiac care – and not necessarily cancer-specific care. While there is ongoing development of Cancer Service Areas (geographic regions based on cancer-related utilization) that may provide more nuanced insight, such work is in its nascent stages.28 However, as HRRs have been used to examine health services in various specialties including urology,23 we consider HRRs suitable units of analysis.
In spite of these limitations, our study has important strengths. Our findings persisted when restricting analyses to larger HRRs, thereby limiting the effects of random variation in smaller geographic regions. They also reflect the care of more than two thirds of the population 65 and older in the United States,29 as we were able to include 100% of the Medicare fee-for-service population. This study, while offering a preliminary look at diagnostic cystoscopy practices for suspected bladder cancer, is to our knowledge one of the largest most representative studies to date.
In conclusion, while women have lower cystoscopy rates for suspected bladder cancer than men, there is greater variation in cystoscopy procedures among women than men. This key latter finding suggests greater clinical uncertainty on how to best care for women with possible bladder cancer. Future work may entail developing a better population-level understanding of the relationship between diagnostic cystoscopy rates and stage of bladder cancer at the time of diagnosis. Furthermore, we may be able to improve care among women with possible bladder cancer by decreasing unwarranted variation in referral and work-up. This may be accomplished by educating patients to recognize symptoms of bladder cancer and seek care,15,26 acknowledging the value in gender-specific guidelines for hematuria work-up,25,30 and raising awareness about the importance of a hematuria work-up among providers.
Supplementary Material
Acknowledgments
Funding: The data was obtained from The Dartmouth Atlas, which is funded by the Robert Wood Johnson Foundation and The Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). FRS is supported by a Conquer Cancer Foundation Career Development Award and by the Dow-Crichlow Award of the Department of Surgery at the Dartmouth-Hitchcock Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: Opinions expressed in this manuscript are those of the authors and do not constitute official opinions of the Dartmouth Atlas of Health Care or the Department of Veterans Affairs.
References:
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD. :2017. [Google Scholar]
- 2.Kiemeney L, Coebergh J, Koper N, et al. Bladder cancer incidence and survival in the south-eastern part of The Netherlands, 1975–1989. European Journal of Cancer 1994;30(8):1134–1137. [DOI] [PubMed] [Google Scholar]
- 3.Mungan NA, Kiemeney LA, van Dijck JA, van der Poel HG, Witjes JA. Gender differences in stage distribution of bladder cancer. Urology 2000;55(3):368–371. [DOI] [PubMed] [Google Scholar]
- 4.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115(1):68–74. [DOI] [PubMed] [Google Scholar]
- 5.Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR. The national cancer data base report on bladder carcinoma. Cancer. 1996;78(7):1505–1513. [DOI] [PubMed] [Google Scholar]
- 6.Wallace D, Harris D. Delay in treating bladder tumours. The Lancet 1965;286(7407):332–334. [DOI] [PubMed] [Google Scholar]
- 7.Abdollah F, Gandaglia G, Thuret R, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer epidemiology 2013;37(3):219–225. [DOI] [PubMed] [Google Scholar]
- 8.Burge F, Kockelbergh R. Closing the Gender Gap: Can We Improve Bladder Cancer Survival in Women?-A Systematic Review of Diagnosis, Treatment and Outcomes. Urologia internationalis 2016;97(4):373–379. [DOI] [PubMed] [Google Scholar]
- 9.Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Paper presented at: Urologic Oncology: Seminars and Original Investigations 2004. [DOI] [PubMed]
- 10.Dobruch J, Daneshmand S, Fisch M, et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. European urology 2016;69(2):300–310. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JA, Vekhter B, Lyttle C, Steinberg GD, Large MC. Sex disparities in diagnosis ofbladder cancer after initial presentation with hematuria: A nationwide claims‐based investigation. Cancer 2014;120(4):555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mommsen S, Aagaard J, Sell A. Presenting symptoms, treatment delay and survival in bladder cancer. Scandinavian journal of urology and nephrology 1983;17(2):163–167. [DOI] [PubMed] [Google Scholar]
- 13.Månsson Å, Anderson H, Colleen S. Time lag to diagnosis of bladder cancer–influence of psychosocial parameters and level of health-care provision. Scandinavian journal of urology and nephrology 1993;27(3):363–369. [DOI] [PubMed] [Google Scholar]
- 14.Cooper CP, Polonec L, Stewart SL, Gelb CA. Gynaecologic cancer symptom awareness, concern and care seeking among US women: a multi-site qualitative study. Family practice 2012;30(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henning A, Wehrberger M, Madersbacher S, et al. Do differences in clinical symptoms and referral patterns contribute to the gender gap in bladder cancer? BJU international 2013;112(1):68–73. [DOI] [PubMed] [Google Scholar]
- 16.Johnson EK, Daignault S, Zhang Y, Lee CT. Patterns of hematuria referral to urologists: does a gender disparity exist? Urology 2008;72(3):498–502. [DOI] [PubMed] [Google Scholar]
- 17.Garg T, Pinheiro LC, Atoria CL, et al. Gender disparities in hematuria evaluation and bladder cancer diagnosis: a population based analysis. The Journal of urology 2014;192(4):1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman DC, Brownlee S, Chang C, Fisher ES. Regional and racial variation in primary care and the quality of care among Medicare beneficiaries. Lebanon, NH: Dartmouth Atlas of Health Care; 2010. [PubMed] [Google Scholar]
- 19.Cheema ZA, Khwaja SA. Implications of miscoding urological procedures in an era of financial austerity–„Every Penny Counts‟. JRSM open 2015;6(6):2054270415593463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. The Journal of urology 2016;196(4):1021–1029. [DOI] [PubMed] [Google Scholar]
- 21.Feltz CJ, Miller GE. An asymptotic test for the equality of coefficients of variation from k populations. Statistics in medicine 1996;15(6):646–658. [DOI] [PubMed] [Google Scholar]
- 22.Tests for the equality of coefficients of variation for multiple groups. v. 0.1.3 [computer program]. 2016.
- 23.Welch HG, Skinner JS, Schroeck FR, Zhou W, Black WC. Regional variation of computed tomographic imaging in the United States and the risk of nephrectomy. JAMA internal medicine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace D, Bryan R, Dunn J, Begum G, Bathers S. Delay and survival in bladder cancer. BJU international 2002;89(9):868–878. [DOI] [PubMed] [Google Scholar]
- 25.Davis R, Jones JS, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. The Journal of urology 2012;188(6):2473–2481. [DOI] [PubMed] [Google Scholar]
- 26.Dyson B DS, Fenton K. Forthcoming national Be Clear on Cancer „Blood in Pee‟ symptom awareness campaign 2015; http://www.cancerresearchuk.org/health-professional/awareness-and-prevention/be-clear-on-cancer/blood-in-pee-campaign.
- 27.Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. The Lancet 2013;382(9898):1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onega T “Automated Delineation of Cancer Service Areas.” Dartmouth College. National Cancer Institute: Division of Cancer Control & Population Services https://maps.cancer.gov/overview/DCCPSGrants/abstract.jsp?applId=9387606&term=CA212687#Abstract; 2017. Accessed July 8, 2018.
- 29.The Henry J Kaiser Family Foundation. Medicare Advantage 2017; https://www.kff.org/medicare/fact-sheet/medicare-advantage/. Accessed March 16, 2018.
- 30.Sung M, Whiteside JL. COMMITTEE OPINION SUMMARY. Obstet Gynecol 2017;129:e168–172.28368896 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


