To the Editor:
It is increasingly well known that specific immunoglobulin (Ig) E antibodies to galactose-α-1,3-galactose (alpha-gal) are associated with delayed anaphylaxis to mammalian meat and gelatin-based products.1 Recently, we reported that gelatin or bovine calf-serum containing vaccines are capable of inducing immediate hypersensitivity and anaphylaxis in alpha-gal allergic adult patients, though not all patients have reactions.2–4 Importantly, multiple vaccines utilized during the routine series of pediatric immunizations contain gelatin and potentially alpha-gal.5 We report a case of vaccine-induced anaphylaxis associated with alpha-gal allergy in a child.
The patient is a 5 year old male with a history of alpha-gal allergy who presented one week after an allergic reaction upon receipt of MMR (Merck), Varicella (Merck), and DTaP/IPV(GSK) vaccines. Five minutes after receiving the vaccines, he developed shortness of breath, wheezing, disseminated urticaria, and angioedema of the face and oropharynx, prompting an emergency room visit where he received epinephrine, diphenhydramine, prednisone and famotidine with relief of symptoms within ten minutes. Eight months prior to this episode he had been diagnosed with alpha-gal allergy (alpha-gal sIgE of 8.19 kU/L, total IgE of 149 kU/L) after several episodes of urticaria and facial angioedema 4–6 hours after ingestion of mammalian meat, following a tick bite, and was avoiding mammalian meat at the time of his vaccine reaction. He had no history of egg, latex, dairy, or gelatin allergy, and had uneventfully received all prior childhood vaccinations.
We repeated his sIgE titers, demonstrating an increase in the galactose-α-1,3-galactose sIgE concentration to 26.9 kU/L, a beef sIgE of 14 kU/L, a lamb/mutton sIgE of 5.64 kU/L, a pork sIgE of 11.2 kU/L, porcine gelatin sIgE of 0.78 kU/L, and a bovine gelatin sIgE of 0.22 kU/L(reference for all <0.35 kU/L).
We next reviewed the ingredients for the vaccines he had received. Both MMR and varicella vaccines contain large amounts of gelatin per dose when compared to other vaccines (14,500 μg per 0.5 ml dose and 12,500 μg per 0.5ml dose, respectively). MMR, Varicella, and DTaP/IPV combination vaccine also contain bovine calf serum, quantity unreported. Skin prick testing was performed to the vaccines he had received, plus DTaP (without IPV) vaccine from the same manufacturer and porcine gelatin (Figure 1).5 He demonstrated positive skin test responses to MMR vaccine, varicella vaccine, and porcine gelatin on undiluted prick.
Figure 1:
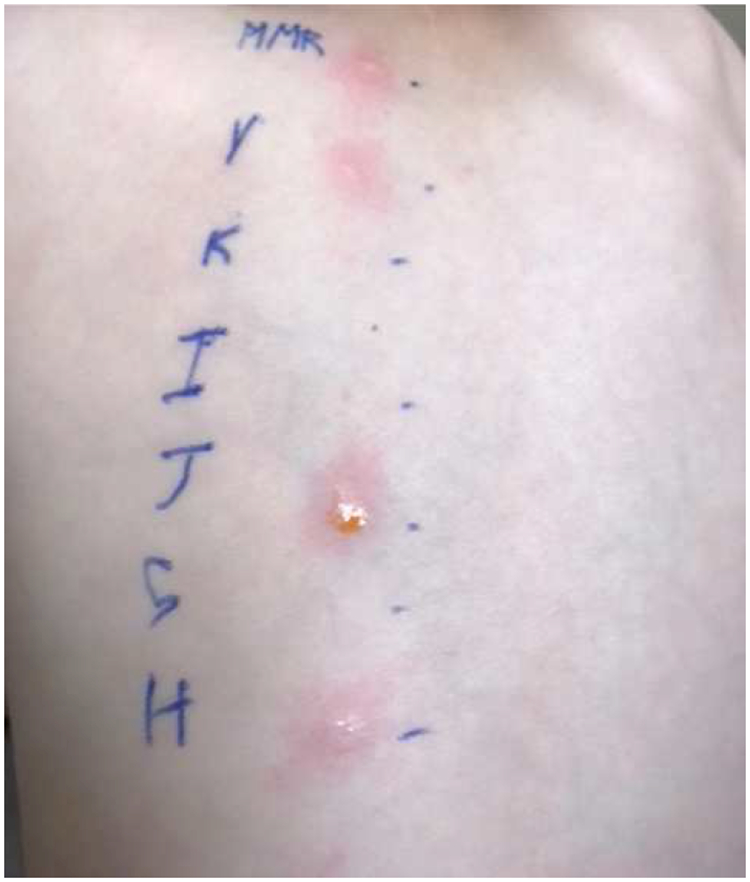
The patient was skin tested to undiluted vaccines and gelatin. Key: MMR= Measles, Mumps, Rubella vaccine, V= Varicella vaccine, K= DTaP/IPV combination vaccine, I= DTaP only vaccine, J= porcine gelatin, S= saline control, and H= histamine control. Skin prick tests were placed and read at 15 minutes, with appropriate controls. MMR was positive with a 5mm wheal and 14mm erythema, Varicella was positive with a 4mm wheal and 12 mm erythema. Porcine gelatin was positive with a 6 mm wheal and 18mm erythema. The Histamine control had a 7mm wheal and 20mm of erythema. DTaP/IPV and DTaP only vaccines were deemed negative on skin prick testing and at 1:100 intradermal testing (not shown).
We reviewed publicly available data from the Vaccine Adverse Event Reporting System (VAERS) database from 2009 onward for severe adverse events occurring on the same day of administration of the MMR or varicella vaccines, along with the keywords “beef”, “pork”, or “alpha-gal”.6 We found 10 reactions consistent with allergy to either MMR or varicella vaccines in patients with preexisting beef, pork, alpha-gal, or gelatin allergy. Six of these cases were consistent with anaphylaxis, as defined by the World Health organization (Online Table I).
We proceeded to assay seven candidate vaccines for alpha-gal antigen related interactions with our patient’s serum (Online Table II). Our previous investigation had suggested that alpha-gal antigen in vaccines might be due to content of either bovine/porcine-derived gelatin or bovine calf serum.2, 5, 7
We performed direct biotinylation of each vaccine in full prescribed dose, after which protein concentration was determined and 5 μg of biotinylated antigen was added to each streptavidin ImmunoCAP, in two identical trials. Forty microliters of undiluted serum from our index patient was used in each sIgE assay to assess for IgE binding to the vaccines or gelatin (commercially available ImmunoCAP assay c74), similar to previously published methods.1, 8 Serum from the subject was also pre-incubated with 50 μg of galactose-alpha-1,3-gal (Product code NGP1203, Dextra Labs, Reading, UK), coupled to sepharose bead slurry to deplete alpha-gal sIgE. Assays for binding to biotinylated vaccines were then repeated in two trials to determine whether binding decreased following pre-incubation with alpha-gal, which would suggest that any observed binding was due to alpha-gal epitopes present in a vaccine.8
The largest direct binding responses that could be removed by the presence of alpha-gal were to MMR (2.33 IU/ml), zoster (1.74 IU/ml), MMRV combination (1.15 IU/ml), and varicella vaccines (0.89 IU/ml) (Table IA). The direct binding “vaccine caps” method suggests the presence of an epitope in these gelatin containing vaccines that is recognized by alpha-gal sIgE in sera from our patient (Table IA).
Table I:
Serologic Assays for Alpha-gal in Selected Vaccines
| IA: IgE binding (IU/mL) to biotinylated vaccines assayed with alpha-gal positive sera from the patient, with and without alpha-gal to deplete alpha gal sIgE | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DTaP | HiB | Yellow Fever | MMR | MMRV | Varicella | Zoster | Bovine Gelatin Immunocap c74 | Cat Serum Albumin (rFel d 2) | |
| Patient | <0.1 | 0.11 | 0.12 | 2.33 | 1.15 | 0.89 | 1.74 | 0.16 | 1.68 |
| w/Alpha-Gal Beads | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.13 | 1.7 |
| w/PBS | <0.1 | 0.10 | 0.13 | 2.29 | 1.19 | 0.84 | 1.84 | 0.14 | 1.65 |
| IB: Serum alpha-gal IgE (IU/mL) levels at baseline and after overnight incubation with vaccines, gelatins, and PBS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | DTaP | HiB | Yellow Fever | MMR | MMRV | Varicella | Zoster | Porcine Gelatin | Bovine Gelatin | PBS | |
| Patient | 29.3 | 29.1 | 28.7 | 29 | 9.21 | 13.6 | 21.7 | 19.4 | 28.3 | 27.5 | 28.9 |
Table IA:All values are in units of IU/mL. Alpha-gal coated beads were used in 4 hour pre-incubation with serum at room temp, followed by immunoassay for each respective biotinylated vaccine or immunocap. PBS= Phosphate Buffered Saline
Table IB:PBS= Phosphate Buffered Saline. All values in units of IU/mL. Baseline values are the patient’s serum alpha-gal IgE values prior to overnight incubation.
We next measured the baseline alpha-gal sIgE titers in sera from our patient. To ascertain the presence of vaccine epitopes that would bind/remove alpha-gal specific IgE in excess of that expected for gelatin alone, we incubated sera samples from the index patient overnight, separately, with 100 μg from each of the seven vaccines, bovine gelatin, porcine gelatin and then re-measured alpha-gal IgE titers (Table IB). The effect by phosphate buffered saline (PBS) on the measurement of alpha-gal and gelatin sIgE was estimated to be approximately 1.4%.
Incubation of the sera samples overnight showed partial depletion of the alpha-gal sIgE response in sera pre-incubated with MMR, MMRV, zoster vaccine and varicella vaccines, greater than that for gelatin alone. We did not observe any evidence of epitope binding to alpha-gal sIgE binding with DTaP, Hib, or Yellow Fever vaccine.
Based upon agreement between skin and ex vivo testing, MMR and/or varicella vaccines appear to have been the cause of our alpha-gal allergic patient’s vaccine-associated anaphylaxis. We have previously demonstrated that MMR and zoster vaccine can bind and deplete sIgE to alpha-gal.2 We now show that MMRV and varicella vaccine also demonstrate binding and depletion of alpha-gal sIgE. Evaluating our previous findings in light of new information in this report suggests that increased gelatin content, and not bovine calf serum, may determine the alpha-gal content in vaccines. Bovine gelatin has already been demonstrated to contain alphagal as an additional allergenic epitope.1 Yellow fever vaccine does not appear to bind alpha-gal sIgE from patients as consistently,2 but has one-half the gelatin content of the other vaccines, and an unreported gelatin type. Our current patient’s lower total concentration of alpha-gal sIgE may have made our previous report more sensitive to differences.2
Both this alpha-gal allergic child and our previous alpha-gal allergic adult who reacted to alpha-gal/gelatin containing vaccines had low serum concentrations of gelatin sIgE.2 Notably, alpha-gal allergic patients have been reported to react to gelatin even with negative gelatin sIgE.9 A previous study by Mullins et al showed 30/40 (75%) red meat allergic patients with positive skin testing to gelatin, but only 12/40 (30%) reacted to gelatin upon clinical challenge.1 Hence alpha-gal allergy may confound the clinical diagnosis and testing of gelatin allergy. While porcine and bovine gelatin sensitivity has been described in children with anaphylaxis to gelatin containing vaccines in vitro,5 this is the first case of vaccine-induced anaphylaxis in a pediatric patient that implicates alpha-gal allergy as another possible contributory mechanism. The co-presence of gelatin sIgE may indicate the context in which an alpha-gal allergic patient will react to vaccines, as not all alpha-gal allergic patients will react to gelatin-containing vaccines.2–4 It needs to be determined if alpha-gal content may vary by gelatin concentration, gelatin source (bovine, porcine) or by manufacturer. Going forward, a priori skin testing and measurement of gelatin sIgE should be evaluated to determine if these tests can predict the safety of gelatin containing vaccines in individual alpha-gal allergic patients. Caution should be used in parenteral administration of gelatin containing products in alpha-gal allergic patients.1 It also needs to be determined whether reduction in vaccine gelatin content might increase vaccine safety for alpha-gal allergic patients.
Supplementary Material
Acknowledgments
Funding Sources:
Dr. Stone receives funding from NIH/NHLBI T32 HL87738
Dr. Phillips receives funding related to this project from: National Institutes of Health (1P50GM115305–01, 1R01AI103348–01, 1P30AI110527–01A1), National Health and Medical Research Foundation of Australia and the Australian Centre for HIV and Hepatitis Virology Research.
Dr. Commins receives funding related to this project from: NIH R56AI113095
IRB: This study was done under IRB approved protocols from Vanderbilt University and the University of North Carolina.
IRB approved study: Vanderbilt IRB 161455
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Implications: We report a pediatric patient with preexisting alpha-gal allergy who developed anaphylaxis immediately after receiving his routine 5 year vaccinations. Further investigation implicates alpha-gal allergy as another mechanism in pediatric anaphylaxis to gelatin containing vaccines.
Conflicts of Interest: The authors declare that they have no conflicts of interest to disclose.
References:
- 1.Mullins R, James H, Platts-Mills T, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1,3-galactose. J Allergy and Clin Immunology 2012; 129:1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone C Jr., Hemler J, Commins S, Schuyler A, Phillips E, Peebles R Jr., et al. Anaphylaxis after zoster vaccine: Implicating alpha-gal allergy as a possible mechanism. J Allergy Clin Immunol 2017; 139:1710–3 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinson M, Waibel K. Safe administration of a gelatin-containing vaccine in an adult with galactose-α-1,3-galactose allergy. Vaccine 2015; 33:1231–2. [DOI] [PubMed] [Google Scholar]
- 4.Retterer M, Workman L, Bacon J, Platts-Mills T. Specific IgE to gelatin as a cause of anaphylaxis to zoster vaccine. J Allergy Clin Immunol 2018:Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelso J, Greenhawt M, Li J, Nicklas R, Bernstein D, Blessing-Moore J, et al. Adverse reactions to vaccines practice parameter update. J Allergy and Clin Immunology 2012; 130:25–43. [DOI] [PubMed] [Google Scholar]
- 6.Vaccine Adverse Event Reporting System. https://vaers.hhs.gov/data.html: Department of Health and Homeland Security; 2017.].
- 7.Vaccine Excipient & Media Summary. Centers for Disease Control and Prevention; 2015] Available from https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf.
- 8.Commins S, Satinover SM, Hosen J, Mozena L, Borish L, Lewis BD. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy and Clin Immunology 2009; 123:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uyttebroek A, Sabato V, Bridts C, De Clerck L, Ebo D. Anaphylaxis to succinylated gelatin in a patient with a meat allergy: galactose-alpha(1, 3)-galactose (alpha-gal) as antigenic determinant. J Clin Anesth 2014; 26:574–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


