Abstract
Background:
Influenza immunization is universally recommended during pregnancy to protect mothers and their offspring. However, pregnancy-induced shifts in vaccine responsiveness remain poorly defined.
Methods:
Quantitative and qualitative shifts in the serological response to influenza vaccination were evaluated in healthy women throughout the course of pregnancy. Serum was obtained before and after vaccination among 71 pregnant and 67 non-pregnant women during the 2011–12 and 2012–13 influenza seasons. Serum hemagglutination inhibition (HAI) assay was used to investigate anti-influenza antibody responses by comparing pre-vaccine and post-vaccine geometric mean titers (GMTs) between groups for each antigen. IgG1, IgG2, IgG3, and IgG4 anti-influenza titers were also evaluated by enzyme-linked immunosorbent assay (ELISA). Pregnancy induced shifts in HAI titers and levels of each anti-influenza antibody isotype were evaluated using linear regression models.
Results:
Post-vaccine GMTs at day 28 were significantly reduced for women vaccinated during pregnancy for A/California (H1N1) in 2011 (p = 0.027), A/Perth (H3N2) in 2011 (p = 0.037), and B/Wisconsin in 2012 (p = 0.039). Vaccine responses progressively declined with the initiation of vaccination later in pregnancy. Anti-H1N1 IgG1, IgG2, and IgG3 titers were reduced in pregnant women compared to non-pregnant controls, and these titers declined with pregnancy progression. The most striking differences were found for anti-H1N1 IgG1, where titers decreased by approximately 7% each week throughout pregnancy.
Conclusions:
HAI responses elicited by immunization were significantly reduced during pregnancy for three different influenza vaccine antigens. Anti-H1N1 IgG1 was significantly lower in pregnant women and decreased throughout the course of pregnancy. Waning serological responsiveness to influenza vaccination with the progression of human pregnancy has important translational implications for when immunization should be optimally administered during pregnancy.
Keywords: Influenza, Influenza vaccine, Pregnancy, Maternal immunization
1. Introduction
Influenza virus vaccine is universally recommended during pregnancy to prevent adverse maternal and fetal outcomes of influenza infection, including increased maternal mortality and prematurity [1]. Maternal vaccination has been shown to confer significant reductions in influenza illness among women and their infants [2,3]. In a cohort of 340 Bangladeshi women, infants of mothers who received influenza vaccine were 63% less likely to have influenza infection [3]. These protective benefits were confirmed in a South African cohort of over 2000 pregnant women with an approximate 50% reduction in influenza disease amongst vaccinated women during pregnancy [2]. Influenza immunization during pregnancy also protects against adverse fetal and infant health outcomes, including prematurity, small for gestational age (SGA) births, and low birthweight [4–7]. A cohort analysis of U.S. surveillance data showed infants of vaccinated mothers born during the influenza season had 69% reductions in the incidence of SGA and 40% reduced rates of premature birth compared to infants of unvaccinated women [7].
Despite these efficient protective benefits of maternal influenza immunization on the health of mothers and infants, influenza virus still causes increased mortality with infection during pregnancy [8–11]. The susceptibility is not uniform throughout pregnancy, since the greatest risk of complications (e.g. pneumonia, heart failure, myocarditis) from influenza virus infection occurs during the second and third pregnancy trimesters [12,13]. Hospitalizations from influenza-related illness also progressively rise throughout pregnancy, with four-fold increased rates in the third pregnancy trimester [12,13]. Thus, pregnancy confers susceptibility to influenza virus infection, with increased risk most pronounced during the latter half of pregnancy.
It remains unclear whether waning host defense against natural influenza infection during pregnancy parallels a similarly skewed response to vaccination. This line of investigation has direct translational relevance because if pregnancy, and the progression of pregnancy, dampens responsiveness to influenza immunization, reproductive age women should be aggressively vaccinated as early as possible in each influenza season. We previously demonstrated that pregnant women had an approximately 50% reduction in hemagglutination inhibition (HAI) antibody response to influenza vaccine compared to non-pregnant controls [14], demonstrating that the vaccine has diminished protection against influenza during pregnancy. Given functional specialization of individual IgG antibody isotypes, shifts in their response to maternal immunization may further inform the reasons functional HAI antibody titers decline during pregnancy. Accordingly, the objective of this study was to evaluate both quantitative and qualitative shifts in antibody response to influenza immunization in healthy pregnant women in comparison with non-pregnant controls.
2. Materials and methods
2.1. Study participants
This prospective study enrolled 138 healthy pregnant (n = 71) and non-pregnant (n = 67) women between the ages of 18 and 39 at Cincinnati Children’s Hospital Medical Center (CCHMC). Fifty-one women were enrolled between October 3, 2011 and October 17, 2011 (Season 1), and 87 new women were enrolled between September 24, 2012 and December 11, 2012 (Season 2).
Eligibility criteria included age 18–39 years and women who did not previously receive the influenza vaccine during the study period. Participants reported their age, date of birth, race/ethnicity, medical insurance, and previous receipt of influenza vaccines. Pregnant participants also reported on gravidity, parity, last menstrual period, estimated due date, and method of calculating due date (last menstrual period versus ultrasound). Women diagnosed with cancer, immunological disorders (including autoimmune disorders and immunodeficiencies), receiving immunomodulatory therapy, or otherwise suffering from chronic disease were excluded.
2.2. Study procedures
At the enrollment visit, approximately 20 mL of blood by venipuncture was obtained prior to receipt of the 2011–2012 triva-lent influenza immunization, Fluarix® (lot number, AFLUA004BC; GlaxoSmithKline Biologicals), intramuscularly, or the 2012–2013 trivalent influenza immunization, Fluzone® (lot number, UT4470AA; Sanofi Pasteur), intradermally. These differing routes of vaccine administration were due to a policy change at the hospital where the vaccine was administered and thus were analyzed separately. The 2011–2012 vaccine included antigens similar to A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008. The 2012–2013 vaccine included antigens similar to A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2), and B/Wisconsin/1/2010 (Yamagata lineage). Participants were observed for 15 min after receipt of vaccine to monitor for adverse reactions or events. Participants returned at day 28 (range 24–31 days) post-immunization for repeat blood draws. If the participant gave birth between visits, she was asked to provide the infant’s date of birth, birth weight, gestational age, and if the infant had any history of fever (>100.4° Fahrenheit) since birth.
2.3. Hemagglutination inhibition
Hemagglutination inhibition (HAI) assay was performed on serum from day 0 (pre-vaccine) and day 28 (post-vaccine) samples from each individual by the Clinical Laboratory Improvement Amendments (CLIA) and the College of American Pathologists (CAP) certified Laboratory for Specialized Clinical Studies at CCHMC using standard methods [15]. In brief, sera were treated with Receptor-Destroying Enzyme (RDE; Denka-Seiken, Japan) to remove non-specific inhibitors of hemagglutination prior to testing. Following RDE treatment, the samples were further diluted to 1:10 in Phosphate Buffered Saline (PBS). The sera were then treated with packed red blood cells (RBCs) to remove nonspecific inhibitors. The RBCs were spun out of the sera and the samples were ready for testing. Starting at 1:10 dilution, the sera were diluted two-fold through 1:2560 in V-bottom microtiter plates.
2.4. Antibody isotype responses
Post-immunization responses were evaluated by ELISA for anti-influenza specific IgG1, IgG2, IgG3, and IgG4 isotypes. In addition to plates coated with multiple influenza antigens representing seasonal vaccines, ELISAs were also performed for all samples on plates coated with individual influenza antigens specific to the 2011–2012 and 2012–2013 vaccines (one antigen per plate) to evaluate responses to specific influenza vaccine antigens.
All serum samples were serially diluted four-fold, from 1:10 to 1:640, in dilution buffer (25 mM Tris-HCl, 150 mM NaCl, 0.01% Tween, and 10% SuperBlock™ T20 (TBS) Blocking Buffer, Thermo Scientific). All dilutions were titered for each isotype. Biotinylated mouse monoclonal antibodies to human IgG1, IgG2, IgG3 and IgG4 (clones G17–1, G18–21, HP6047, and G17–4, respectively) were used to determine isotype levels of specific antibody. Peroxidase-labelled goat anti-mouse IgG (1:5000, Bio-Rad) was used as sandwich antibody and ortho-phenylenediamine (1 tablet/10 mL OPD buffer + 3 μl H2O2) was used as substrate. The enzyme reaction was stopped with 2.5 M sulphuric acid, and the optical density at 490 nm measured with an E-max spectrophotometer (Molecular Devices Corp, CA). Titration curves (serum dilution vs. OD490) were established for each isotype for each serum, and titers were defined at the reciprocal of the serum dilution required to yield a specified OD490.
2.5. Data analysis
The sample size estimation was based on our preliminary analyses of anti-influenza HAI and IgG1 titers in pregnant and non-pregnant women. A sample size of 51 per group was estimated to have 80% power to detect at least a 50% increase in HAI or IgG1 titers using a two sample t-test with an alpha of 0.05.
Demographics were compared between pregnant and non-pregnant women using Fisher exact test and Wilcoxon-rank sum test as appropriate. A two-sample t-test was performed to compare geometric mean titers (GMT) of hemagglutination inhibition titers between pregnant and non-pregnant women before (day 0) and after influenza immunization (day 28). The geometric mean ratio (GMR), or the fold rise in HAI titers before and after immunization were calculated and compared with pregnant and non-pregnant women using a paired t-test. The percent of seroconverted (greater than or equal to a fourfold rise in titer) and seroprotected (HAI titer ≥ 1:40) participants in each group were compared using Fisher exact test. P-values <0.05 were considered statistically significant. Mean titers of individual IgG isotypes at day 28 between pregnant and non-pregnant women were evaluated using the two-sample t-test. Linear regression models were used to estimate the association between log-transformed IgG titers and gestational week. Models were adjusted for baseline titers. Statistical analysis was conducted using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA).
2.6. Study approval
Informed consent was obtained from each participant at the enrollment visit. The protocol was approved by the Institutional Review Board (IRB) of Cincinnati Children’s Hospital Medical Center. Informed consent was obtained from participants, and human experimentation guidelines of the United States Department of Health and Human Services and those of Cincinnati Children’s Hospital Medical Center were followed in the conduct of clinical research.
3. Results
Of the 138 participants enrolled in the study, 51 women were enrolled in 2011, and 87 women were enrolled in 2012. Of these 138 women, 71 were pregnant at the time of influenza immunization (13 in the first trimester, 43 in the second trimester, and 15 in the third trimester). Including all women enrolled over both seasons, pregnant and non-pregnant women had similar characteristics. When analyzed separately by influenza season, pregnant women were more likely to be Caucasian and older in 2011 (Table 1). Most participants were previously vaccinated against influenza, and pregnant and non-pregnant women were equally likely to have received influenza vaccine during the 2008–2009, 2009–2010, and 2010–2011 influenza seasons. Since all but one participant in each group received H1N1 vaccine during the A/California/7/2009 (H1N1) pandemic, antibodies to this serotype reflect a boosted response to H1N1 vaccination. Booster responses to H1N1 are expected to reflect vaccination response patterns within the general population, as H1N1 antigen has been included in all seasonal vaccines since 2010.
Table 1.
Characteristics of study participants.
| 2011 | 2012 | ||||||
|---|---|---|---|---|---|---|---|
| Pregnant (N = 29) | Not pregnant (N = 22) | P-Value | Pregnant (N = 42) | Not pregnant (N = 45) | P-value | ||
| N (%)a | N (%) | N (%) | N (%) | ||||
| Race | Caucasian | 28 (96.6) | 16(72.7) | 0.04b | 35 (83.3) | 39 (86.7) | 0.77b |
| Asian | 1 (3.45) | 2 (9.1) | 3(7.1) | 4 (8.9) | |||
| Hispanic | 0 | 2 (9.1) | 1 (2.4) | 1 (2.2) | |||
| African American | 0 | 2 (9.1) | 1 (2.4) | 1 (2.2) | |||
| Other | 0 | 0 | 2 (4.8) | 0 | |||
| Age | 32 (29,34) | 28 (26, 32) | 0.02c | 30.5 (28, 35) | 31 (27, 34) | 0.87 | |
| Weeks of gestation at enrollment | 18.3 (15.1, 27.3) | 0 | 21.29 (14.7,27.9) | 0 | |||
| Trimester at enrollment | 1 | 4 (7.8) | 0 | 9 (21.4) | 0 | ||
| 2 | 19 (37.2) | 0 | 24 (57.1) | 0 | |||
| 3 | 6 (11.8) | 0 | 9 (21.4) | 0 | |||
| Previous receipt of influenza vaccine | 2008–2009 | 22 (75.9) | 13 (59.1) | 0.2 | 21 (50) | 26 (57.8) | 0.47 |
| 2009–2010 | 25 (86.2) | 18 (81.8) | 0.71b | 23 (54.8) | 35 (77.8) | 0.02 | |
| 2010–2011 | 28 (96.6) | 21 (95.5) | 0.99b | 34 (81.0) | 40 (88.9) | 0.3 | |
| 2011–2012 | N/A | N/A | 34 (81.0) | 42 (93.3) | 0.08 | ||
| Prior receipt of H1N1 vaccine | 15 (57.7) | 15 (68.2) | 0.24 | 17 (40.5) | 24 (53.3) | 0.23 | |
Number of subjects (percent in each group).
Fisher exact test.
Wilcoxon-rank sum test, median (IQR).
3.1. Hemagglutination inhibition titers
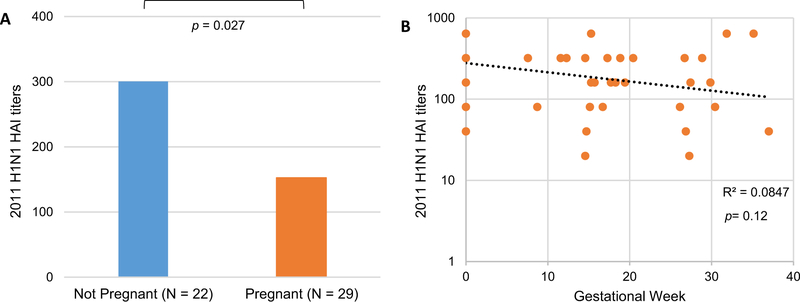
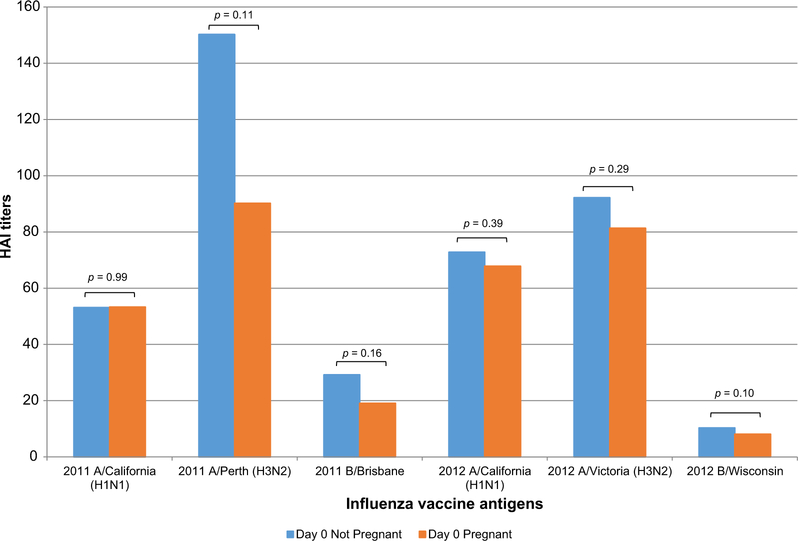
Day 28 post-vaccine HAI GMTs were approximately 50% lower in pregnant women for 2011 H1N1 (p = 0.027, Fig. 1A). Analyzing by the time during pregnancy when vaccine was administered, we found a steady decline in H1N1 HAI GMTs with the progression of pregnancy (p = 0.12, Fig. 1B). Day 28 post-vaccine HAI GMTs were also significantly lower in pregnant women for 2011 H3N2 (p = 0.037, Fig. 2A), with a 2.4% decrease with every week of gestational age (p = 0.15, Fig. 2B). Day 28 post-vaccine HAI GMTs were decreased in pregnant compared to non-pregnant women for 2012 H1N1 (Fig. 3A) and H3N2 (Fig. 3B) vaccine antigens. Prevaccine HAI GMTs were similar between pregnant and non-pregnant women for all 2011–12 and 2012–13 vaccine antigens (Fig. 4). However, though not statistically significant, the prevaccine difference in 2011 H3N2 HAI GMTs may have contributed to the higher post-vaccine HAI GMTs and IgG titers in non-pregnant women.
Fig. 1.
Reduced 2011 H1N1 hemagglutination inhibition (HAI) titers after influenza vaccination during pregnancy. 2011 H1N1 HAI geometric mean titers 28 days after influenza immunization comparing pregnant women with not pregnant controls (A), or by linear regression for pregnant women based on gestational week, with not pregnant controls at gestational week zero (B). R2 and p values are shown.
Fig. 2.
Reduced 2011 H3N2 hemagglutination inhibition (HAI) titers after influenza vaccination during pregnancy. 2011 H3N2 HAI geometric mean titers 28 days after influenza immunization comparing pregnant women with not pregnant controls (A), or by linear regression for pregnant women based on gestational week, with not pregnant controls at gestational week zero (B). R2 and p values are shown.
Fig. 3.
Reduced 2012 H1N1 and H3N2 hemagglutination inhibition (HAI) titers after influenza vaccination during pregnancy. 2012 H1N1 HAI geometric mean titers (A) and 2012 H3N2 HAI geometric mean titers (B) 28 days after influenza immunization comparing pregnant women with not pregnant controls. p values are shown.
Fig. 4.
Hemagglutination inhibition (HAI) titers prior to influenza vaccination are similar in pregnant and not pregnant women. HAI geometric mean titers prior to influenza vaccination in pregnant and not pregnant women for all vaccine antigens in the 2011–12 and 2012–13 vaccines. p values are shown.
Despite a range of responses varying by vaccine antigen and influenza season, there were no differences in seroconversion (≥4-fold increase in titer) between pregnant and non-pregnant women. After receiving the 2011 vaccine containing A/California (H1N1), A/Perth (H3N2), and B/Brisbane, 10.3% (3 of 29) of pregnant and 9.1% (2 of 22) of non-pregnant participants seroconverted to H3N2, while 41.4% (12 of 29) of pregnant and 50% (11 of 22) of non-pregnant participants seroconverted to H1N1. After receiving the 2012 influenza vaccine containing A/California (H1N1), A/Victoria (H3N2), and B/Wisconsin, only 16.7% (7 of 42) of pregnant and 25% (11 of 44) of non-pregnant participants seroconverted to H1N1, while 33.3% (14 of 42) of pregnant and 40.9% (18 of 44) of non-pregnant participants seroconverted to B/Wisconsin. The percentages of participants who seroconverted generally increased when women were vaccinated with influenza antigens for the first time (2012 H3N2 and B/Wisconsin), as has been shown in other influenza vaccine studies [16,17]. This is likely due to the fact that vaccinees start with lower pre-vaccine GMTs when previously unexposed to new influenza vaccine antigens and are therefore more likely to demonstrate a 4-fold increase in titer.
There were no differences in seroprotection (HAI ≥ 1:40) between pregnant and non-pregnant women during either influenza season. Greater than 90% of pregnant and non-pregnant participants were seroprotected against 2011 and 2012 influenza A antigens (H1N1 and H3N2), but only 58.6% (17 of 29) of pregnant and 68.2% (15 of 22) of non-pregnant participants (2011B/Brisbane) and 35.7% (15 of 42) of pregnant and 43.2% (19 of 44) of non-pregnant participants (2012B/Wisconsin) were seroprotected against influenza B antigens. This lower seroprotection against influenza B antigens may reflect lower pre-vaccine GMTs (Fig. 4) [17].
3.2. IgG isotype responses
To investigate how pregnancy induced reductions in HAI titers correlated with anti-influenza IgG isotypes, titers of anti-influenza IgG1, IgG2, IgG3 and IgG4 were further evaluated for 2011 A/California (H1N1), 2011 A/Perth (H3N2), and 2012B/Brisbane, vaccine antigens with significantly reduced HAI responses for pregnant women compared with non-pregnant controls. Anti-influenza IgG1, IgG2, IgG3, and IgG4 isotype titers after influenza vaccination with 2011 A/California (H1N1), 2011 A/Perth (H3N2), and 2012B/Wisconsin antigens were compared in pregnant versus non-pregnant women (Fig. 5A–D).
Fig. 5.
Anti-influenza IgG isotype titers after influenza vaccination in pregnant and not pregnant women. IgG1 (A), IgG2 (B), IgG3 (C), and IgG4 (D) mean antibody titers at 28 days after influenza vaccination to 2011 A/California (H1N1), 2011 A/Perth (H3N2), and 2012B/Wisconsin influenza vaccine antigens.
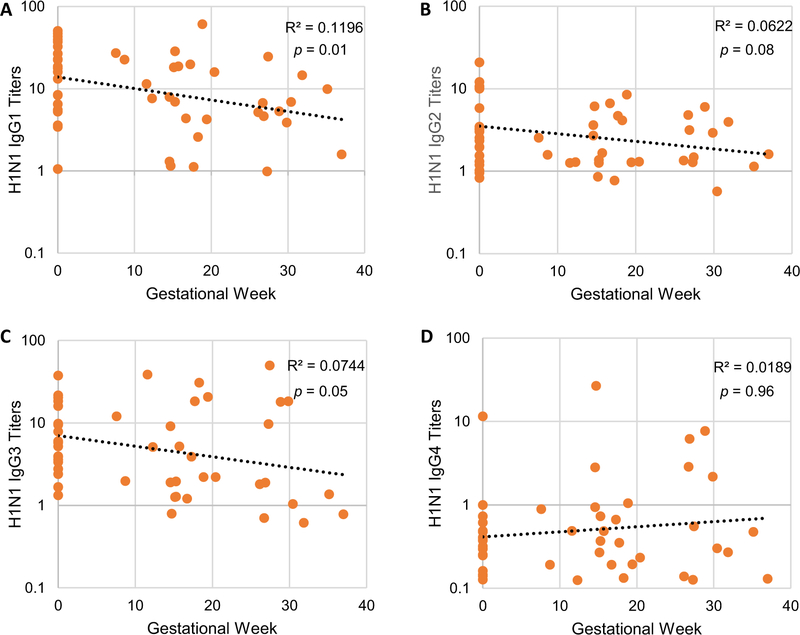
Anti-influenza IgG1 was 1.6-fold lower in pregnant compared to non-pregnant women for 2011 H1N1 antigen (p = 0.037). For every additional week of gestational age, there was a significant decline of 7.12% (p = 0.01) in anti-influenza 2011 H1N1 IgG1 titers (Fig. 6A). There were no 2011 H3N2-specific differences in antibody isotypes. However, isotype declines over time were noted for the 2011 H3N2 antigen; anti-influenza IgG1 decreased an average of 2.44% with every week of gestation (p = 0.40), and anti-influenza IgG3 decreased 5.1% (p = 0.27).
Fig. 6.
Altered 2011 H1N1 anti-influenza IgG1–4 responses after influenza vaccination during pregnancy. IgG1 (A), IgG2 (B), IgG3 (C), and IgG4 (D) mean antibody titers at 28 days after influenza vaccination comparing pregnant women based on gestational week, with non-pregnant controls at gestational week zero using linear regression models. R2 and p values are shown.
Anti-influenza IgG2 (Fig. 6B) and IgG3 (Fig. 6C) also decreased throughout pregnancy; there was a 6.6% (p = 0.05) decrease in anti-influenza 2011 H1N1 IgG3 for every additional week of gestational age. There was a slight increase in anti-influenza IgG4 as delivery approached (Fig. 6D). Pregnant and non-pregnant women had statistically similar results for anti-influenza B/Brisbane IgG1, IgG2, IgG3, and IgG4 in the 2012–13 influenza vaccine.
4. Conclusions
Protective immunity induced by influenza vaccination has historically been attributed to antibodies that recognize the viral hemagglutinin protein and neutralize viral infectivity, measured with the standardized HAI assay. While HAI titers in our analysis were significantly lower in pregnant compared to non-pregnant women for some vaccine antigens, these titers also decreased as women were vaccinated later in pregnancy with an average 4% decline with every additional week of gestational age. This suggests that pregnant women produce a diminished antibody response to some influenza antigens when vaccinated during the later stages of pregnancy, correlating with the increased rate of influenza hospitalizations and complications seen as delivery approaches [12,13].
An HAI titer of 1:40 is considered a seroprotective titer, but this titer is associated with only 50% protection against influenza infection [18]. In spite of this limitation, HAI titers are the only standardized measure of protection against influenza at present. Both pregnant and non-pregnant women in this study demonstrated HAI antibody responses above 1:40, but these HAI titers declined as women were vaccinated later in pregnancy. Decreasing HAI titers are associated with decreasing probability of clinical protection against influenza illness [19], suggesting that pregnant women vaccinated later in pregnancy will be more susceptible to influenza infection despite vaccination. This diminished HAI response may play a role in the multifactorial process that leads to increased mortality secondary to influenza infection during the later stages of pregnancy.
5. Discussion
Accumulating evidence suggests that vaccines induce protection through several additional mechanisms beyond antibody neutralization of viral hemagglutinin protein [20,21], such as antibody-mediated activation of inflammatory cells [22] and aggregation of influenza virus on the cell surface. This aggregation is responsible for reducing the amount of virus released from infected cells [23]. In humans, these unique protective mechanisms of antibodies are predominantly mediated by four individual IgG isotypes (IgG1, IgG2, IgG3, and IgG4). IgG1 and IgG3 are predominantly produced in response to viral infections and induce the aforementioned effector functions, while IgG3 stimulates potent anti-inflammatory responses [24]. We know that influenza vaccination of pregnant women induces a pro-inflammatory response less robust than but similar to infection, with significantly increased C-reactive protein and tumor necrosis factor-alpha post-vaccination [25]. This suggests that the immunological milieu associated with vaccination may mimic that of viral infections, resulting in similar alterations in isotype response. If IgG1 and IgG3 are expected to increase after vaccination but are suppressed by pregnancy, the decline in these isotypes may produce a suboptimal response to influenza vaccine. Anti-influenza IgG1, IgG2, and IgG3 responses were generally decreased in the pregnant subjects in our study, and these diminished responses were most pronounced for women vaccinated during the later stages of pregnancy.
Preclinical models show significantly higher mortality rates and a 4-fold lower hemagglutinin neutralizing antibody titers after influenza virus infection during pregnancy [26], as well as an elevated level of viral replication and cytokine imbalances that lead to a less effective immune response [27]. These data suggest that shifts in maternal antibody responses required for maintaining fetal tolerance may dampen the priming and activation of non-fetal antigen-specific immunological responses. Pregnancy is associated with reduced Th1 responses and reciprocally increased maternal Th2 and regulatory T cell (Treg) differentiation. These pregnancy-induced shifts are expected to increase IgG4 secretion at the expense of IgG1 and IgG3. IgG1 and IgG3 are the predominant IgG isotypes produced in response to viral and protein antigens [28,29], raising the possibility that these isotypes may contribute to increased anti-viral activity. IgG2 is primarily responsible for responses to bacterial capsular polysaccharide antigens, suggesting that levels of this isotype may not be affected by influenza vaccination. Alternatively, the global suppression of IgG1, IgG2, and IgG3 may diminish functional antibody response to influenza immunization of pregnant women, including HAI antibody responses. IgG4 is relatively less abundant than other isotypes [30], and differences may be more difficult to detect due to low titers for most participants. However, our results interestingly demonstrate a slight increase in IgG4 as pregnancy progresses, which may negatively impact virus opsonization and neutralization.
Pregnancy is sustained, in part, by an intricate balance between immune stimulatory and suppressive CD4+ T cell subsets at the maternal/fetal interface [31]. Production of unique effector cytokines is a hallmark feature of CD4+ T cell subset differentiation. These cytokines promote fetal survival by inhibiting maternal Th1 and cell-mediated immune responses [32,33]. Studies suggest that a shift to Th2 cytokine production decreases the risk of abortion or preterm labor and supports the maintenance of a healthy pregnancy [34]. Anti-inflammatory and Th2 cytokines at the maternal/fetal interface include IL-4 and IL-13. Treg responses are also increased in human placental trophoblasts, though their role in pregnancy is still poorly understood [35]. Our results demonstrating decreasing IgG1, IgG2, and IgG3 levels in response to influenza vaccination as pregnancy progresses suggest that these cytokine responses, which are centered in the placenta [31], affect systemic immune responses to immunogens such as influenza vaccine. Peripheral blood basal cytokine expression may affect IgG isotype switching, qualitatively decreasing antibody responses to immunization of pregnant women. Interestingly, the decreased effector functions associated with suppressed IgG iso-type responses during pregnancy would be expected to decrease protection against influenza infection in the highly vulnerable population of pregnant women.
Pregnancy induced shifts in the serological response to vaccination were most significant for the 2011 H1N1 antigen, and these results correlate with the previously published HAI titers for 2011 H1N1 antigen [14]. The significance of this particular antigen correlates with a recent case-control study demonstrating a postive association between spontaneous abortions and maternal influenza vaccination with vaccine containing H1N1pdm09 in the preceding 28 days and in the previous influenza season [36]. H1N1pdm09-infected pregnant ferrets had higher levels of inflammatory cytokines in their pulmonary tracts compared to non-pregnant ferrets [27]. Total CD8+ T cell counts and H1N1pdm09-specific B-cell responses in blood were significantly lower in pregnant compared to non-pregnant ferrets. Further study is warranted to determine if previous or recent exposure to the H1N1pdm09 antigen influenced these dramatic results.
Strengths of our study include direct comparison with a non-pregnant control group and linear regression models that eliminate the subjective nature of trimesters. Since commonly used designations that sub-divide pregnancy into trimesters (defined as weeks 1 to 12, 13 to 27, and 28 to birth) have nearly exclusively been used to stratify the increasing susceptibility to infection during pregnancy, the key immunological changes that occur as pregnancy advances have likely been obscured as well. For example, pregnancy induced immunological changes are likely to be more similar between weeks 12 and 13 than between weeks 13 and 27, even though the latter are within the same trimester. Our linear regression model allows analysis of pregnancy duration as a continuous variable.
Possible limitations of this study include the small sample size during each influenza season and the lack of vaccine efficacy data, limiting our ability to detect small differences in IgG antibody titers and clinical effectiveness. Some of the decreased antibody titers could be associated with the dilutional effect of increasing physiologic volume expansion during pregnancy, and future studies could alleviate this limitation by sampling another serologic component at different gestational time periods. Additionally, two different routes of influenza vaccine administration (intramuscular and intradermal) were used in our hospital’s annual influenza vaccine campaigns in 2011 and 2012, respectively. Though most studies have demonstrated similar antibody responses in intradermal compared with intramuscular vaccinees [37–39], some studies suggest higher or lower responses in older adults [40,41]; the effect of these differing routes of administration on immune responses during pregnancy are unknown.
In conclusion, our study provides evidence that pregnancy quantitatively and qualitatively diminishes systemic antibody responses to viral antigens administered via vaccination, and these responses change as pregnancy progresses from conception to delivery. These results suggest a need to reconsider approaches to the timing of vaccination for pregnant women to maximize protection for mothers and their infants, as earlier immunization of pregnant women may lead to better antibody responses to vaccine antigen and improved protection against influenza.
Acknowledgements
We wish to thank all of the women who participated in this study.
7. Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number 5 K12 HD028827], the National Center for Advancing Translational Sciences [grant number 8 UL1 TR000077], and the Common Fund [grant number DP1AI120202] at the National Institutes of Health (NIH); and the March of Dimes Foundation [grant number FY-15–254]. SSW is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease, and a Howard Hughes Medical Institute Faculty Scholar. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
6. Potential conflicts of interests
The authors do not have a commercial or other association that might pose a conflict of interest.
References
- [1].Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009;58:1–52. [PubMed] [Google Scholar]
- [2].Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014;371:918–31. [DOI] [PubMed] [Google Scholar]
- [3].Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008;359:1555–64. [DOI] [PubMed] [Google Scholar]
- [4].Dodds L, Macdonald N, Scott J, Spencer A, Allen VM, McNeil S. The association between influenza vaccine in pregnancy and adverse neonatal outcomes. J Obstet Gynaecol Can 2012;34:714–20. [DOI] [PubMed] [Google Scholar]
- [5].Legge A, Dodds L, MacDonald NE, Scott J, McNeil S. Rates and determinants of seasonal influenza vaccination in pregnancy and association with neonatal outcomes. CMAJ 2014;186:E157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adedinsewo DA, Noory L, Bednarczyk RA, Steinhoff MC, Davis R, Ogbuanu C, et al. Impact of maternal characteristics on the effect of maternal influenza vaccination on fetal outcomes. Vaccine 2013;31:5827–33. [DOI] [PubMed] [Google Scholar]
- [7].Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, Stoll BJ, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med 2011;8:e1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bland PB. Influenza in its relation to pregnancy and labor. Am J Obstet 1919;79:184–97. [Google Scholar]
- [9].Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol 1959;78:1172–5. [DOI] [PubMed] [Google Scholar]
- [10].Ashley J, Smith T, Dunell K. Deaths in Great Britain associated with the influenza epidemic of 1989/90. Populations Trends 1991;65:16–20. [Google Scholar]
- [11].Louie JK, Acosta M, Jamieson DJ, Honein MA, Working California Pandemic, Severe G. H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2009;2010(362):27–35. [DOI] [PubMed] [Google Scholar]
- [12].Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998;148:1094–102. [DOI] [PubMed] [Google Scholar]
- [13].Dodds L, McNeil SA, Fell DB, Allen VM, Coombs A, Scott J, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ 2007;176:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schlaudecker EP, McNeal MM, Dodd CN, Ranz JB, Steinhoff MC. Pregnancy modifies the antibody response to trivalent influenza immunization. J Infect Dis 2012;206:1670–3. [DOI] [PubMed] [Google Scholar]
- [15].Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Altaye M, et al. Influenza immunization in pregnancy–antibody responses in mothers and infants. N Engl J Med 2010;362:1644–6. [DOI] [PubMed] [Google Scholar]
- [16].Huang KA, Chang SC, Huang YC, Chiu CH, Lin TY. Antibody responses to trivalent inactivated influenza vaccine in health care personnel previously vaccinated and vaccinated for the first time. Sci Rep 2017;7:40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seidman JC, Richard SA, Viboud C, Miller MA. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respir Viruses 2012;6:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979;35:69–75. [DOI] [PubMed] [Google Scholar]
- [19].Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 2010;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Subbramanian RA, Basha S, Shata MT, Brady RC, Bernstein DI. Pandemic and seasonal H1N1 influenza hemagglutinin-specific T cell responses elicited by seasonal influenza vaccination. Vaccine 2010;28:8258–67. [DOI] [PubMed] [Google Scholar]
- [21].Quinnan GV, Ennis FA, Tuazon CU, Wells MA, Butchko GM, Armstrong R, et al. Cytotoxic lymphocytes and antibody-dependent complement-mediated cytotoxicity induced by administration of influenza vaccine. Infect Immun 1980;30:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol 2001;166:7381–8. [DOI] [PubMed] [Google Scholar]
- [23].Sylte MJ, Suarez DL. Influenza neuraminidase as a vaccine antigen. Curr Top Microbiol Immunol 2009;333:227–41. [DOI] [PubMed] [Google Scholar]
- [24].Ferrante A, Beard LJ, Feldman RG. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr Infect Dis J 1990;9:S16–24. [PubMed] [Google Scholar]
- [25].Christian LM, Iams JD, Porter K, Glaser R. Inflammatory responses to trivalent influenza virus vaccine among pregnant women. Vaccine 2011;29:8982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chan KH, Zhang AJ, To KK, Chan CC, Poon VK, Guo K, et al. Wild type and mutant 2009 pandemic influenza A (H1N1) viruses cause more severe disease and higher mortality in pregnant BALB/c mice. PLoS One 2010;5:e13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yoon SW, Wong SS, Zhu H, Chen R, Li L, Zhang Y, et al. Dysregulated T-Helper Type 1 (Th1):Th2 cytokine profile and poor immune response in pregnant ferrets infected With 2009 Pandemic Influenza A(H1N1) virus. J Infect Dis 2018;217:438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Spinsanti LI, Farias AA, Aguilar JJ, del Pilar Diaz M, Contigiani MS. Immunoglobulin G subclasses in antibody responses to St. Louis encephalitis virus infections. Arch Virol 2011;156:1861–4. [DOI] [PubMed] [Google Scholar]
- [29].Hammarstrom L, Smith CI. IgG subclasses in bacterial infections. Monogr Allergy 1986;19:122–33. [PubMed] [Google Scholar]
- [30].Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014;5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sacks GP, Clover LM, Bainbridge DR, Redman CW, Sargent IL. Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta 2001;22:550–9. [DOI] [PubMed] [Google Scholar]
- [32].Liu F, Guo J, Tian T, Wang H, Dong F, Huang H, et al. Placental trophoblasts shifted Th1/Th2 balance toward Th2 and inhibited Th17 immunity at fetomaternal interface. APMIS 2011;119:597–604. [DOI] [PubMed] [Google Scholar]
- [33].Piccinni MP. T cell tolerance towards the fetal allograft. J Reprod Immunol 2010;85:71–5. [DOI] [PubMed] [Google Scholar]
- [34].Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 2010;63:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shao L, Jacobs AR, Johnson VV, Mayer L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J Immunol 2005;174:7539–47. [DOI] [PubMed] [Google Scholar]
- [36].Donahue JG, Kieke BA, King JP, DeStefano F, Mascola MA, Irving SA, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010–11 and 2011–12. Vaccine 2017;35:5314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Belshe RB, Newman FK, Wilkins K, Graham IL, Babusis E, Ewell M, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine 2007;25:6755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Van Damme P, Arnou R, Kafeja F, Fiquet A, Richard P, Thomas S, et al. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect Dis 2010;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marra F, Young F, Richardson K, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses 2013;7:584–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med 2004;351:2286–94. [DOI] [PubMed] [Google Scholar]
- [41].Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis 2008;198:650–8. [DOI] [PubMed] [Google Scholar]