Abstract
The field of cancer immunotherapy has been revolutionized with the use of immune checkpoint blockade antibodies such as anti-programmed cell death 1 protein (PD-1) and chimeric antigen receptor T cells. Significant clinical benefits are observed in different cancer types with these treatments. While considerable efforts are made in augmenting tumor-specific T cell responses with these therapies, other immunotherapies that actively stimulate endogenous anti-tumor T cells and generating long-term memory have received less attention. Given the high cost of cancer immunotherapies especially with chimeric antigen receptor T cells, not many patients will have access to such treatments. The next-generation of cancer immunotherapy could entail in vivo cancer vaccination to activate both the innate and adaptive anti-tumor responses. This could potentially be achieved via in vivo targeting of dendritic cells which are an indispensable link between the innate and adaptive immunities. Dendritic cells highly expressed toll-like receptors for recognizing and eliminating pathogens. Synthetic toll-like receptors agonists could be synthesized at a low cost and have shown promise in preclinical and clinical trials. As different subsets of human dendritic cells exist in the immune system, activation with different toll-like receptor agonists could exert profound effects on the quality and magnitude of anti-tumor T cell responses. Here, we reviewed the different subsets of human dendritic cells. Using published preclinical and clinical cancers studies available on PubMed, we discussed the use of clinically approved and emerging toll-like receptor agonists to activate dendritic cells in vivo for cancer immunotherapy. Finally, we searched www.clinicaltrials.gov and summarized the active cancer trials evaluating toll-like receptor agonists as an adjuvant.
Introduction
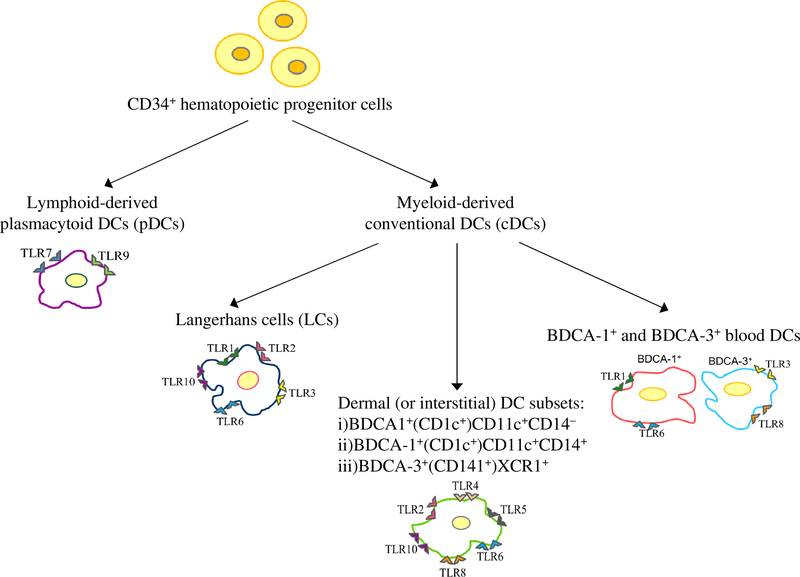
Compelling evidences now demonstrated that the human immune system plays an important role in tumor surveillance and suppression. Dendritic cells (DCs) are the first-line of defense against invading pathogens and actively participate in tumor surveillance by removing damaged tissues in the microenvironment. Being potent antigen-presenting cells (APCs), DCs can initiate and regulate immune responses including anti-tumor responses. DCs follows two major developmental pathways from CD34+ hematopoietic progenitor cells to become either lymphoid-derived plasmacytoid DCs (pDCs) or myeloid-derived conventional DCs [1, 2]. The myeloid-derived DCs are further classified based on their tissue location, phenotype, cytokine and chemokine production, and the immune responses they elicit. (Figure 1). Myeloid monocyte-derived DCs are most widely used for tumor immunotherapy. They are differentiated ex vivo from peripheral monocytes with recombinant granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin (IL)-4. They are highly efficient in antigen phagocytosis and production of IL-12 [3, 4], as well as eliciting anti-tumor T cell responses. However, their ex vivo production is laborious and costly. An attractive alternative is to target DCs in vivo with appropriate tumor antigens and activating them to produce proinflammatory cytokines.
Figure 1.
Schematic diagram showing the major human DC subsets.
Toll-like receptors (TLRs) are an integral part of the innate immunity for recognizing and eliminating invading pathogens [5–7]. They are predominantly expressed by immune cells such as DCs, macrophages and monocytes. Activation of TLRs by their corresponding ligands (e.g. natural conserved pathogen-associated molecular patterns [PAMPs] or synthetic) leads to inflammatory cytokines and chemokines productions that exert multiple effects on both innate and adaptive immunities [8, 9]. Thus, TLR activation in the context of cancer could potentially influence the activation, magnitude and quality of anti-tumor T cell responses. Ten different TLRs have been characterized in the human immune system – TLR1, 2, 4, 5, 6 and 10 are expressed on the cell surface, whereas TLR7, 8 and 9 are expressed in endosomal/lysosomal membranes of the cell. TLR3 is expressed in different cell types including immune cells [10–14]. It is interesting to note that some of the most effective vaccines, such as the live-attenuated yellow fever vaccine 17D (YF-17D), owe their effectiveness through simultaneous activation of different DC subsets to produce proinflammatory cytokines including IL-12, IL-6 and interferon (P)-α that stimulate T helper (Th) 1 and 2 responses [15, 16]. Table 1 summarizes a selection of TLR agonists that have been used for in vivo DC targeting.
Table 1.
Selected toll-like receptor (TLR) agonists for potential in vivo targeting of dendritic cells (DCs).
| TLR | Synthetic agonists tested In clinics | Potential targeted DC subset(s) | References |
|---|---|---|---|
| 2 | LPS, MPLA (in AS01–04 adjuvant systems), amino alkyl glucosamlmide phosphates (In Dynavax®), GLA, BCG (used together with MPLA in Melacine®), MALP-2 |
LCs, Dermal DCs | 136, 137, 139–149, 153 |
| 3 | Poly I:C, Poly(I:C12U) [Ampligen®], Poly-ICLC [Hiltonol®] | LCs, BDCA-3+ blood DCs | 117–124 |
| 4 | LPS, MPLA, amino alkyl glucosamlmide phosphates, GLA, BCG | Dermal DCs | 136, 137, 139–149, 153 |
| 5 | Flagellln | Dermal DCs | 103 |
| 6 | MALP-2 | LCs, dermal DCs, BDCA-1+ blood DCs |
153, 154 |
| 7 | Imlquimod (R-837), 852A (3M-001), Reslquimod (R-848; S-28463), Gardlqulmod | pDCs | 47–72 |
| 8 | Resiqulmod (R-848, S-28463), VTX-2337 (Motollmod), VTX-294 | Dermal DCs, BDCA-3+ blood DCs |
68–72, 157, 158 |
| 9 | CpG ODN 7909 (Agatollmod; PF-3512676; Promune®), SD-101 | pDCs | 97–102 |
Abbreviations: LC (Langerhans cells), pDCS (plasmacytod dendritic cells), LPS (lipopolysaccharides); MPLA (monophosphoryl lipid A; GLA (glucopyranosyl lipid A); BCG (Mycobacterium bovis bacillus Calmette-Guérin); MALP-2 (macrophage activating lipopeptide-2); VTX-2237 and VTX-294 (VentiRx Pharmaceuticals, USA); CpG ODN (CpG-oligodeoxynucleotides).
Lymphoid-derived plasmacytoid DCs
Lymphoid-derived plasmacytoid DCs (tns) is a unique subset that localize in the lymph nodes (LNs), spleen, blood, mucosal-associated tissues, thymus and liver in normal physiological condition. Under pathological conditions including lymphoid hyperplasia of the skin [17], cutaneous systemic lupus erythematosus (SLE), psoriasis vulgaris, contact dermatitis and allergic mucosa [18], pDCs are also found in the skin. Unlike their myeloid counterparts, pDCs does not depend on GM-CSF for differentiation. Instead, they follow a distinct developmental pathway that uses IL-3 and Fms-like tyrosine kinase 3 ligand (Flt3L) [19]. pDCs play an important role against viral infections. They are the main producers of Type I IFN-α and -β against invading viruses, and modulate different aspects of innate, adaptive and humoral immune responses via these cytokines [20]. The IFN-α/β secreted by pDCs could exert numerous effects including promoting pDC survival, inducing the differentiation of myeloid-derived DCs [21], facilitating crosspresentation and crosspriming of DCs [22], shaping both T helper (Th) 1 and CD8+ T cell responses, activating natural killer (NK) cells, and inducing the generation of plasma cells and primary antibody responses against viruses [23, 24]. pDCs also efficiently expanded antigen-specific CD8+ T effector cells, demonstrating their importance in adaptive immunity against subsequent viral infections [25].
In cancers, pDCs are shown to play contrasting roles. In murine B16 melanoma, pDCs were activated by TLR agonists to express tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), granzymes B and C for potential tumor killing. The activated pDCs also produced IFN-α to promote tumor cell lysis by cytotoxic T cells (CTLs) and NK cells [26–28]. In a murine orthotropic mammary tumor model, pDCs were stimulated in vivo via TLR7 agonist administration to elicit anti-tumor responses [29]. In a melanoma trial, treated subjects showed tumor-specific CD4+ and CD8+ T cell responses after receiving ex vivo generated blood-derived pDCs that were loaded with tumor-associated antigens (TAAs) [30]. On the other hand, some studies indirectly showed that tumor-infiltrating pDCs were defective in Type I IFN secretion [31–35], and produced immunosuppressive factors that assisted in tumor progression [36]. Also, tumor-infiltrating pDCs were significantly impaired in their TLR9 expression and responsiveness [34, 37, 38]. Moreover, tumor-infiltrating pDCs could be polarized by immunosuppressive indoleamine 2,3-dioxygenase (IDO) in the tumor microenvironment to secret IL-10 for T regulatory (Treg) cell proliferation [39–42].
Targeting pDCs with TLR7/8 agonists
Human pDCs distinctly express blood-derived cell antigen (BDCA)-2, BDCA-4 (CD304/Neuropilin), ILT-7 and IL-3Rα chain (CD123) [43]. They also express TLR7 and 9 for recognizing viral nucleic acid and bacterial DNA with unmethylated cytosine-phosphateguanine (CpG) sequences, respectively [44–46]. Imiquimod (R-837), a US Food and Drug Administration (FDA)-approved synthetic TLR7 agonist, has been used successfully as a topical treatment for superficial basal cell carcinomas [47–49], human papilloma virus (HPV)-mediated external genital warts and actinic keratosis [50–53]. It effectively induces strong infiltrations of IFN-α producing pDCs, T and NK cells in the treated carcinomas [54, 55]. In metastatic melanoma, Imiquimod given topically with intradermal injection of NY-ESO-1 protein induced activation of pDCs, NK cells, and elicited IFN-γ secretion from CD4+ T cells [56, 57]. In vulvar intraepithelial neoplasia, Imiquimod given topically elicited CD8+ T cells and NK cells responses and 35% of the subjects remained disease-free for more than a year [58]. In metastatic breast cancer, clinical responses were observed in subjects who applied Imiquimod topically 5 days per week for 8 weeks to their chest wall [59]. Six out of 8 subjects showed partial responses or stable diseases, and tumor regression was associated with the presence of tumor-infiltrating CD4+ and CD8+ T cells [59]. In a Phase II breast cancer trial, 15 subjects applied Imiquimod topically to targeted lesions on 4 consecutive days per week for 12 weeks [60]. Albumin bound paclitaxel (100mg/m2) was also given intravenously once weekly for 3 weeks and repeated every 28 days for 12 weeks. Ten subjects experienced complete and partial responses [60]. The clinical response was short-lived, suggesting that longer course of treatment was needed. Nevertheless, these results demonstrated that Imiquimod is well-suited as a topical agent to activate pDCs and T cell responses via TLR7. Two clinical trials are currently evaluating the use of Imiquimod with radiotherapy and/or chemotherapy (Table 2).
Table 2.
Use of TLR7 agonists as adjuvant in active clinical trials (*www.clinicaltrials.gov).
| Number | *NCT number and title | Cancer type(number of subjects to enroll) | Intervention(s) | Trial type | Status | Sponsor/collaborators |
|---|---|---|---|---|---|---|
| 1 |
NCT01421017 Toll-like Receptor(TLR)7Agonist, Cyclophosphamide, and Radiotherapy for Breast Cancer With Skin Metastases |
Metastatic and recurrent breast cancer (55) | • TLR7 agonist-Imiquimod • Radiotherapy • Cyclophosphamide |
• Phase I/II, non- randomized and open- labeled |
• Active, not recruiting • Start date: Aug 2011 • Completion date: Feb 2019 |
• New York University School of Medicine • National Cancer Institute (NCI) |
| 2 |
NCT03196180 Topical Fluorouracil and Imiquimod in Treating Patients With High-Grade Cervical Intraepithelial Neoplasia |
High grade cervical intraepithelial neoplasia, and p16-positive neoplastic cells present (34) |
• TLR7 agonist-Imiquimod • Topical fluorouracil |
• Phase I, single group assignment and open- label. |
• Active, not recruiting • Start date: 30th Sept 2018 • Completion date: 31st Jan 2021 |
• NCI |
| 3 |
NCT03416335 A Study of DSP-0509 in Patients With Advanced Solid Tumors to Determine the Safety and the Pharmacokinetic Profile |
Neoplasms (44) | • TLR7 agonist-DSP-0509 | • Phase I, single group assignment and open- label. |
• Recruiting. • Start date: 1st June 2018 • Completion date: July 2020 |
• Boston Biomedical, Inc • Syneos Health |
| 4 |
NCT00960752 Tumor and Vaccine Site With a Toll Like Receptor (TLR) Agonist |
Melanoma (71) | • TLR7/8 agonist-Resiquimod (R 848)gel • Gp100 and MAGE-3 peptides |
• Phase II, randomized factorial assignment and open-label. |
• Active, not recruiting • Start date: May 2010 • Completion date: May 2019 |
• M.D. Anderson Cancer Center |
| 5 |
NCT02556463 A Study of MEDI9197 in Subjects With Solid Tumors or CTC Landin Combination With Durvalumab and/ or Palliative Radiation in Subjects With Solid Tumors |
Solid Tumors and early-stage cutaneous T Cell lymphoma [CTCL] (135) |
• TLR7/8 agonist-MEDI9197 • Durvalumab (Fc optimized anti programmed cell death-ligand 1 [PD-L1] monoclonal antibody) • Radiation |
• Phase I, non- randomized and open- labeled |
• Recruiting. • Study Start date: 4th Nov 2015 • Completion date: 19th Aug 2020 |
• MedImmune LLC |
| 6 |
NCT03435640 A Study of NKTR-262 in Combination With NKTR 214 and With NKTR-214 Plus Nivolumab in Patients With Locally Advanced or Metastatic Solid Tumor |
Melanoma, Merkel cell carcinoma, triple Negative breast cancer, ovarian carcinoma, renal cell carcinoma, colorectal cancer, urothelial carcinoma and sarcoma(393) |
• TLR7/8 agonist-NKTR-262 • CD122-biased agonist-NKTR-214 • Nivolumab (anti-PD-L1 monoclonal antibody) |
• Phase I/II, non- randomized and open- label |
• Recruiting • Start date: 15th Mar 2018 • Completion date: Dec 2019 |
• Nektar Therapeutics |
| 7 |
NCT03301896 Study of the Safety and Efficacy of LHC165 Single Agent and in Combination With PDR001 in Patients With Advanced Malignancies |
Solid tumors (200) | • TLR7/8 agonist-LHC165 • PDR001 (PD-1 checkpoint inhibitor) |
• Phase I, non- randomized and open- labeled. |
• Recruiting • Start date: 31st Jan 2018 • Completion date: 20th Dec 2021 |
• Novartis |
| 8 |
NCT02431559 A Phase 1/2 Study of Motolimod (VTX-2337)and MEDI4736 in Subjects With Recurrent, Platinum Resistant Ovarian Cancer for Whom Pegylated Liposomal Doxorubicin (PLD) is Indicated |
Ovarian carcinoma (53) | • TLR8 agonist-VTX2337 • Durvalumab • Pegylated liposomal doxorubicin (PLD) |
• Phase I/II, non- randomized and open- label. |
• Active, not recruiting • Start date: Nov 2015 • Completion date: Mar 2019 |
• Ludwig Institute for Cancer Research • MedImmune LLC • VentiRx Pharmaceuticals Inc. • Cancer Research Institute, New York City |
852A (3M-001), like Imiquimod, is a small-molecule imidazoquinoline and synthetic analogue of DNA/RNA oligonucleotide designed to bind specifically to the TLR7 of pDCs. This binding mimics the recognition of single-stranded (ss) RNA in viral infection leading to the production of IFN-α by pDCs, and eventual priming of specific immune responses [61–64]. Compared to Imiquimod, 852A is several-folds more potent for TLR7 and almost 40 times more water-soluble at physiologic pH. Hence, 852A is well-suited for intravenous administration as it is less extensively metabolized and eliminated than Imiquimod [65]. Oral, subcutaneous and intravenous have been investigated for 852A administration in humans. However, the oral route showed very little efficacy compared to other routes and was not discussed here. In a Phase I trial, 852A was given subcutaneously twice a week for 12 weeks to 15 subjects with recurrent ovarian, cervical and breast cancers. Increased sera IP-10 and IL-1 receptor antagonist (IL-1RA) were observed, and one ovarian cancer subject experienced stable disease and a cervical cancer subject remained disease-free for 18 months [65]. In a Phase I trial of refractory metastatic melanoma, 852A was given intravenously 3 times a week for 12 weeks. Four out of 21 subjects experienced disease stabilization for more than 3 months [66]. Three clinical trials evaluating the use of 852A in refractory solid cancers (NCT00095160) and melanoma (NCT00189332, NCT00091689) have been completed, and the results are pending.
Resiquimod (R-848; S-28463) interacts with TLR7 and 8 to induce secretions of IFN-α, IL-6, −8, −12, and TNF-α from pDCs, and stimulating Th1 responses [67]. In a Phase I trial of early-stage cutaneous T cell lymphoma (CTCL), 9 out of 12 subjects treated with 0.03% or 0.06% topical Resiquimod gel showed improved or complete lesion clearance [68]. T cell receptor sequencing revealed a reduction or complete elimination of the malignant T cells in the responders. Significantly more IFN-γ and TNF-α producing CD4+ T cells were detected in the high responders compared to the low responders [68]. In melanoma, subjects were given full-length NY-ESO-1 protein emulsified in Montanide (100µg) subcutaneously and topical application of 1g of 0.2% Resiquimod gel 3 times a week [69]. All the subjects showed humoral responses against NY-ESO-1, and 16 out of the 20 evaluable subjects showed CD4+ T cells responses. In another study, 90% of the subjects with actinic keratosis had complete clearance of their lesions after topical Resiquimod treatment [70]. These results demonstrated the feasibility of using topical Resiquimod to activate pDCs for cancer therapy. Resiquimod is being assessed as an adjuvant in melanoma-derived peptide vaccinations and in autologous tumor lysate-pulsed DC vaccination in brain tumors [Table 2 and 4]. Given the promising results of Imiquimod and Resiquimod, one could envisage using them topically to activate pDCs in the skin and mucosa-associated tissues to elicit humoral and cellular anti-tumor responses. As more results become available attesting the effectiveness of 852A, one could envisage giving it subcutaneously or intravenously to target pDCs residing in the blood, spleen and LNs. An emerging TLR7 agonist, Gardiquimod which is structurally similar to Imiqumod and Resiquimod, could exert anti-tumor effects in preclinical studies including inhibiting tumor cell growth [71] and triggering their apoptosis [72], and together with Imiquimod augmented tumor-lysate loaded DC therapy in B16 murine melanoma [71]. Other novel TLR7/8 agonists including DSP-0509, MEDI9197, NKTR-262 and LHC165 are currently being investigated in combination with immune checkpoint inhibitors, radiotherapy and/or chemotherapy (Table 2).
Table 4.
Use of TLR3 agonists as adjuvant in a selection of active clinical trials (*www.clinicaltrials.gov).
| Number | *NCT number and title | Cancer type(number of subjects to enroll) | Intervention(s) | Trial type | Status | Sponsor/collaborators |
|---|---|---|---|---|---|---|
| 1 |
NCT02643303 A Phase 1/2 Study of In Situ Vaccination With Tremelimumab and IV Durvalumab Plus Poly-ICLC in Subjects With Advanced, Measurable, Biopsy- accessibleCancers |
Head and neck squamous cell carcinoma, Breast, prostate, renal and bladder carcinomas, melanoma sarcoma, Merkel cell and cutaneous T cell lymphoma (102) |
• TLR3 agonist-Poly-ICLC • Durvalumab • Tremelimumab (human IgG2 anti- cytotoxic T-lymphocyte - associated antigen 4 [CTLA-4] monoclonal antibody) |
• Phase I/II, non- randomized and open- label. |
• Recruiting. • Start date: 28th Dec 2016 • Completion date: Aug 2022 |
• Ludwig Institute for Cancer Research • MedImmune LLC • Cancer Research Institute, New York City |
| 2 |
NCT03162562 The Safety and Antitumor Activity of the Combination of Oregovomab and Hiltonol in Recurrent Advanced Ovarian Cancer |
Stage III-IV and recurrent ovarian carcinoma (10) |
• TLR3 agonist-Poly-ICLC • Oregovomab (murineanti-CA125 monoclonal antibody) |
• Phase I, single group and open-label |
• Recruiting. • Start date: 30th May 2017 • Completion date: Sept 2021 |
• OncoQuest Inc. |
| 3 |
NCT02834052 Pembrolizumab + Poly-ICLC in MRP Colon Cancer |
Metastatic colorectal carcinoma (42) | • TLR3 agonist-Poly-ICLC • Pembrolizumab |
• Phase I/II, non- randomized single group assignment and open- label. |
• Recruiting. • Start date: 10th Jan 2018 • Completion date: Jan 2021 |
• Asha Nayak • Oncovir, Inc. • Merck Sharp & Dohme Corp. • Augusta University |
| 4 |
NCT02126579 Phase I/II Trial of a Long Peptide Vaccine (LPV7) Plus TLR Agonists |
Melanoma (58) | • TLR3 agonist - Poly-ICLC and TLR7/8 agonist - Resiquimod • Peptide vaccine (LPV7) + Tetanus peptide • Incomplete Freund’s adjuvant (IFA) |
• Phase I/II, randomized and open-label |
• Recruiting. • Study Start: April 2014 • Completion date: Dec 2018 |
• Craig L Slingluff, Jr • University of Virginia |
| 5 |
NCT01976585 In Situ Vaccine for Low-Grade Lymphoma: Combination of Intratumoral Flt3L and Poly-ICLC With Low-Dose Radiotherapy |
Low-Grade B-cell Lymphoma (30) | • TLR3 agonist - Poly-ICLC • Recombinant human (rhu) Flt3 ligand (CDX-301) |
• Phase I/II, single group assignment and open- label. |
• Recruiting. • Start date: Dec 2013 • Completion date: Nov 2018 |
• Joshua Brody • Icahn School of Medicine at Mount Sinai |
| 6 |
NCT01720836 Study of the Immune Response of MUC1 (Mucin1) Peptide Vaccine for Non-small Cell Lung Cancer |
Non-small cell lung cancer (NSCLC) (30) | • TLR3 agonist - Poly-ICLC (Hiltonol®) • Mucin 1 (MUC1) peptide |
• Phase I/II, non- randomized and open- label |
• Recruiting • Start date: Nov 2012 • Completion date: Sept 2020 |
• Olivera Finn • University of Pittsburgh |
| 7 |
NCT03262103 Neoadjuvant Hiltonol® (PolyICLC) for Prostate Cancer |
Prostate cancer (24) | • TLR3 agonist - Poly-ICLC (Hiltonol®)given intratumorally (0.5 mg or 1.0 mg) or intramuscularly • Radical prostatectomy |
• Phase I, non-randomized and open-label |
• Recruiting • Start date: May 2017 • Completion date: May 2020 |
• Ashutosh Kumar Tewari • Oncovir, Inc. • Icahn School of Medicine at Mount Sinai |
| 8 |
NCT02721043 Safety and Immunogenicity of Personalized Genomic Vaccine to Treat Malignancies |
Solid tumors (20) | • TLR3 agonist - Poly-ICLC (Hiltolon®) • Synthetic peptides • Lenalidomide (a thalidomide analog) |
• Phase I, single • group assignment and open-label. |
• Recruiting • Start date: April 2016 • Completion date: July 2020 |
• Nina Bhardwaj • Icahn School of Medicine at Mount Sinai |
| 9 |
NCT02061449 PolyICLC, Radiation, and Romidepsin for Advanced Cutaneous T Cell Lymphoma |
Cutaneous T-cell Lymphoma (3) | • TLR3 agonist - Poly-ICLC (Hiltonol®) • Romidepsin (antibiotic) • Focal lesional radiation |
• Phase I/II, non- randomized and open- labeled |
• Active, not recruiting. • Start date: Mar 2014 • Completion date: June 2018 |
• New York University, School of Medicine • Ludwig Institute for Cancer Research |
| 10 |
NCT01204684 Dendritic Cell Vaccine for Patients With Brain Tumors |
Glioma, glioblastoma, anaplastic astrocytoma and Astrooligodendro-glioma (60) |
• Autologous tumor lysate-pulsed DC vaccination in combination with either • TLR3 agonist - Poly-ICLC (Hiltonol®), or • 0.2% Resiquimod |
• Phase II, randomized and open-labeled |
• Active, not recruiting. • Start date:8th Oct 2010 • Completion date: Oct 2019 |
• Jonsson Comprehensive Cancer Center |
Targeting pDCs with TLR9 agonists
pDCs express TLR9 for specific recognition of unmethylated CpG motifs in bacteria and DNA viruses [73–77]. Upon stimulation with CpG, pDCs upregulated CD40, CD54, CD80, CD86 and MHC class II [78–80], and produced IFN-α, IL-1, IL-6, IL-12, IL-18 and TNF-α [81] to activate other immune cells. pDCs also differentiated into potent APCs and increased their resistance to IL-4-induced apoptosis [82]. As CpG has powerful effects on pDCs and subsequent Th1 responses, it shows promise as a cancer adjuvant [21, 83–86]. Four classes of synthetic CpG-oligodeoxynucleotides (CpG ODNs) ligands, i.e. Class A, B, C and P, are generated to mimic the immunostimulatory activity of CpG on pDCs [76, 87]. They differ in nucleotide sequences and lengths, and exhibit different functional properties. Class A (D-type) enters the lysosome compartments of pDCs to stimulate strong IFN-α production, as well as activate NK cells via its palindromic structure. Class B (K-type) traffics to the endosomal compartments of pDCs to stimulate their maturation and antigen-presentation and induces upregulation of costimulatory molecules on B cells. However, it elicits poor IFN-α production due to its nonpalindromic sequences [87–89]. Class C exhibits stimulatory properties of both Class A and B CpG ODNs [90, 91]. Class P contains two palindromic sequences and thus has greater ability than Class C in inducing IFN-α secretion [92, 93]. All of them elicit strong Th1 responses [94].
In IGROV-1 human ovarian adenocarcinoma, tumor-bearing athymic mice receiving repeated intraperitoneal injections of ODN1826 (Class B) showed ascites regression and increased survival [95]. Mice treated intraperitoneally survived significantly longer than mice treated subcutaneously or intravenously with the same CpG ODNs [96]. Moreover, mice receiving CpG ODNs intraperitoneally showed higher level of sera and tissue C-X-C motif chemokine ligand 1 (CXCL1), and increased circulating NK cells and neutrophils [96]. Subcutaneous administration of CpG ODN 7909 (Class B; Agatolimod; PF-3512676; ProMune®) in healthy volunteers elicited strong IFN-α, IL-12p40, IL-6 and IP-10 secretions at the injection site and draining LNs for 2 weeks. Interestingly, intravenous administration of CpG ODN 7909 even at 128-fold higher dose did not induce any immune responses [97]. In a Phase I melanoma trial, increased circulating MART-1 specific CD8+ effector memory T cells was detected in the subjects after 4 monthly subcutaneous vaccinations of CpG ODN 7909, MART-1 peptide and incomplete Freund’s adjuvant [98]. Similarly, CpG ODN 7909 co-administered with NY-ESO-1 peptides/proteins and Montanide ISA-51 subcutaneously led to an overall increased in antigen-specific CD8+ T cells [57, 99, 100] that persisted for 3 years after vaccinations [100]. Furthermore, administrating CpG ODN 7909 subcutaneously activated both pDCs and myeloid DCs and priming Th1-related cytokines and anti-tumor CD8+ T cell responses [97, 101]. In a Phase I/II B cell lymphoma trial, 13 subjects treated intratumorally with SDS-101 (Class C CpG ODN) and low-dose radiation showed decreased tumor size and increased CD8+ T cell in the treated lesions (NCT02254772). SDS-101 is currently being investigated in cancer trials in combination with anti-TNF receptor superfamily member 4 (OX40) antibody, pembrolizumab and ibrutinib [Table 3]. Clinical evaluation is also warranted for intraperitoneal administration of CpG ODNs as this route has shown promise in preclinical tumor models.
Table 3.
Use of TLR9 agonists as adjuvant in registered clinical trials (*information taken from Clinicaltrials.gov).
| Number | *NCT number and tide | Cancer type (number of subjects to enroll) |
Intervention(s) | Trial type | Status | Sponsor/collaborators |
|---|---|---|---|---|---|---|
| 1 |
NCT02668770 Ipilimumab (Immunotherapy) and MGN1703 (TLR Agonist) in Patients With Advanced Solid Malignancies |
Advanced solid cancers including melanoma (60) |
• TLR9 agonist - MGN1703 • Ipilimumab (recombinant human IgGl anti-CTLA-4 monoclonal antibody) |
• Phase II, non-randomized and open-label. |
• Recruiting • Start date: 11 th May 2016 • Completion date: May 2020 |
• M.D. Anderson Cancer Center • Mologen AG |
| 2 |
NCT02452697 Ph2 NK Cell Enriched DCls vv/vvo RI.R9 Agonist, DUKCPG-001 From Donors Following Allogeneic SCT |
Myeloid and lymphoid malignancies (100) |
• TI-R9 agonist - DUK-CPG-001 • NK Cell enriched-DLI |
• Phase II, randomized and open-label. |
• Recruiting • Start date: 8th June 2016 • Completion date: July 2023 |
• David Rizzieri, MD • Agilent Technologies, Inc. • Duke University |
| 3 |
NCT03326752 Phase lb DV281 With an AntiPD-1 Inhibitor in NSCLC |
Advanced NSCLC (80) | • TLR9 agonist - DV281 (synthetic, aerosolized C-class CpG ODN; RP2D) • Anti-PD-1 Inhibitor |
• Phase I, non-randomized and open-label. |
• Recruiting • Start date: 20th Sept 2017 • Completion date: 30th Aug 2020 |
• Dynavax Technologies Corporation |
| 4 |
NCT03618641 CMP-001 in Combo With Nivolumab in Stage 1IIB/C/D Melanoma Patients With Clinically Apparent Lymph Node Disease |
Melanoma and lymph node cancer(20) |
• TLR9 agonist - CMP-001 (Virus-like particle-encapsulated TLR9 agonist) • Nivolumab |
• Phase II, sequential assignment and open- label. |
• Recruiting • Start date: 8th Aug 2018 • Completion date: 15th July 2023 |
• Diwakar Davar • Checkmate Pharmaceuticals • University of Pittsburgh |
| 5 |
NCT03507699 Combined Immunotherapy and Radiosurgery for Metastatic Colorectal Cancer |
Colorectal carcinoma and liver metastases (19) |
• TI-R9 agonist - CMP-001 • Liver radiotherapy • Nivolumab • Ipilimumab |
• Phase I, non-randomized and open-label. |
• Active, not yet recruiting • Star date: 1st Jul 2018 • Completion date: 31st May 2021 |
• Sheba Medical Center • Checkmate Pharmaceuticals • Bristol-Myers Squibb |
| 6 |
NCT03445533 A Study of IMO-2125 in Combination With Ipilimumab Versus Ipilimumab Alone in Subjects With Anti-PD-1 Refractory Melanoma (ILLUMINATE-301) |
Metastatic melanoma (308) | • TLR9 agonist - IMO-2125 • Ipilimumab |
• Phase III, randomized and open-label. |
• Recruiting • Start date: 28th Feb 2018 • Completion date: 1st Jul 2020 |
• Idera Pharmaceuticals, Inc. • Bristol-Myers Squibb |
| 7 |
NCT03410901 TLR9 Agonist SDS-101, Anti-OX40 Antibody BMS 986178, and radiotherapy in Treating Patients With Low Grade B-Cell Non-Hodgkin Lymphomas |
B-cell Non-Hodgkin lymphoma (15) |
• TLR9 Agonist - SDS-101 • Anti-necrosis factor receptor superfamily member 4 (0X40) antibody [BMS 986178) • radiotherapy |
• Phase I, single group assignment and open- label. |
• Recruiting • Start date: 9th April 2018 • Completion date: 9th Oct 2020 |
• Ronald Levy • NCI • Stanford University |
| 8 |
NCT03007732 Pembrolizumab in Combination With Intratumoral SDS- 101 Therapy |
Prostate cancer (42) | • TLR9 agonist - SDS-101 • Pembrolizumab • Leuprolide acetate and abiraterone acetate • Prednisone (synthetic glucocorticoid) • Stereotactic body radiotherapy |
• Phase I, randomized and open-label. |
• Recuiting • Start date: 27th April 2017 • Completion date: April 2022 |
• Lawrence Fong • University of California, San Francisco |
| 9 |
NCT02927964 TLR9 Agonist SDS-101, Ibrutinib, and radiotherapy in Treating Patients With Relapsed or Refractory Grade 1- 3A Follicular Lymphoma |
Follicular lymphoma including recurrent and recurrent forms (30) |
• TLR9 agonist - SDS-101 • Ibrutinib (small-molecule inhibitor of Bruton’s tyrosine kinase) • radiotherapy |
• Phase I/II, single group assignment and open- label. |
• Recruiting • Start date: Nov 2016 • nov.16 • Completion date: Nov 2021 |
• Robert Lowsky • NCI • Stanford University |
IMO-2125, a novel synthetic TLR9 agonist, was shown to suppress murine A20 lymphoma and CT26 colon carcinoma [102]. An increased in CD3+ tumor-infiltrating T cells (TILs) was observed following intratumoral IMO-2125 administration, and further studies showed that CD8+ T cells were required for tumor control. Increased immune checkpoint gene expressions including programmed cell death protein (PD)-1, PD-ligand 1 (PD-L1), OX40 and OX40 ligand, and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) were observed following IMO-2125 treatment, suggesting that immune checkpoint therapies could be combined with IMO-2125 for improved efficacy. Currently, 3 clinical trials are assessing the efficacy of IM0-2125 with ipilimumab and/or pembrolizumab in metastatic melanoma [Table 3]. Numerous novel TLR9 agonists, including MGN1703 (lefitolimod; DNA-based agonist), DUK-CPG-001 (synthetic CpG-rich oligonucleotide), DV281 (aerosolized synthetic C-class ODN for lung cancer treatment), and CMP-001 (virus-like particle-encapsulated agonist with unmethylated CpG motif-rich G10 oligonucleotides) are being tested in cancer trials (Table 3).
CpG ODNs could synergize with other TLR agonists to increase their overall potencies through activation of multiple DC subsets. CpG ODNs synergized with flagellin, a TLR5 agonist that binds to TLR5 on dermal DCs, to induce anti-tumor immunity in mice [103]. CpG synergized with Poly I:C (polyinosinic-polycytidylic acid), a TLR3 agonist recognized by TLR3 on Langerhans cells (LCs), to induce IL-6 and IL-12 productions from the DCs and promote anti-tumor responses [104]. The adjuvant AS15, which contains a TLR4 agonist monophosphoryl lipid A (MPLA) and CpG ODN 7909, is used to boost anti-tumor responses elicited by full-length recombinant PRAME (PReferentially Expressed Antigen of Melanoma) in non-small cell lung carcinoma (NSCLC) [NCT01853878].
Langerhans cells
Langerhan cells (LCs) reside in the basal and supra-basal layers of the epidermis, where they form a dense network of first-line defense against invading pathogens [105]. LCs and dermal DCs are the two major DC subsets found in the human skin. Although they develop from a common bone-marrow myeloid precursor, these DC subsets express distinct phenotypic markers and induce predominantly different types of immune responses. LCs express Langerin (CD207), Birbeck granules, DEC-205 (CD205), DCIR (DC immunoreceptor)/CLEC4A and TLR1, 2, 3, 6 and 10 [106]. LCs seem to regulate cellular immunity, while dermal DCs distinctly regulate the humoral immunity. This notion was supported by murine studies showing that activated dermal DCs migrated into the outer paracortex beneath the B cell follicles and LCs entered the T cell-rich inner paracortex [107]. In human, LCs pulsed with tumor or viral peptides were superior to CD14+ dermal DCs in priming high-avidity antigen-specific CD8+ CTLs that expressed higher levels of granzymes and perforin [108]. This might be due to LCs preferentially produce strong level of IL-15 that is known to enhance naïve CD8+ T cell proliferation and stimulate memory T cell development [109]. Interestingly, the presence of LCs in tumor lesions was positively correlated with better prognosis in gastric carcinoma [110]. Similarly, more LCs were found in low-stage breast and uterine carcinomas than in advanced-stage cancers [111, 112]. These results suggested that human LCs play a role in tumor immunity.
Targeting LCs with TLR3 agonists
Poly I:C, a synthetic double-stranded (ds) RNA that binds to TLR3, can be used to target LCs as well as BDCA-3+CD141+ myeloid DCs in the blood, LNs and other secondary lymphoid organs via their TLR3. Poly I:C can mature these DCs leading to productions of IL-12p70, IFN-α, IFN-β, and IFN-inducible chemokines, including RANTES (CCL5) and IL-6 [113]. As Poly I:C causes serious side-effects including shock, renal failure, and hypersensitivity reactions [114], Poly(I:C12U) [Ampligen®, Hemispherx] is developed by introducing unpaired bases (uracil and guanine) to create regions of mismatches for accelerated hydrolysis. Poly(I:C12U) shows markedly reduced systemic toxicity as it binds only to TLR3, while Poly I:C binds to both TLR3 and melanoma differentiation-association antigen-5 [115]. Poly(I:C12U) was as effective as Poly I:C in stimulating IL-12 secretion in mature DCs ex vivo [116]. In a randomized placebo-controlled double-blinded trial, HIV-positive subjects were given Poly(I:C12U) intravenously with minimal side-effects [117]. Significantly, fewer treated subjects showed disease progression compared to the placebo group [117]. Poly(I:C12U) was given intravenously to treat chronic fatigue syndrome in a randomized, placebo-controlled, double-blind study. It improved the symptoms of the treated subjects [118, 119].
Poly-ICLC, a RNAse-resistant Poly I:C stabilized with poly-lysine (Hiltonol®, Oncovir), could upregulate type I and II IFNs, and activate the complement system and inflammasome signaling [120]. In malignant glioma, 38 subjects were given 2 or 3 low doses of poly-ICLC (~1–2mg) intramuscularly for 56 months with little toxicity [121]. Twenty subjects experienced tumor regression or stabilization, with the median survival of 8 years and 19 months for subjects who had anaplastic astrocytoma and malignant gliomas, respectively. In an open-label, single-arm Phase II trial, 55 subjects with recurrent supratentorial anaplastic glioma treated with Poly-ICLC (20μg/kg/intramuscularly) thrice weekly in a 4-week cycle showed a partial objective radiographic response (5 subjects) and stable diseases (18 subjects) [122]. In a Phase I/II trial, subjects with glioblastoma multiforme, anaplastic astrocytoma, oligodendroglioma and oligoastrocytoma were treated with peptide-pulsed myeloid monocyte-derived DCs [1 or 3 million/dose at 2-week intervals] and Poly-ICLC (20μg/kg/intramuscularly twice weekly) [123]. Significant upregulation of type-1 cytokines and chemokines, including IFN-α and CXCL10 was observed, and 9 subjects achieved progression-free status lasting 12 months. In a Phase I trial of 45 subjects with advanced malignancies, CDX-1401 (monoclonal anti-DEC-205 fused to full-length NY-ESO-1 injected intracutaneously to the dermal and subcutaneous layers) together with Resiquimod and/or Poly-ICLC given topically or subcutaneously [124]. Humoral and cellular responses to NY-ESO-1 were detected in the subjects, and 13 subjects had disease stabilization of a median duration of 6.7 months. Six out of 8 subjects who received anti-CTLA-4 within 3 months after CDX-1401 treatment showed objective tumor regression. Currently, 23 active cancer trials are evaluating Poly-ICLC (www.clinicaltrials.gov) and a selection was listed in Table 4.
Dermal DCs
Dermal (also called interstitial) DCs are found in the papillary dermis layer of the skin (120). Three different subsets existed – 1) BDCA-1+(CD1c+)CD11c+CD14−; 2) BDCA-1+(CD1c+)CD11c+CD14+ ([125]); and 3) BDCA-3+(CD141+)XCR1+ [126]. The CD14+ dermal DC subset expresses many C-type lectins including DC-SIGN (DC-specific intercellular adhesion molecule-3-grabbing non-integrin), LOX-1 (lectin-like oxidized LDL receptor-1), CLEC-6 (C-type lectin domain family 1 member-6), dectin-1, DCIR /CLEC4A, and TLR2, 4, 5, 6, 8 and 10 [106, 127]. They distinctly regulate B cell differentiation via the secretion of IL-12 that activates CD4+ T cells. The activated CD4+ T cells would secrete IL-21 to stimulate B cell differentiation and antibody class switching from IgM to IgG and IgA [128, 129] and produce CXCL13 to promote B cell homing to the follicular center [108]. Compare to LCs, CD14+ dermal DCs produce various cytokines including IL-1β, IL-6, IL-8, IL-10, IL-12, GM-CSF, monocyte chemoattractant protein (MCP) and transforming growth factor (TGF)-β after CD40 stimulation [108]. The recently identified BDCA-3+(CD141+)XCR1+ DCs might play a critical role in maintaining tissue homeostasis, inducing immune tolerance, and mounting responses against invading pathogens [126, 130, 131]. They could present self-antigens, produce high levels of IL-10 to activate Treg cells [126], and crosspresent soluble antigens [130, 132].
Targeting dermal DCs with TLR2, 4 and 6 agonists
Monophosphoryl lipid A (MPLA) is a chemically modified, detoxified version of the lipopolysaccharides (LPS) derived from the outer membrane of the Gram-negative Salmonella Minnesota [133–135]. Both MPLA and LPS interact with the TLR2 and 4 on CD14+ dermal DCs to induce Th1-priming cytokine secretions including IL-12p70 and IFN-γ-inducible protein-10 (IP-10). MPLA is the first and only TLR agonist used in licensed human vaccines, e.g. Cervarix® a prophylactic vaccine against HPV types 16 and 18. GlaxoSmithKline has developed a series of adjuvant systems (i.e. AS01–04) for evaluation against malaria, tuberculosis, leishmania, HIV, vesicular stomatitis virus and cancers [136]. AS01 is formulated with liposomes and MPLA for inducing antibody and CTL responses, while AS02 is an oil-in-water emulsion containing MPLA and QS-21 (a purified component of Quil-A) to stimulate strong antibody and Th1 responses. AS04 is an aqueous formulation of MPLA and alum for eliciting higher specific antibody titers with fewer injections [136]. A TLR4 agonist, amino alkyl glucosamimide phosphates, has been licensed for use in a Hepatitis B Virus vaccine (Dynavax®). Emerging TLR4 agonist, synthetic glycolipid GSK1795091 (CRX-601), is being evaluated in healthy volunteers and advanced solid tumors [Table 5]. Another TLR4 agonist, glucopyranosyl lipid A (GLA), is a synthetic derivative of the LPS lipid A tail that shows limited toxicity. GLA-stable emulsion (GLA-SE; G100), is being evaluated for intramuscular injections with NY-ESO-1 recombinant protein in metastatic cancers (NCT02015416) and MART-1 antigen peptide in resected melanoma (NCT02320305) [136]. It is also tested intratumorally in combination with radiotherapy in soft tissue sarcomas, and pembrolizumab in Follicular non-Hodgkin’s lymphoma [Table 5]. Using a human skin explant model, intradermal injection of GLA-SE or LPS enhanced the emigration of LCs and induced the maturation of both LCs and CD14– dermal DCs [137]. These activated DCs in turn stimulated a strong T cell proliferation. Hence, more studies are warranted to evaluate the use of GLA-SE intradermally in cancer treatment.
Table 5.
Use of TLR4 agonist as adjuvant in active clinical trials (*www.clinicaltrials.gov).
| Number | *NCT number and title | Cancer type(number Of subjects to enroll) |
Intervention | Trial ype | Status | Sponsor/collaborators |
|---|---|---|---|---|---|---|
| 1 |
NCT02798978 A Phase I, 2-part (Part 1 Being a Single Dose Escalation and Part2, a Parallel Group) Study of Toll-like Receptor (TLR4) Agonist (GSK1795091) in Healthy Subjects |
Neoplasms (42) | • TLR4 agonist - GSK1795091 (CRX- 601) |
• Phase I, randomized and double-blind |
• Completed, no results posted. • Sart date:10th Jan 2017 • Completion date: 13th Oct 2017 |
• GlaxoSmithKline |
| 2 |
NCT03447314 Study of a Combination of GSK1795091 and Immunotherapies in Subjects With Advanced Solid Tumors |
Neoplasms (54) | • TLR4 agonist-GSK1795091 • Humanized IgG1 anti-OX40 agonistic monoclonal antibody -GSK3174998 |
• Phase I, non-randomized, sequential assignment and open-label. |
• Recruiting. • Sart date: 26th Mar 2018 • Completion date: 14th Feb 2020 |
• GlaxoSmithKline • IQVIA |
| 3 |
NCT02180698 TLR4 Agonist GLA-SE and radiotherapy in Treating Patients With Soft Tissue Sarcoma That Is Metastatic or Cannot Be Removed by Surgery |
Stage III and IV adult Soft tissues arcoma (16) |
• TLR4 agonist–glucopyranosyl lipid A stable emulsion (GLA-SE; G100) • radiotherapy |
• Phase I, single group assignment |
• Active, not recruiting. No results posted. • Start date: 17th Nov 2014 • Completion date: 1st Mar 2018 |
• Fred Hutchinson Cancer Research Center • NCI |
| 4 |
NCT02320305 MART-1 Antigen With or Without TLR4 Agonist GLA-SE in Treating Patients With Stage II-IV Melanoma That Has Been Removed by Surgery |
Stage II-IV melanoma (23) |
• TLR4 Agonist – GLA-SE • MART-1 antigen peptide |
• Pilottrial. Randomized and open-label |
• Active, not recruiting. • Start date: 27th Jan 2015 • Completion date: 1st Jan 2019 |
• Mayo Clinic • NCI |
| 5 |
NCT02501473 Study of Intratumoral G100 With Or Without Pembrolizumab In Patients With Follicular Non-Hodgkin’s Lymphoma |
Follicular non- Hodgkin’slymphoma (51) |
• TLR4 agonist – GLA-SE (G100) • Pembrolizumab (humanized IgG4 anti-PD-1 monoclonal antibody) |
• Phase I/II, randomized and open-label |
• Active, not recruiting. • Start date: 1st June 2015 • Completion date: Mar 2019 |
• Immune Design • Merck Sharp & Dohme Corp |
Mycobacterium bovis Bacillus Calmette-Guérin (BCG), a bacterium closely related to the bacterial strain that causes tuberculosis in human, could activate DCs via TLR2, 4 and nucleotide binding oligomerization domain (NOD)-like receptor (NLR)-2 through interactions with its peptidoglycan cell wall skeleton [138]. BCG has been approved by FDA for treating bladder cancer for more than 30 years [139], as wells as an adjuvant with MPLA in an allogeneic cell-based vaccine (Melacine®) for melanoma [140]. In Phase III colorectal carcinoma trials, subjects were treated successfully with an autologous whole tumor lysate vaccine and BCG as adjuvant [141–143]. In the largest study, 412 subjects received 3 intradermal vaccinations (1×107 irradiated tumor cells/dose/week) 4 weeks after surgery together with 1×107 BCG in the first two vaccines. The magnitude of the DTH response was correlated with improved prognosis [144]. Meta-analysis of these trials showed that the treatment provided significant clinical benefits in a subset of subjects with stage II colorectal carcinoma [143]. In contrast, renal cell carcinoma subjects treated the same way developed severe reactions and skin ulceration at the injection sites [145]. The 5-year overall survival between the treated (69%) and control (78%) subjects did not reach statistical significance. No clinical benefits were observed in a Phase III renal cell carcinoma trial using the same treatment [141]. Instead, several Phase III renal cell carcinoma trials using autologous tumor cell lysate vaccine alone showed promising results [146–148]. In melanoma, subjects receiving both BCG and Imiquimod showed encouraging clinical responses [149]. These results suggested that BCG might exert different effects in different cancer types and should be investigated carefully.
Macrophage-activating lipopeptide (MALP)-2, a synthetic lipopeptide analogue of the mycoplasma cell wall lipoprotein M161Ag, could bind to both TLR2 and 6 to activate NF-κ and cytokines and chemokines synthesis in mouse [150] and human DCs [151], as well as stimulating DC maturation [152]. In a Phase I/II pancreatic carcinoma trial, 10 subjects treated with MALP-2 (20 to 30μg/intratumorally/dose) and post-operative gemcitabine showed a mean survival of 17.1±4.2 months and 2 patients remained alive 31 months post-treatment [153]. Another dual TLR2 and 6 agonist, CBLB612, is a synthetic lipoprotein that has been evaluated in a Phase II trial for protection against neutropenia while on doxorubicin and cyclophosphamide treatments in breast cancer (NCT02778763). Its anti-tumor property was not investigated in the trial. CADI-05, a novel TLR2 agonist targeting desmocollin-3 (DSC3)-expressing cancer cells, was assessed in preclinical and clinical studies. In preclinical models, CADI-05 effectively suppressed small DSC3-expressing tumors, and synergized with chemotherapy and immune checkpoint inhibitors to increase IFN-γ secreting TILs and macrophages [154, 155]. In clinical studies, CADI-05 given together with chemotherapy \in NSCLC led to improved outcome [156]. In a randomized, multicenter trial, CADI-05 was evaluated for synergistic effects with cisplatin and paclitaxel in NSCLC [157]. No significant survival benefit was observed between subjects receiving chemotherapy and chemotherapy plus CADI-05 [208 versus 196 days; hazard ratio (HR)=0.86; 95% confidence interval (CI) 0.63–1.19; P=0.3804]. However, subgroup analysis showed that squamous NSCC subjects receiving chemotherapy plus CADI-05 experienced a slight improvement in median survival by 127 days (HR=0.55; 95% CI 0.32–0.95; P=0.046). It has been shown that TLR2 ligation could promote tumor-infiltrating Treg cells and IL-10 production [158], thus raising concerns of using TLR2 agonists as cancer adjuvants. More preclinical studies are needed to clarify this observation.
BDCA-1+ and BDCA-3+ blood DCs, and targeting them with TLR8 agonists
BDCA-1+ DCs constitute the majority of the myeloid-derived DCs in the LNs, and express CD1a, CD11c, CD11b and CD172a (signal-regulatory protein [SIRP]-α). The BDCA-3+ DCs are present as a smaller population and uniquely express CLEC9A which is a C-type lectin with ITAM-like motif [159], CADM1 (cellular adhesion molecule-1; NECL2), XCR1, CD1a and CD11c. They also show distinct TLR expressions, i.e. BDCA-1+ DC express TLR1 and 6, while BDCA-3+ DCs express TLR3 and 8. The BDCA-3+ DCs could be activated by TLR3 stimulation to secrete IL-12 [131], and efficiently crosspresented antigens to CD8+ T cells [130]. Due to their low frequencies, the functions of these DCs have not been well-characterized. Nevertheless, based on their TLR expressions, they might specialize in mounting immune responses against different types of pathogens. BDCA-1+ DCs could be targeted with TLR6 agonists, while BDCA-3+ DCs could be targeted with TLR3 and 8 agonists.
A novel small molecule TLR8 agonist, VTX-2337 (Motolimod; VentiRx Pharmaceuticals, USA), could specifically stimulate TNF-α and IL-12 secretions from human myeloid DCs and monocytes. It also induced NK cells to produce IFN-γ and augmented antibody dependent cell-mediated cytotoxicity (ADCC) by rituximab and trastuzumab [160]. In a Phase I open-label trial, 38 subjects with advanced solid tumors and lymphoma were treated with ascending dose of VTX-2337 (0.1–3.9mg/m2/subcutaneously on Day 1, 5 and 8 on each 28-day cycle) [161]. Subjects who received doses of ≥0.4mg/m2 showed elevated plasma granulocyte-colony stimulating factor (G-CSF), MCP-1, macrophage inflammatory protein (MIP)-1β and TNF-α. Eight subjects experienced stable diseases (median duration of 54.5 days). VTX-2337 was also evaluated in combination with cetuximab in a Phase Ib, open-label, dose-escalation study of squamous cell carcinoma of the head and neck (SCCHN) [162]. Thirteen subjects with recurrent or metastatic SCCHN were given VTX-2337 at 2.5, 3.0 or 3.5 mg/m2 on day 1, 8 and 15 together with a fixed weekly dose of cetuximab in 28-day cycles. The maximum tolerated dose of VT-2337 was determined to be at 3.0 mg/m2. Two subjects achieved partial responses, and 5 subjects experienced stable diseases. An increased in the frequency of activated circulating NK cells were also detected. A Phase II, randomized double-blind trial was conducted to further evaluate the anti-tumor effects of VTX-2337 in a cohort of 195 SCCHN subjects [163]. The subjects received 6 chemotherapy cycles consisting of platinum (carboplatin or cisplatin), fluorouracil, cetuximab, and either placebo or VTX-2337 intravenously every 3 weeks. Then, each subject continued to receive weekly cetuximab with either placebo or VTX-2337 every 4 weeks. There were no significant differences in the progression-free survival (PFS) [6.1 versus 5.9 months; HR=0.99; 1-sided 90% CI, 0.00–1.22; P=0.47] and overall survival (OS) [13.5 versus 11.3 months; HR=0.95; 1-sided 90% CI, 0.00–1.22; P=0.4] between the VTX-2337 and the placebo-treated groups. However, a subgroup analysis revealed that HPV-positive subjects treated with VTX-2337 and not placebo showed a significantly longer PFS (7.8 versus 5.9 months; P=0.046) and OS (15.2 versus 12.6 months; P=0.03).
VTX-2337 was also being evaluated in combination with pegylated liposomal doxorubicin (PLD) in ovarian carcinomas (NCT01666444 and Table 2). In a Phase II randomized, double-blind trial, 297 subjects with recurrent epithelial ovarian carcinoma were treated with PLD (for inducing immunogenic tumor cell death) in combination with either VTX-2337 or placebo for 28-day cycles until disease progression. [164]. It was shown that PLD and VTX-2337 combination did not significantly improve the PFS [4.8 versus 5.2 months in the PLD plus placebo; log rank one-sided P=0.943, HR=1.21] and OS [18.1 versus 18.9 months in PLD plus placebo; log rank one-sided P=0.923, HR=1.22] of the treated subjects. Evaluation of TILs, TLR8 single-nucleotide polymorphisms, autoantibody biomarkers, BRCA and DNA repair gene mutation status did not correlate with the PFS and OS of the subjects. Interestingly, significantly longer OS was observed in subjects who experienced an injection site reaction (ISR) after VTX-2337 treatment compared to the subjects who did not experience an ISR after VTX-2337 treatment (19.8 versus 13.3 months; P=0.067). Further analysis also revealed that subjects who had higher baseline responses of IFN-γ (P=0.049), TNF-α (P=0.041), or IL-12p40 (P=0.024) to ex vivo VTX-2337 stimulation of their PBMCs prior to VTX-2337 treatment survived longer than subjects who had low baseline responses of these cytokines prior to VTX-2337 treatment. These data suggested that the immune fitness of the cancer subjects (e.g. the presence of a strong pre-existing immunity against the tumor) might be important in the VTX-2337 treatment outcome. Additionally, viral infection in cancer might create a proinflammatory milieu that enhances VTX-2337 treatment (e.g. prolonged PFS of HPV-positive SCCHN subjects as described earlier). These data underscore the complexity of the immune responses of cancer patients, and screening methods could be tailored to help select suitable subjects for future VTX-2337 cancer studies. Another novel benzazepine TLR8 agonist, VTX-294, was found to be more potent than MPLA, R848 or CL075 (a synthetic TLR7/8 agonist) in inducing TNF-α and IL-1β production from newborn cord blood and upregulated HLA-DR and CD86 in newborn monocyte-derived DCs [165]. Production of these cytokines was further enhanced when VTX-294 was combined with MPLA, thus suggesting that VTX-294 is suitable as a vaccine adjuvant in neonates.
Conclusions
In vivo cancer vaccination could be achieved via in vivo targeting of DCs with different synthetic TLR agonists in different tissue compartments to elicit the type of immune response desired. Several synthetic TLR agonists such as Imiquimod, CpG ODNs, MPLA and BCG have been evaluated in the clinics and showed promising results against cancers. Several novel TLR agonists have also been developed, and are currently being tested in cancer clinical trials. Different TLR agonists could potentially synergized to increase the overall efficacy of the treatment. In vivo targeting of DCs with TLR agonists could serve as a low-cost alternative or even complement existing immunotherapies such as immune checkpoint blockade antibodies. Indeed, several ongoing clinical trials are evaluating different TLR agonists in combination with anti-PD-1, anti-CTLA-4 and anti-PD-L1 for synergistic anti-tumor effects.
Highlights.
The next-generation of cancer immunotherapy could entail in vivo cancer vaccination to elicit both innate and adaptive anti-tumor responses.
This could be achieved via in vivo targeting of dendritic cells (DCs) that serve as an indispensable link between the innate and adaptive immunities.
Toll-like receptors (TLRs) expressed on human DCs play an important role in recognizing and eliminating pathogens. In vivo targeting of DCs with TLR agonists such as Imiquimod, CpG-oligodeoxynucleotides (CpG ODNs), polyinosinic-polycytidylic acid (Poly I:C) and monophosphoryl lipid A (MPLA) have shown promise in preclinical and clinical cancer studies.
Different TLR agonists could synergize to exert profound effects on the quality and magnitude of anti-tumor T cell responses.
Synthetic TLR agonists could be synthesized at a low cost and could potentially be a cost effective stand-alone treatment or complimentary to immune checkpoint blockade therapy.
Acknowledgments
This work was supported by National Cancer Institute P01-CA83638 Ovarian Specialized Program of Research Excellence (SPORE), the Immunotherapy Initiative for Ovarian Cancer (ITI-OC), and the Markus Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declared no conflict of interest.
Formatted references
- 1.Kushwah R, Hu J Complexity of dendritic cell subsets and their function in the host immune system. Immunology 2011;133:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu L, Liu Y-J Development of dendritic-cell lineages. Immunity 2007;26:741–750. [DOI] [PubMed] [Google Scholar]
- 3.Chiang CL, Hagemann AR, Leskowitz R, Mick R, Garrabrant T, Czerniecki BJ et al. Day-4 myeloid denritic cells pulsed with whole tumor lysate are highly immunogenic and elicit potent anti-tumor responses. PloS ONE 2011;6:e28732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang CL, Kandalaft L, Tanyi JL, Hagemann AR, Motz GT, Svoronos N et al. A Dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res 2013;19:4801–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ et al. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother 2010;59:1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer S, Hangel D, Yu P Immunobiology of toll-like receptors in allergic disease. Immunobiology 2007;212:521–233. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Kaisho T, Akira S Toll-like receptors. Annu Rev Immunol 2003;21:335–376. [DOI] [PubMed] [Google Scholar]
- 8.Tsuneyasu K, Shizuo A Regulation of dendritic cell function through toll-like receptors. Curr Mol Med 2003;3:373–385. [DOI] [PubMed] [Google Scholar]
- 9.Hochrein H, O’Keeffe M Dendritic cell subsets and toll-like receptors. Handb Exp Pharmacol 2008;183:153–179. [DOI] [PubMed] [Google Scholar]
- 10.Ueta M, Hamuro J, Kiyono H, Kinoshita S Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem Biophys Res Commun 2005;331:285–294. [DOI] [PubMed] [Google Scholar]
- 11.Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol 2006;176:1733–1740. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol 2003;171:3154–3162. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun 2002;293:1364–1369. [DOI] [PubMed] [Google Scholar]
- 14.Hewson CA, Jardine A, Edwards MR, Laza-Stanca V, Johnston SL Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J Virol 2005;79:12273–12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med 2006;203:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulendran B Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol 2009;9:741–747. [DOI] [PubMed] [Google Scholar]
- 17.Eckert F, Schmi U Identification of plasmacytoid t cells in lymphoid hyperplasia of the skin. Arch Dermatol 1989;125:1518–1524. [PubMed] [Google Scholar]
- 18.Wollenberg A, Wagner M, Gunther S, Towarowski A, Tuma E, Moderer M et al. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol 2002;119:1096–1102. [DOI] [PubMed] [Google Scholar]
- 19.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, Liu Y-J The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 1997;185:1101–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S et al. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999;284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 21.Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol 1998;161:3042–3049. [PubMed] [Google Scholar]
- 22.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol 2003;4:1009–1015. [DOI] [PubMed] [Google Scholar]
- 23.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 2001;14:461–470. [DOI] [PubMed] [Google Scholar]
- 24.Jego G, Palucka AK, Blanck J-P, Chalouni C, Pascual V, Banchereau J Plasmacytoid dendritic cells induce plasma cell differentiation through Type I interferon and interleukin 6. Immunity 2003;19:225–234. [DOI] [PubMed] [Google Scholar]
- 25.Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol 2008;9:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardi VC, Khaiboullina SF, Rizvanov AA Plasmacytoid dendritic cells, a role in neoplastic prevention and progression. Eur J Clin Invest 2015;45:1–8. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel J, Bekisch B, Uerlich M, Haller O, Bieber T, Tüting T Type I interferon–associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesions. Am J Clin Pathol 2005;124:37–48. [DOI] [PubMed] [Google Scholar]
- 28.Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL et al. Plasmacytoid dendritic cells prime IFN-γ-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol 2003;33:1052–1062. [DOI] [PubMed] [Google Scholar]
- 29.Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res 2013;73:4629–4640. [DOI] [PubMed] [Google Scholar]
- 30.Tel J, Aarntzen EHJG, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res 2013;73:1063–1075. [DOI] [PubMed] [Google Scholar]
- 31.Treilleux I, Blay J-Y, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla J-P et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res 2004;10:7466–7474. [DOI] [PubMed] [Google Scholar]
- 32.Faith A, Peek E, McDonald J, Urry Z, Richards DF, Tan C et al. Plasmacytoid dendritic cells from human lung cancer draining lymph nodes induce Tc1 responses. Am J Respir Cell Mol Biol 2007;36:360–367. [DOI] [PubMed] [Google Scholar]
- 33.Bontkes HJ, Ruizendaal JJ, Kramer D, Meijer CJLM, Hooijberg E Plasmacytoid dendritic cells are present in cervical carcinoma and become activated by human papillomavirus type 16 virus-like particles. Gynecol Oncol 2005;96:897–901. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch I, Caux C, Hasan U, Bendriss-Vermare N, Olive D Impaired Toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends Immunol 2010;31:391–397. [DOI] [PubMed] [Google Scholar]
- 35.Vermi W, Bonecchi R, Facchetti F, Bianchi D, Sozzani S, Festa S et al. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol 2003;200:255–268. [DOI] [PubMed] [Google Scholar]
- 36.Francesca F, Gizzi S, Mosci P, Grohmann U, Puccetti P Tryptophan catabolism in IDO+ plasmacytoid dendritic cells. Curr Drug Metab 2007;8:209–216. [DOI] [PubMed] [Google Scholar]
- 37.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pachéco Y et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol 2007;178:2763–2769. [DOI] [PubMed] [Google Scholar]
- 38.Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B et al. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res 2003;63:6478–6487. [PubMed] [Google Scholar]
- 39.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res 2005;65:5020–5026. [DOI] [PubMed] [Google Scholar]
- 40.Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med 2001;7:1339–1346. [DOI] [PubMed] [Google Scholar]
- 41.Gerlini G, Di Gennaro P, Mariotti G, Urso C, Chiarugi A, Pimpinelli N et al. Indoleamine 2,3-dioxygenase+ cells correspond to the BDCA2+ plasmacytoid dendritic cells in human melanoma sentinel nodes. J Invest Dermatol 2010;130:898–901. [DOI] [PubMed] [Google Scholar]
- 42.Ito T, Yang M, Wang Y-H, Lande R, Gregorio J, Perng OA et al. Plasmacytoid dendritic cells prime IL-10–producing T regulatory cells by inducible costimulator ligand. J Exp Med 2007;204:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G et al. Plasmacytoid dendritic cell–specific receptor ILT7–FcεRIγ inhibits Toll-like receptor–induced interferon production. J Exp Med 2006;203:1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swiecki M, Colonna M Accumulation of plasmacytoid DC: Roles in disease pathogenesis and targets for immunotherapy. Eur J Immunol 2010;40:2094–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saadeh D, Kurban M, Abbas O Plasmacytoid dendritic cell role in cutaneous malignancies. J Dermatol Sci 2016;83:3–9. [DOI] [PubMed] [Google Scholar]
- 46.Swiecki M, Colonna M The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 2015;15:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urosevic M, Dummer R, Conrad C, Beyeler M, Laine E, Burg G et al. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst 2005;97:1143–1153. [DOI] [PubMed] [Google Scholar]
- 48.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med 2007;204:1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf IH, Kodama K, Cerroni L, Kerl H Nature of inflammatory infiltrate in superficial cutaneous malignancies during topical imiquimod treatment. Am J Dermatopathol 2007;29:237–241. [DOI] [PubMed] [Google Scholar]
- 50.Turza K, Dengel LT, Harris RC, Patterson JW, White K, Grosh WW et al. Effectiveness of imiquimod limited to dermal melanoma metastases, with simultaneous resistance of subcutaneous metastasis. J Cutan Pathol 2010;37:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schulze HJ, Cribier B, Requena L, Reifenberger J, Ferrándiz C, Garcia Diez A. et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from a randomized vehicle-controlled phase III study in Europe. Br J Dermatol 2005;152:939–947. [DOI] [PubMed] [Google Scholar]
- 52.Ondo AL, Mings SM, Pestak RM, Shanler SD Topical combination therapy for cutaneous squamous cell carcinoma in situ with 5-fluorouracil cream and imiquimod cream in patients who have failed topical monotherapy. J Am Acad Dermatol 2006;55:1092–1094. [DOI] [PubMed] [Google Scholar]
- 53.Moore RA, Edwards J, Hopwood J, Hicks D Imiquimod for the treatment of genital warts: a quantitative systematic review. BMC Infect Dis 2001;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 2003;4:694–709. [DOI] [PubMed] [Google Scholar]
- 55.Mouriès J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood 2008;112:3713–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams S, O’Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol 2008;181:776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A 2007;104:8947–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Seters M, van Beurden M, ten Kate FJW, Beckmann I, Ewing PC, Eijkemans MJC et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med 2008;358:1465–1473. [DOI] [PubMed] [Google Scholar]
- 59.Adams S, Kozhaya L, Martiniuk F, Meng T-C, Chiriboga L, Liebes L et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res 2012;18:6748–6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salazar LG, Lu H, Reichow JL, Childs JS, Coveler AL, Higgins DM et al. Topical imiquimod plus nab-IFN for breast cancer cutaneous metastases: a phase 2 clinical trial. JAMA Oncol 2017;3:969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witt PL, Ritch PS, Reding D, McAuliffe TL, Westrick L, Grossberg SE et al. Phase I trial of an oral immunomodulator and interferon inducer in cancer patients. Cancer Res 1993;53:5176–5180. [PubMed] [Google Scholar]
- 62.Schön MP, Schön M TLR7 and TLR8 as targets in cancer therapy. Oncogene 2008;27:190–199. [DOI] [PubMed] [Google Scholar]
- 63.Smits EL, Ponsaerts P, Berneman ZN, Van Tendeloo VF The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist 2008;13:859–875. [DOI] [PubMed] [Google Scholar]
- 64.Meyer T, Stockfleth E Clinical investigations of Toll-like receptor agonists. Expert Opin Investig Drugs 2008;17:1051–1065. [DOI] [PubMed] [Google Scholar]
- 65.Dudek AZ, Yunis C, Harrison LI, Kumar S, Hawkinson R, Cooley S et al. First in human phase I trial of 852A, a novel systemic toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer. Clin Cancer Res 2007;13:7119–7125. [DOI] [PubMed] [Google Scholar]
- 66.Dummer R, Hauschild A, Becker JC, Grob J-J, Schadendorf D, Tebbs V et al. An exploratory study of systemic administration of the toll-like receptor-7 agonist 852A in patients with refractory metastatic melanoma. Clin Cancer Res 2008;14:856–864. [DOI] [PubMed] [Google Scholar]
- 67.Chang BA, Cross JL, Najar HM, Dutz JP Topical resiquimod promotes priming of CTL to parenteral antigens. Vaccine 2009;27:5791–5799. [DOI] [PubMed] [Google Scholar]
- 68.Rook AH, Gelfand JM, Wysocka M, Troxel AB, Benoit B, Surber C et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood 2015;126:1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sabado RL, Pavlick A, Gnjatic S, Cruz CM, Vengco I, Hasan F et al. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high-risk melanoma. Cancer Immunol Res 2015;3:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szeimies RM, Bichel J, Ortonne JP, Stockfleth E, Lee J, Meng TC A phase II dose-ranging study of topical resiquimod to treat actinic keratosis. Br J Dermatol 2008;159:205–210. [DOI] [PubMed] [Google Scholar]
- 71.Ma F, Zhang J, Zhang J, Zhang C The TLR7 agonists imiquimod and gardiquimod improve DC-based immunotherapy for melanoma in mice. Cell Mol Immunol 2010;7:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber A, Zimmermann C, Mausberg AK, Kieseier BC, Hartung HP, Hofstetter HH Induction of pro-inflammatory cytokine production in thymocytes by the immune response modifiers Imiquimod and Gardiquimod™. Int Immunopharmacol 2013;17:427–431. [DOI] [PubMed] [Google Scholar]
- 73.Le Hello C, Cohen P, Bousser MG, Letellier P, Guillevin L Suspected hepatitis B vaccination related vasculitis. J Rheumatol 1999;26:191–194. [PubMed] [Google Scholar]
- 74.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995;374:546–549. [DOI] [PubMed] [Google Scholar]
- 75.Takeshita F, Leifer CA, Gursel I, Ishii KJ, Takeshita S, Gursel M et al. Cutting Edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol 2001;167:3555–3558. [DOI] [PubMed] [Google Scholar]
- 76.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000;408:740–745. [DOI] [PubMed] [Google Scholar]
- 77.Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A 2001;98:9237–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol 2003;170:4465–4475. [DOI] [PubMed] [Google Scholar]
- 79.Hartmann G, Weiner GJ, Krieg AM CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci U S A 1999;96:9305–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol 2001;31:3388–3393. [DOI] [PubMed] [Google Scholar]
- 81.Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev 2008;60:795–804. [DOI] [PubMed] [Google Scholar]
- 82.Krieg AM CpG Motifs in bacterial DNA and their immune effects. Annu Rev Immunol 2002;20:709–760. [DOI] [PubMed] [Google Scholar]
- 83.Liu HM, Newbrough SE, Bhatia SK, Dahle CE, Krieg AM, Weiner GJ Immunostimulatory CpG oligodeoxynucleotides enhance the immune response to vaccine strategies involving granulocyte-macrophage colony-stimulating factor. Blood 1998;92:3730–3736. [PubMed] [Google Scholar]
- 84.Kochenderfer JN, Chien CD, Simpson JL, Gress RE Synergism between CpG-containing oligodeoxynucleotides and IL-2 causes dramatic enhancement of vaccine-elicited CD8+ T Cell responses. J Immunol 2006;177:8860–8873. [DOI] [PubMed] [Google Scholar]
- 85.Sandler AD, Chihara H, Kobayashi G, Zhu X, Miller MA, Scott DL et al. CpG oligonucleotides enhance the tumor antigen-specific immune response of a granulocyte macrophage colony-stimulating factor-based vaccine strategy in neuroblastoma. Cancer Res 2003;63:394–399. [PubMed] [Google Scholar]
- 86.Li J, Song W, Czerwinski DK, Varghese B, Uematsu S, Akira S et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol 2007;179:2493–2500. [DOI] [PubMed] [Google Scholar]
- 87.Ishii KJ, Takeshita F, Gursel I, Gursel M, Conover J, Nussenzweig A et al. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA–induced immune activation. J Exp Med 2002;196:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 2002;168:4531–4537. [DOI] [PubMed] [Google Scholar]
- 89.Gürsel M, Verthelyi D, Gürsel I, Ishii KJ, Klinman DM Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotide. J Leukoc Biol 2002;71:813–820. [PubMed] [Google Scholar]
- 90.Wooldridge JE, Weiner GJ CpG DNA and cancer immunotherapy: orchestrating the antitumor immune response. Curr Opin Oncol 2003;15:440–445. [DOI] [PubMed] [Google Scholar]
- 91.Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol 2004;34:251–262. [DOI] [PubMed] [Google Scholar]
- 92.Samulowitz U, Weber M, Weeratna R, Uhlmann E, Noll B, Krieg AM et al. A novel class of immune-stimulatory CpG oligodeoxynucleotides unifies high potency in type I interferon induction with preferred structural properties. Oligonucleotides 2010;20:93–101. [DOI] [PubMed] [Google Scholar]
- 93.Kuramoto E, Yano O, Kimura Y, Baba M, Makino T, Yamamoto S et al. Oligonucleotide sequences required for natural killer cell activation. Jpn J Cancer Res 1992;83:1128–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krieg AM Development of TLR9 agonists for cancer therapy. J Clin Invest 2007;117:1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Cesare M, Sfondrini L, Campiglio M, Sommariva M, Bianchi F, Perego P et al. Ascites regression and survival increase in mice bearing advanced-stage human ovarian carcinomas and repeatedly treated intraperitoneally with CpG-ODN. J Immunother 2010;33:8–15. [DOI] [PubMed] [Google Scholar]
- 96.De Cesare M, Calcaterra C, Pratesi G, Gatti L, Zunino F, Mènard S et al. Eradication of ovarian tumor xenografts by locoregional administration of targeted immunotherapy. Clin Cancer Res 2008;14:5512–5518. [DOI] [PubMed] [Google Scholar]
- 97.Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-Class CpG oligodeoxynucleotide TLR9 agonist. J Immunother 2004;27:460–471. [DOI] [PubMed] [Google Scholar]
- 98.Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 2005;115:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fourcade J, Kudela P, Andrade Filho P.A., Janjic B, Land SR, Sander C et al. Immunization with analog peptide in combination with CpG and Montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother 2008;31:781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karbach J, Gnjatic S, Bender A, Neumann A, Weidmann E, Yuan J et al. Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide® ISA-51: association with survival. Int J Cancer 2010;126:909–918. [DOI] [PubMed] [Google Scholar]
- 101.Molenkamp BG, Sluijter BJ, van Leeuwen PA, Santegoets SJ, Meijer S, Wijnands PG et al. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res 2008;14:4532–4542. [DOI] [PubMed] [Google Scholar]
- 102.Wang D, Jiang W, Zhu F, Mao X, Agrawal S Modulation of the tumor microenvironment by intratumoral administration of IMO-2125, a novel TLR9 agonist, for cancer immunotherapy. Int J Oncol 2018;53:1193–1203. [DOI] [PubMed] [Google Scholar]
- 103.Sfondrini L, Rossini A, Besusso D, Merlo A, Tagliabue E, Mènard S et al. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J Immunol 2006;176:6624–6630. [DOI] [PubMed] [Google Scholar]
- 104.Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D et al. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res 2004;64:5850–5860. [DOI] [PubMed] [Google Scholar]
- 105.Valladeau J, Saeland S Cutaneous dendritic cells. Semin Immunol 2005;17:273–283. [DOI] [PubMed] [Google Scholar]
- 106.Flacher V, Bouschbacher M, Verronèse E, Massacrier C, Sisirak V, Berthier-Vergnes O et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and gram-positive bacteria. J Immunol 2006;177:7959–7967. [DOI] [PubMed] [Google Scholar]
- 107.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhé C, Perrin P, Romani N et al. Dynamics and function of Langerhans Cells In vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans Cells. Immunity 2005;22:643–654. [DOI] [PubMed] [Google Scholar]
- 108.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity 2008;29:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA IL-15/IL-15Rα-mediated avidity maturation of memory CD8+ T cells. Proc Nat Acad Sci USA 2004;101:15154–15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carralot JP, Probst J, Hoerr I, Scheel B, Teufel R, Jung G et al. Polarization of immunity induced by direct injection of naked sequence-stabilized mRNA vaccines. Cell Mol Life Sci 2004;61:2418–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol 2005;174:3798–807. [DOI] [PubMed] [Google Scholar]
- 112.Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res 2003;63:2127–2133. [PubMed] [Google Scholar]
- 113.Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJ, Brand A et al. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol 1999;163:57–61. [PubMed] [Google Scholar]
- 114.Robinson RA, DeVita VT, Levy HB, Baron S, Hubbard SP, AS L A phase I-II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patients with leukemia or solid tumors. J Natl Cancer Inst 1976;57:599–602. [DOI] [PubMed] [Google Scholar]
- 115.Gowen BB, Wong MH, Jung KH, Sanders AB, Mitchell WM, Alexopoulou L et al. TLR3 is essential for the induction of protective immunity against Punta Toro Virus infection by the double-stranded RNA (dsRNA), poly(I:C12U), but not Poly(I:C): differential recognition of synthetic dsRNA molecules. J Immunol 2007;178:5200–5208. [DOI] [PubMed] [Google Scholar]
- 116.Navabi H, Jasani B, Reece A, Clayton A, Tabi Z, Donninger C et al. A clinical grade poly I:C-analogue (Ampligen®) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine 2009;27:107–115. [DOI] [PubMed] [Google Scholar]
- 117.Thompson KA, Strayer DR, Salvato PD, Thompson CE, Klimas N, Molavi A et al. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:PolyC12U in the treatment of HIV infection. Eur J Clin Microbiol Infect Dis 1996;15:580–587. [DOI] [PubMed] [Google Scholar]
- 118.Strayer DR, Carter WA, Brodsky I, Cheney P, Peterson D, Salvato P et al. A controlled clinical trial with a specifically configured RNA drug, poly(I).poly(C12U), in chronic fatigue syndrome. Clin Infect Dis 1994;18:S88–S95. [DOI] [PubMed] [Google Scholar]
- 119.Thompson KA, Strayer DR, Salvato PD, Thompson CE, Klimas N, Molavi A et al. Results of a double-blind placebo-controlled study of the double-stranded RNA drug polyI:PolyC12U in the treatment of HIV infection. Eur J Clin Microbiol Infect Dis 1996;15:580–587. [DOI] [PubMed] [Google Scholar]
- 120.Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med 2011;208:2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salazar AM, Levy HB, Ondra S, Kende M, Scherokman B, Brown D et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery 1996;38:1096–1104. [PubMed] [Google Scholar]
- 122.Butowski N, Lamborn K, Lee B, Prados M, Cloughesy T, DeAngelis L et al. A North American brain tumor consortium phase II study of poly-ICLC for adult patients with recurrent anaplastic gliomas. J Neurooncol 2009;91:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE et al. Induction of CD8+ T-cell responses against novel glioma–associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 2011;29:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med 2014;6:232ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zaba LC, Krueger JG and Lowes MA Resident and “Inflammatory” dendritic cells in human skin. J Invest Dermatol 2008;129:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chu C-C, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med 2012;209:935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB Cutting Edge: Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol 2007;178:1986–1990. [DOI] [PubMed] [Google Scholar]
- 128.Kuchen S, Robbins R, Sims GP., Sheng C, Phillips TM, Lipsky PE et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T Cell-B cell collaboration. J Immunol 2007;179:5886–5896 [DOI] [PubMed] [Google Scholar]
- 129.Rasheed MAU, Latner DR, Aubert RD, Gourley T, Spolski R, Davis CW et al. IL-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J Virol 2013;87:7737–7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 2012;37:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010;207:1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Villadangos JA, Shortman K Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med 2010;207:1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baldridge JR, Crane RT Monophosphoryl Lipid A (MPL) formulations for the next generation of vaccines. Methods 1999;19:103–107. [DOI] [PubMed] [Google Scholar]
- 134.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007;316:1628–1632. [DOI] [PubMed] [Google Scholar]
- 135.Martin M, Michalek SM, Katz J Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun 2003;71:2498–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Garçon N, Van Mechelen M Recent clinical experience with vaccines using MPL- and QS-21-containing Adjuvant Systems. Expert Rev Vaccines 2011;10:471–486. [DOI] [PubMed] [Google Scholar]
- 137.Schneider LP, Schoonderwoerd AJ, Moutaftsi M, Howard RF, Reed SG, de Jong EC et al. Intradermally administered TLR4 agonist GLA-SE enhances the capacity of human skin DCs to activate T cells and promotes emigration of Langerhans cells. Vaccine 2012;30:4216–4224. [DOI] [PubMed] [Google Scholar]
- 138.Uehori J, Matsumoto M, Tsuji S, Akazawa T, Takeuchi O, Akira S et al. Simultaneous blocking of human toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by mycobacterium bovis Bacillus Calmette-Guerin peptidoglycan. Infect Immun 2003;71:4238–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brandau S, Suttmann H Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: A success story with room for improvement. Biomed Pharmacother 2007;61:299–305. [DOI] [PubMed] [Google Scholar]
- 140.Sosman JA, Sondak VK Melacine®: an allogeneic melanoma tumor cell lysate vaccine. Expert Rev Vaccines 2003;2:353–368. [DOI] [PubMed] [Google Scholar]
- 141.Hoover HC Jr., Brandhorst JS, Peters LC, Surdyke MG, Takeshita Y, Madariaga J et al. Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J Clin Oncol 1993;11:390–399. [DOI] [PubMed] [Google Scholar]
- 142.Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet 1999;353:345–350. [DOI] [PubMed] [Google Scholar]
- 143.Hanna MG Jr., Hoover HC Jr., Vermorken JB, Harris JE, Pinedo HM Adjuvant active specific immunotherapy of stage II and stage III colon cancer with an autologous tumor cell vaccine: first randomized phase III trials show promise. Vaccine 2001;19:2576–2582. [DOI] [PubMed] [Google Scholar]
- 144.Harris JE, Ryan L, Hoover HC Jr, Stuart RK, Oken MM, Benson AB et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol 2000;18:148–157. [DOI] [PubMed] [Google Scholar]
- 145.Galligioni E, Quaia M, Merlo A, Carbone A, Spada A, Favaro D et al. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and Bacillus Calmette-Guèrin: five-year results of a prospective randomized study. Cancer 1996;77:2560–2566. [DOI] [PubMed] [Google Scholar]
- 146.Doehn C, Richter A, Lehmacher W, Jocham D Adjuvant autologous tumour cell-lysate vaccine versus no adjuvant treatment in patients with M0 renal cell carcinoma after radical nephrectomy: 3-year interim analysis of a German multicentre phase-III trial. Folia Biol (Praha) 2003;49:69–73. [PubMed] [Google Scholar]
- 147.Repmann R, Goldschmidt AJ, Richter A Adjuvant therapy of renal cell carcinoma patients with an autologous tumor cell lysate vaccine: a 5-year follow-up analysis. Anticancer Res 2003;23:969–74. [PubMed] [Google Scholar]
- 148.Jocham D, Richter A, Hoffmann L, Iwig K, Fahlenkamp D, Zakrzewski G et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet 2004;363:594–599. [DOI] [PubMed] [Google Scholar]
- 149.Kidner TB, Morton DL, Lee DJ, Hoban M, Foshag LJ, Turner RR et al. Combined intralesional Bacille Calmette-Guérin (BCG) and topical imiquimod for in-transit melanoma. J Immunother 2012;35:716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Deiters U, Mühlradt PF Mycoplasmal lipopeptide MALP-2 induces the chemoattractant proteins macrophage inflammatory protein-1alpha (MIP-1alpha), monocyte chemoattractant protein 1, and MIP-2 and promotes leukocyte infiltration in mice. Infect Immun 1999;67:3390–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kaufmann A, Mühlradt PF, Gemsa D, Sprenger H Induction of cytokines and chemokines in human monocytes by mycoplasma fermentans-derived lipoprotein MALP-2. Infect Immun 1999;67:6303–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schneider C, Schmidt T, Ziske C, Tiemann K, Lee K-M, Uhlinsky V et al. Tumour suppression induced by the macrophage activating lipopeptide MALP-2 in an ultrasound guided pancreatic carcinoma mouse model. Gut 2004;53:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schmidt J, Welsch T, Jäger D, Mühlradt PF, Büchler MW, Märten A Intratumoural injection of the toll-like receptor-2/6 agonist ‘macrophage-activating lipopeptide-2’ in patients with pancreatic carcinoma: a phase I/II trial. Br J Cancer 2007;97:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ahmad F, Mani J, Kumar P, Haridas S, Upadhyay P, Bhaskar S Activation of Anti-Tumor Immune Response and Reduction of Regulatory T Cells with Mycobacterium indicus pranii (MIP) Therapy in Tumor Bearing Mice. PloS ONE 2011;6:e25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Banerjee S, Halder K, Ghosh S, Bose A, Majumdar S The combination of a novel immunomodulator with a regulatory T cell suppressing antibody (DTA-1) regress advanced stage B16F10 solid tumor by repolarizing tumor associated macrophages in situ. OncoImmunology 2015;4:e995559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pant MC, Chakraborty BS, Patel N, Bisht SS, Verma VP, Singh S et al. P3–022: Role of Cadi-05 as an adjuvant therapy in advanced Non Small Cell Lung Cancer. J Thor Oncol 2007;2:S616. [Google Scholar]
- 157.Belani CP, Chakraborty BC, Modi RI, Khamar BM A randomized trial of TLR-2 agonist CADI-05 targeting desmocollin-3 for advanced non-small-cell lung cancer. Ann Oncol 2017;28:298–304. [DOI] [PubMed] [Google Scholar]
- 158.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP et al. Toll-like receptor 2–dependent induction of vitamin A–metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med 2009;15:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huysamen C, Willment JA, Dennehy KM, Brown GD CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem 2008;283:16693–16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lu H, Dietsch GN, Matthews MA, Yang Y, Ghanekar S, Inokuma M et al. VTX-2337 is a novel TLR8 agonist that activates NK Cells and augments ADCC. Clin Cancer Res 2012;18:499–509. [DOI] [PubMed] [Google Scholar]
- 161.Northfelt DW, Ramanathan RK, Cohen PA, Von Hoff DD, Weiss GJ, Dietsch GN et al. A phase I dose-finding study of the novel toll-like receptor 8 agonist VTX-2337 in adult subjects with advanced solid tumors or lymphoma. Clin Cancer Res 2014;20:3683–3691. [DOI] [PubMed] [Google Scholar]
- 162.Chow LQM, Morishima C, Eaton KD, Baik CS, Goulart BH, Anderson LN et al. Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin Cancer Res 2017; 23:2442–2450. [DOI] [PubMed] [Google Scholar]
- 163.Ferris RL, Saba NF, Gitlitz BJ, Haddad R, Sukari A, Neupane P et al. Effect of adding motolimod to standard combination chemotherapy and cetuximab treatment of patients with squamous cell carcinoma of the head and neck: The active8 randomized clinical trial. JAMA Oncol 2018; doi: 10.1001/jamaoncol.2018.1888 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 164.Monk BJ, Brady MF, Aghajanian C, Lankes HA, Rizack T, Leach J et al. A phase 2, randomized, double-blind, placebo-controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: a Gynecologic Oncology Group partners study. Ann Oncol 2017;28:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dowling DJ, Tan Z, Prokopowicz ZM, Palmer CD, Matthews MA, Dietsch GN et al. The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes. PLoS ONE 2013;8:e58164. [DOI] [PMC free article] [PubMed] [Google Scholar]