Abstract
We previously reported that microvesicles (MV) released by human mesenchymal stem cells (MSC) were as effective as the cells themselves in both Escherichia coli (E.coli) LPS and live bacterial induced acute lung injury (ALI) mice models. However, it remained unclear whether the biological effect of MSC MV can be applied to human ALI. In the current study, we tested the therapeutic effects of MSC MVs in a well-established ex vivo perfused human model of bacterial pneumonia. Using human donor lungs not used for transplantation, we instilled E.coli bacteria intra-bronchially and, 1 h later, administered MSC MVs into the perfusate as therapy. After 6 h, instillation of E.coli bacteria caused influx of inflammatory cells which resulted in significant inflammation, lung protein permeability and pulmonary edema formation. Administration of MSC MV significantly increased alveolar fluid clearance and reduced protein permeability and numerically lowered the bacterial load in the injured alveolus. The beneficial effect on bacterial killing was more pronounced with pretreatment of MSCs with a TLR3 agonist, Poly (I:C), prior to the isolation of MVs. Isolated human alveolar macrophages had increased antimicrobial activity with MSC MV treatment in vitro as well. Although oxygenation and lung compliance levels were similar between injury and treatment groups, administration of MSC MVs numerically decreased median pulmonary artery pressure at 6 h. In summary, MSC MVs increased alveolar fluid clearance and reduced lung protein permeability, and pretreatment with Poly (I:C) enhanced the antimicrobial activity of MVs in an ex vivo perfused human lung with severe bacteria pneumonia.
Keywords: Acute lung injury, E.coli pneumonia, Ex vivo perfused human lung, Mesenchymal stem cell, Microvesicles.
INTRODUCTION
Acute respiratory distress syndrome due to bacterial pneumonia and/or sepsis remains a common cause of respiratory failure in critically ill ICU patients with high mortality rates despite appropriate antibiotic use and supportive care1, 2. New therapeutic modalities are needed to improve morbidity and mortality. Multiple pre-clinical studies have demonstrated significant therapeutic potential with mesenchymal stem/stromal cells (MSC) for ALI despite limited engraftment rates of 1 ~ 5%3, 4, 5. The therapeutic potential of MSCs appeared to arise in part from the secretion of growth factors such as keratinocyte growth factors (KGF)6, anti-inflammatory products such as PGE2 or Lipoxin A47, 8, anti-permeability factors such as angiopoietin-1 (Ang1)9, 10, and antimicrobial products such as LL-37 or Lipocalin211, 12. Based on the significant potential of MSC, several Phase I and II clinical trials focused on safety are underway (NCT02097641, NCT01902082, Clinicaltrials.gov.)13. However, there remains some concerns with allogeneic stem cell therapy such as tumor formation and hemodynamic instability due to their physical properties.
Recently, stem cell derived therapy, such as MSC conditioned media (CM) or extracellular vesicles, have generated considerable interest due to similar therapeutic properties without the inherent challenges of using live cells. Ionoscu et al. demonstrated that MSC CM reduced lung inflammation and injury following lipopolysaccharide (LPS) induced ALI in a mouse14. Using LPS induced ALI in an ex vivo perfused human lung, we also found that MSC CM reduced extravascular lung water, improved lung endothelial barrier permeability and restored alveolar fluid clearance (AFC) in part by KGF secretion by MSCs6. More recently, we demonstrated that MSC derived extracellular vesicles were as biologically active as MSCs in E.coli LPS and bacterial induced ALI in mice in part through the transfer of mRNA and microRNA to target cells which resulted in improved resolution of pulmonary edema and increased antimicrobial activity15, 16. We and other investigators also found that pretreatment of MSCs, such as with Poly (I:C)17, carbon monoxide18, hypoxia19, etc., may be a viable method to enhance the therapeutic properties of the cells and its released MVs.
Extracellular vesicles, which are released by most cell types, can be broadly classified into exosomes, microvesicles (MV) and apoptotic bodies based largely on their size and origin. Interestingly, extracellular vesicles contain mRNA, microRNA, protein, surface receptors and potentially organelles which may be involved in their function20–22. However, whether or not stem cell derived therapy such as extracellular vesicles can be translated to large animal or even human ALI models is unknown. In the current study, we hypothesized that MSC MVs could be effective in reducing inflammation and total bacterial load in a severe pneumonia model in the ex vivo perfused human lung and that preconditioning MSCs with Poly (I:C) would enhance the antimicrobial properties of the released MVs.
METHODS
Ex Vivo Perfused Human Lung and Measurement of Alveolar Fluid Clearance
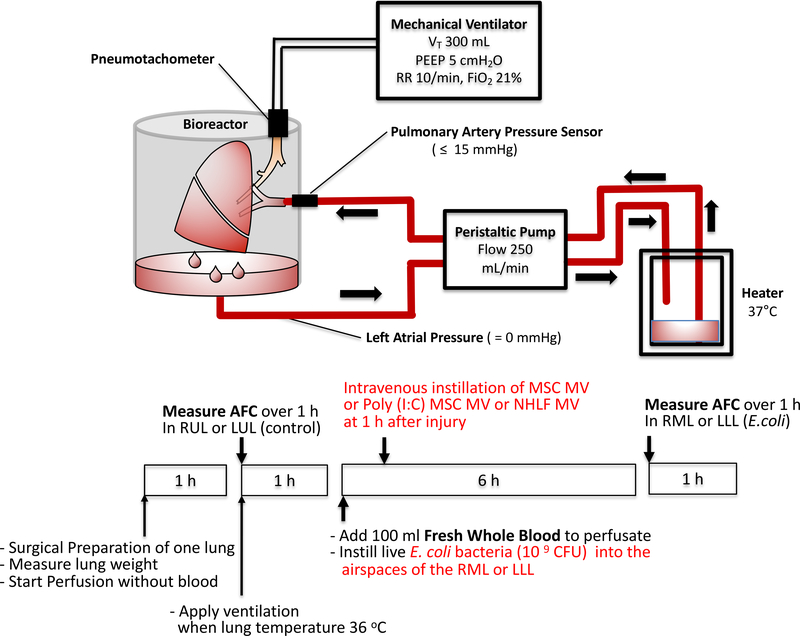
Either the right or left lung, from the Northern California Transplant Donor Network, was perfused slowly with DMEM without Phenol Red + 5% bovine serum albumin until a cardiac output of 0.25 L/min was reached and then ventilated (Harvard Apparatus, tidal volume 300 ml, respiratory rate = 10/min, FiO2 = 21%, and PEEP = 5 cmH2O) (Figure 1)23. AFC rate was then measured in the control lung lobe by the change in protein concentration of a BAL fluid over 30 min using the following equation: AFC (%/h) = 2(1-Ci/Cf) x 100 (Ci and Cf = protein concentration at 5 and 35 min respectively). If 0 <AFC <10%/h, 109 CFU of E.coli bacteria was instilled into the lower lobe, and fresh human blood (100 ml) was added to the perfusate. One hour after injury, either 200 μl of MSC MV (1X), 400 μl of MSC MV (2X), 200 μl of Toll-like receptor 3 (TLR-3) agonist polyinosinic:polycytidylic acid (Poly(I:C)) pretreated MSC MV, or 200 μl of normal human lung fibroblasts (NHLF) MV as cellular control was administered intravenously in a random fashion. At 6 h, the AFC rate of the injured lobe was measured.
Figure 1. Schematic of the Ex Vivo Perfused Human Lung Preparation.
Marginal human lungs which were not used for lung transplantation were obtained from the Northern California Transplantation Donor Network. Using a large organ Harvard bioreactor, the pulmonary artery of the right or left lung was initially cannulated and gently perfused with a crystalloid solution containing 5% albumin at a rate up to 250 ml/min. Once rewarmed, the lung was ventilated using a tidal volume of 300 ml with 5 cmH20 of PEEP and a respiratory rate 10 breaths per minute in room air. AFC rate was first measured in the upper control lobe. If AFC > 0 and <10 %/h, 10 9 CFU E.coli bacteria was administered into the lower lung lobe, and 100 ml of fresh human blood was added to the perfusate. After 6 h, AFC was measured in the lower lobe as the primary endpoint. In separate experiments and randomly, 200 or 400 μl MSC derived microvesicles (MVs), Poly (I:C) pretreated 200 μl MSC MVs, or 200 μl NHLF derived MVs was administered into the perfusate at 1 h.
Isolation of Microvesicles Derived from Human Mesenchymal Stem Cells
MVs were isolated from the conditioned medium of human bone marrow derived MSCs24, obtained from a NIH repository at Texas A&M Health Science Center, and NHLFs using ultracentrifugation. Briefly, MSCs or NHLFs were grown in a T75 flask until 90% confluency and then serum starved in αMEM or FBM supplemented with 0.5% Bovine Albumin Fraction (MP Biomedical LLC). After 48 h, the conditioned medium was collected and centrifuged at 3000 rpm for 20 min to remove cellular debris and then at 100,000 g (Beckman Coulter Optima L-100XP Ultracentrifuge) to isolate the MVs for 1 h twice at 4°C. The final sediment was resuspended in PBS and stored in −80°C. Ten μl of the resuspension were equivalent to MVs released by 1 million MSCs or NHLFs over 48 h.
TLR3 Priming of MSC
In order to switch MSC towards a more immunoregulatory phenotype, TLR3 agonist, Poly (I:C) (1 μg/mL, Sigma Aldrich), was cultured with MSCs for 1 h17. The cells were then washed twice in PBS and serum starved over 48 h prior to isolation of MVs.
MSC MV Characterization
MSC MVs were labelled with PKH26 (Sigma-Aldrich) to separate out vesicles from debris or with antibodies for CD9-FITC, control IgG1 k-FITC, CD44-FITC or control IgG2b k-FITC (eBioscience Inc. & BD Biosciences) for flow cytometry. A BD FACSAria™ Fusion Special Order (SORP) cell sorter (BD Biosciences) with 100 nm nozzle and ND filter 1 was used. The threshold was set on the SSC 200. Data were analyzed by Diva software (BD Biosciences). For fluorescence detection, we used a 586/15 band pass filter for PKH26 and 525/50 band pass filter for FITC labelled antibodies. An unstained sample was used to detect auto-fluorescence and set the photomultiplier for all the channels. Standard silica beads (Apogee Mix for Flow Cytometer, Apogee Flow Systems Ltd), with a similar refractive index of vesicles, was used to gate the MSC MVs. MSC MVs were also characterized by scanning electron microscopy and by using NanoSight NS 300 (Malvern Instruments).
Measurement of Lung Protein Permeability Using Evans Blue
At time T5 h, we injected 400 mg of Evans Blue (Sigma-Aldrich) into the perfusate. At time T6.5 h, 1 ml of perfusate was removed, and then two liters of PBS was used to flush the lung. Several pieces (1 – 2 gm) from both control and injured lung lobes were then collected and incubated with formamide for 3 days to release the dye. We measured the conc. of Evans blue dye from each sample to calculate lung protein permeability.
Isolation and Bacterial Killing Effect of Human Alveolar Macrophage
Human alveolar macrophages were isolated following BAL with warm normal saline and plated with RPMI 1640 media in a transwell plate (200,000 cell/well) after washing twice with HBSS solution and stimulated with 1 μg/ml LPS with or without 90 μl of MSC MV. The next day, the cells were cultured with opsonized 107 CFU of E.coli bacteria for 90 min. The CFU of E.coli in the medium was then counted.
Statistical Analysis
Results were expressed as the mean ± SD or median with 25–75% percentile (interquartile range [IQR]), as appropriate. Whether or not the samples were normally distributed was determined by D’Agostino & Pearson or Shapiro-Wilk normality test. Comparisons between several groups were made using the analysis of variance (ANOVA) with Bonferroni correction if normally distributed or by Kruskal Wallis Test followed by post hoc Dunn’s tests if not. We used the software GraphPad Prism.
RESULTS
Baseline Demographics and Clinical Data for Donor Human Lungs
The demographic and clinical data for 37 donor lungs used are listed in the Table. There appeared to be no major differences between the MV treatment groups compared with E.coli injury group in terms of age, sex, PaO2/FiO2 (P/F) ratio, lung ischemic time and lung injury score (LIS).
Table.
Clinical Data of Donor Lungs
| E.coli Pneumonia(N= 9) | + MSC MV 1X(N = 6) | + MSC MV 2X(N = 6) | + Poly (I:C) MSC MV (N = 11) | + NHLF MV(N = 5) | |
|---|---|---|---|---|---|
| Mean Age (Year) | 44 ± 10 | 54 ± 16 | 52 ± 21 | 50 ± 13 | 48 ± 22 |
| Male % (Number) | 56% (5) | 83% (5) | 67% (4) | 73% (8) | 80% (4) |
| Donor PaO2/FiO2 Ratio (mmHg) | 221 ± 13 | 112 ± 33 | 238 ± 44 | 251 ± 38 | 156 ± 70 |
| Ischemic Time (h:min) | 36:38 ± 8:40 | 32:36 ± 7:42 | 24:55 ± 11:05 | 29:09 ± 6:38 | 28:40 ± 3:26 |
| Lung Injury Score (LIS) | 1.4 ± 0.6 | 1.9 ± 0.4 | 1.2 ± 0.4 | 1.3 ± 0.4 | 1.9 ± 0.7 |
Data is presented as mean ± SD.
MSC MV Characterization
Scanning electron microscopy showed that the isolation technique yielded homogeneous population of spheroid particles (Supplemental Figure 1). NanoSight analyses showed MSC MV mean size was 180 ± 14 nm with a concentration at 4.7 ± 0.2 ×108 particles per ml. For flow cytometry, we labelled MSC MV with PKH26 to quantify only membrane bound vesicles and to exclude debris. As a percentage of PKH26 labelled MSC MV, we found that 43 ± 8% was CD44 positive. An IgG Ab was used as a control to remove background staining. For CD9, only 0.1 ± 0.1% of PKH26 MSC MVs was labelled positively, suggesting that the majority of vesicles were MVs. However, since there is significant overlap between exosomes and MVs in terms of size, it is possible that the total number of exosome were under-represented. The MSC MV dose chosen was initially based on prior studies using MSCs in the ex vivo perfused human lung and our observation that MVs were approximately 5–10x less potent than live cells23. Consequently, we administered 200 μl of MSC MVs or the MVs released by 20 million MSCs over 48 h; previous studies using the ex vivo human lung used 5 to 10 million MSCs as a therapeutic.
MSC MVs Improved Alveolar Fluid Clearance in Lungs Injured Following E.coli Pneumonia
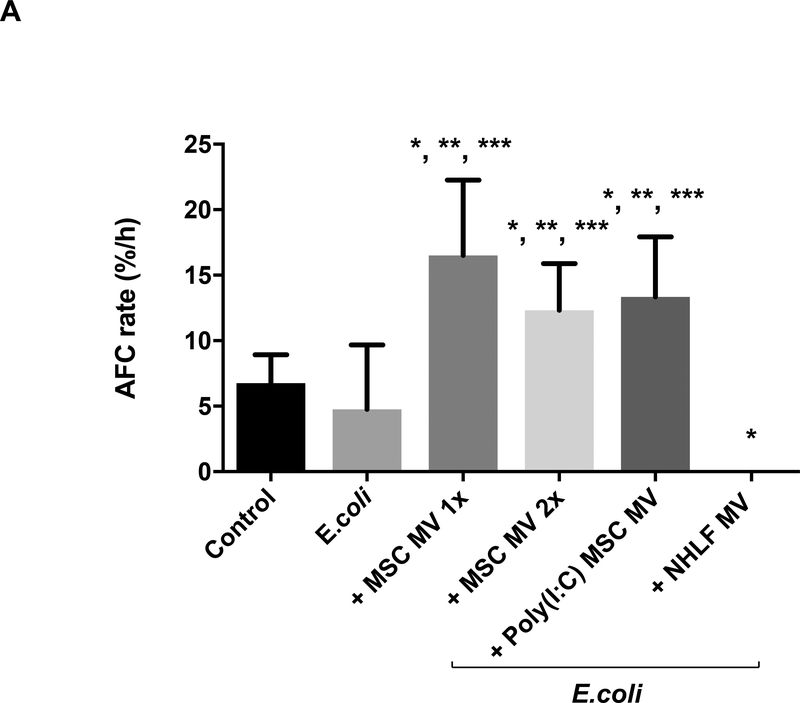
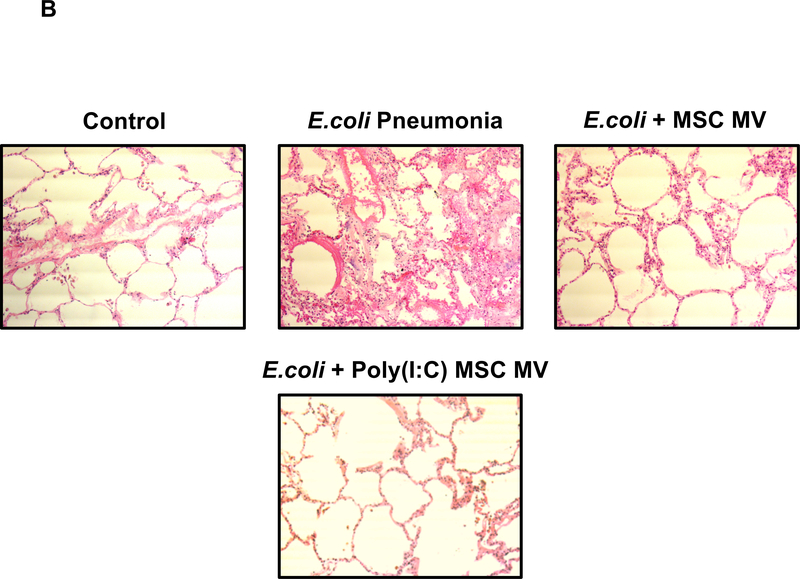
Administration of either human MSC MVs (1X or 2X) or the Poly (I:C) MSC MVs into the perfusate at 1 h following E.coli induced ALI significantly increased AFC at 6 h by 247% (P<0.001), 159% (P= 0.001) or 180% (P<0.001) respectively. The AFC values were also significantly elevated compared to the control lung lobe by 144% (P<0.001), 82% (P=0.008) or 97% (P<0.001) respectively, suggesting not only a restoration but an improvement. In contrast, administration of NHLF MVs had no benefit and actually significantly decreased AFC compared to the control group (P=0.002) (Figure 2A). Surprisingly, there was no dose effect with increasing MSC MV by 2X on any parameter, suggesting that there was a therapeutic ceiling to the MVs. By histology, instillation of intra-bronchial E.coli bacteria caused significant injury as depicted by an increase in cellularity and blood, edema and interstitial thickening which was substantially reduced with the administration of either MSC MV (1X or 2X) or Poly (I:C) MSC MVs (Figure 2B).
Figure 2. Therapeutic Effect of MSC MVs on Alveolar Fluid Clearance Rate following Severe Escherichia coli Pneumonia in Ex Vivo Perfused Human Lung.
(A) Administration of MSC MVs or Poly (I:C) pretreated MSC MVs intravenously significantly increased the AFC rate in the lung lobe injured by E.coli pneumonia at 6 h. Administration of NHLF MVs had no beneficial effect on AFC rate. AFC was measured by the change in protein concentration of a 5% albumin instillate in the lung lobe over 1 h and expressed as mean AFC (% per h, per 150 ml of BAL fluid) with 95% confidence intervals for each condition. N = 9 for E.coli injured lung lobe, N = 6 for E.coli injured lung lobe treated with MSC MVs (200 μl or 400 μl) or N = 11 for 200 μl MSC MVs pretreated Poly (I:C) or N = 5 for 200 μl NHLF MVs. Overall P Value < 0.001, * p is significant vs. Control, ** p is significant vs. E.coli injured, and *** p is significant vs. NHLF MVs by ANOVA (Bonferroni). P values and confidence intervals for individual pair comparisons are shown in Supplementary Table. (B) Human lungs injured with E.coli bacteria with and without MSC MVs 1X or Poly (I:C) pretreated MSC MVs were fixed in 10% formalin at 6 h. Sections were stained with hematoxylin and eosin. Administration of human MSC MVs 1X or Poly (I:C) pretreated MSC MVs 1 h after E.coli pneumonia injury reduced the level of hemorrhage, edema, and cellularity in the injured lung lobe at 6 h following E.coli pneumonia.
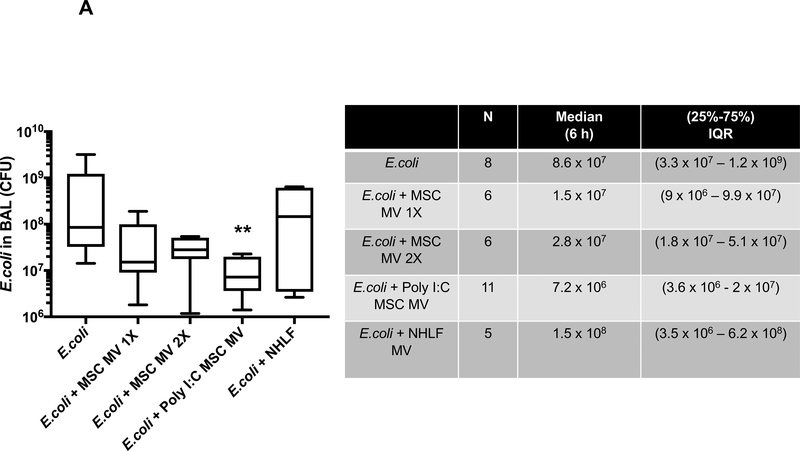
Poly (I:C) MSC MVs Decreased Bacterial Count in the Injured Alveolus
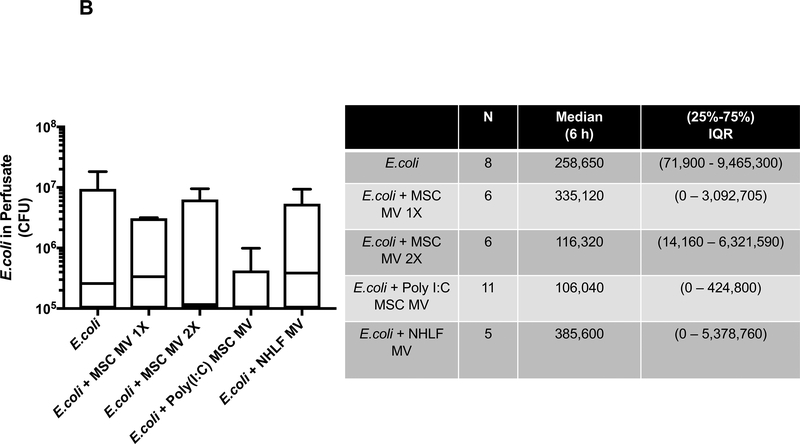
Bacterial colony forming units (CFU) were measured in the BAL fluid following instillation of 109 E. coli bacteria with or without MV treatment at 6 h. Although the median values of CFU levels in the MSC MV 1X and 2X treatment groups were numerically lower than the E.coli injury group, only the administration of Poly (I:C) MSC MVs significantly decreased CFU counts compared to the E.coli injured group at 6 h by post hoc Dunn’s analyses following Kruskal Wallis test (P=0.007). (Figure 3A). Administration of NHLF MVs had no significant effect. The bacterial CFU counts in perfusate were also numerically lower in the MSC MV 2X and Poly (I:C) MSC MV groups compared to E.coli group at 6 h but not statistically significant (Figure 3B).
Figure 3. Therapeutic Effect of MSC MVs on Bacterial Levels following Severe Escherichia coli Pneumonia in Ex Vivo Perfused Human Lung.
Administration of Poly (I:C) pretreated MSC MVs decreased (A) bacterial counts in the BAL fluid at 6 h. Administration of NHLF MVs had no beneficial effect. Data is expressed in the box plot as median bacterial CFU counts with IQR for bars in the BAL fluid, Overall P value = 0.016, ** p is significant vs. E.coli injury group by Post-Hoc Dunn’s Analyses Following Kruskal Wallis Test. (B) Although not statistically significant, administration of MSC MVs or Poly (I:C) pretreated MSC MVs numerically decreased bacterial CFU counts in the perfusate. Data is expressed in the graph as median bacterial CFU counts with IQR for bars in the perfusate. Overall P value = 0.624. (C) Co-incubation of MSC MVs with freshly isolated human alveolar macrophages also increased E.coli bacterial phagocytosis by the macrophages. Data is expressed in the graph as median bacterial CFU counts (as % of LPS control) with IQR for bars. Overall P value = 0.026, * p is significant vs. LPS treated group by Post-Hoc Dunn’s Analyses Following Kruskal Wallis test. P values and confidence intervals for individual pair comparisons are shown in Supplementary Table.
When freshly isolated human alveolar macrophages were co-cultured with E.coli bacteria with or without LPS and/or MSC MVs, bacterial phagocytosis by alveolar macrophages significantly increased with MSC MV treatment (P=0.044) (Figure 3C).
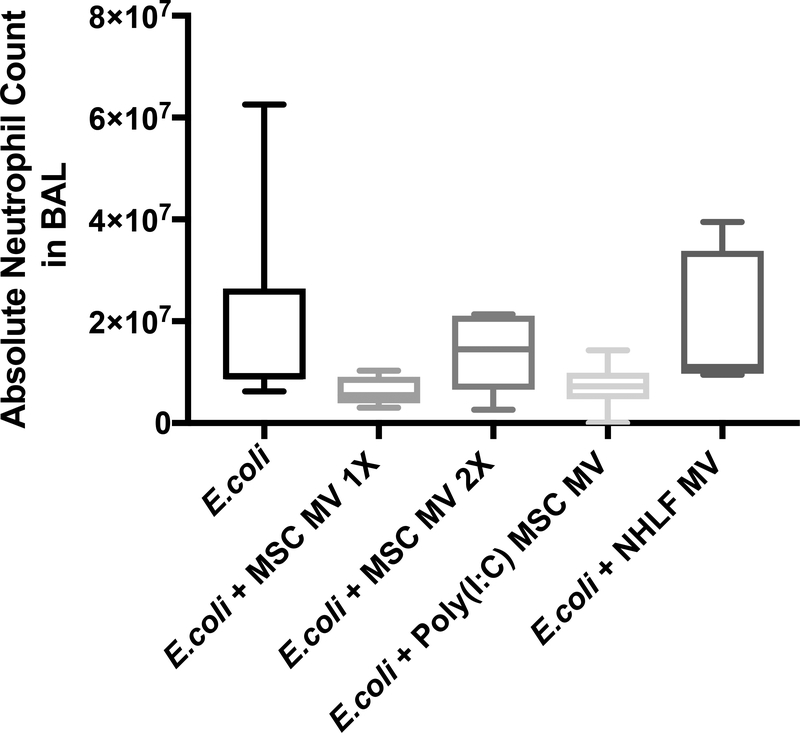
MSC MVs Decreased Absolute Neutrophil Count in the Injured alveolus
Absolute neutrophil counts in the BAL fluid following E.coli pneumonia were numerically lower following administration of MSC MV (1X) or Poly (I:C) MSC MV at 6 h (median 9.1 × 106 with IQR of 8.6 × 106 – 2.6 × 107 for E.coli, median 5.5 × 106 with IQR of 3.9 × 106 – 9.1 × 106 for + MSC MV 1X, median 7.2 × 106 with IQR of 4.7 × 106 – 9.9 × 106 for + Poly (I:C) MSC MV, N = 6 – 11), with overall P-value of 0.024 (Figure 4A). However, no pair comparisons showed statistical significance after Dunn’s correction. There were also no significant differences in absolute neutrophil counts in the perfusate (data not shown).
Figure 4. Effect of MSC MVs on the Influx of Inflammatory Cells following Severe Escherichia coli Pneumonia in Ex Vivo Perfused Human Lung.
Instillation of MSC MVs numerically decreased the influx of inflammatory cells, specifically neutrophils, into the lung lobe injured by E.coli pneumonia at 6 h. Absolute neutrophil count is expressed as median with IQR for bars for each condition, N = 9 for E.coli injured lung lobe, N = 6 – 11 for E.coli–injured lung lobe treated with 200 μl or 400 μl of MSC MVs or 200 μl of Poly (I:C) pretreated MSC MVs. Overall P value = 0.024, P values and confidence intervals for individual pair comparisons are shown in Supplementary Table.
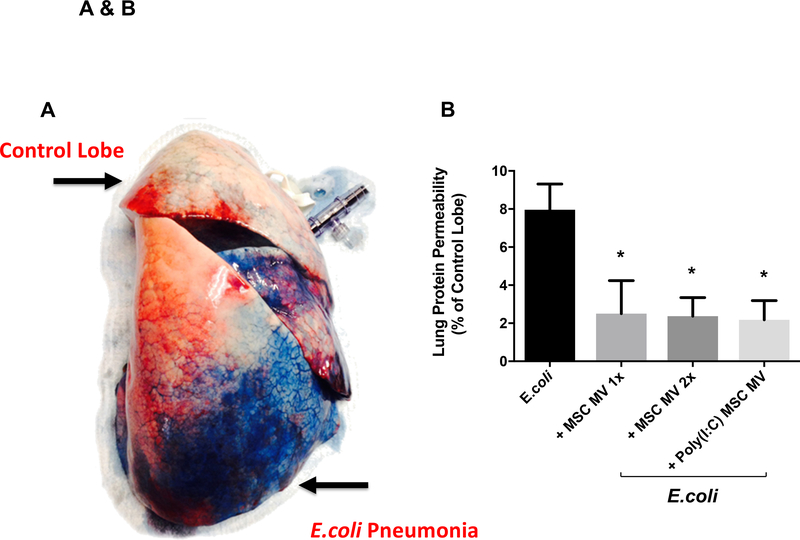
MSC MVs Decreased Lung Protein Permeability
Lung protein permeability was measured in all lung lobes using the extravasation of Evans Blue dye, similar to the technique used in mice (Figure 5A). Administration of MSC MVs (1X or 2X) or Poly (I:C) MSC MVs significantly reduced lung protein permeability (P=0.001–0.003) (Figure 5B).
Figure 5. Therapeutic Effect of MSC MVs on Lung Protein Permeability following Severe Escherichia coli Pneumonia in Ex Vivo Perfused Human Lung.
Lung protein permeability at 6 h as measured by Evan’s Blue extravasation. (A) Administration of Evan’s Blue intravenously demonstrated albumin protein leak into the E.coli bacteria injured lung lobe. (B) Compared to E.coli bacteria injured lung lobe, administration of MSC MVs or Poly (I:C) pretreated MSC MVs significantly reduced lung protein permeability. Data is presented as mean change in permeability with 95% confidence intervals. Overall P value = 0.001, * P is significant vs. E.coli by ANOVA (Bonferroni). P values and confidence intervals for individual pair comparisons are shown in Supplementary Table.
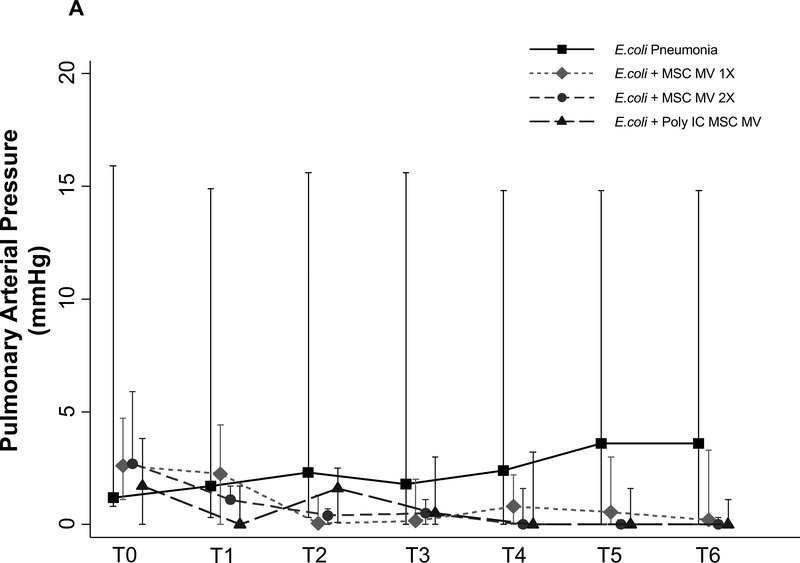
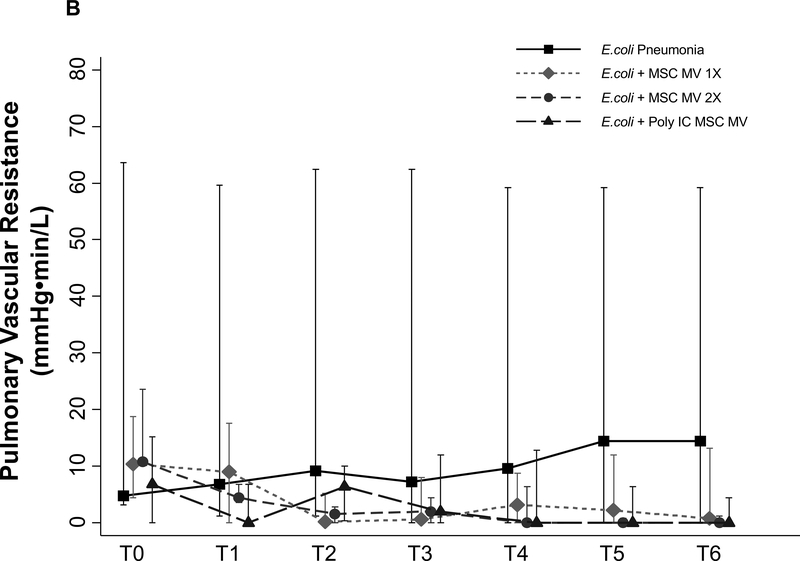
Effects of MSC MVs on Pulmonary Arterial Pressure and Pulmonary Vascular Resistance
PAP was measured throughout the duration of the experiment. Although not statistically significant, administration of MSC MVs or Poly (I:C) MSC MVs lowered PAP at T6 h compared to the E.coli injury group (median 3.6 with IQR of 0 – 18 for E.coli, median 0.2 with IQR of 0 – 3.3 for + MSC MV 1X, median 0 with IQR of 0 – 1.4 for + MSC MV 2X, median 0 with IQR of 0 – 1.1 for + Poly (I:C) MSC MV, N = 6 – 11) (Figure 6A). PVR followed a similar trend (Figure 6B).
Figure 6. Effect of MSC MVs on Pulmonary Arterial Pressure and Vascular Resistance following Severe Escherichia coli Pneumonia in Ex Vivo Perfused Human Lung.
Administration of MSC MVs or Poly (I:C) pretreated MSC MVs numerically decreased PAP (A) or PVR (B) at T6 h compared to E.coli injury group. Data is presented as median PAP or PVR with IQR as bars. P = 0.20 – 0.66 for each time point by Kruskal Wallis tests (A). P = 0.20 – 0.63 for each time point by Kruskal Wallis tests (B).
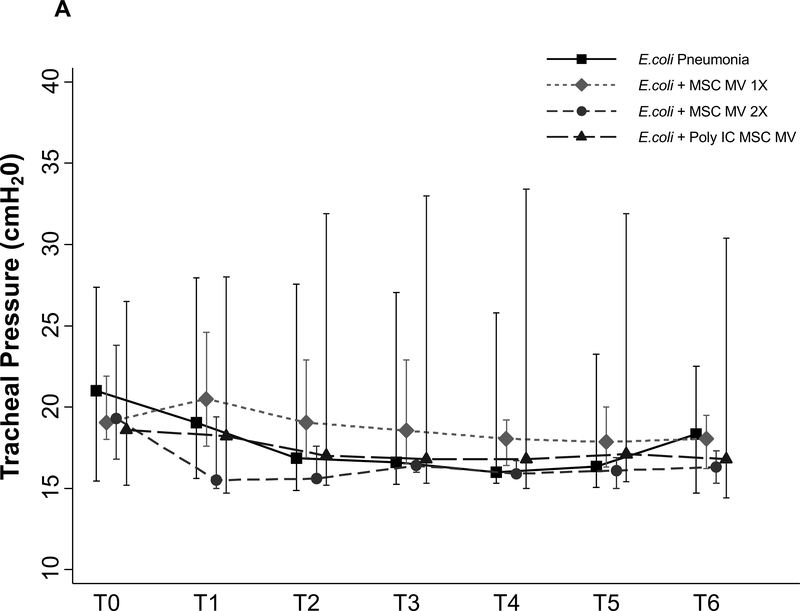
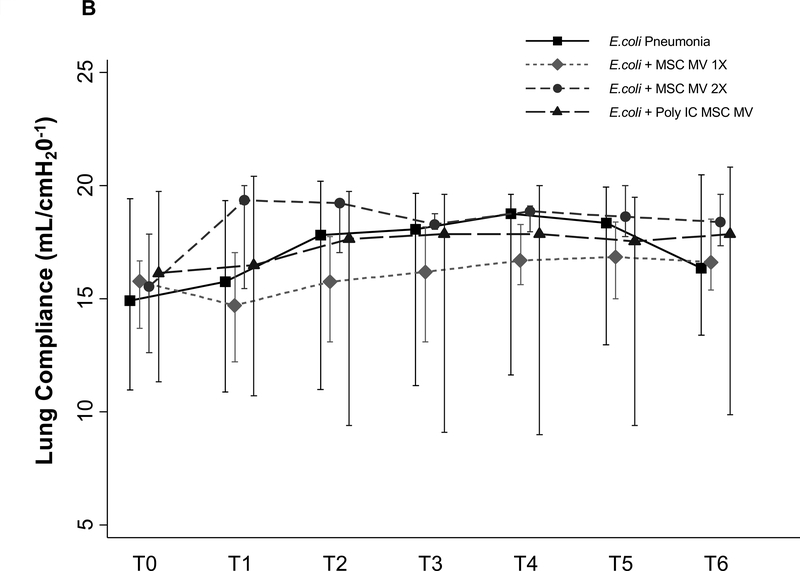
No Effect of MSC MVs on Tracheal Pressure, Lung compliance or Oxygenation
There were no significant differences in tracheal pressure or lung compliance between control and treatment groups (Figure 7A & B). There was also no change in PaO2 between groups (Figure 7C), possibly due to the short duration of the experiment and the technique utilized to measure AFC.
Figure 7. Lack of a Therapeutic Effect of MSC MVs on Lung Compliance or Oxygenation following Severe Escherichia coli Pneumonia in Ex Vivo Perfused Human Lung.
Administration of MSC MVs or Poly (I:C) pretreated MSC MVs had no significant effect on tracheal pressure (A), lung compliance (B) or oxygenation (C) over 6 h. Data is presented as median Tracheal pressure or Lung Compliance or PaO2 with IQR as bars. P = 0.56 – 0.98 for each time point by Kruskal Wallis tests (A). P = 0.56 – 0.98 for each time point by Kruskal Wallis tests (B). P = 0.10 – 0.92 for each time point by Kruskal Wallis tests (C).
Effect of MSC MV on TNFα Levels in the Injured Alveolus.
We measured the inflammatory cytokine, tumor necrosis factor (TNFα), in the BAL fluid. Although not statistically significant, administration of human MSC MV 1X after E.coli pneumonia numerically reduced the levels of TNFα by 72% (median 5285 with IQR of 3446 – 13409 for E.coli, median 1454 with IQR of 681 – 8097 for + MSC MV 1X, median 5105 with IQR of 3263 – 9285 for + MSC MV 2X, median 6123 with IQR of 1766 – 9152 for + Poly (I:C) MSC MV, N = 6 – 11).
Discussion
The main findings of these studies can be summarized as follows: (1) Intravenous administration of MSC MVs significantly increased the rate of AFC and decreased lung protein permeability and numerically lowered the CFU bacteria counts and inflammation in the injured alveolus following severe E.coli pneumonia in an ex vivo perfused human lung (Figure 2, 3 & 5); (2) Poly (I:C) pretreatment of MSCs significantly enhanced the antimicrobial property of the released MVs in the injured alveolus (Figure 3); (3) Increased antimicrobial effect of Poly (I:C) MSC MVs was associated with a numerically lower influx of inflammatory cells (i.e. neutrophils) in the injured lung (Figure 4); (4) MSC MVs and Poly (I:C) MSC MVs treatment groups had numerically lower median PAP and PVR levels at 6 h (Figure 6). However, MSC MVs and Poly (I:C) MSC MVs did not improve tracheal pressure, lung compliance or oxygenation (Figure 7); (5) Freshly isolated human alveolar macrophage had enhanced antimicrobial properties with MSC MV treatment (Figure 3); (6) And, more significantly, the pre-clinical therapeutic findings seen with MSC MVs in mice were apparent in a clinically relevant human lung injury model, especially in the effect of Poly (I:C) MSC MVs, making it a possible viable alternative to MSCs as a therapy.
Most all cell types release MVs25 which can be biologically active as mediators of cell-to-cell communication or through direct effects on target cells26–28. Recently, MVs derived from MSC have been reported to exert therapeutic effects on various organ injury models29, 30. We previously found that MSC MV significantly reduced pulmonary edema in mice injured with LPS or E.coli pneumonia15, 16 and an ex vivo perfused human lung model of ischemia/reperfusion31. However, the underlying mechanisms were not fully known. In the current study, both MSC MVs and Poly (I:C) MSC MVs improved AFC to a near normal level at 6 h following E.coli pneumonia (Figure 2). We previously demonstrated that MSC and/or MSC MVs increased AFC rate in part through enhanced KGF expression; KGF secreted from MSCs upregulated the key sodium channel (αENaC) in alveolar epithelial cells, increasing fluid absorption6.
We also found that Poly (I:C) MSC MVs enhanced bacterial killing in vivo and MSC MVs in vitro (Figure 3) and numerically reduced the influx of neutrophils (Figure 4) into the injured alveolus. Several potential mechanisms may account for these observations: 1) MSC and MSC MVs secrete or enhance the expression of antimicrobial soluble factors. Krasnodembskaya et al. found that MSCs secreted LL-37, a known antimicrobial peptide, and Gupta et al.11, 12 demonstrated that MSCs enhanced survival and bacterial clearance through upregulation of Lipocalin2 by MSCs in a mice model of E.coli pneumonia; 2) Raffaghello et al.32 demonstrated that MSCs inhibited apoptosis of neutrophils by MSC through downregulation of reactive oxygen species and Stat-3 phosphorylation; 3) And multiple groups demonstrated that MSC and MSC MVs enhanced alveolar macrophage survival via mitochondria transfer (via tunneling nanotubes) and phenotypic change from the pro-inflammatory M1 to the anti-inflammatory M233–35. Both Nemeth et al and Mei et al.7, 36 found that MSCs upregulated macrophage phagocytic activity which improved bacterial clearance in a cecal ligation and puncture model of sepsis. In addition, recent studies indicated that TLR3 activation with Poly (I:C) can enhance the immunomodulatory potential of MSC, while TLR4 activation could reduce it17. In a mice model of E.coli pneumonia, we found that Poly (I:C) pretreatment of MSC MV enhanced macrophage/monocyte phenotypic M2 change, as indicated by decreased inflammation and increased bacteria phagocytosis16.
MSC MVs and Poly (I:C) MSC MVs both significantly reduced lung protein permeability (Figure 5) which may be attributed to its angiopoietin-1 content, a protein with well-known anti-inflammatory, anti-permeability, and endothelial protective characteristics. Both MSCs and MSC MVs have significant levels of angiopoietin-1 mRNA. In LPS induced ALI in mice, MSC or MSC transfected with the human Ang1 gene reduced pulmonary vascular injury and the recruitment of inflammatory cells into the lung10. More recently, Tang37 et al. found that Ang1 in MSC MV was important in reducing inflammation and pulmonary edema in LPS induced ALI in mice.
Our study has some limitations: (1) Lack of an intact lymphatic system for lung interstitial fluid clearance which may be relevant if AFC is measured over longer periods; (2) Lack of other immune organs such as the spleen or liver which may participate in injury and/or repair; (3) Short duration of lung injury which limits assessment of whether the effects of MSC MVs can be sustained; (4) And limited sample size due to lack of availability of lungs for research.
Despite the limitations, the importance of the current study was in establishing the use of MVs as a possible viable alternative to using live cells in ALI. The benefits include ease of isolation and storage, avoiding dimethyl sulfoxide for preservation and the need for a bone marrow transplant facility, avoidance of using live stem cells with tumorigenic potential and which may cause hemodynamic instability with administration, and potential to modify MVs with pretreatment of the cells to enhance the therapeutic effects. However, the major obstacle remains in scaling the production of MSC MVs since the potency is approximately 5–10x less than the cells. Given that a typical MSC dose is 5 million cells/kg or 500 million cells for a 100 kg patient, future clinical trials with MSC MVs may require isolating MVs released from up to 5 billion cells per patient, which may be prohibitive. Studies are on-going to address this critical issue.
In conclusion, our study demonstrated that MSC MVs was therapeutic in a clinically relevant human model of severe E.coli pneumonia in lungs with baseline injury. Based on these promising results, further studies are warranted in a large animal with pneumonia and or sepsis to provide further biological rationale and additional preclinical data for possible clinical trials.
Supplementary Material
MSC MVs were analyzed by electron microscopy, NanoSight analyses and Flow Cytometry. (A) Electron microscopy demonstrated a fairly homogenous population of small membrane bound vesicles roughly 100–200 nm in length. (B) NanoSight analyses demonstrated approximately 4.7 × 108 particles per ml with a mean diameter of 180 nm ± 14 nm, N = 3 separate isolations. In the ex vivo perfused human lung, approximately 200 – 400 μl or 9.4 – 18.8 × 107 MVs were administered as therapy. (C) Representative flow cytometry figures showed that a large proportion of the vesicles were CD44 positive. We labelled the vesicles with PKH26 prior to flow cytometry to eliminate artifacts and debris.
What is the Key Question?
Can the therapeutic effects of MSC microvesicles, as reported in preclinical small animal models of acute lung injury, be applicable to human lung injury models?
What is the bottom line?
Human bone marrow derived MSC microvesicles reduced multiple indices of acute lung injury in an ex vivo perfused human lung injured with severe bacterial pneumonia.
Why read on?
Based on promising results, further studies are warranted in a large animal model of pneumonia with or without sepsis to provide additional preclinical data for possible clinical trials.
ACKNOWLEGMENTS
We thank Hideya Kato, MD, for technical assistance.
Acknowledgment of grants:
This work was supported by the National Institute of Health National Heart, Lung, and Blood Institute grant number HL-113022 (Dr. JW Lee), and HL-51856 and HL-131621 (Dr. MA Matthay)
Footnotes
Competing Interests: No author declared any competing interests.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest.
REFERENCES
- [1].Ware LB, Matthay MA: The acute respiratory distress syndrome. N Engl J Med 2000, 342:1334–49. [DOI] [PubMed] [Google Scholar]
- [2].Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, Ware LB: Clinical Predictors of Hospital Mortality Differ Between Direct and Indirect ARDS. Chest 2017, 151:755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kotton DN, Fabian AJ, Mulligan RC: Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005, 33:328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ: Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med 2006, 173:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR: Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284:143–7. [DOI] [PubMed] [Google Scholar]
- [6].Lee JW, Fang X, Gupta N, Serikov V, Matthay MA: Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A 2009, 106:16357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E: Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009, 15:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, Matthay MA: Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J Immunol 2015, 195:875–81. [DOI] [PubMed] [Google Scholar]
- [9].Fang X, Neyrinck AP, Matthay MA, Lee JW: Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem 2010, 285:26211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ: Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 2007, 4:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA: Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010, 28:2229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA: Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012, 67:533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu KD, Wilson JG, Zhuo H, Caballero L, McMillan ML, Fang X, Cosgrove K, Calfee CS, Lee JW, Kangelaris KN, Gotts JE, Rogers AJ, Levitt JE, Wiener-Kronish JP, Delucchi KL, Leavitt AD, McKenna DH, Thompson BT, Matthay MA: Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome. Ann Intensive Care 2014, 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thebaud B: Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 2012, 303:L967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW: Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem cells 2014, 32:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW: Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med 2015, 192:324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM: A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 2010, 5:e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tsoyi K, Hall SR, Dalli J, Colas RA, Ghanta S, Ith B, Coronata A, Fredenburgh LE, Baron RM, Choi AM, Serhan CN, Liu X, Perrella MA: Carbon Monoxide Improves Efficacy of Mesenchymal Stromal Cells During Sepsis by Production of Specialized Proresolving Lipid Mediators. Crit Care Med 2016, 44:e1236–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Han KH, Kim AK, Kim MH, Kim DH, Go HN, Kim DI: Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia. Cell Biol Int 2016, 40:27–35. [DOI] [PubMed] [Google Scholar]
- [20].Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G: Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant 2012, 27:3037–42. [DOI] [PubMed] [Google Scholar]
- [21].Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, Kwong A, Mitsialis SA, Kourembanas S: Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med 2018, 197:104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matthay MA, Pati S, Lee JW: Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. Stem Cells 2017, 35:316–24. [DOI] [PubMed] [Google Scholar]
- [23].Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA: Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 2013, 187:751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E: Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8:315–7. [DOI] [PubMed] [Google Scholar]
- [25].Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, Gores GJ, Malhi H: Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology 2016, 64:2219–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schorey JS, Harding CV: Extracellular vesicles and infectious diseases: new complexity to an old story. J Clin Invest 2016, 126:1181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pitt JM, Kroemer G, Zitvogel L: Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest 2016, 126:1139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zappulli V, Friis KP, Fitzpatrick Z, Maguire CA, Breakefield XO: Extracellular vesicles and intercellular communication within the nervous system. J Clin Invest 2016, 126:1198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G: Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 2011, 26:1474–83. [DOI] [PubMed] [Google Scholar]
- [30].Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK: Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010, 4:214–22. [DOI] [PubMed] [Google Scholar]
- [31].Gennai S, Monsel A, Hao Q, Park J, Matthay MA, Lee JW: Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am J Transplant 2015, 15:2404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V: Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 2008, 26:151–62. [DOI] [PubMed] [Google Scholar]
- [33].Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J: Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012, 18:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA: Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 2015, 6:8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, Krasnodembskaya AD: Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med 2017, 196:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ: Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 2010, 182:1047–57. [DOI] [PubMed] [Google Scholar]
- [37].Tang XD, Shi L, Monsel A, Li XY, Zhu HL, Zhu YG, Qu JM: Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 mRNA. Stem Cells 2017, 35:1849–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MSC MVs were analyzed by electron microscopy, NanoSight analyses and Flow Cytometry. (A) Electron microscopy demonstrated a fairly homogenous population of small membrane bound vesicles roughly 100–200 nm in length. (B) NanoSight analyses demonstrated approximately 4.7 × 108 particles per ml with a mean diameter of 180 nm ± 14 nm, N = 3 separate isolations. In the ex vivo perfused human lung, approximately 200 – 400 μl or 9.4 – 18.8 × 107 MVs were administered as therapy. (C) Representative flow cytometry figures showed that a large proportion of the vesicles were CD44 positive. We labelled the vesicles with PKH26 prior to flow cytometry to eliminate artifacts and debris.